INTRODUCTION: GENE THERAPY IN 2017
Gene therapy has changed dramatically in the 28 years since the first human gene transfer experiment in 1989. Alipogene tiparvovec, GlyberaR®, a recombinant adeno‐associated virus (rAAV) product for lipoprotein lipase deficiency, and Strimvelis®, a lentivirus vector for severe combined immune deficiency are approved in Europe. An rAAV2 product for a congenital form of blindness is currently under review in the United States, likely to be followed by numerous other gene therapies.
Nonviral gene transfer
The success of gene therapy has largely been driven by improvements in nonviral and viral gene transfer vectors. An array of physical and chemical nonviral methods have been used to transfer DNA and mRNA to mammalian cells and a substantial number of these have been developed as clinical stage technologies for gene therapy, both ex vivo and in vivo.
Cationic liposome technology is based on the ability of amphipathic lipids, possessing a positively charged head group and a hydrophobic lipid tail, to bind to negatively charged DNA or RNA and form particles that generally enter cells by endocytosis. Some cationic liposomes also contain a neutral co‐lipid, thought to enhance liposome uptake by mammalian cells.4, 5, 6, 7 Similarly, other polycations, such as poly‐l‐lysine and polyethylene‐imine, complex with nucleic acids via charge interaction and aid in the condensation of DNA or RNA into nanoparticles, which are then substrates for endosome‐mediated uptake.8 Several of these cationic‐nucleic acid complex technologies have been developed as potential clinical products, including complexes with plasmid DNA (pDNA), oligodeoxynucleotides, and various forms of synthetic RNA.9, 10, 11
Modified (and unmodified or “naked”) DNA, RNA, and oligonucleotides have also been shown to mediate successful gene transfer in a number of circumstances. These include the use of pDNA by direct intramuscular injection for DNA vaccines, the use of intratumoral injection of pDNA to deliver cytokine and/or suicide genes, systemic (s.c. or i.v.) injection of antisense nucleotides to induce RNAse H1 or exon‐skipping.12, 13, 14 The most recent of these developed for induction of RNAi are discussed in a later section.
Ex vivo introduction of pDNA and/or other nucleotides using physical methods has been well developed for certain cell types, including T lymphocytes.15 Electroporation techniques have become the standard with T cells for the introduction of a variety of molecular cargoes, including ribonucleoproteins composed of Cas9 and short‐guide RNAs for genome editing (see section below) and transposons for long‐term integration of transgenes.
Gammaretrovirus and lentivirus vectors
Many within the gene therapy field consider viruses as the ultimate vectors for the delivery of therapeutic tools, and the number of gene therapy clinical trials reflects this bias.16, 17, 18, 19, 20 Retroviruses were the first class of viruses to be harnessed for mammalian and human gene transfer, and they are at the leading edge of products that show clinical efficacy1(Figure 1).
Figure 1.
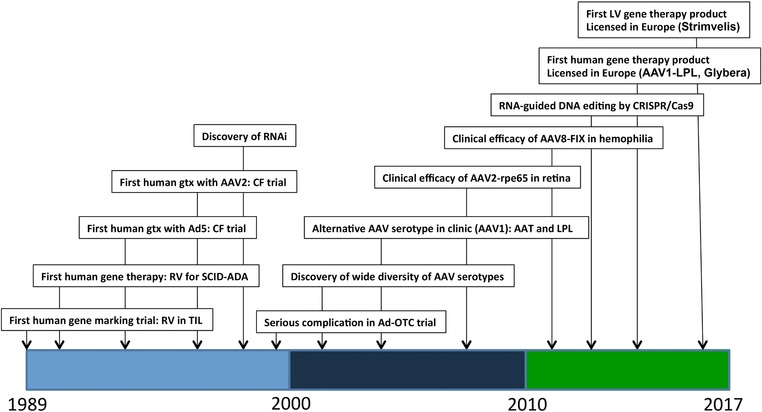
Timeline of major events in clinical gene therapy. A few selected key dates in the history of human gene therapy are depicted, with the dates indicated on the x‐axis. AAV2, adeno‐associated virus type 2; Ad5, adenovirus type 5; CF, cystic fibrosis; CRISPR, clustered regularly interspaced short palindromic repeats; FIX, clotting factor IX; LPL, lipoprotein lipase; OTC, ornithine transcambamylase; RNAi, RNA inhibition; RPE65, 65 kilo‐Dalton retinal pigment epithelial protein; RV, gammaretrovirus; SCID‐ADA, severe combined immune deficiency due to adenosine deaminase deficiency; TIL, tumor‐infiltrating lymphocytes.
For example, direct clinical benefit with chimeric antigen receptor T (CAR‐T) cells is a promising novel therapy for many malignancies. CAR‐T cells are produced by ex vivo transduction of T cells with lentiviral vectors.21, 22, 23 Exciting results with B‐cell lymphomas and leukemia eradication was seen when CAR‐T cells are directed against the B‐cell surface antigen, CD19.24, 25 However, because CD19 is a pan‐B cell marker, one side effect is normal B‐cell depletion. Thus, to try and restrict normal B‐cell depletion after CAR‐T cell administration, a recent study refined CD19 CAR‐T cells to recognize κ‐restricted cells, thereby excluding normal B‐cells from targeted destruction.26 In addition, other tumor‐associated antigens have been targeted with some clinical success.21, 27, 28 Although most of these trials have utilized autologous T cells, one recent report showed efficacy in “off‐the‐shelf” (TCR−/CD52−) allogeneic anti‐CD19 CAR‐T cells. These T‐cells not only are transduced with the lentivirus expressing a chimeric antigen receptor, but they also have their endogenous T‐cell receptor knockout via transcription activator‐like effector nuclease TALEN‐mediated genome editing.29, 30
Another prominent example of clinically effective gene therapy with gammaretrovirus and lentivirus vectors is ex vivo transduction of hematopoietic stem cells to treat conditions such as severe combined immunodeficiency (SCID). These include both X‐linked SCID gammaretrovirus31 and lentivirus32 therapies, as well as SCID due to adenosine deaminase‐SCID deficiency. In fact, the lentiviral StimvelisR recently received European Market Authorization to treat patients with adenosine deaminase‐SCID deficiency.33 In addition, similar clinical effectiveness was seen in X‐linked adrenoleukodystrophy between patients treated with ex vivo lentiviral correction and those treated with allogeneic hematopoietic cell transplantation (Table 1).19 Other promising retroviral hemopoietic stem‐cell gene therapies include lentiviral therapies for metachromatic leukodystrophy34 and both gammaretroviral and lentiviral therapies for Wiskott‐Aldrich syndrome. In Wiskott‐Aldrich syndrome, lentiviral therapies showed a safer profile than gammaretrovirus vectors, relative to the risk of insertional mutagenesis.35, 36
Table 1.
Gene therapy clinical trials for monogenic diseases in phases III/IV
| Disease | Vector | Outcomes | Location of the Trial | References |
|---|---|---|---|---|
| LPLD | AAV‐Glybera | Improved lipid profile and decreased pancreatitis | The United States | 113, 114, 115 |
| X‐linked adrenoleukodystrophy | Lentivirus | Improved neurologic development | France | 19 |
| Thalassemia major | Lentivirus | Decreased transfusion need | The United States | 16 |
| X‐linked/ choroideremia rentinal disease (REP1) | AAV2 | Improved vision | The United States‐ multiple countries | 17, 116 |
| LCA | AAV2 | Improved low‐light vision | The United States | 20, 117, 118 |
| Leber hereditary optic neuropathy | AAV2 | Improved vision | France ‐ multiple countries including US | 18, 119 |
AAV, adeno‐associated virus; LCA, Leber congenital amaurosis type 2; LPLD, lipoprotein lipase deficiency.
Adenoviruses and oncolytic viruses
Adenoviruses (Ads) were also used early on in gene therapy clinical trials, and are one of the most studied and published viral vectors (Figure 1). Ads have robust transduction profiles, particularly in the liver, but they were also accompanied by robust immune responses. Different levels of attenuation of the virus can be achieved by removing different components, including complete removal of all genetic information – the so‐called “gutless” vectors.37 Unfortunately, early clinical trials for gene correction using Ads did not have many clinical successes, and one trial resulted in a tragic fatality.38 Additional hurdles seen with systemic delivery include nonspecific binding to blood components leading to viral inactivation. In addition, a majority of adults have antibodies against common Ad5 serotypes.39, 40 Further modifications of Ad vectors, such as making chimeric vectors, and chemical modifications have helped overcome some of the early challenges with liver targeting and host immunity.37 However, Ads have recently been used in cancer treatment as oncolytic viruses. A number of clinical trials using Ad to target a number of different cancers, such as prostate, ovarian, bladder, and refractory solid tumors, have been promising.41, 42, 43, 44, 45 In this type of therapy, robust immune responses are beneficial for therapeutic outcomes. Many other viruses have been used as oncolytic viruses, such as: vaccine virus; herpes virus; Coxsackievirus, reovirus, parvovirus, vesicular stomatitis virus, Newcastle disease, measles virus, polio virus, and Seneca Valley virus.46 The first clinically approved oncolytic virus is Talimogene Laherparepvec (Imlygic®; Amgen, South San Francisco, CA), which is a genetically modified herpes virus expressing human granulocyte‐macrophage colony‐stimulating factor. Talimogene Laherparepvec has been approved for the treatment of advanced melanoma.47, 48, 49, 50 Thus, Ad, one of the earliest vectors in the gene therapy field, may find a new role in cancer gene therapy along with other oncolytic viruses.
Recombinant adeno‐associated virus vectors
Recombinant adeno‐associated virus (rAAV) vector‐mediated gene therapy has also proven to be efficacious in certain conditions.2 Hundreds of clinical trials have been performed using rAAV viral vectors for recessive monogenic disorders, with the first human rAAV injection performed almost 25 years ago.51 Examples of clinical efficacy with rAAV include data from trials with hemophilia B,52, 53 spinal muscular atrophy (unpublished), alpha 1 antitrypsin,54, 55 and Leber congenital amaurosis.56 In hemophilia B, a single systemic administration of rAAV carrying the human factor IX gene resulted in a multiyear sustained expression of factor IX levels at 1–6% of normal.52, 53 Similarly, in alpha 1 antitrypsin, intramuscular injections of AAV1 carrying the AAT gene resulted in sustained AAT expression for 5 years (Gruntman et al., unpublished). Ongoing clinical trial in spinal muscular atrophy resulted in improved survival in patients with spinal muscular atrophy type 1. Patients with Leber congenital amaurosis had partial restoration of their vision after receiving therapy with rAAV2 vector carrying the human retinal pigment epithelium 65kDa gene.55
Other rAAV vectors are on their way through preclinical and clinical proof‐of‐concept studies Table 1. Recently, a number of proof‐of‐concept studies have been completed using rAAV technology for correction of single gene disorders targeting a wide variety of tissues. The clinical success of the Leber congenital amaurosis trial, have led to a number of studies targeting genetic diseases of the retina.3 Diseases of the central nervous system have also had some important proof‐of‐concept studies, along with metabolic and skeletal diseases.57, 58, 59, 60, 61, 62 Additionally, rAAVs have been used to treat diseases other than monogenic disorders, such as interferon‐beta delivery to treat the aggressive brain cancer glioblastoma multiforme,63 and to provide treatment for infectious diseases, such as human immunodeficiency virus64 and influenza.65
Despite these many successes with rAAV and other viral gene therapies, the future of the field of gene therapy may lie in new technologies. Examples of these technologies stem from the discovery of RNA interference in C. elegans by Fire et al.,66 discovery of the host defense system CRISPR/Cas9 in S. pyogenes,67 and finally from the discovery of new vectors through co‐evolution and directed evolution.
Novel adeno‐associated virus capsids
The rAAV is a simple and ubiquitous wildtype virus that occurs naturally in humans (Figure 2). Even wildtype adeno‐associated virus (AAV) is naturally a vector given its replication dependence on helper viruses. However, antibodies against AAV can decrease the efficacy of rAAV‐mediated gene therapy. A lot of work has recently gone into updating the existing repertoire of natural variants of AAV viruses by both rational design and directed evolution. For example, pioneering work by Gao et al.,68, 69 greatly added hundreds of natural variants to the gene therapists tool kits and new AAV variants are still being discovered. In addition, many novel vectors have been rationally engineered. These new vectors allow avoidance of the immune system and enable tissue‐specific tropism to target exact organs involved in the disease of interest. Rationally engineered viruses, such as AAV2g9, have been developed to exploit the benefits from parental variants, such as the galactose receptor footprint from AAV9, while creating unique transduction profiles, such as central nervous system‐restricted transduction.70, 71 Furthermore, directed evolution was used to create unique transduction profiles. Using a cre‐recombination‐based adeno‐associated virus‐targeted evolution (CREATE) strategy, novel AAV variants can now achieve widespread expression through the central nervous system in mice.72 However, improved transduction efficiency is not only limited to the CNS but has also been more efficient in muscles,73 as well as in human and murine livers.74
Figure 2.
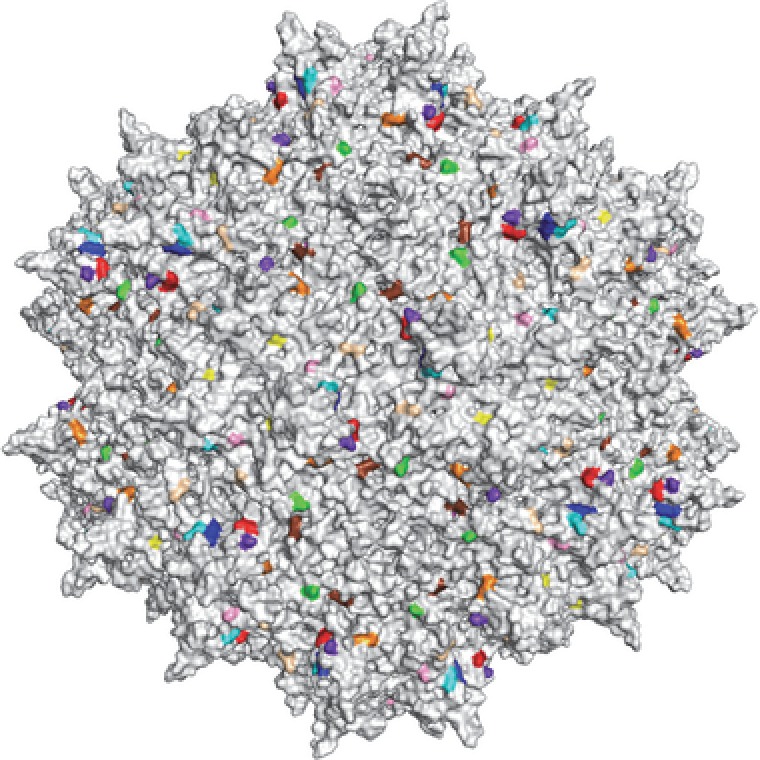
Structure of adeno‐associated virus 2 (AAV2).113 The icosahedral structure of the virus capsid is shown. Note that this structure is symmetrical across a twofold, threefold, and fivefold axis of symmetry. The ability of the AAV vectors to transduce various cell types largely depends on variation in amino acid moieties highlighted in color.
RNAi
The discovery of RNAi allowed for a shift from gene therapy focused on gene augmentation to a focus on downregulation of gene expression for diseases in which pathology is caused by toxic gain of function. Some of the first clinical therapies used small‐interfering RNA in diseases of the liver; small‐interfering RNA sequences were developed to target hepatocytes75 in which knockdown of gene expression have a therapeutic effect on disease pathogenesis. Examples of this include therapy for transthyretin‐mediated amyloidosis76 and complement‐mediated diseases.77
However, in order to achieve long‐term knockdown, a more stable approach is to use another RNAi pathway, specifically delivery of synthetic microRNA (miRNA), which can be continuously expressed by viral vectors. Interestingly, the discovery of exogenous RNA‐mediated downregulation by Fire et al.,66 was preceded by the discovery of miRNA in C. elegans by Lee et al.78 and Wightman et al.79 Both discoveries utilize the same enzymatic pathway that allows for exploitation of natural miRNA in mammalian systems for therapeutic purposes. The rAAV delivery platform can be used to continually express synthetic miRNA “genes” to downregulate a gene by sequence‐specific targeting. This technology has been used to treat dominant disorders, such as Huntington's disease, in which a toxic gain of function causes neurological disease by increased number of CAG repeats in the huntingtin's gene.80, 81, 82 It has also been used to treat SOD1‐mediated amyotrophic lateral sclerosis in a murine model of amyotrophic lateral sclerosis, in which motor neuron disease is caused by toxic gain of function of the SOD1 protein.58, 83 Furthermore, this system can be used to treat diseases in which both a loss‐of‐function and a toxic gain of function is associated with a certain mutation. For example, in alpha‐1 antitrypsin (AAT) deficiency, a mutant AAT protein (the PiZ protein) causes both liver disease by toxic accumulation of the protein and emphysematous lung disease by an absence of AAT. For AAT, rAAV was used to deliver a miRNA designed to knock‐down expression of PiZ causing liver disease while simultaneously augmenting expression of the normal AAT transgene to prevent lung disease.84
CRISPR/Cas9 genome editing
Novel exciting gene editing tools offer a more elegant and precise method of treating genetic diseases. There have been efforts on this front through ex vivo homologous recombination, TALENS and Zinc Finger Nucleases. However, none have the promise of the recently discovered clustered regularly interspersed short palindromic repeats (CRISPR)/crispr‐associated protein 9 (Cas9).67 CRISPR/Cas9 has transformed biomedical research. This technology was initially discovered in bacteria and archaea as a means for these organisms to defend themselves against invading viruses.85 Using CRISPR/Cas9, many are targeting specific regions of the human genome in an attempt to achieve a therapeutic effect.86, 87, 88 Targeting sequence specificity, similar to that of the RNAi approach, allows for the success and efficiency of CRISPR/Cas9.89, 90, 91 In order for the system to work, synthetic short‐guide RNAs are delivered in combination with a Cas‐like enzyme, which allows for double‐stranded breaks in the host DNA in a specific manner. Expression of genes can be disrupted by host DNA repair mechanisms in which a small insertion and/or deletion (indel) of two to six nucleotides generally results in a frameshift mutation and termination.86, 87 However, genes can also be repaired by providing a ds‐DNA template with the short‐guide RNA and Cas‐like enzyme, allowing homology‐dependent recombination to occur.
Although CRISPR/Cas9 therapies are still in early development, some therapeutic approaches have already been demonstrated for genetic diseases and for Duchenne muscular dystrophy92 and liver disease fumarylacetoacetate hydrolase deficiency.93 One of the major challenges is in the delivery of the components necessary to complete the editing process. Viral vectors like rAAV have been suggested, but long‐term expression, one advantage of rAAV, in augmentation or downregulation therapy becomes a disadvantage in the CRISPR/Cas9 genome editing in which short‐term expression is all that is necessary to make the genetic changes. Conversely, vectors like adenovirus, which have robust expression albeit only in the short term, would be ideal if immune responses were not robust and led to clearance of virally targeted cells. However, the remaining challenges should not dissuade scientists from perusing these types of therapies as the potential for several clinical applications are impressive.
CONCLUSION/FUTURE DIRECTIONS
The knowledge gained within the field over the past several decades provides much hope for the future of gene therapy. The exciting possibility of treating many genetic and infectious disorders is now close to a reality with the success of rAAV in bench‐work and clinical trials, novel vector engineering, and the recent discoveries of miRNAs, and CRISPR/Cas9. We are on the brink of having therapies approved for clinical usage in the United States, and the European Medicines Agency already has two approved therapies. AAV encoded miRNAs will soon be tested in clinical trials, whereas technologies, such as CRISPR/Cas9, are still in proof‐of‐concept stages but hold massive clinical promise.
Moving forward, the ability to exploit new molecular tools, such as RNAi and CRISPR/Cas9, should be able to make use of some of the clinical development paradigms from earlier gene therapy trials. Examples of this might include basic preclinical and clinical study designs to examine biodistribution of vector components and risk for carcinogenesis. One important distinction exists, however, between conventional viral gene therapy vectors and newer RNA‐guided mechanisms. The toxicity observed with viral vectors has generally been consistent with toxicity of the viruses on which each vector is based.94 This is true of both Ad vector‐mediated inflammation and gammaretrovirus vector‐mediated leukemia.95, 96, 97 In the case of CRISPR/Cas9, a system is being used that does not occur in mammalian cells at all, as far as is currently known.67, 98 Likewise, although miRNA‐mediated gene regulation is seen in mammalian cells, the understanding of miR‐based diseases is at a fairly early stage. Thus, the modelling possible toxicities from such therapies are based more on theoretical concerns than on past experiences.
Nonetheless, it seems likely that certain genetic diseases will be approachable with CRISPR/Cas9 and RNAi‐based therapy that were not approachable with prior methods.86, 87, 88, 92 Specifically, the ability to treat autosomal dominant disorders is a feature of both of these methods. In addition, CRISPR/Cas9 presents the ability to repair a gene in situ, allowing for preservation of all of the elements required for normal physiologic regulation of the gene of interest by its own promoter and enhancers.99, 100
This has led some to speculate that CRISPR/Cas9 could actually enable a permanent and definitive germ‐line correction of a genetic disorder.101, 102 One such study, performed in nonviable human embryos, demonstrated the feasibility of doing so in addressing hemoglobinopathies.103 Clearly, such an approach is not currently deemed to fall within ethical guidelines,102 although some have pointed out that therapeutic transfer of whole mitochondria has been allowed even though mitochondrial DNA will likely be passed down in the germ line. It is conceivable, however, that a purely therapeutic approach, intended to cure a disease rather than to enhance, could be allowable in the future if appropriate questions about safety and efficacy can be addressed. This was anticipated by the joint statement from the National Academy of Sciences and the National Academy of Medicine.104 If that were to transpire in the future, it could represent the most definitive treatment for families with a genetic defect that has ever been attempted.
More near‐term, the ability to control gene expression in the context of gene transfer may be a more realistic goal. A number of promoter systems inducible by small molecule drugs have shown excellent dynamic range for gene regulation in cell culture and in animal models. Among these are the tetracycline inducible and repressible systems, and those based on modified estrogen and progesterone receptors.105, 106, 107, 108 None of these has achieved clinical translation to date, primarily due to the immune responses to transcriptional activator domains that are not human in origin.109, 110, 111 The field of synthetic biology has produced two‐component RNA‐based systems, so‐called “toehold switches” that may have superior characteristics in certain circumstances.112 The added complexity of these multicomponent systems could be particularly useful for transgene products with a relatively narrow therapeutic window of expression. This sort of adaptation of different gene therapy platforms to different disease targets will likely represent the primary challenge and opportunity facing gene therapy researchers in the coming years.
Conflict of Interest
This work was funded by National Institutes of Health (NIH) grants 1K08HD077040‐01A1 NIH/NICHD to M.K.E., 1R21NS098131‐01 NIH/NINDS to M.K.E., and P01HL 131471‐01 NIH/NHLBI to T.R.F. and M.K.E. T.R.F. is a member of the scientific advisory board and paid consultant for Dimension Therapeutics, a paid consultant for Editas Medicine, a scientific founder of AGTC, and has previously been a paid consultant for Alnylam Therapeutics and Biogen Corporation. M.K.E. and A.M.K. have no conflict of interest to declare.
References
- 1. Rosenberg, S.A. , Aebersold, P. , Cornetta, K. , Kasid, A. , Morgan, R.A. , Moen, R. et al Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor‐infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 323, 570–578 (1990). [DOI] [PubMed] [Google Scholar]
- 2. Watanabe, N. , Yano, K. , Tsuyuki, K. , Okano, T. & Yamato, M . Re‐examination of regulatory opinions in Europe: possible contribution for the approval of the first gene therapy product Glybera. Mol. Ther. Methods Clin. Dev. 2, 14066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pierce, E.A. & Bennett, J . The status of RPE65 gene therapy trials: safety and efficacy. Cold Spring Harb. Perspect. Med. 5, a017285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Felgner, P.L. et al Lipofection: a highly efficient, lipid‐mediated DNA‐transfection procedure. Proc. Natl. Acad. Sci. USA 84, 7413–7417 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. San, H. , Yang, Z.Y. , Pompili, V.J. , Jaffe, M.L. , Plautz, G.E. , Xu, L. et al Safety and short‐term toxicity of a novel cationic lipid formulation for human gene therapy. Hum. Gene Ther. 4, 781–788 (1993). [DOI] [PubMed] [Google Scholar]
- 6. Xu, Y. & Szoka, F.C. Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 35, 5616–5623 (1996). [DOI] [PubMed] [Google Scholar]
- 7. Legendre, J.Y. & Szoka, F.C. Jr. Delivery of plasmid DNA into mammalian cell lines using pH‐sensitive liposomes: comparison with cationic liposomes. Pharm. Res. 9, 1235–1242 (1992). [DOI] [PubMed] [Google Scholar]
- 8. Boussif, O. , Lezoualc'h, F. , Zanta, M.A. , Mergny, M.D. , Scherman, D. , Demeneix, B. et al A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92, 7297–7301 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodrigo, G. , Landrain, T.E. & Jaramillo, A . De novo automated design of small RNA circuits for engineering synthetic riboregulation in living cells. Proc. Natl. Acad. Sci. USA 109, 15271–15276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oishi, M. , Nagatsugi, F. , Sasaki, S. , Nagasaki, Y. & Kataoka, K . Smart polyion complex micelles for targeted intracellular delivery of PEGylated antisense oligonucleotides containing acid‐labile linkages. Chembiochem. 6, 718–725 (2005). [DOI] [PubMed] [Google Scholar]
- 11. Bhatt, P. , Khatri, N. , Kumar, M. , Baradia, D. & Misra, A . Microbeads mediated oral plasmid DNA delivery using polymethacrylate vectors: an effectual groundwork for colorectal cancer. Drug Deliv. 22, 849–861 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Crooke, S.T. Molecular mechanisms of antisense drugs: RNase H. Antisense Nucleic Acid Drug Dev. 8, 133–134 (1998). [DOI] [PubMed] [Google Scholar]
- 13. Ulmer, J.B. , Deck, R.R. , DeWitt, C.M. , Friedman, A. , Donnelly, J.J. & Liu, M.A . Protective immunity by intramuscular injection of low doses of influenza virus DNA vaccines. Vaccine 12, 1541–1544 (1994). [DOI] [PubMed] [Google Scholar]
- 14. Heinzerling, L. , Burg, G. , Dummer, R. , Maier, T. , Oberholzer, P.A. , Schultz, J. et al Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum. Gene Ther. 16, 35–48 (2005). [DOI] [PubMed] [Google Scholar]
- 15. June, C.H. , Blazar, B.R. & Riley, J.L . Engineering lymphocyte subsets: tools, trials and tribulations. Nat. Rev. Immunol. 9, 704–716 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Negre, O. , Eggimann, A.V. , Beuzard, Y. , Ribeil, J.A. , Bourget, P. , Borwornpinyo, S. et al Gene therapy of the β‐hemoglobinopathies by lentiviral transfer of the β(A(T87Q))‐globin gene. Hum. Gene Ther. 27, 148–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards, T.L. , Jolly, J.K. , Groppe, M. , Barnard, A.R. , Cottriall, C.L. , Tolmachova, T. et al Visual acuity after retinal gene therapy for choroideremia. N. Engl. J. Med. 374, 1996–1998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feuer, W.J. , Schiffman, J.C. , Davis, J.L. , Porciatti, V. , Gonzalez, P. , Koilkonda, R.D. et al Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology 123, 558–570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cartier, N. , Hacein‐Bey‐Abina, S. , Bartholomae, C.C. , Veres, G. , Schmidt, M. , Kutschera, I. et al Hematopoietic stem cell gene therapy with a lentiviral vector in X‐linked adrenoleukodystrophy. Science 326, 818–823 (2009). [DOI] [PubMed] [Google Scholar]
- 20. Testa, F. , Maguire, A.M. , Rossi, S. , Pierce, E.A. , Melillo, P. , Marshall, K. et al Three‐year follow‐up after unilateral subretinal delivery of adeno‐associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 120, 1283–1291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown, C.E. , Alizadeh, D. , Starr, R. , Weng, L. , Wagner, J.R. , Naranjo, A. et al Regression of glioblastoma after chimeric antigen receptor T‐cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turtle, C.J. , Hanafi, L.A. , Berger, C. , Hudecek, M. , Pender, B. , Robinson, E. et al Immunotherapy of non‐Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19‐specific chimeric antigen receptor‐modified T cells. Sci. Transl. Med. 8, 355ra116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali, S.A. , Shi, V. , Maric, I. , Wang, M. , Stroncek, D.F. , Rose, J.J. et al T cells expressing an anti‐B‐cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128, 1688–1700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davila, M.L. , Riviere, I. , Wang, X. , Bartido, S. , Park, J. , Curran, K. , Chung, S.S. et al Efficacy and toxicity management of 19‐28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porter, D.L. , Hwang, W.T. , Frey, N.V. , Lacey, S.F. , Shaw, P.A. , Loren, A.W. et al Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramos, C.A. , Savoldo, B. , Torrano, V. , Ballard, B. , Zhang, H. , Dakhova, O. et al Clinical responses with T lymphocytes targeting malignancy‐associated κ light chains. J. Clin. Invest. 126, 2588–2596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katz, S.C. , Burga, R.A. , McCormack, E. , Wang, L.J. , Mooring, W. , Point, G.R. et al Phase I hepatic immunotherapy for metastases study of intra‐arterial chimeric antigen receptor‐modified T‐cell therapy for CEA+ liver metastases. Clin. Cancer Res. 21, 3149–3159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beatty, G.L. , Haas, A.R. , Maus, M.V. , Torigian, D.A. , Soulen, M.C. , Plesa, G. et al Mesothelin‐specific chimeric antigen receptor mRNA‐engineered T cells induce anti‐tumor activity in solid malignancies. Cancer Immunol. Res. 2, 112–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ratner, M. Off‐the‐shelf CAR‐T therapy induces remission in child with ALL. Nat. Biotechnol. 34, 12 (2016). [DOI] [PubMed] [Google Scholar]
- 30. Poirot, L. , Philip, B. , Schiffer‐Mannioui, C. , Le Clerre, D. , Chion‐Sotinel, I. , Derniame, S. et al Multiplex genome‐edited T‐cell manufacturing platform for "off‐the‐shelf" adoptive T‐cell immunotherapies. Cancer Res. 75, 3853–3864 (2015). [DOI] [PubMed] [Google Scholar]
- 31. Hacein‐Bey‐Abina, S. , Pai, S.Y. , Gaspar, H.B. , Armant, M. , Berry, C.C. , Blanche, S. et al A modified γ‐retrovirus vector for X‐linked severe combined immunodeficiency. N. Engl. J. Med. 371, 1407–1417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Ravin, S.S. , Wu, X. , Moir, S. , Anaya‐O'Brien, S. , Kwatemaa, N. , Littel, P. et al Lentiviral hematopoietic stem cell gene therapy for X‐linked severe combined immunodeficiency. Sci. Transl. Med. 8, 335ra57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cicalese, M.P. , Ferrua, F. , Castagnaro, L. , Pajno, R. , Barzaghi, F. , Giannelli, S. et al Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 128, 45–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sessa, M. et al Lentiviral haemopoietic stem‐cell gene therapy in early‐onset metachromatic leukodystrophy: an ad‐hoc analysis of a non‐randomised, open‐label, phase 1/2 trial. Lancet 388, 476–487 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Hacein‐Bey Abina, S. , Lorioli, L. , Fumagalli, F. , Acquati, S. , Redaelli, D. , Baldoli, C. et al Outcomes following gene therapy in patients with severe Wiskott‐Aldrich syndrome. JAMA 313, 1550–1563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Braun, C.J. , Boztug, K. , Paruzynski, A. , Witzel, M. , Schwarzer, A. , Rothe, M. et al Gene therapy for Wiskott‐Aldrich syndrome–long‐term efficacy and genotoxicity. Sci. Transl. Med. 6, 227ra33 (2014). [DOI] [PubMed] [Google Scholar]
- 37. Capasso, C. , Garofalo, M. , Hirvinen, M. & Cerullo, V . The evolution of adenoviral vectors through genetic and chemical surface modifications. Viruses 6, 832–855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raper, S.E. , Chirmule, N. , Lee, F.S. , Wivel, N.A. , Bagg, A. , Gao, G.P. et al Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158 (2003). [DOI] [PubMed] [Google Scholar]
- 39. Hendrickx, R. , Stichling, N. , Koelen, J. , Kuryk, L. , Lipiec, A. & Greber, U.F . Innate immunity to adenovirus. Hum. Gene Ther. 25, 265–284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nwanegbo, E. , Vardas, E. , Gao, W. , Whittle, H. , Sun, H. , Rowe, D. et al Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11, 351–357 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hemminki, O. et al Immunological data from cancer patients treated with Ad5/3‐E2F‐Δ24‐GMCSF suggests utility for tumor immunotherapy. Oncotarget 6, 4467–4481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freytag, S.O. , Parviainen, S. , Juhila, J. , Turkki, R. , Linder, N. , Lundin, J. et al Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus‐mediated cytotoxic gene therapy in intermediate‐risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 89, 268–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim, K.H. , Dmitriev, I.P. , Saddekni, S. , Kashentseva, E.A. , Harris, R.D. , Aurigemma, R. et al A phase I clinical trial of Ad5/3‐Δ24, a novel serotype‐chimeric, infectivity‐enhanced, conditionally‐replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 130, 518–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burke, J.M. , Lamm, D.L. , Meng, M.V. , Nemunaitis, J.J. , Stephenson, J.J. , Arseneau, J.C. et al A first in human phase 1 study of CG0070, a GM‐CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 188, 2391–2397 (2012). [DOI] [PubMed] [Google Scholar]
- 45. Pesonen, S. , Diaconu, I. , Cerullo, V. , Escutenaire, S. , Raki, M. , Kangasniemi, L. et al Integrin targeted oncolytic adenoviruses Ad5‐D24‐RGD and Ad5‐RGD‐D24‐GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int. J. Cancer 130, 1937–1947 (2012). [DOI] [PubMed] [Google Scholar]
- 46. Kaufman, H.L. , Kohlhapp, F.J. & Zloza, A . Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andtbacka, R.H. , Ross, M. , Puzanov, I. , Milhem, M. , Collichio, F. , Delman, K.A. et al Patterns of clinical response with talimogene laherparepvec (T‐VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann. Surg. Oncol. 23, 4169–4177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rehman, H. , Silk, A.W. , Kane, M.P. & Kaufman, H.L . Into the clinic: talimogene laherparepvec (T‐VEC), a first‐in‐class intratumoral oncolytic viral therapy. J. Immunother. Cancer 4, 53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andtbacka, R.H. , Agarwala, S.S. , Ollila, D.W. , Hallmeyer, S. , Milhem, M. , Amatruda, T. et al Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte‐macrophage colony‐stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma. Head Neck 38, 1752–1758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andtbacka, R.H. , Kaufman, H.L. , Collichio, F. , Amatruda, T. , Senzer, N. , Chesney, J. et al Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015). [DOI] [PubMed] [Google Scholar]
- 51. Flotte, T. , Carter, B. , Conrad, C. , Guggino, W. , Reynolds, T. , Rosenstein, B. et al A phase I study of an adeno‐associated virus‐CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 7, 1145–1159 (1996). [DOI] [PubMed] [Google Scholar]
- 52. Nathwani, A.C. , Reiss, U.M. , Tuddenham, E.G. , Rosales, C. , Chowdary, P. , McIntosh, J. et al Long‐term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 371, 1994–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nathwani, A.C. , Tuddenham, E.G. , Rangarajan, S. , Rosales, C. , McIntosh, J. , Linch, D.C. et al Adenovirus‐associated virus vector‐mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flotte, T.R. , Trapnell, B.C. , Humphries, M. , Carey, B. , Calcedo, R. , Rouhani, F. et al Phase 2 clinical trial of a recombinant adeno‐associated viral vector expressing ɑ1‐antitrypsin: interim results. Hum. Gene Ther. 22, 1239–1247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cideciyan, A.V. , Jacobson, S.G. , Beltran, W.A. , Sumaroka, A. , Swider, M. , Iwabe, S. et al Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl. Acad. Sci. USA 110, E517–E525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bainbridge, J.W. , Mehat, M.S. , Sundaram, V. , Robbie, S.J. , Barker, S.E. , Ripamonti, C. et al Long‐term effect of gene therapy on Leber's congenital amaurosis. N. Engl. J. Med. 372, 1887–1897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stoica, L. , Todeasa, S.H. , Cabrera, G.T. , Salameh, J.S. , ElMallah, M.K. , Mueller, C. et al Adeno‐associated virus‐delivered artificial microRNA extends survival and delays paralysis in an amyotrophic lateral sclerosis mouse model. Ann. Neurol. 79, 687–700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borel, F. , Gernoux, G. , Cardozo, B. , Metterville, J.P. , Toro Cabreja, G.C. , Song, L. et al Therapeutic rAAVrh10 mediated SOD1 silencing in adult SOD1(G93A) mice and nonhuman primates. Hum. Gene Ther. 27, 19–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Elmallah, M.K. , Falk, D.J. , Nayak, S. , Federico, R.A. , Sandhu, M.S. , Poirier, A. et al Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in Pompe mice. Mol. Ther. 22, 702–712 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kattenhorn, L.M. , Tipper, C.H. , Stoica, L. , Geraghty, D.S. , Wright, T.L. , Clark, K.R. et al Adeno‐associated virus gene therapy for liver disease. Hum. Gene Ther. 27, 947–961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nance, M.E. & Duan, D . Perspective on adeno‐associated virus capsid modification for Duchenne muscular dystrophy gene therapy. Hum. Gene Ther. 26, 786–800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Keeler, A.M. , Conlon, T. , Walter, G. , Zeng, H. , Shaffer, S.A. , Dungtao, F. et al Long‐term correction of very long‐chain acyl‐coA dehydrogenase deficiency in mice using AAV9 gene therapy. Mol. Ther. 20, 1131–1138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. GuhaSarkar, D. , Su, Q. , Gao, G. & Sena‐Esteves, M . Systemic AAV9‐IFNβ gene delivery treats highly invasive glioblastoma. Neuro. Oncol. 18, 1508–1518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gardner, M.R. et al AAV‐expressed eCD4‐Ig provides durable protection from multiple SHIV challenges. Nature 519, 87–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adam, V.S. , Kattenhorn, L.M. , Kondur, H.R. , von Schaewen, M. , Dorfman, T. , Chiang, J.J. et al Adeno‐associated virus 9‐mediated airway expression of antibody protects old and immunodeficient mice against influenza virus. Clin. Vaccine Immunol. 21, 1528–1533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fire, A. , Xu, S. , Montgomery, M.K. , Kostas, S.A. , Driver, S.E. & Mello, C.C . Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998). [DOI] [PubMed] [Google Scholar]
- 67. Sontheimer, E.J. & Barrangou, R . The bacterial origins of the CRISPR genome‐editing revolution. Hum. Gene Ther. 26, 413–424 (2015). [DOI] [PubMed] [Google Scholar]
- 68. Gao, G. , Alvira, M.R. , Somanathan, S. , Lu, Y. , Vandenberghe, L.H. , Rux, J.J. et al Adeno‐associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. USA 100, 6081–6086 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gao, G.P. , Alvira, M.R. , Wang, L. , Calcedo, R. , Johnston, J. & Wilson, J.M . Novel adeno‐associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99, 11854–11859 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Murlidharan, G. , Sakamoto, K. , Rao, L. , Corriher, T. , Wang, D. , Gao, G. et al CNS‐restricted transduction and CRISPR/Cas9‐mediated gene deletion with an engineered AAV vector. Mol. Ther. Nucleic Acids 5, e338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shen, S. , Horowitz, E.D. , Troupes, A.N. , Brown, S.M. , Pulicherla, N. , Samulski, R.J. et al Engraftment of a galactose receptor footprint onto adeno‐associated viral capsids improves transduction efficiency. J. Biol. Chem. 288, 28814–28823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deverman, B.E. , Pravdo, P.L. , Simpson, B.P. , Kumar, S.R. , Chan, K.Y. , Banerjee, A. et al Cre‐dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Choudhury, S.R. , Fitzpatrick, Z. , Harris, A.F. , Maitland, S.A. , Ferreira, J.S. , Zhang, Y. et al In vivo selection yields AAV‐B1 capsid for central nervous system and muscle gene therapy. Mol. Ther. 24, 1247–1257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lisowski, L. , Dane, A.P. , Chu, K. , Zhang, Y. , Cunningham, S.C. , Wilson, E.M. et al Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nair, J.K. , Willoughby, J.L. , Chan, A. , Charisse, K. , Alam, M.R. , Wang, Q. et al Multivalent N‐acetylgalactosamine‐conjugated siRNA localizes in hepatocytes and elicits robust RNAi‐mediated gene silencing. J. Am. Chem. Soc. 136, 16958–16961 (2014). [DOI] [PubMed] [Google Scholar]
- 76. Butler, J.S. , Chan, A. , Costelha, S. , Fishman, S. , Willoughby, J.L. , Borland, T.D. et al Preclinical evaluation of RNAi as a treatment for transthyretin‐mediated amyloidosis. Amyloid 23, 109–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chakraborti, S. , Lewis, L.A. , Cox, A.D. , St Michael, F. , Li, J. , Rice, P.A. et al Phase‐variable heptose I glycan extensions modulate efficacy of 2C7 vaccine antibody directed against Neisseria gonorrhoeae Lipooligosaccharide. J. Immunol. 196, 4576–4586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee, R.C. , Feinbaum, R.L. & Ambros, V . The C. elegans heterochronic gene lin‐4 encodes small RNAs with antisense complementarity to lin‐14. Cell 75, 843–854 (1993). [DOI] [PubMed] [Google Scholar]
- 79. Wightman, B. , Ha, I. & Ruvkun, G . Posttranscriptional regulation of the heterochronic gene lin‐14 by lin‐4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993). [DOI] [PubMed] [Google Scholar]
- 80. Keeler, A.M. , Sapp, E. , Chase, K. , Sottosanti, E. , Danielson, E. , Pfister, E. et al Cellular analysis of silencing the Huntington's disease gene using AAV9 mediated delivery of artificial micro RNA into the striatum of Q140/Q140 mice. J. Huntingtons Dis. 5, 239–248 (2016). [DOI] [PubMed] [Google Scholar]
- 81. Dufour, B.D. , Smith, C.A. , Clark, R.L. , Walker, T.R. & McBride, J.L . Intrajugular vein delivery of AAV9‐RNAi prevents neuropathological changes and weight loss in Huntington's disease mice. Mol. Ther. 22, 797–810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stanek, L.M. , Sardi, S.P. , Mastis, B. , Richards, A.R. , Treleaven, C.M. , Taksir, T. et al Silencing mutant huntingtin by adeno‐associated virus‐mediated RNA interference ameliorates disease manifestations in the YAC128 mouse model of Huntington's disease. Hum. Gene Ther. 25, 461–474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stoica, L. & Sena‐Esteves, M . Adeno associated viral vector delivered RNAi for gene therapy of SOD1 amyotrophic lateral sclerosis. Front. Mol. Neurosci. 9, 56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mueller, C. , Tang, Q. , Gruntman, A. , Blomenkamp, K. , Teckman, J. , Song, L. et al Sustained miRNA‐mediated knockdown of mutant AAT with simultaneous augmentation of wild‐type AAT has minimal effect on global liver miRNA profiles. Mol. Ther. 20, 590–600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bolotin, A. , Quinquis, B. , Sorokin, A. & Ehrlich, S.D . Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151 (Pt 8), 2551–2561 (2005). [DOI] [PubMed] [Google Scholar]
- 86. Mali, P. , Yang, L. , Esvelt, K.M. , Aach, J. , Guell, M. , DiCarlo, J.E. et al RNA‐guided human genome engineering via Cas9. Science 339, 823–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cong, L. , Ran, F.A. , Cox, D. , Lin, S. , Barretto, R. , Habib, N. et al Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cho, S.W. , Kim, S. , Kim, J.M. & Kim, J.S . Targeted genome engineering in human cells with the Cas9 RNA‐guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013). [DOI] [PubMed] [Google Scholar]
- 89. Barrangou, R. & Doudna, J.A . Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 34, 933–941 (2016). [DOI] [PubMed] [Google Scholar]
- 90. Jinek, M. , Chylinski, K. , Fonfara, I. , Hauer, M. , Doudna, J.A. & Charpentier, E . A programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gasiunas, G. , Barrangou, R. , Horvath, P. & Siksnys, V . Cas9‐crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 109, E2579–E2586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nelson, C.E. , Hakim, C.H. , Ousterout, D.G. , Thakore, P.I. , Moreb, E.A. , Castellanos Rivera, R.M. et al In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yin, H. , Xue, W. , Chen, S. , Bogorad, R.L. , Benedetti, E. , Grompe, M. et al Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 32, 551–553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thomas, C.E. , Ehrhardt, A. & Kay, M.A . Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4, 346–358 (2003). [DOI] [PubMed] [Google Scholar]
- 95. Muruve, D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15, 1157–1166 (2004). [DOI] [PubMed] [Google Scholar]
- 96. Wold, W.S. & Toth, K . Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 13, 421–433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maetzig, T. , Galla, M. , Baum, C. & Schambach, A . Gammaretroviral vectors: biology, technology and application. Viruses 3, 677–713 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barrangou, R. , Fremaux, C. , Deveau, H. , Richards, M. , Boyaval, P. , Moineau, S. et al CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007). [DOI] [PubMed] [Google Scholar]
- 99. Anders, C. , Niewoehner, O. , Duerst, A. & Jinek, M . Structural basis of PAM‐dependent target DNA recognition by the Cas9 endonuclease. Nature 513, 569–573 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Josephs, E.A. , Kocak, D.D. , Fitzgibbon, C.J. , McMenemy, J. , Gersbach, C.A. & Marszalek, P.E . Structure and specificity of the RNA‐guided endonuclease Cas9 during DNA interrogation, target binding and cleavage. Nucleic Acids Res. 43, 8924–8941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ishii, T. Germline genome‐editing research and its socioethical implications. Trends Mol. Med. 21, 473–481 (2015). [DOI] [PubMed] [Google Scholar]
- 102. Flotte, T.R . Therapeutic germ line alteration: has CRISPR/Cas9 technology forced the question? Hum. Gene Ther. 26, 245–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liang, P. , Xu, Y. , Zhang, X. , Ding, C. , Huang, R. , Zhang, Z. et al CRISPR/Cas9‐mediated gene editing in human tripronuclear zygotes. Protein Cell 6, 363–372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. National Research Council; Academies of Science EaM . Guidelines for human embryonic stem cell research. (The National Academies Press, Washington, DC, 2005).
- 105. Hubner, E.K. , Lechler, C. , Kohnke‐Ertel, B. , Zmoos, A.F. , Sage, J. , Schmid, R.M. et al An in vivo transfection system for inducible gene expression and gene silencing in murine hepatocytes. J. Gene Med. 19, 1–2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Marro, B.S. , Grist, J.J. & Lane, T.E . Inducible expression of CXCL1 within the central nervous system amplifies viral‐induced demyelination. J. Immunol. 196, 1855–1864 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bina, X.R. , Wong, E.A. , Bina, T.F. & Bina, J.E . Construction of a tetracycline inducible expression vector and characterization of its use in Vibrio cholerae. Plasmid 76, 87–94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Buluwela, L. , Pike, J. , Mazhar, D. , Kamalati, T. , Hart, S.M. , Al‐Jehani, R. et al Inhibiting estrogen responses in breast cancer cells using a fusion protein encoding estrogen receptor‐alpha and the transcriptional repressor PLZF. Gene Ther. 12, 452–460 (2005). [DOI] [PubMed] [Google Scholar]
- 109. Ginhoux, F. , Turbant, S. , Gross, D.A. , Poupiot, J. , Marais, T. , Lone, Y. et al HLA‐A*0201‐restricted cytolytic responses to the rtTA transactivator dominant and cryptic epitopes compromise transgene expression induced by the tetracycline on system. Mol. Ther. 10, 279–289 (2004). [DOI] [PubMed] [Google Scholar]
- 110. Lena, A.M. , Giannetti, P. , Sporeno, E. , Ciliberto, G. & Savino, R . Immune responses against tetracycline‐dependent transactivators affect long‐term expression of mouse erythropoietin delivered by a helper‐dependent adenoviral vector. J. Gene Med. 7, 1086–1096 (2005). [DOI] [PubMed] [Google Scholar]
- 111. Le Guiner, C. , Stieger, K. , Snyder, R.O. , Rolling, F. & Moullier, P . Immune responses to gene product of inducible promoters. Curr. Gene Ther. 7, 334–346 (2007). [DOI] [PubMed] [Google Scholar]
- 112. Green, A.A. , Silver, P.A. , Collins, J.J. & Yin, P . Toehold switches: de‐novo‐designed regulators of gene expression. Cell 159, 925–939 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li, B. , Ma, W. , Ling, C. , Van Vliet, K. , Huang, L.Y. , Agbandje‐McKenna, M. et al Site‐Directed Mutagenesis of Surface‐Exposed Lysine Residues Leads to Improved Transduction by AAV2, But Not AAV8, Vectors in Murine Hepatocytes In Vivo. Human gene therapy methods. 26(6), 211–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gaudet, D. , Stroes, E.S. , Methot, J. , Brisson, D. , Tremblay, K. , Bernelot Moens, S.J. et al Long‐Term Retrospective Analysis of Gene Therapy with Alipogene Tiparvovec and Its Effect on Lipoprotein Lipase Deficiency‐Induced Pancreatitis. Hum Gene Ther. 27(11), 916–25 (2016). [DOI] [PubMed] [Google Scholar]
- 115. Ferreira, V. , Twisk, J. , Kwikkers, K. , Aronica, E. , Brisson, D. , Methot, J. et al Immune responses to intramuscular administration of alipogene tiparvovec (AAV1‐LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 25(3), 180–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gaudet, D. , Methot, J. , Dery, S. , Brisson, D. , Essiembre, C. , Tremblay, G. et al Efficacy and long‐term safety of alipogene tiparvovec (AAV1‐LPLS447X) gene therapy for lipoprotein lipase deficiency: an open‐label trial. Gene Ther. 20(4), 361–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Simonelli, F. , Maguire, A.M. , Testa, F. , Pierce, E.A. , Mingozzi, F. , Bennicelli, J.L. , et al Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 18(3), 643–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Maguire, A.M. , High, K.A. , Auricchio, A. , Wright, J.F. , Pierce, E.A. , Testa, F. , et al Age‐dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose‐escalation trial. Lancet. 374(9701), 1597–605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Koilkonda, R.D., Yu, H., Chou, T.H., Feuer, W.J., Ruggeri, M., Porciatti, V., et al Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 132(4), 409–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


