Abstract
Nickel-catalyzed coupling reactions provide exciting tools in chemical synthesis. However, most methodologies in this area require high catalyst loadings, which commonly range from 10–20 mol % nickel. Through an academic-industrial collaboration, we demonstrate that kinetic modeling can be used strategically to overcome this problem, specifically within the context of the Ni-catalyzed conversion of amides to esters. The successful application of this methodology to a multigram-scale coupling, using only 0.4 mol % Ni, highlights the impact of this endeavor.
Keywords: nickel, catalysis, cross-coupling, amides, kinetic modeling
New synthetic methodologies have the potential to greatly impact pharmaceutical manufacturing, which, in turn, can have a positive effect on human health. Although there is no shortage of new chemical transformations being reported each year, the likelihood of any of these being adopted in a pharmaceutical manufacturing process remains low. Indeed, process chemists often rely on a handful of common transformations that proceed reliably and efficiently, and, as such, the barrier for adopting a new methodology in a large-scale pharmaceutical manufacturing process can be substantial.1 A key hurdle lies in practical gaps between the typical academic methodology and an economical manufacturing process. For instance, the pressures of manufacturing deadlines may prohibit industrial optimization of published academic methodologies. As such, the earlier a methodology can be rendered scalable and efficient, the more likely it is to be implemented in drug synthesis.
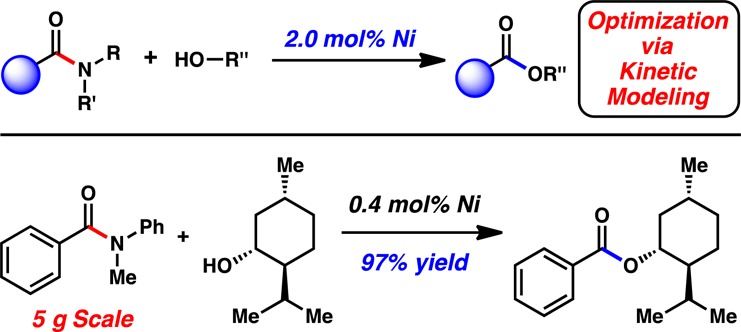
One burgeoning area of academic research that is, in principle, well-suited for large-scale manufacturing is the field of nickel-catalyzed cross-couplings. This is not only because of the high natural abundance, low cost, and low CO2 footprint of nickel, but also because of its unique ability to effect novel or challenging transformations (Figure 1).2 However, nickel-catalyzed cross-couplings reported by academic laboratories often employ high catalyst loadings. For example, as shown in Figure 1, upon surveying >80 manuscripts published in selected top journals since 2015 involving nickel-catalyzed cross-couplings, we found that the vast majority of methodologies use ≥5 mol % nickel, with greater than half of those methodologies employing 10–20 mol % nickel.3 Indeed, examples that require <5 mol % nickel are uncommon. In our own experience, the high catalyst loadings in part stem from the desire to identify broadly applicable reaction conditions and pressures to publish before potential competitors. Although these burdens are not likely to subside, the greater attention to developing process-friendly variants of nickel-catalyzed couplings by academic laboratories could only lead to better chances of such methodologies being adopted industrially.
Figure 1.
Features of nickel catalysis and the most frequently employed catalyst loadings in nickel-catalyzed cross-coupling reactions published January 2015–April 2017.
Prompted by discussions with industrial colleagues, we established a collaboration targeted at rendering a recently developed nickel-mediated coupling more catalytically efficient. The reaction that we chose to pursue is the nickel-catalyzed conversion of amides to esters, which represents a unique and challenging transformation.4−9 An example of this reaction is depicted in Figure 2, wherein benzamide 1 is coupled with (−)-menthol (2) to furnish ester 3 in 88% yield. Notably, this reaction proceeds at 80 °C using both 10 mol % Ni(cod)2 and 10 mol % SIPr in toluene (0.66 M).4,10 At the time this reaction was developed, initial reaction optimization efforts to lower the catalyst loading were unsuccessful. We sought to revisit this challenge through an academic/industrial collaboration that relied on a combination of experiments and kinetic modeling, the latter of which is a tool commonly employed industrially, but less often in academic pursuits.11−13 In this manuscript, we describe the success of these efforts, which allow for amide esterification to occur using catalyst loadings as low as 0.4 mol % Ni.
Figure 2.
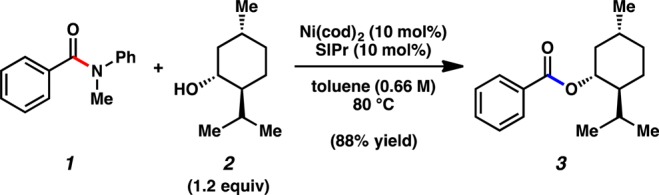
Previously reported nickel-catalyzed coupling of benzamide 1 with (−)-menthol (2) to furnish ester 3 using 10 mol % Ni.
To initiate our studies, we identified the coupling of benzamide 1 with (−)-menthol (2) as a practical reaction choice for several reasons, including (a) the high purity to which (−)-menthol (2) can be obtained by recrystallization, (b) the robustness of the reaction, and (c) the low volatility of all reagents under the reaction conditions. Initial attempts to reduce the reaction temperature from the reported 80 °C revealed that the coupling had reached >90% conversion after ∼8 h at 40 °C (Table 1, entry 1). DynoChem software14 was used to derive rate information from this coupling, and roughly one dozen further exploratory experiments were then designed to probe the sensitivity of the observed reaction rate to changes in several reaction variables. Parameters that were examined included (a) the ligand-to-metal ratio, (b) equivalents of (−)-menthol (2), (c) presence of product/byproduct spikes, (d) length of time holding the catalyst at a given temperature prior to substrate addition, (e) catalyst loading, and (f) reaction concentration.15 With the guidance of the software used, it was determined that only a small number of these experiments involved changes to kinetically relevant reaction variables (Table 1). It was demonstrated that changes in temperature, concentration, and catalyst loading had a marked impact on the reaction rate (entries 2–5 in Table 1).16 However, the stoichiometry of the alcohol, in addition to numerous other variables, did not influence the reaction rate.
Table 1. Experiments Used To Train the Kinetic Modela.
| entry | Ni(cod)2 content (mol %) | temperature (°C) | concentration (M) | maximum conversionb (%) | time (h) |
|---|---|---|---|---|---|
| 1 | 10.0 | 40 | 0.66 | 92 | 8 |
| 2 | 10.0 | 33 | 0.66 | 70 | 6 |
| 3 | 10.0 | 50 | 0.66 | 91 | 4 |
| 4 | 0.5 | 65 | 1.16 | 77 | 8 |
| 5 | 0.1 | 80 | 1.16 | 13 | 1 |
All reactions were performed on a 0.50–1.00 mmol scale, with respect to amide 1, using 1.2 equiv (−)-menthol (2) and a 1:1 ratio of Ni(cod)2:SIPr in toluene.10
Conversion was determined by SFC analysis, using biphenyl as an internal standard.
The data in Table 1 were utilized to build a kinetic model, and a simplified reaction pathway was constructed based on prior computational studies from the Houk laboratory, as well as extensive literature precedent (Figure 3).4,17 The fitted model supports three fundamental steps, which are in agreement with the literature:4 oxidative addition (k1), ligand exchange (k2), and reductive elimination (k3). The model fitting implicates oxidative addition as the rate-determining step (k1), which is consistent with previously reported computational predictions (23.0 kcal/mol DynoChem vs 26.0 kcal/mol DFT calculations).4 In addition, the presence of a catalyst degradation pathway (k4) was also found. These degradation kinetics (k4) were represented by a simplified first-order pathway from the catalyst resting state (NiL). Although details of the catalyst degradation pathway are unknown, NiL was selected as the most abundant catalyst species in the reaction, as oxidative addition is rate-limiting. The regressed rate constants and associated activation energies are depicted in Figure 3. Since the rate of ligand exchange (k2) and reductive elimination (k3) were not found to be rate-limiting, an arbitrary fast rate was used for fitting. Further independent experiments were then conducted under atypical reaction conditions in order to verify the model prediction capabilities, and such experiments were found to be successful in validating the model.18
Figure 3.
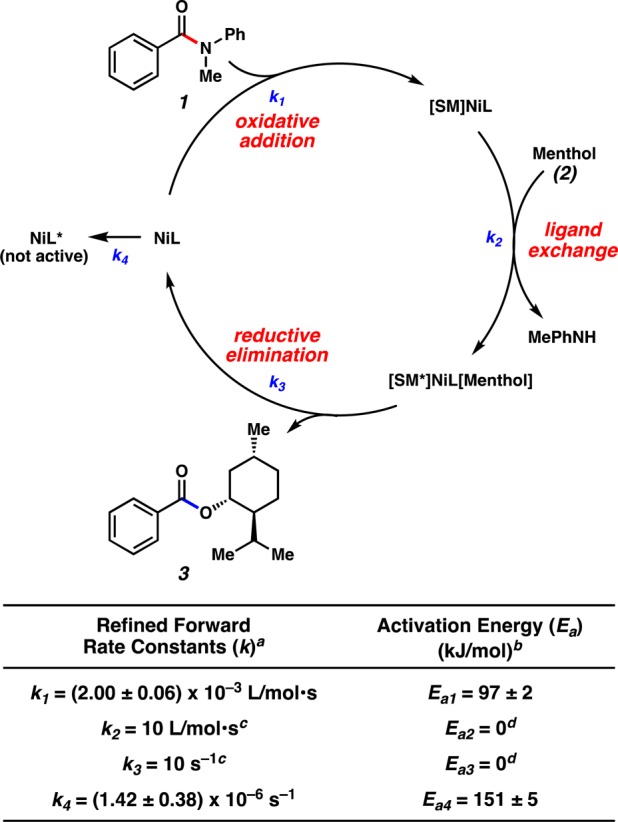
Simplistic reaction pathway, calculated rate constants, and energies of activation for the esterification reaction. [Footnotes in figure: aRate constants are reported at 40 °C; the ± values represent the 95% confidence interval obtained from the DynoChem fitting of the data to the kinetic model. bFor comparison, the corresponding values in kcal/mol are as follows: Ea1 = 23.0 ± 0.5 kcal/mol, Ea4 = 36.1 ± 1.0 kcal/mol. cThis reaction is fast and not rate-limiting; therefore, an arbitrary fast rate of 10 was selected for subsequent fitting. dReaction rate was a weak function of temperature within the explored temperature range.]
With a working kinetic model in hand, thousands of in silico simulations were performed in a matter of minutes in order to visualize the multidimensional relationships between concentration, temperature, and catalyst loading (Figure 4). Based on these calculations, 2.0 mol % Ni catalyst at 60 °C in toluene (∼1.04 M)10 was chosen as an optimal set of conditions that would provide a balance between reaction conversion and catalyst degradation. These conditions were then used to further probe the generality of the coupling.19
Figure 4.
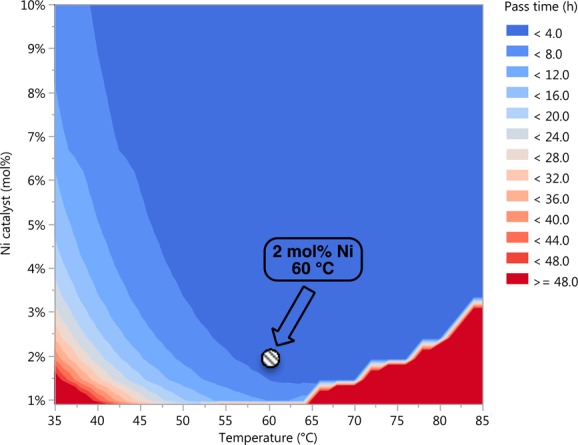
In silico simulations of reaction pass time (95% conversion) as a function of Ni catalyst (mol %) and temperature (°C) for overall reaction concentrations of 1.00–1.30 M.10 Contour plot depicts the result of several thousand simulations.
Having projected suitable conditions that would require only 2.0 mol % Ni, efforts turned to verifying this prediction (Figure 5). These conditions were found to be broadly applicable to several amide substrates 4 and alcohol coupling partners 5 to furnish ester products 6 with high efficiencies. For example, methyl benzoate (7) could be obtained in good yields from benzamide derivatives possessing either N-Me,Ph or N-Bn,Boc nitrogen substitutions. In addition, extended aromatic systems were tolerated, as demonstrated by the formation of 8 in 92% yield. Notably, the conditions were found to be tolerant of heterocycles, as suggested by the preparation of isoquinoline derivative 9 in 66% yield. The alcohol coupling partner was also varied, permitting the generation of interesting ester products such as cyclopropane 10 in 75% yield. Moreover, secondary alcohol nucleophiles were found to be competent in the coupling, allowing for the formation of 11 and 3 in quantitative yields. Finally, an ester derived from a tertiary alcohol could also be accessed, as demonstrated by the production of adamantyl ester 12. As shown, yields were generally comparable to those reported in the literature using 10 mol % Ni.4
Figure 5.
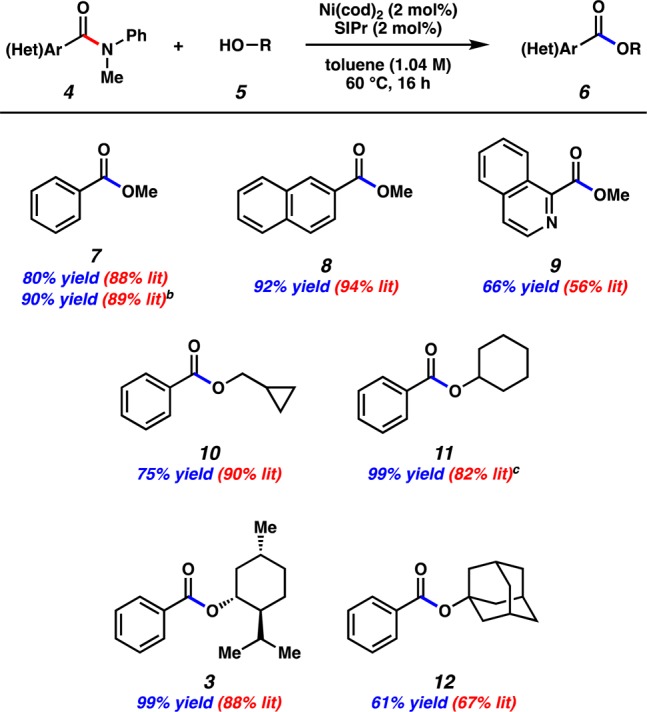
Exploration of scope in the esterification. [Footnote in figure: aAll reactions were performed on 0.50 mmol scale using 1.2 equiv alcohol, 2.0 mol % Ni(cod)2, and 2.0 mol % SIPr in toluene (1.04 M) at 60 °C for 16 h. Yields determined by 1H NMR analysis using hexamethylbenzene as an external standard. bCoupling performed with the corresponding N-Bn,Boc benzamide. c97% isolated yield obtained after silica gel chromatography.]
With the aim of minimizing the catalyst loading further, additional simulations were performed using <1.0 mol % Ni.18 The simulation results predicted that the esterification of benzamide 1 with (−)-menthol (2) could reach nearly full conversion within <56 h if performed at 45 °C with 0.4 mol % Ni in toluene at high concentrations (1.52 M)10 (see Figure 6).20 These predicted reaction conditions using only 0.4 mol % Ni were thus attempted on a 5 g scale to test the scalability of the coupling. To our delight, this effort afforded ester 3 in almost-quantitative yield.21 Compared to our original disclosure, this reaction uses 25-fold less Ni(cod)2 and >10-fold less of the SIPr ligand. If each reaction variable had been tested independently, this result would have likely been discovered in a much less concise manner, if at all. However, by employing a kinetic model, a catalyst degradation pathway was identified that informed the careful tuning of the reaction conditions, in turn permitting an efficient coupling to happen. This example, which showcases the rare use of <0.5 mol % Ni in a catalytic coupling, underscores the value of kinetic modeling and bodes well for the increasingly widespread adoption of nickel catalysis in industry.
Figure 6.
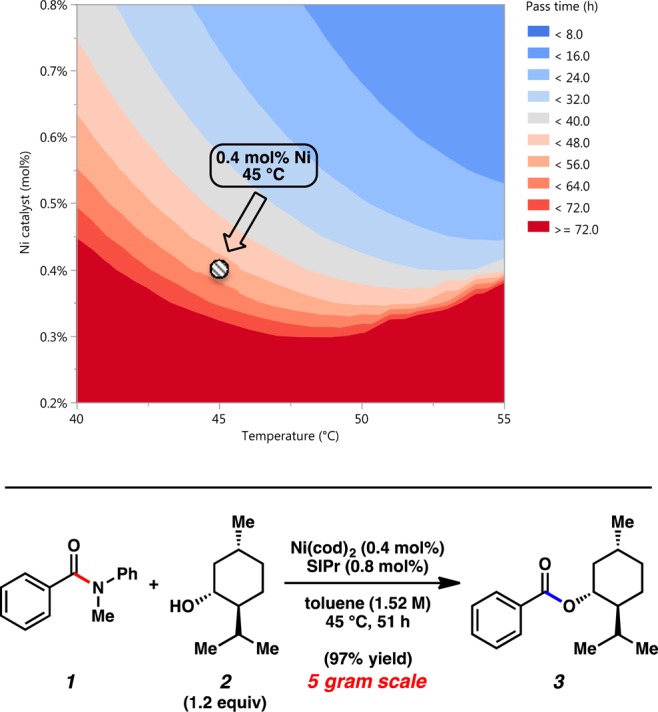
In silico simulations of reaction pass time (95% conversion), as a function of Ni catalyst (mol %) and temperature (°C) for overall reaction concentrations of 1.44–1.74 M10 and 5 g scale coupling of benzamide 1 with (−)-menthol (2) using 0.4 mol % Ni. [Footnote in figure: aContour plot depicts the result of several thousand simulations. Conditions: 5.00 g amide 1, 1.2 equiv (−)-menthol (2), 0.4 mol % Ni(cod)2, and 0.8 mol % SIPr in toluene (1.52 M) at 45 °C for 51 h. Yield refers to isolated yield after column chromatography.]
In summary, we have developed a kinetic model that allowed for the optimization of the nickel-catalyzed esterification of amides. The model-predicted reaction conditions, involving a 5-fold reduction in catalyst loading to 2.0 mol % Ni, were tested and deemed suitable for a variety of coupling partners. Further simulations using the kinetic model predicted the coupling of benzamide 1 and (−)-menthol (2) could then occur using as little as 0.4 mol % Ni. This forecast was validated, as demonstrated by a multigram scale coupling that proceeded in an almost-quantitative yield. Thus, guided by reaction kinetics, the esterification of amides was optimized in a concise manner and was rendered substantially more efficient. These studies are expected to facilitate the adoption of kinetic modeling as a powerful tool in academic methodology design for the expedited translation of those methodologies into industry.
Acknowledgments
The authors are grateful to the NIH-NIGMS (No. R01 GM117016 for N.K.G.), the UCLA Gold Shield Alumnae, and the University of California, Los Angeles for financial support. N.A.W. acknowledges the National Science Foundation (No. DGE-1144087) and the Foote Family for fellowship support. These studies were also supported by shared instrumentation grants from the NSF (No. CHE-1048804) and the National Center for Research Resources (No. S10RR025631).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.7b01444.
Detailed experimental and compound characterization data (PDF)
Author Contributions
§ These authors contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Shioiri T., Izawa K., Konoike T., Eds. Pharmaceutical Process Chemistry; Wiley–VCH: Weinheim, Germany, 2010; pp 1–526. [Google Scholar]; b Tomioka K., Shioiri T., Sajiki H., Eds. New Horizons of Process Chemistry; Springer: Singapore, 2017; pp 1–285 (DOI: 10.1007/978-981-10-3421-3). [DOI] [Google Scholar]
- For pertinent reviews on nickel catalysis, see:; a Rosen B. M.; Quasdorf K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Chem. Rev. 2011, 111, 1346–1416. 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tasker S. Z.; Standley E. A.; Jamison T. F. Nature 2014, 509, 299–309. 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mesganaw T.; Garg N. K. Org. Process Res. Dev. 2013, 17, 29–39. 10.1021/op300236f. [DOI] [Google Scholar]; d Ananikov V. P. ACS Catal. 2015, 5, 1964–1971. 10.1021/acscatal.5b00072. [DOI] [Google Scholar]
- SciFinder search for the research topic “nickel, cross-coupling” yielded hits corresponding to 83 original manuscripts in select top journals since 2015. (accessed April 20, 2017). See the Supporting Information for details and a complete list of Digital Object Identifiers (DOIs) corresponding to sampled manuscripts.
- Hie L.; Fine Nathel N. F.; Shah T.; Baker E. L.; Hong X.; Yang Y.-F.; Liu P.; Houk K. N.; Garg N. K. Nature 2015, 524, 79–83. 10.1038/nature14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the nickel-catalyzed esterification of aliphatic amides, see:Hie L.; Baker E. L.; Anthony S. M.; Desrosiers J.-N.; Senanayake C.; Garg N. K. Angew. Chem., Int. Ed. 2016, 55, 15129–15132. 10.1002/anie.201607856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For other nickel-catalyzed reactions involving cleavage of the amide C–N bond, see:; a Weires N. A.; Baker E. L.; Garg N. K. Nat. Chem. 2015, 8, 75–79. 10.1038/nchem.2388. [DOI] [PubMed] [Google Scholar]; b Baker E. L.; Yamano M. M.; Zhou Y.; Anthony S. M.; Garg N. K. Nat. Commun. 2016, 7, 11554. 10.1038/ncomms11554. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Simmons B. J.; Weires N. A.; Dander J. E.; Garg N. K. ACS Catal. 2016, 6, 3176–3179. 10.1021/acscatal.6b00793. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Dander J. E.; Weires N. A.; Garg N. K. Org. Lett. 2016, 18, 3934–3936. 10.1021/acs.orglett.6b01758. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Shi S.; Szostak M. Org. Lett. 2016, 18, 5872–5875. 10.1021/acs.orglett.6b02952. [DOI] [PubMed] [Google Scholar]; f Shi S.; Szostak M. Chem.—Eur. J. 2016, 22, 10420–10424. 10.1002/chem.201602202. [DOI] [PubMed] [Google Scholar]; g Dey A.; Sasmal S.; Seth K.; Lahiri G. K.; Maiti D. ACS Catal. 2017, 7, 433–437. 10.1021/acscatal.6b03040. [DOI] [Google Scholar]; h For a recent review, see:Dander J. E.; Garg N. K. ACS Catal. 2017, 7, 1413–1423. 10.1021/acscatal.6b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]; I Ni S.; Zhang W.; Mei H.; Han J.; Pan Y. Org. Lett. 2017, 19, 2536–2539. 10.1021/acs.orglett.7b00831. [DOI] [PubMed] [Google Scholar]; j Medina J. M.; Moreno M.; Racine S.; Du S.; Garg N. K. Angew. Chem., Int. Ed. 2017, 56, 6567–6571. 10.1002/anie.201703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For palladium-catalyzed C–C bond forming reactions of amides, see:; a Li X.; Zou G. Chem. Commun. 2015, 51, 5089–5092. 10.1039/C5CC00430F. [DOI] [PubMed] [Google Scholar]; b Li X.; Zou G. J. Organomet. Chem. 2015, 794, 136–145. 10.1016/j.jorganchem.2015.07.009. [DOI] [Google Scholar]; c Meng G.; Szostak M. Org. Biomol. Chem. 2016, 14, 5690–5705. 10.1039/C6OB00084C. [DOI] [PubMed] [Google Scholar]; d Meng G.; Szostak M. Org. Lett. 2016, 18, 796–799. 10.1021/acs.orglett.6b00058. [DOI] [PubMed] [Google Scholar]; e Meng G.; Szostak M. Angew. Chem., Int. Ed. 2015, 54, 14518–14522. 10.1002/anie.201507776. [DOI] [PubMed] [Google Scholar]; f Meng G.; Szostak M. Org. Lett. 2015, 17, 4364–4367. 10.1021/acs.orglett.5b02209. [DOI] [PubMed] [Google Scholar]; g Liu C.; Meng G.; Liu Y.; Liu R.; Lalancette R.; Szostak R.; Szostak M. Org. Lett. 2016, 18, 4194–4197. 10.1021/acs.orglett.6b01836. [DOI] [PubMed] [Google Scholar]; h Lei P.; Meng G.; Szostak M. ACS Catal. 2017, 7, 1960–1965. 10.1021/acscatal.6b03616. [DOI] [Google Scholar]; i Liu C.; Liu Y.; Liu R.; Lalancette R.; Szostak R.; Szostak M. Org. Lett. 2017, 19, 1434–1437. 10.1021/acs.orglett.7b00373. [DOI] [PubMed] [Google Scholar]; j Liu C.; Meng G.; Szostak M. J. Org. Chem. 2016, 81, 12023–12030. 10.1021/acs.joc.6b02294. [DOI] [PubMed] [Google Scholar]; k Meng G.; Shi S.; Szostak M. ACS Catal. 2016, 6, 7335–7339. 10.1021/acscatal.6b02323. [DOI] [Google Scholar]; l Cui M.; Wu H.; Jian J.; Wang H.; Liu C.; Daniel S.; Zeng Z. Chem. Commun. 2016, 52, 12076–12079. 10.1039/C6CC06428K. [DOI] [PubMed] [Google Scholar]; m Wu H.; Li Y.; Cui M.; Jian J.; Zeng Z. Adv. Synth. Catal. 2016, 358, 3876–3880. 10.1002/adsc.201600555. [DOI] [Google Scholar]
- For the nickel-catalyzed decarbonylative coupling of amides with arylboronic esters, see:Shi S.; Meng G.; Szostak M. Angew. Chem., Int. Ed. 2016, 55, 6959–6963. 10.1002/anie.201601914. [DOI] [PubMed] [Google Scholar]
- For the nickel-catalyzed decarbonylative borylation of amides, see:Hu J.; Zhao Y.; Liu J.; Zhang Y.; Shi Z. Angew. Chem., Int. Ed. 2016, 55, 8718–8722. 10.1002/anie.201603068. [DOI] [PubMed] [Google Scholar]
- Concentration was calculated by approximating volume as the sum of the masses of all reactants, reagents, and solvent with an assumed overall density of 0.87 g/mL (toluene). Experimentally measured densities for reaction mixtures ranged from 0.85–0.91 g/mL.
- For reviews of kinetic modeling in homogeneous catalytic processes, see:; a Chaudhari R. V.; Seayad A.; Jayasree S. Catal. Today 2001, 66, 371–380. 10.1016/S0920-5861(00)00633-7. [DOI] [Google Scholar]; b Blackmond D. G. J. Am. Chem. Soc. 2015, 137, 10852–10866. 10.1021/jacs.5b05841. [DOI] [PubMed] [Google Scholar]
- For selected examples of kinetic modeling in industry, see:; a Changi S. M.; Wong S.-W. Org. Process Res. Dev. 2016, 20, 525–539. 10.1021/acs.oprd.5b00281. [DOI] [Google Scholar]; b Susanne F.; Smith D. S.; Codina A. Org. Process Res. Dev. 2012, 16, 61–64. 10.1021/op200202k. [DOI] [Google Scholar]; c Burt J. L.; Braem A. D.; Ramirez A.; Mudryk B.; Rossano L.; Tummala S. J. Pharm. Innov. 2011, 6, 181–192. 10.1007/s12247-011-9109-3. [DOI] [Google Scholar]; d Hallow D. M.; Mudryk B. M.; Braem A. D.; Tabora J. E.; Lyngberg O. K.; Bergum J. S.; Rossano L. T.; Tummala S. J. Pharm. Innov. 2010, 5, 193–203. 10.1007/s12247-010-9094-y. [DOI] [Google Scholar]; e Massari L.; Panelli L.; Hughes M.; Stazi F.; Maton W.; Westerduin P.; Scaravelli F.; Bacchi S. Org. Process Res. Dev. 2010, 14, 1364–1372. 10.1021/op100176u. [DOI] [Google Scholar]
- For selected examples of kinetic modeling in academia, see:; a Rosner T.; Le Bars J.; Pfaltz A.; Blackmond D. G. J. Am. Chem. Soc. 2001, 123, 1848–1855. 10.1021/ja003191e. [DOI] [PubMed] [Google Scholar]; b Ji Y.; Plata R. E.; Regens C. S.; Hay M.; Schmidt M.; Razler T.; Qiu Y.; Geng P.; Hsiao Y.; Rosner T.; Eastgate M. D.; Blackmond D. G. J. Am. Chem. Soc. 2015, 137, 13272–13281. 10.1021/jacs.5b01913. [DOI] [PubMed] [Google Scholar]; c Ruiz-Castillo P.; Blackmond D. G.; Buchwald S. L. J. Am. Chem. Soc. 2015, 137, 3085–3092. 10.1021/ja512903g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DynoChem, by Scale-up Systems, is a leading process development and scale-up software for scientists and engineers working in the pharmaceutical industry and interfaces directly with Microsoft Excel.
- Reactions were performed in an inert atmosphere glovebox; aliquots were taken periodically and analyzed by SFC using biphenyl as an internal standard in order to monitor reaction progress. In general, five aliquots were taken per experiment to chart the reaction profile.
- Lower catalyst loadings (i.e., entries 4 and 5) were run at higher temperatures and concentrations solely to achieve conversion in a reasonable time frame.
- Although the kinetic model can itself provide insight into possible mechanistic steps, it is helpful to have some understanding of the mechanism of the reaction in question prior to optimization.
- See the Supporting Information for details.
- Although the esterification of benzamide 1 with (−)-menthol (2) could be optimized further, conditions using 2.0 mol% Ni at 60 °C with the extended reaction time of 16 h were selected for additional explorations of scope.
- It was observed empirically that a 2:1 ligand:metal ratio facilitated this coupling at catalyst loadings below 1.0 mol% Ni, likely helping to stabilize the catalyst and impede degradation. The mechanism of the catalyst degradation is not yet well understood.
- This outcome is the result of direct optimization of the esterification of benzamide 1 with (−)-menthol (2). To achieve similar efficiencies with other coupling partners, independent optimizations would likely have to be carried out based on individual reaction kinetics.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


