Abstract
5-Hydroxymethylcytidine (hm5C) was recently identified as a direct metabolite of m5C in RNA. We investigated the stability of hm5C in human cells using bio-isotopologues and LC-MS/HRMS. This has led to the discovery of a second oxidative metabolite of m5C in RNA, namely 2′-O-methyl-5-hydroxymethylcytidine (hm5Cm). Subsequent quantitative analysis of total RNA from higher organisms revealed varying levels and TET-independent formation of this new RNA modification.
There is a wide chemical diversity of ribonucleoside modifications in RNA.1,2 While epigenetic events such as DNA methylation and histone modifications are understood to be dynamic and reversible processes, RNA modifications, have long been considered relatively static and stable marks. However, it was recently shown that the enzyme FTO mediates the oxidative demethylation of m6A via N6-hydroxy- and N6-formylcytidine in mRNA.3,4 This first example of reversible RNA methylation has opened up the possibility that RNA modifications may also be dynamic, with potential regulatory roles analogous to reversible epigenetic modifications. In support of this, we recently showed that m5C undergoes similar oxidative metabolism in RNA to produce hm5C and that the latter modification is conserved across Archaea, Bacteria and Eukarya.5 Furthermore, Fu et al. reported the ability of TET enzymes to oxidize m5C to hm5C in synthetic RNA strands in vitro and showed the dependency of hm5C on the TET3 enzyme in an in vivo knockout mouse model.6 Together, these studies established hm5C as a new RNA modification that is introduced through active, enzyme-catalyzed oxidation, rather than passive, reactive oxygen species-mediated oxidation of m5C.
In contrast with m6A, which is predominantly an mRNA modification, we had determined by quantitative LC-MS/HRMS that hm5C is enriched in tRNA fractions (Figure S1). The turnover of m5C into hm5C is of particular interest as the extent of m5C modification at specific tRNA sites plays a key role in regulating the cellular stress response. For example, the absence of m5C triggers increased stress-induced cleavage of tRNAs and sensitizes organisms to oxidative stress.7,8 Furthermore, tRNA wobble modifications can change as a result of exposure to toxic agents and thereby trigger stress-specific enhancement of translation of proteins critical to the cell stress response.9,10 These response mechanisms require a dynamic control of tRNA modifications which can either be achieved through their reversible introduction or specific tRNA turnover/degradation.
We investigated whether hm5C is indeed subject to dynamic turnover and looked for the existence of additional, novel oxidative metabolites of m5C to examine the presence of an active cytidine-C5 demethylation pathway in RNA.
As a means to study the relative stabilities of m5C and hm5C in tRNA-enriched fractions, as compared to tRNA turnover, we selected stable isotope tracing monitored by mass spectrometry (Figure 1). We adapted methods previously reported by us in which we studied both RNA and DNA methylation and their oxidative pathways.5,11,12 Briefly, human HEK293T cells were cultured in the presence of stable isotope labeled (SIL) methionine, 13CD3-l-methionine, to metabolically 13CD3 label the methyl group of m5C in RNA (Figure 1). The medium was then replaced with medium containing unlabeled methionine and SIL labeled 1,3-15N2-cytidine (Figure 1, t = 0) and cells were collected at hourly intervals and subjected to total RNA isolation, over the course of 15 h, approximately a complete cell cycle of a HEK293T cell. The total RNA fractions were each subsequently enriched for tRNAs by fractional precipitation, enzymatically digested into nucleosides and subjected to mass spectrometric analysis to quantify the SIL forms of both m5C (13CD3-m5C, 15N2-m5C) and hm5C (13CD2-hm5C, 15N2-hm5C). 1,3-15N2-cytidine was included to ensure the differential labeling of RNA synthesized during (Figure 1, before t = 0) and after (Figure 1, from t = 0 onward) the 13CD3-l-methionine labeling. This distinguishes the apparent 13CDn decay as a result of isotope dilution due to cell proliferation or tRNA turnover, rather than modification turnover.
Figure 1.
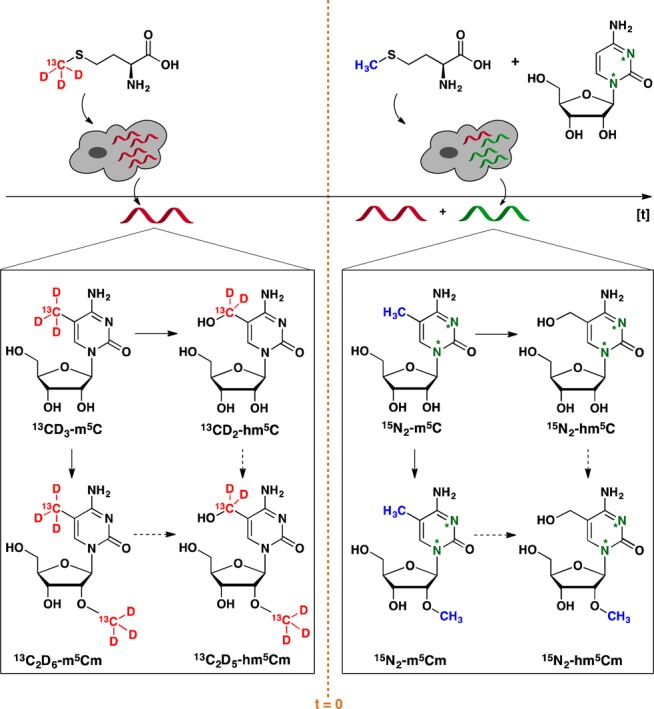
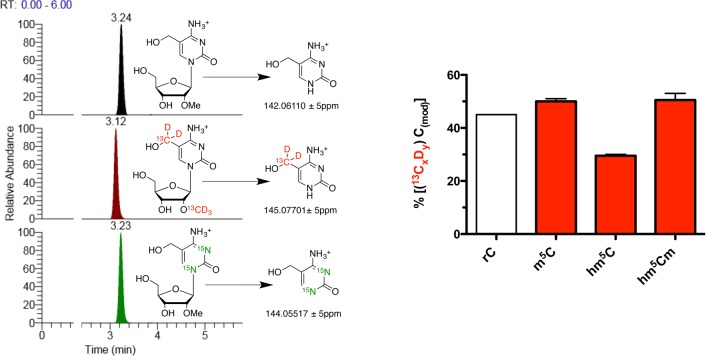
Overview of the stable isotope, dual-labeling approach. Cells were grown in the presence of [methyl-13CD3]-l-methionine until near quantitative isotope incorporation for m5C was observed by LC-MS/HRMS. Heavy methionine (red) was then removed and a mixture of unlabeled l-methionine (blue) and 1,3-15N2-cytidine (green) was added.
Thus, we measured the abundances of the different isotopologues of C, m5C and hm5C in the tRNA-enriched digests and calculated the amount of their 13CDn labeled (m5C and hm5C) and unlabeled (C) fractions relative to the sum of the total amounts of any given modification (Figure 2).
Figure 2.
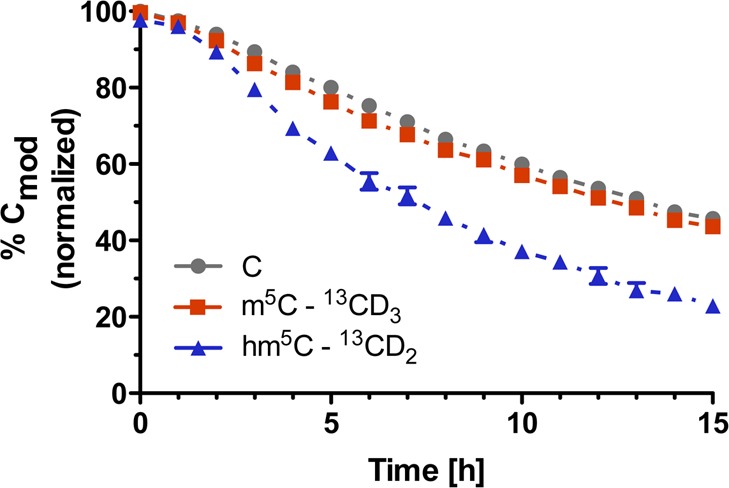
Amounts of the 13CDn-labeled fractions of a modification relative to the sum of the total amounts of the same modification in small RNAs from 13CDn-labeled HEK293T cells grown in the presence of 1,3-15N2-cytidine and absence of 13CD3-l-methionine as a function of time (i.e., for m5C: % 13CD3-m5C = {[13CD3-m5C]/([13CD3-m5C] + [15N2-m5C] + [m5C])} × 100).
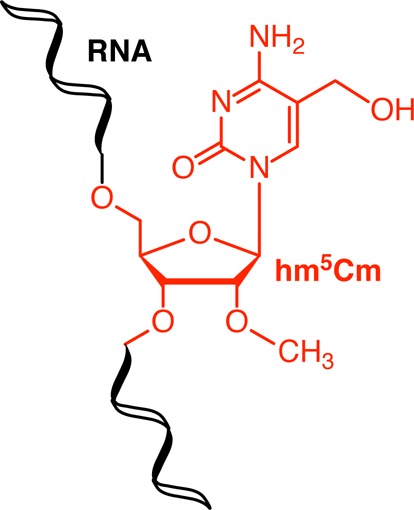
We observed a 50% decrease of unlabeled cytidines (Figure 2, gray trace), consistent with the cell population and total RNA doubling in this time, which dilutes the 15N2 label by 50% due to the addition of 1,3-15N2-cytidine at t = 0. We observed 5-methylcytidine (Figure 2, red trace) had comparable stability to cytidine, indicating the bulk of m5C residues was not subject to active turnover. Because only a small fraction of m5C residues is converted to hm5C (∼0.1%), this turnover was not sufficient to detect by our approach. However, when we considered the turnover of hm5C (Figure 2, blue trace), we observed a strikingly steeper initial slope for its decay and a much lower relative abundance of the 13CD2 isotopologue after 15 h as compared to m5C. Consequently, hm5C-containing RNA transcripts could either be unstable and subject to accelerated degradation, or hm5C itself could be actively metabolized within its RNA transcript. To explore the latter hypothesis, we screened the dual SIL RNA samples for other oxidative derivatives of m5C. Recently, it was shown that the Fe(II)-dependent oxygenase ALKBH1/ABH1 oxidizes m5C at position 34 in human mitochondrial tRNAMet to f5C and hm5C was not observed as an intermediate in this study.13 We therefore considered the hitherto unknown 2′-OH methylated derivative of hm5C, 2′-O-methyl-5-hydroxymethylcytidine (hm5Cm), as a potential downstream product of hm5C metabolism in subsequent LC-MS/HRMS analyses. 2′-O-Methylation has been observed in several RNA classes14 and close examination of previous extracted ion counts and fragmentation patterns of hm5C led us to hypothesize the presence of hm5Cm in RNA. Thus, using the tRNA-enriched digests from the 8 h time point, we targeted the mass spectrometry for 2′-O-[13CD3-methyl]-5-[13CD2-hydroxymethyl]-cytidine, 2′-O-methyl-1,3-[15N2]-5-hydroxymethylcytidine and the minor, completely unlabeled hm5Cm isotopologues. As depicted in Figure 3 (left), we could extract all the corresponding product ions. This, together with the observed coelution of all the isotopologues during liquid chromatography, provided the first evidence for the presence of hm5Cm in RNA. The slightly earlier elution of deuteriated compounds, as observed for 13C2D5 labeled hm5Cm (Figure 3, left, red trace), is commonly observed in liquid chromatography of deuterium labeled compounds due to their different polarity, polarizability and molecular volume compared to their lighter isotopologues.15
Figure 3.
(Left) Differential labeling of hm5Cm. LC-MS/HRMS analysis of RNA obtained from HEK293T cells grown in regular (top), [methyl-13CD3]-l-methionine- (middle) or 1,3-15N2-cytidine-supplemented (bottom) medium. Extracted ion counts are shown for hm5C, 13CD2-hm5C and 15N2-hm5C. Right) Analysis of the levels of 13CxDy labeled modifications after 15 h.
To assess the stability of this novel RNA modification in relation to C, m5C and hm5C we determined the levels of 13CxDy-labeled modifications after 15 h, from our previous time decay study. As shown in Figure 3 (right), around 50% of all hm5Cm are still 13C2D5-labeled after a complete HEK293T cell cycle. This is comparable to that observed for rC (45%) and m5C (50%), two residues that we identified as stable. In contrast, 13CD2-hm5C accounts for only 29% of all hm5C residues after 15 h. These data demonstrate that hm5Cm is a stable modification. To establish unequivocally hm5Cm as a novel RNA modification, we synthesized a reference standard for hm5Cm by sodium persulfate-mediated oxidation of commercially obtained m5Cm16 and performed quantitative LC-MS/HRMS analysis of total RNA samples form a variety of organisms. Thereby, we measured the abundance of m5C, hm5C, m5Cm and hm5Cm (Figure 4). We selected HEK293T cells and murine brain tissue as human and mammalian examples, respectively. Furthermore, we chose models with previously reported low (Caenorhabditis elegans), high (Arabidopsis thaliana) and undetermined (Drosophila melanogaster) absolute levels of hm5C.5,17 As shown in Figure 5, we measured hm5C levels that agreed with those previously described by us and others.5,6A. thaliana RNA exhibited the highest hm5C levels (130 ppm), whereas C. elegans RNA showed the lowest (<10 ppm). Although the presence of hm5C in D. melanogaster total RNA was previously demonstrated by dot blot experiments, we could not verify these results by LC-MS/HRMS.17 On the other hand, we could readily observe the 2′-OH methylated form of hm5C, hm5Cm, in the latter organism. This may indicate that currently used antibodies cannot discriminate between hm5C and hm5Cm.17 In general, organisms exhibiting a very low or undetectable level of hm5C, actually showed a relatively high, detectable level of the 2′-OH methylated form, hm5Cm. In addition to D. melanogaster, this is exemplified by C. elegans, which contains 30 ppm hm5Cm in total RNA. For the human cells and mouse brain the abundances of methylated and unmethylated hm5C are comparable. These results suggest that oxidation of C5-methylated cytidines (m5C and/or m5Cm) is a widespread process and eukaryotes seem to select largely for either the 2′-OH methylated or unmetylated derivative with only mammalian RNA containing both forms of C5-hydroxymethylation (hm5C and hm5Cm).
Figure 4.
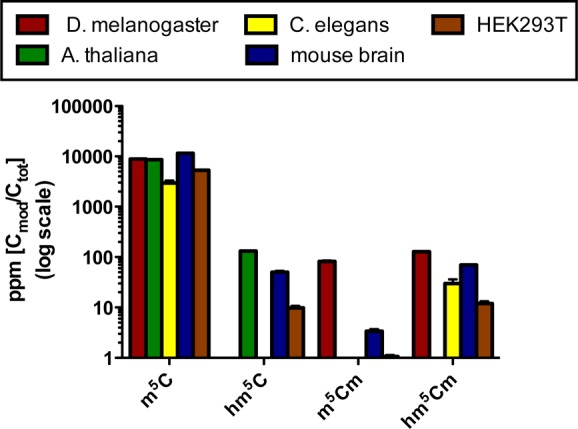
Abundance of m5C and its derivatives as determined by quantitative LC-MS/HRMS.
Figure 5.
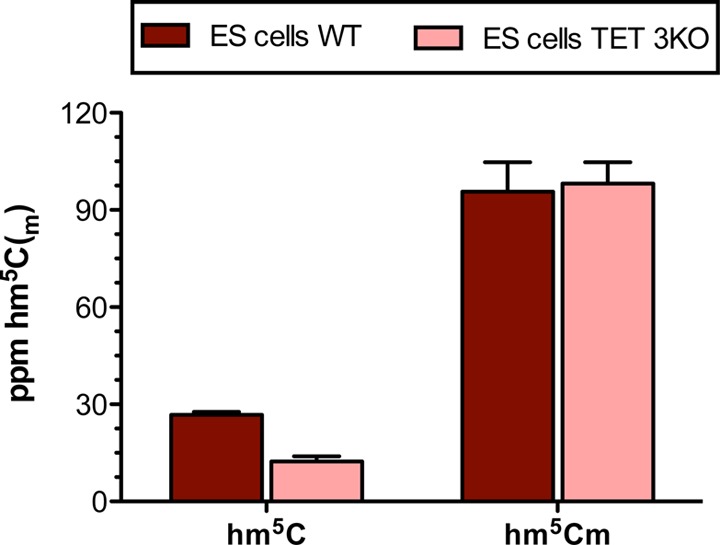
Abundance of hm5C and hm5Cm as determined by quantitative LC-MS/HRMS in mouse embryonic WT and TET triple KO stem cells.
Although further studies are required to fully discern the function of hm5Cm, it should be noted that methylation of 2′-hydroxyl groups in tRNA molecules has been previously observed to occur at the first position of the anticodon to promote codon–anticodon interaction.18 Furthermore, 2′-O-methylation can block the ability of the 2′-position of the nucleoside to serve as a proton donor and therefore prevents RNA hydrolysis, increasing the lifetime of the RNA.19 We therefore propose that hm5Cm may promote the stability of tRNAs themselves and the stability of duplex formation with complementary RNA molecules. It is noteworthy that the oxidative derivative of hm5Cm, 2′-O-methyl-5-formylcytidine is already known to be present at the wobble position of cytoplasmic tRNAs.20
The TET family of enzymes was previously reported to be capable of oxidizing m5C to hm5C in RNA both in vitro and in vivo.6,17 To shed light on whether hm5Cm is also TET-dependent, we measured its levels in TET triple knockout (TKO) mouse embryonic stem cells that have been mutated in the catalytic domain of all three TET enzymes and therefore have no residual TET activity (Figure 5).21 Interestingly, as we show here, hm5Cm is not TET-dependent. RNA obtained from TET wild type and TET TKO cells show equal amounts of the 2′-O-methylated version of hm5C. This shows that hm5Cm is generated by an enzyme other than TET, which is in accordance with the findings that hm5Cm is highest in organisms that do not express TET (C. elegans) or express TETs at a reduced level (D. melanogaster).
In conclusion, we have identified a novel derivative of C5-methylated ribonucleosides in RNA from mammalian cells, tissue and several organisms. The exact functional roles of hm5C and hm5Cm and the relationship between them will be the subject of future studies.
Acknowledgments
This work was supported by a Wellcome Trust Senior Investigator award to SB (grant no. 099232/z/12/z). The Balasubramanian group is supported by core funding from Cancer Research U.K. (C14303/A17197). Prof. Daniel St. Johnston is thanked for the provision of D. melanogaster for total RNA isolation.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b12180.
Detailed experimental procedures, supporting figures and tables (PDF)
Author Contributions
§ These authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Cantara W. A.; Crain P. F.; Rozenski J.; Mc Closkey J. A.; Harris K. A.; Zhang X.; Vendeix F. A.; Fabris D.; Agris P. F. Nucleic Acids Res. 2011, 39, D195–D201. 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka M. A.; Milanowska K.; Osman Oglou O.; Purta E.; Kurkowska M.; Olchowik A.; Januszewski W.; Kalinowski S.; Dunin-Horkawicz S.; Rother K. M.; Helm M.; Bujnicki J. M.; Grosjean H. Nucleic Acids Res. 2013, 41, D262–D267. 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G.; Fu Y.; Zhao X.; Dai Q.; Zheng G.; Yang Y.; Yi C.; Lindahl T.; Pan T.; Yang Y. G.; He C. Nat. Chem. Biol. 2011, 7, 885–887. 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.; Jia G.; Pang X.; Wang R. N.; Wang X.; Li C. J.; Smemo S.; Dai Q.; Bailey K. A.; Nobrega M. A.; Han K. L.; Cui Q.; He C. Nat. Commun. 2013, 4, 1798. 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. M.; van Delft P.; Mendil L.; Bachman M.; Smollett K.; Werner F.; Miska E. A.; Balasubramanian S. ChemBioChem 2015, 16, 752–755. 10.1002/cbic.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L.; Guerrero C. R.; Zhong N.; Amato N. J.; Liu Y.; Liu S.; Cai Q.; Ji D.; Jin S. G.; Niedernhofer L. J.; Pfeifer G. P.; Xu G. L.; Wang Y. J. Am. Chem. Soc. 2014, 136, 11582–11585. 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M.; Pollex T.; Hanna K.; Tuorto F.; Meusburger M.; Helm M.; Lyko F. Genes Dev. 2010, 24, 1590–1595. 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco S.; Dietmann S.; Flores J. V.; Hussain S.; Kutter C.; Humphreys P.; Lukk M.; Lombard P.; Treps L.; Popis M.; Kellner S.; Hölter S. M.; Garrett L.; Wurst W.; Becker L.; Klopstock T.; Fuchs H.; Gailus-Durner V.; Hrabë de Angelis M.; Káradóttir R. T.; Helm M.; Ule J.; Gleeson J. G.; Odom D. T.; Frye M. EMBO J. 2014, 33, 2020–2039. 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. T.; Dyavaiah M.; De Mott M. S.; Taghizadeh K.; Dedon P. C.; Begley T. J. PLoS Genet. 2010, 6, e1001247. 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. T.; Pang Y. L.; Deng W.; Babu I. R.; Dyavaiah M.; Begley T. J.; Dedon P. C. Nat. Commun. 2012, 3, 937. 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman M.; Uribe-Lewis S.; Yang X.; Williams M.; Murrell A.; Balasubramanian S. Nat. Chem. 2014, 6, 1049–1055. 10.1038/nchem.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman M.; Uribe-Lewis S.; Yang X.; Burgess H. E.; Iurlaro M.; Reik W.; Murrell A.; Balasubramanian S. Nat. Chem. Biol. 2015, 11, 555–557. 10.1038/nchembio.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag S.; Sloan K. E.; Ranjan N.; Warda A. S.; Kretschmer J.; Blessing C.; Hübner B.; Seikowski J.; Dennerlein S.; Rehling P.; Rodnina M. V.; Höbartner C.; Bohnsack M. T. EMBO J. 2016, 35, 2104–2119. 10.15252/embj.201694885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T. Cell 2002, 109, 145–148. 10.1016/S0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- Wade D. Chem.-Biol. Interact. 1999, 117, 191–217. 10.1016/S0009-2797(98)00097-0. [DOI] [PubMed] [Google Scholar]
- Itahara T. Chem. Lett. 1991, 20, 1591–1594. 10.1246/cl.1991.1591. [DOI] [Google Scholar]
- Delatte B.; Wang F.; Ngoc L. V.; Collignon E.; Bonvin E.; Deplus R.; Calonne E.; Hassabi B.; Putmans P.; Awe S.; Wetzel C.; Kreher J.; Soin R.; Creppe C.; Limbach P. A.; Gueydan C.; Kruys V.; Brehm A.; Minakhina S.; Defrance M.; Steward R.; Fuks F. Science 2016, 351, 282–285. 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- Satoh A.; Takai K.; Ouchi R.; Yokoyama S.; Takaku H. RNA 2000, 6, 680–686. 10.1017/S1355838200000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin Y.; Helm M. Biochemistry 2010, 49, 4934–4944. 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- Païs de Barros J. P.; Keith G.; El Adlouni C.; Glasser A. L.; Mack G.; Dirheimer G.; Desgrès J. Nucleic Acids Res. 1996, 24, 1489–1496. 10.1093/nar/24.8.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Zhang L.; Mao S.-Q.; Li Z.; Chen J.; Zhang R.-R.; Wu H.-P.; Gao J.; Guo F.; Liu W.; Xu G.-F.; Dai H.-Q.; Shi Y.-G.; Li X.; Hu B.; Tang F.; Pei D.; Xu G.-L. Cell Stem Cell 2014, 14, 512–522. 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.