Abstract
The aim of our study was to compeer levels of antioxidative agent -total SH groups and the finale product of lipid peroxidationmalondialdehyde (MDA) in serum, and glutathione (GSH) and MDA in nucleocortical parts of lens after extracapsular extraction of cataract. Patient were (38 with cataract and 38 controls) matched by sex and years of life. Diagnosis of cataract was established by complete ocular examination. All results are expressed as mean ± S.D. A Student’s t-test was used to estimate differences between the groups. The level of significance was p<0,05. Total sulfhydryl groups were determined in serum by the method of Ellman as well as GSH content in nucleocortical parts of lenses using the method of Sedlak and Lindsay. Lipid peroxidation, evidenced by formation of thiobarbituric acid reactive substances (TBARS), was determined in nucleocortical parts of the lens and in serum. Our results show a statistical significance in concentration of total SH groups (225,37±82,19μmol/L, controls 311,03±60,37μm0l/L p<0,05) and MDA (20,24±8,12, and controls 8,73±2,53μmol/L, p<0,001) in serum among patients with age related cataract and controls. There was no statistical significance in concentration of total SH groups and MDA in serum among patients with different type of age related cataract and in nucleocortical parts of lens. The present study concludes that there is a statistical significance in concentration of total SH groups and MDA in serum among patients with age related cataract and controls, but there were no statistical significance in concentration of GSH and MDA in serum and nucleocortical parts of lens in patient with different type of cataract.
Keywords: cataract age related, oxidative stress, GSH, MDA
INTRODUCTION
Age related cataract is a vision - imparing disease. It is one of the leading causes of reversible blindness in the world today. The pathophysiology behind age related cataract is complex and yet to be fully understood. It is belived that oxidation is a very early or initiating event in overall process in the sequence of events leading to cataract (1,2,3,4,5,6,7). Oxidative stress may result from an imbalance between the production of reactive oxygen species and the cellular antioxidant defence mechanisms. In the cells of the eyes reactive oxygen species may initiate a surge of toxic biochemical reactions such as peroxidation of membrane lipids and extensive damage to proteins causing intracellular protein aggregation and precipitation (2,8,9,10). Oxidative insult probably originates at cell membranes (11). The most important oxidants are free radicals. Free radicals are molecules that have unpaired electrons in their outer shell. This unpaired state makes them very unstable and prone to react with other molecules to eighter gain or lose electrons. A major source of free radicals is the partial reduction of di oxygen by heme proteins and flavoproteins during mitochondrial electron transport (11).
Glutathione (GSH) is the principal lenticular antioxidant of the lens and it is synthesized and regenerated on the lens cortex (12). The remarkably high concentration of this compound in the lens also suggest that it provides a ready target for oxidizing agent, thus protecting more vulnerable molecules. Careful examination of glutathione in lens epithelial cells suggests that under normal conditions, 1% or less of the glutathione is in the oxidized form (12). Even after oxidative attack, when more than 50% of the glutathione may be in the oxidized form, GSSG has within a few minutes plummeted to normal trace levels. The hydroxyl radical can cause lipid peroxidation chain reaction with polyunsaturated fatty acids to form lipid peroxides (12,13,14). It is widely accepted that lipid peroxidation associated with oxidation of membrane proteins can cause a breakdown of transmembranes ion gradients and loss of cellular viability. One of the byproducts of lipid peroxidation is the toxic compound malondialdehyde (MDA), whose involvement in cataractogenesis has been suggested, manly due to its cross-linking ability (15, 16). The aim of our study was to compeer levels of antioxidative agent -total sulfhydryl groups(SH) and the finale product of lipid peroxidationMDA in serum and GSH and MDA nucleocortical parts of lens after extracapsular extraction of cataract (ECCE).
MATERIALS AND METHODS
Patient, matched by sex and years of life (SD±3 years), were divided in two groups: patients with lens opacities and without lens opacities. During selection of the patients from both groups, controls and patient with cataract it was made sure that they were without previous medical history from any chronic disease or metabolic disorder. Diagnosis of cataract was established by complete ocular examination (visual acuity, slit lamp examination, direct and indirect ophthalmoscopy, ultrasonography and tonometry). Patients with cataract were divided into 3 main types, due to The Lens Opacities Classification System, Verso III (LOCS III): nuclear cataract, cortical cataract, and posterior subcapsular cataract (PSC) (20). The lens was observed with slitlamp in dark room with pupilla dilatated. Solutio Phenylephrine 10%, drops topically, were used for mydriasis. The pupilla was dilatated to 6 mm. The LOCS III was used for grading lens opacities (20). The biochemical determinations were carried out in serum and 10% homogenates of nucleocortical parts of lenses after ECCE. Total sulfhydryl groups were determined in serum by the method of Ellman (21) as well as glutathione content (GSH) in nucleocortical parts of lenses using the method of Sedlak and Lindsay (22). Lipid peroxidation, evidenced by formation of thiobarbituric acid reactive substances (TBARS), was determined in nucleocortical parts of the lens and in serum (23). Protein content in tissue homo-ge-nates was determined according to the method of Lowry (24). In blood serum of 76 patients, 38 controls and 38 patients with cataract we measured total SH groups and MDA. Total -SH concentration was determined by using 5-5’-dithio-bis (2-nitrobenzoic acid) (DTNB) as described by Ellman (1959)(21). Absorbance were measured at 412 nm against blank samples without and expressed as mmol/l. Concentration of SH groups is expressed in μmol/L. Serum MDA levels were determined by spectrophotometry at 532 nm after boiling the sample and condensing it with thiobarbituric acid (TBA) (23). The results were expressed as μmol/l. In 38 patients, after ECCE, in nucleocortical parts of lens we measured concentration of glutathione and MDA. Lens tissue samples were homogenized in Elvenjem-Potter homogenizer (10% homogenate).
Concentration of GSH in lens was detected in 10% homogenous in 0,02M EDTA with DTNB(ɛ=13,6Mcm- 1-412nm). Content of GSH in 10% homogenates of tissues was determined by a modification method of Sedlak and Lindsday (1968). Ellmans reagent (5,5”-di- thiobis-(2-nitrobenzoic acid), reacting with sulfhydryl groups with maximal absorbance at 412 nm. Concentration of GSH expressed as nmol/mg proteins. All results are expressed as mean ± S.D. A Student’s t-test was used to estimate differences between the groups. The significance was p<0,05.
RESULTS
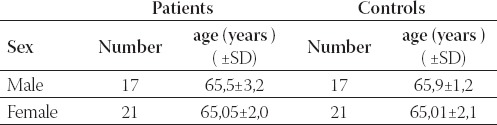
The mean age and sex in patients and controls are presented in Table 1.
TABLE 1.
Mean age and sex in patients and controls
Concentration of total SH groups and MDA in serum of patients with age related cataract and controls are summarized in Table 2.
TABLE 2.
Concentration of total SH and MDA in serum

There is a statistical significance in concentration of total SH groups and MDA in serum among patients with age related cataract and controls Table 3. We divided 38 patients with age related cataract in 3 groups, depending on a type of cataract. The concentration of GSH and MDA are summarized in Table 3.
TABLE 3.
Concentration of total SH groups and MDA in blood serum in different type of age related cataract

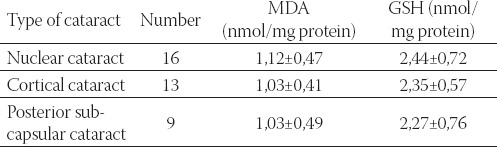
In Table 4. is presented concentration of MDA and GSH in nucleocortical parts of cataracts lenses. There was no statistical significance in concentration of total SH groups and MDA in nucleocortical parts of cataract lenses among patients with different type of age related cataract.
TABLE 4.
Concentration of MDA and GSH in nucleocortical parts of cataract lenses
DISCUSSION
There are 3 metabolically distinguishable zones of the lens: the epithelium, the cortex, and the lens nucleus or core (8, 9). Epithelial cells are found just under the collagenous capsule that surrounds the lens (8). These are the most recently formed cells and they are the most metabolically active. Some of these cells divide to form lens fibres cells. It is in these cells that the major gene products of the lens, the crystalline, are elaborated (8, 9, 10). The outer layers of such fibres cells comprise the cortex. Buried under the cortical cells are the oldest lens cells, called nuclear or core cells. Thus, there is a gradient, with the most recently elaborated proteins in the epithelium and the oldest proteins, which were elaborated during embryonic stages, in the nuclear cells (4). Posterior subcapsular (PSC) opacities are primarily due to aberrations in the outermost layers of the lens (9, 10, 11). Cortical opacities involve inner and outer cortical tissue. Many cataracts involve the cortex. Nuclear opacities are found in the central and oldest zone, which is metabolically quiescent. Because of these metabolic distinctions, some investigators think that opacification in these 3 zones has different etiology, and most epidemiologic studies treat the 3 zones separately (17, 18, 19). A normal lens is well equipped with protective agents and systems to combat oxidative stress, over decades, chronic exposure to active forms of oxygen may lead to the gradual erosion of the antioxidant protective mechanisms of the lens. The major antioxidants in lens are GSH and ascorbic acid (11, 12, 13, 14). Depletion of antioxidants renders makes the lens susceptible to oxidative damage. It results in accumulation of oxidized residues in the long-lived lens proteins and enzymes. The effect is a loss of normal metabolic function and derangement of the organization of the normal intracellular protein matrix necessary for transparency. Low concentration of H2O2 may be responsible for the oxidative modification of the lens proteins during the development of age related nuclear cataracts (12). Nourmohammadi among fourth-five patients with age related cataract and 35 controls (selected and matched), indicated that the total antioxidant status of the patients and controls has a significant difference between controls and patients) (12). Kao proved that the concentration of azotmonoxide (NO), as one of the product of oxidative stress, is a higher in humor aqueous in patients with age related cataract (13). A common feature of nuclear cataract is the low concentration of GSH in the centre of the lens (13, 19).
Towardi proves that the levels of reactive substances with thiobarbituric acid in plasma is higher in patients with age related cataract and also the levels of MDA are higher in red blood cells and plasma in patients with age related cataract then in controls (25). GSH is the obvious compound for defending the lens against oxidative insult being directly involved in reducing disulfides, being a pivotal cofactor in detoxication of H202 and acting as a free-radical quencher. Such data may be interpreted as suggesting that the intracellular environment of the epithelial cells is primarily a reducing environment. The deleterious nature of the presence of GSSG is apparent form the reports of protein GSH mixed disulfides formed as a result of the presence of the GSSG. Such mixed disulfides can lead to protein disulfides and further modification. MDA is one of the byproducts of lipid peroxidation, whose involvement in cataractogenesis has been suggested, mainly due to its cross-linking ability. The lens MDA may be the result of lipid peroxidation of the lens cells membranes or may represent the consequence of its migration from the readily peroxidized retina or from the central body compartment. Our results prove that there is a statistical significance in concentration of total SH groups and MDA in serum between controls in serum and the patients with age related cataract. There was no statistical significance in concentration of total SH groups and MDA in plasma among patients with different type of age related cataract. Oxidative stress can be present or initiating factor in all three types of cataract: cortical, nuclear and posterior subcapsular.
CONCLUSION
The pathophysiology behind age related cataract is complex and yet not understood. Oxidation may be a very early or initiating event in overall process in the sequence of events leading to cataract.
List of Abbreviations
GSH - reduced form of glutathione
MDA - malondialdehyde
SH - sulfhydryl groups
ECCE - extracapsular extraction of cataract
GSSG - oxidized form of glutathione
TBA - thiobarbituric acid
DTNB - 5’-dithio-bis (2-nitrobenzoic acid)
REFERENCES
- 1.Knekt P, Heliovaara M, Rissanen A, Aromaa A, Aaran R. Serum antioxidant vitamins and risk of cataract. BMJ. 1992;305:1392–1394. doi: 10.1136/bmj.305.6866.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datiles MB, Kinoshita JH. Modification by Oxidative Damage. 4th ed. Vol. 73. Duans Ophthalmogy Lippincott - Raven; 1998. pp. 11394–11660. [Google Scholar]
- 3.Chitikara DK. Cataracta Formation Mechanisms. In: Yanoff M, Duker JS, editors. Ophthalmology. second ed. Vol. 4. Mosby; 2004. pp. 273–279. [Google Scholar]
- 4.Boulton M, Saxby LA. Age Changes. In: Yanoff M, Duker JS, editors. Ophthalmology. Second ed. Vol. 4. Mosby; 2004. pp. 261–268. [Google Scholar]
- 5.Taylor A, Jacques P, Chylack LT, Hankinson SE, Khu PM, Rogerset G, et al. Long-term intake of vitamins and carotenoids and odds of early age-related cortical and posterior subcapsular lens opacities1-4. J. Am. Clin. Nutr. 2002;75:540–549. doi: 10.1093/ajcn/75.3.540. [DOI] [PubMed] [Google Scholar]
- 6.Taylor A. Nutritional and environmental influences on risk for cataract. In: Taylor A, editor. Nutritional and environmental influenceson vision. Boca Raton, FL: CRC Press; 1999. pp. 53–93. [Google Scholar]
- 7.Leske MC, Chylack LT, Jr, Wu SY. The Lens Opacities CaseControl Study. Risk factors for cataract. Arch. Ophthalmol. 1991;109:244–251. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 8.Blondin J, Taylor A. Measures of leucine aminopeptidase can be used to anticipate UV-induced age-related damage to lens proteins: ascorbate can delay this damage. Mech. Ageing Dev. 1987;41:39–46. doi: 10.1016/0047-6374(87)90052-2. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer DA, Berger JJ, Peltier CZ, Taylor A. Protease activities in cultured beef lens epithelial cells peak and then decline upon progressive passage. Exp. Eye Res. 1988;46:579–590. doi: 10.1016/s0014-4835(88)80014-9. [DOI] [PubMed] [Google Scholar]
- 10.Boscia F, Grattagliano I, Vandermiale G, et al. Protein Oxidaton and Lens Opacity in Humans. Invest. Ophthalmol. Vis. Sci. 2000;41:2461–2465. [PubMed] [Google Scholar]
- 11.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other a repair systems in cataract and macular degenerations. Exp. Eye Res. 2009;88(2):195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourmohammadi I, Gohari L, Moddares M, et al. Evaluation of Erythrocytes Glutathione Peroxides, Superoxide Dismutase and Total Antioxidants in Cataract Patients. Arch. of Iranian Med. 2001;4:123–126. [Google Scholar]
- 13.Kyselova Z, Gajdosk A, Ulcina O, Miholova D, Karasu C, Stefek M. Effect of the Piroindole Antioxudant Stobadine on Development of Experimental Diabetic Cataract and on Lens Protein Oxidation in Rats: Comparation with vitamin E and BHT. Mol. Vis. 2005;11:56–65. [PubMed] [Google Scholar]
- 14.Sweeney MH, Truscott RJ. An Impediment to Glutathione Diffusion in Older Normal Lenses a Possible Precondition for Nuclear Cataract. Exp. Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- 15.Mc Namara M, Augusteyn RC. The Effects of Hydrogen Peroxide on Lens Proteins -a Possible model for Nuclear Cataract. Exp. Eye. Res. 1984;38(1):45–56. doi: 10.1016/0014-4835(84)90137-4. [DOI] [PubMed] [Google Scholar]
- 16.Kao CL, Chou CK, Tsai DC, et al. Nitric oxide levels in the Aqueous Humor in Cataract Patients. J. Cataract. Refract. Surg. 2002;28:507–512. doi: 10.1016/s0886-3350(01)01102-6. [DOI] [PubMed] [Google Scholar]
- 17.Jacques PF, Chylack LT., Jr Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am. J. Clin. Nutr. 1991;53:352–355. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- 18.The Italian-American Cataract Study Group. Risk factors for agerelated cortical, nuclear, and posterior subcapsular cataracts. Am. J. Epidemiol. 1991;133:541–553. [PubMed] [Google Scholar]
- 19.Vitale S, West S, Hallfrisch J, et al. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiol. 1993;4:195–203. doi: 10.1097/00001648-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Chylack TL, Wolf JK, Singer DM, et al. The lens opacitates Classification System III. Arch Ophthalmol. 1993;11:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 21.Ellman LG. Tissue Sulphydryl groups. Arch. Biochem. Biophys. 1952;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Sedlak J, Lindsday R. Estimation of total protein bound and nonprotein sulphydryl groups in tissue with Ellman s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 23.Ohkava H, Ohishi N, Taki K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Lorwy OH, Rosenbrough NJ, Farr AL, Randall T. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1955;82:70–77. [PubMed] [Google Scholar]
- 25.Tarwadi K, Agte V. Linkages of Antioxidant, Micronutritient and socioeconomic status with the Degree of Oxidative Stress and Lens Opacity in Indian Cataract patients. Nutrition. 2004;20:261–267. doi: 10.1016/j.nut.2003.11.020. [DOI] [PubMed] [Google Scholar]


