Abstract
Chromium is transported in mainstream tobacco smoke at very low concentrations. However, when chromium is deposited too deeply in the lungs for mucociliary clearance, or is in a particle that is too large to pass directly through tissues, it bioaccumulates in the lungs of smokers. It is important to determine the concentrations of chromium that are transported in mainstream smoke.
Several reliable studies have resulted in reports of chromium concentrations in smoke particulate that were below limits of detection for the instruments and methods employed. In this study, electrothermal vaporization-triple quad-inductively coupled plasma-mass spectrometry (ETV-QQQ-ICP-MS) was chosen for determination of chromium concentrations in mainstream smoke because of the high sensitivity of ETV combined with QQQ-ICP-MS. The smoke from five reference, quality control, and commercial cigarettes was analyzed using ETV-QQQ-ICP-MS with isotope dilution for quantitative determination of chromium. The method limit of detection (LOD) was sufficiently low that chromium concentrations in mainstream smoke could indeed be determined. The chromium concentrations in the smoke particulate were between 0.60 and 1.03 ng/cigarette. The range of chromium concentrations was at or below previously reported LODs.
Determination of the oxidation state of the chromium transported in mainstream smoke would also be important, in consideration of the fact that both chromium(III) and chromium(VI) oxidation states cause inhalation toxicity, but chromium(VI) is also a carcinogen. It was possible to separate the oxidation states using ETV-QQQ-ICP-MS. However, determination of individual species at the levels found in mainstream smoke particulate matter was not possible with the present method.
Introduction
Toxic metals and metalloids constitute one of the five major classes of chemical carcinogens found in mainstream cigarette smoke.1 Chromium(VI) is one of the metals in mainstream smoke particulate considered a contributor to smoking-related health risks. Chromium(VI) is classified by the International Agency for Research on Cancer as a Group 1 human carcinogen,2 and chromium(VI) exposure from smoking has been associated with increased risk for both cancer and non-cancer adverse health outcomes.1 Though the Minimum Risk Level for intermediate inhalation exposure to particulate chromium(III) (exposure for 15 – 364 days, 0.005 mg chromium(III)/m3) is approximately 17 times higher than for carcinogenic chromium(VI) (0.0003 mg chromium(VI)/m3),3 there are health risks from intermediate and chronic inhalation exposure to chromium(III) as well, including sensitization, inflammatory response, and interstitial fibrosis.3,4 Neither chromium(III) nor chromium(VI) is a volatile elemental species like mercury or cadmium, so the transport of total chromium from tobacco into cigarette smoke particulate is low compared with more volatile metals.5,6 Chromium nevertheless bioaccumulates in the lungs of smokers,7,8 indicating that chromium in some nonvolatile form is transferred from tobacco during combustion into mainstream smoke particulate. The resulting deposition of chromium in smokers' lungs is significantly correlated with smoking time, number of pack years, and severity of emphysema.7,8 For this reason, it is important to be able to quantitatively determine the chromium levels in mainstream cigarette smoke.
A variety of methods have been used to measure chromium in cigarette filler. Chromium concentrations in filler tobacco from 28 commercial and research cigarettes available in the U.S. have been determined using graphite furnace atomic absorption spectroscopy (GFAA).9 Microwave digestion systems and inductively coupled plasma-mass spectrometers that are presently available have diminished the difficulty of chromium determination in tobacco, despite analytical interferences. Though an instrument detection limit was used rather than a method detection limit, chromium concentrations in 59 tobacco products available in Pakistan were determined using quadrupole ICP-MS (q-ICP-MS) with a collision cell.10 Chromium concentrations in 52 commercial and research cigarettes available in the U.S.,5 and in 36 smokeless and reference tobacco samples available in the U.S.11 were determined using sector field ICP-MS. Chromium concentrations in tobacco from 17 little cigars and 23 research cigarettes available in the U.S. were determined using QQQ-ICP-MS.12,13 It is apparent that ICP-MS has replaced GFAA as the instrumentation of choice for analysis of chromium in tobacco over the last decade.
Determination of chromium in mainstream cigarette smoke particulate is more challenging than determination of chromium in tobacco. The smoke particulate must be obtained from cigarettes prepared according to accepted standards and smoked according to ISO or Health Canada Intense (HCI) smoking regimens.14-16 To avoid contamination from the trapping medium, electrostatic precipitation with high purity quartz collection tubes is superior to the use of glass or quartz fiber filters.6,17 Even though high purity quartz collection tubes were utilized, Counts et al. reported chromium concentrations in mainstream smoke particulate from 30 research and international commercial cigarettes (20 cigarettes per analysis smoked under ISO conditions) as below GFAA method quantitation limits.17 Counts et al. later reported chromium concentrations in mainstream smoke particulate from 50 Philip Morris USA, Philip Morris International, and research cigarettes (10 to 20 cigarettes smoked per analysis under ISO, Massachusetts Department of Public Health, and HCI smoking conditions) as below GFAA method quantitation limits.18 Counts et al., however, reported chromium concentrations in mainstream smoke particulate obtained from 12 of 28 cigarettes available in the U.S. (ISO smoking regimen, 20 cigarettes per analysis) as slightly above the GFAA method detection limit. The mean results in the latter study ranged from below the limit of quantitation to 3.0 ng/cigarette.9 Pappas et al. reported chromium concentrations in mainstream smoke particulate obtained from 53 commercial and research cigarettes available in the U.S. (10 to 60 cigarettes smoked according to ISO and HCI smoking conditions per analysis) as below q-ICP-MS method quantitation limits (< 0.88 ng/cigarette and < 1.1 ng/cigarette for the respective smoking conditions),6 and below QQQ-ICP-MS method quantitation limits (< 0.85 ng/cigarette) for particulate from 23 research cigarettes (10 cigarettes per analysis, HCI smoking regimen).19 Pappas et al. also reported the observation of random analytical false positives for chromium in the apparent concentration range of 1 to 4 ng per cigarette when He alone was used as q-ICP-MS cell gas with kinetic energy discrimination. The addition of 0.1 mL/min H2 along with He eliminated the false positives.6 In summary, analytical results from studies of chromium concentrations in smoke particulate from a large number of cigarette varieties available in the U.S. and Europe indicate that chromium concentrations were below method quantitation limits to slightly above quantitation limits for GFAA, q-ICP-MS, and QQQ-ICP-MS methods.
Since chromium accumulates in the lungs of smokers,7,8 its measurement in mainstream cigarette smoke is of public health importance. The results cited earlier demonstrate that, because of the very low chromium concentrations in mainstream smoke, determination of chromium concentrations in smoke particulate requires ultraclean sample preparation and a more sensitive analytical methodology. We have previously used highly sensitive electrothermal vaporization (ETV)-ICP-MS with isotope dilution for the determination of ultrafine cadmium particle breakthrough from standard 0.1 μm glass fiber filters.20 A temperature program may be utilized with the ETV to separate the metal analytes from cigarette total particulate matter (TPM) matrix that is predominantly organic in nature. A reactive gas mixture (CCl4–saturated argon) is used in the ETV to convert the metals from nonvolatile forms, such as oxides, to a more volatile form. At temperatures above 600°C, the carbon-halogen bonds in CCl4 dissociate, and the liberated chlorine reacts with metals or metal oxides to produce volatile chlorides, enabling volatilization at lower temperatures than would be possible otherwise. The metal chlorides enter gas phase, are carried by the argon flow from the ETV, and enter the ICP plasma separated from the original sample matrix at higher concentrations than obtained when diluted for introduction by liquid sample flow injection. This increases sensitivity with ETV-ICP-MS methods relative to flow injection liquid sample introduction approaches. For these reasons, we chose ETV-ICP-MS as an optimal choice for the quantification of chromium in mainstream cigarette smoke particulate.
Reliable quantitative and speciation analysis for chromium(III) and chromium(VI) in mainstream cigarette smoke is highly desirable. ETV temperature programs were developed for quantitative analysis of total chromium in mainstream tobacco smoke and for separation of chromium(III) and chromium(VI) before speciation analysis using double species-specific isotope dilution. We report the results of the analyses of chromium in mainstream smoke from a reference cigarette, a quality control cigarette, and three commercial cigarettes.
Experimental
Cigarettes
CORESTA Monitor CM7 cigarettes (CORESTA, Paris France) were obtained from Cerulean (Henrico, Virginia, USA). The 3R4F research reference cigarettes were obtained from University of Kentucky, Lexington, KY, USA. Commercial cigarette varieties were purchased in 2014 from retail outlets in the greater metropolitan Atlanta area in Georgia, USA. The full flavor commercially available cigarette varieties were chosen based on 100 mm length or high tobacco mass and low ventilation for high TPM delivery. Prior to smoking, cigarettes were conditioned according to ISO 3402.15
Cigarette physical parameters were determined using a C2 instrument (Cerulean, Henrico, Virginia, USA) with the exception of dried tobacco mass, rod length, and filter length. Filter and rod lengths were measured manually with a National Institute of Standards and Technology (NIST) traceable ruler. Dried tobacco mass was determined manually with a certified balance.
Reagents
Ultrapure nitric acid was obtained from GFS Chemicals, Powell, OH, USA. Tert-butanol (puriss. p.a.) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Water was brought to ≥ 18 MΩ·cm with combined reverse osmosis and deionized water systems (Aqua Solutions, Jasper, GA, USA). NIST-traceable isotopically natural chromium(III) and chromium(VI) standards were obtained from High Purity Standards (Charleston, SC, USA). Enriched and natural chromium isotopes were obtained from Applied Isotope Technologies (Pittsburgh, PA, USA).
Tobacco chromium concentrations
Concentrations of chromium in the cigarette filler tobacco were determined as described using a fully validated ISO 17025 accredited method.12 These data, along with the smoke data, allowed us to determine what cigarette physical and chemical parameters influence the amount of chromium transferred into mainstream smoke.
Total particulate matter analysis for total chromium with ETV
A Borgwaldt RM20H rotary smoking machine (Borgwaldt KC, Hamburg, Germany) was used to smoke 10 cigarettes per analysis using the HCI smoking regimen16 with filter ventilation blocking cigarette holders. Air flow, puff volume and leak tests were performed daily to assure acceptable performance. The TPM was trapped in high purity quartz tubes by electrostatic precipitation (24.0 kV). The mass difference before and after collection in the quartz tubes was used to determine TPM deliveries. TPM was dissolved in 30.0 mL tert-butanol : water (1:1, v/v) by vortexing capped precipitation tubes. TPM solution (4.5 g) was transferred to precleaned polypropylene tubes and spiked with 100 μL 50Cr3+ (19.85 μg/L in 1% v/v HNO3). Solutions were vortexed. 100μL of 50Cr3+-spiked TPM solution was transferred to glassy carbon ETV boats (HTW Hochtemperatur-Werkstoffe GmbH, Thierhaupten, Germany), followed by evaporation of solvent. Boats were placed in the autosampler of the Spectral Systems ETV 4000 (Fürstenfeldbruck, Germany, distributed by ESI-Meinhard, Golden, CO, USA) that was interfaced with an Agilent 8800 (Tokyo, Japan) QQQ-ICP-MS. The argon carrier and sheath gas flows were 0.715 and 0.266 L/min, respectively. The CCl4-saturated argon reactive gas flow was 3.0 mL/min. The ETV temperature program used for analysis of total chromium was as follows:
10 second ramp to 400°C, hold at 400°C 15 seconds
5 second step to 600°C, hold at 600°C 20 seconds
0 second step to 2500°C, hold at 2500°C 25 seconds.
Total particulate matter analysis for speciated chromium with ETV
Cigarettes were smoked and TPM collected as described for total chromium analysis. The TPM solution (4.5g) was double spiked with 100 μL 50Cr3+ (19.85 μg/L in 1% v/v HNO3) and 100 μL 53Cr6+ (19.92 μg/L in 0.5% NH4OH). Solutions were vortexed. 100μL of double species-spiked solution were transferred to glassy carbon ETV boats, followed by evaporation of solvent. Boats were placed in the ETV system autosampler, which was interfaced with QQQ-ICP-MS. The ETV gas flows were the same as for total chromium analysis. The temperature program used for chromium speciation analysis (chromium(III) and chromium(VI)) was as follows:
10 second ramp to 400 °C, hold at 400 °C 15 seconds
0 second step to 800°C, hold at 800°C 25 seconds
0 second step to 1100°C, hold at 1100°C 25 seconds
0 second step to 1650°C, hold at 1650°C 20 seconds
0 second step to 2400°C, hold at 2400°C 15 seconds
0 second step to 2500°C, hold at 2500°C 15 seconds.
Quantitative analysis for chromium with ETV-QQQ-ICP-MS
Quantitative analysis was accomplished using single isotope dilution (total chromium) or double species-specific isotope dilution (chromium speciation) with direct introduction of volatilized samples from the ETV into the QQQ-ICP-MS injector. The QQQ-ICP-MS was operated at 1550 watts RF power, 15.0 L/min plasma gas, 0.90 L/min auxiliary gas in MS/MS mode. Sample gas was replaced by the combined sheath, carrier, and reactive gas flowing directly from the ETV to the injector. Nickel-plated Pt-tipped cones were essential for resistance to the chlorine from the CCl4 reactive gas in the plasma.
Isobaric interferences were rendered negligible in MS/MS mode using a Q1 entrance voltage of -5.0 V and chromium isotope mass shifts with a 5.0 mL/min flow of 10% electronics grade anhydrous ammonia, 90% ultrapure helium (Airgas, GA, USA). Conditions for the mass shift were -18 V octapole bias, -8 V KED, and 5 ms wait time offset. Sampling position and other lens voltages were optimized.
Chromium isotopes 50Cr, 52Cr, 53Cr focused through the first quad were reacted with NH3 in the octapole cell, then focused through the second quad and counted as the respective [Cr(NH3)2]+ complex ions; i.e., 50Cr was counted as mass 84 [50Cr(NH3)2]+, 52Cr was counted as mass 86 [52Cr(NH3)2]+, and 53Cr was counted as mass 87 [53Cr(NH3)2]+. Dead time was monitored and updated as required. Mass bias was evaluated for every analysis by bracketing analytical samples with the natural chromium in unspiked samples and correcting each respective isotope ratio.
Isotopic Accuracy, Method Limits of Detection
Undiluted and diluted enriched chromium isotope concentration accuracy was periodically verified by reverse isotope dilution with NIST-traceable natural chromium standards. Method (procedural) limit of detection (LOD) for total chromium was determined according to Taylor21 by plotting the standard deviations versus mean chromium results from 30 separate analyses of TPM from 3R4F, CM7, American Spirit® (full bodied, light blue pack), Newport 100 Menthol®, Marlboro 100®, and procedural blanks [obtained by vortexing 30.0 mL tert-butanol : water (1:1, v/v) in electrostatic precipitation tubes, subsampling, spiking with enriched isotopes as described for samples, but with no TPM collected in the quartz tubes]. The standard deviation extrapolated to “0” ng chromium per cigarette concentration according to Taylor was multiplied by three to obtain the LOD. Because of chromium(III) and chromium(VI) species interconversion in TPM at low concentrations, the Taylor method could not be used for individual species. For this reason, species-specific LODs were estimated based on 3 times the standard deviations of the analyses of 30 procedural blanks.
Statistical Analysis
Multivariate and bivariate statistical analyses for correlation of chromium concentrations in TPM with cigarette physical characteristics were performed using JMP software (SAS, Cary, NC, USA).
Results and Discussion
Method limits of detection
The procedural LOD for total chromium in TPM based on the Taylor method21 was 0.031 ng per cigarette. Individual species procedural LODs were 0.051 ng per cigarette chromium(III) and 0.19 ng per cigarette for chromium(VI), based on the analyses of 30 procedural blanks.
Total chromium concentrations in mainstream smoke TPM
Mainstream smoke particulate was obtained using the HCI smoking regimen16 so that the TPM delivery more closely reflects smokers' exposure ranges than would other regimens, such as ISO 3308.14 The TPM trapped using electrostatic precipitation was dissolved and diluted to 30.0 mL with 1:1 tert-butanol : water, a solvent combination that does not reduce chromium(VI). The tert-butanol : water solvent from the 100 μL aliquots transferred to glassy carbon boats was evaporated prior to analysis, leaving only a small TPM residue for analysis. The sample dilution volume was chosen to minimize carbon buildup from the TPM residue on the cones during vaporization by the ETV.
Total chromium concentrations in mainstream smoke TPM from all five brands were above the procedural LOD (Table 1). Though the 3R4F reference cigarette has a ventilated filter, the use of filter perforation-blocking cigarette holders with the HCI smoking regimen minimizes filter ventilation effects on TPM delivery. Consequently, the mean mainstream smoke total chromium delivery of the 3R4F reference cigarette was comparable to the total chromium deliveries of the other unventilated cigarette brands. The mainstream smoke mean total chromium concentrations for the five cigarettes ranged from 0.60 to 1.03 ng chromium per cigarette. All five of the total chromium concentrations are below the procedural LODs that we previously reported for mainstream smoke chromium concentrations obtained using HCI smoking regimen and analyzed using single quadrupole ICP-MS with kinetic energy discrimination.6 However, four of five of these mainstream smoke total chromium concentrations are below the procedural LOD that we reported using QQQ-ICP- MS without ETV.19 The results therefore agree with previous data, at least in the sense that the range of concentrations obtained using QQQ-ETV-ICP-MS would indeed have been less than or close to the LOD for the previously reported Q-ICP-MS and QQQ-ICP-MS methods,6,19 and with results from GFAA studies described in the Introduction.17,18
Table 1.
Mainstream smoke TPM and tobacco chromium concentrations, and physical characteristics of the cigarettes.
| TPM Cr, ng/cigarette | Tobacco Cr concentration, μg/g (dry) | Filter length, mm | Tobacco length, mm | Tobacco mass, mg | Tobacco mass/length, mg/mm | Pressure drop open (mmWG)* | Pressure drop shut (mmWG)* | |
|---|---|---|---|---|---|---|---|---|
| 3R4F | 0.60 ± 0.17 | 2.05 ± 0.15 | 27 | 57 | 663 ± 5 | 13.3 | 137 | 184 |
| American Spirit | 0.63 ± 0.15 | 1.80 ± 0.38 | 27 | 56 | 769 ± 27 | 13.7 | 124 | 145 |
| CM7 | 0.64 ± 0.16 | 2.34 ± 0.30 | 21 | 62 | 656 ± 24 | 10.6 | 143 | 143 |
| Marlboro 100 | 0.84 ± 0.20 | 3.36 ± 0.27 | 27 | 71 | 719 ± 23 | 10.1 | 114 | 130 |
| Newport 100 | 1.03 ± 0.32 | 2.14 ± 0.08 | 27 | 72 | 727 ± 4 | 10.1 | 109 | 132 |
Millimeters water.
Cigarette physical parameters and tobacco chromium concentrations (Table 1) were analyzed for statistical correlations with TPM chromium concentrations to determine which parameters contribute significantly to chromium concentrations in smoke. All cigarettes had similar circumference (24.83 ± 0.17 mm), so circumference was not a variable in TPM chromium deliveries among the cigarettes studied. The results of multivariate data analyses showed no significant correlation between smoke TPM chromium concentrations and tobacco mass, a finding that contrasts with results for more volatile arsenic, cadmium, and lead.6 There was no significant correlation between smoke TPM chromium concentrations and tobacco rod mass or length, pressure drop open, pressure drop shut, or even tobacco chromium concentration (p > 0.05, data in Table 2). The chromium concentrations in mainstream smoke were also independent of the filter lengths of these cigarettes, in contrast with the more volatile element, cadmium.6 TPM chromium concentrations were positively correlated with length of the tobacco rod, as were arsenic and lead, in contrast with the more volatile cadmium.6
Table 2.
Statistical analysis of smoke TPM chromium concentrations correlated with tobacco chromium concentrations and physical characteristics of the cigarettes.
| Variable | Correlation | Probability of Significance |
|---|---|---|
| Filter Length | 0.3274 | 0.5907 |
| Tobacco Length | 0.9150 | 0.0294 |
| Tobacco Mass | -0.0895 | 0.8862 |
| Tobacco Density in Rod | -0.7217 | 0.1687 |
| Pressure Drop Open | -0.8532 | 0.0660 |
| Pressure Drop Shut | -0.6621 | 0.2235 |
| Tobacco Cr Concentration | 0.1060 | 0.5928 |
Comparison of the physical parameters that were correlated with arsenic, cadmium, and lead concentrations in TPM versus those that were correlated with chromium concentrations suggests that chromium is probably not volatilized, but perhaps liberated in particulate from the surrounding matrix during combustion. Appreciable particulate was likely trapped along the tobacco in the rod before reaching the filters, which made filter length insignificantly correlated with TPM chromium concentrations. It is possible that the chromium-containing particulate deposited along the rod, as the longer the tobacco rod, the greater the percentage of tobacco that would undergo combustion. This behavior perhaps increased the opportunities for combustion of leaves on which chromium-containing particles had previously deposited, permitting repeated liberation until some small portion of the total chromium passed through the cigarette. Chromium concentrations in tobacco were not statistically correlated with smoke TPM chromium concentration, but the tobacco chromium concentration range was small.
Chromium speciation in mainstream smoke TPM
Separation of chromium(III) and chromium(VI) was accomplished using QQQ-ETV-ICP-MS with the ETV heating program described in Section 2.5. Chromium(III) and chromium(VI) species are apparent in the vaporizogram of the diluted TPM obtained from a quality control cigarette (Figure 1). The intensities of two separate peaks increased when chromium(III) was spiked into the TPM (Figure 2). This is possibly due to cis and trans chromium coordination complex isomers with at least one bidentate ligand that were volatilized by chlorine in the reactive gas. Volatile cis and trans isomers of chromium(III) with bidentate ligands are separable even at gas chromatographic temperatures.22 Since one or more of the oxygen ligands would have been replaced with chloride ligands in order to volatilize chromium(III), it is also possible that the two peaks derived from the chromium(III) spike represent chromium(III) with different combinations of chlorine and oxygen ligands, or possibly the formation of a higher oxidation state such as chromium(IV) or chromium (V) as a result of oxidation by chlorine generated from CCl4 in the ETV. Evidence favoring the latter interpretation comes from the reversal of the peak intensities between the second and third peaks when the sample was spiked with natural chromium(VI) (Figure 3).
Figure 1.
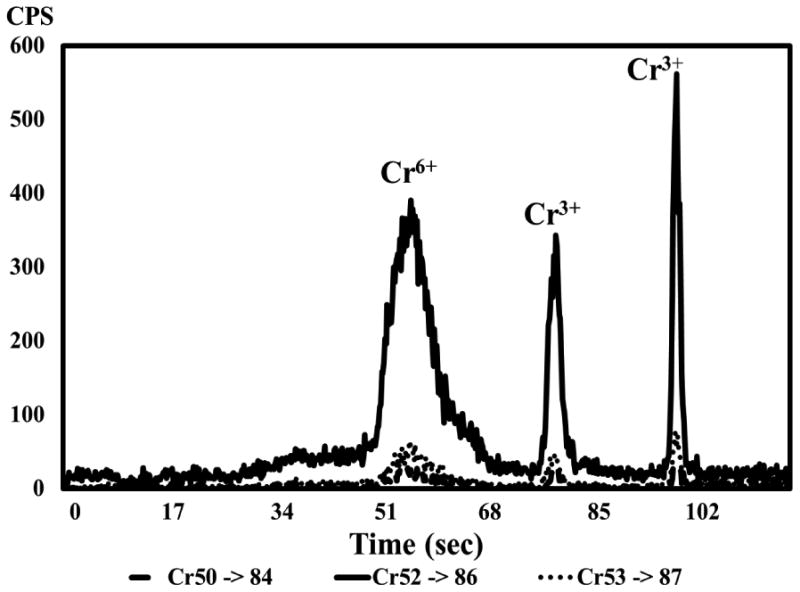
ETV-ICP-MS vaporizogram of unspiked chromium in diluted particulate matter from mainstream smoke of a CORESTA Monitor 7 cigarette.
Figure 2.
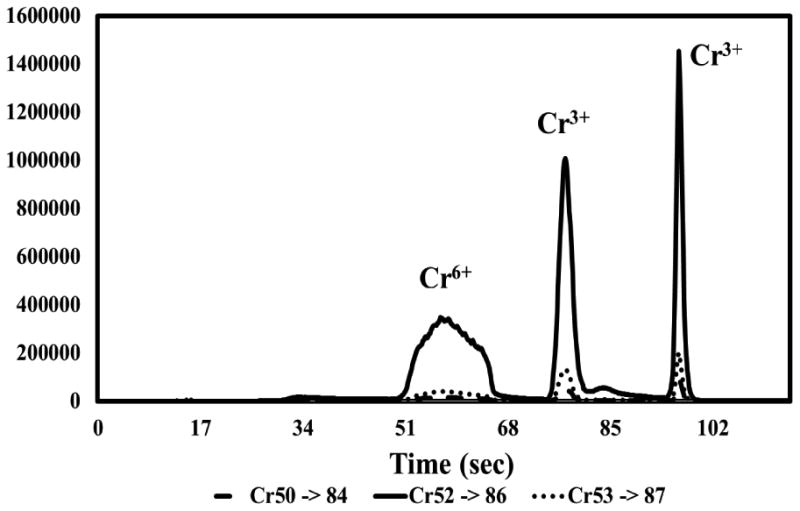
ETV-ICP-MS vaporizogram of chromium in diluted particulate matter from mainstream smoke of a CORESTA Monitor 7 cigarette spiked with 67ng natural Cr3+.
Figure 3.

ETV-ICP-MS vaporizogram of chromium in diluted particulate matter from mainstream smoke of a CORESTA Monitor 7 cigarette spiked with 67 ng natural Cr6+.
The separation of the chromium species with ETV-ICP-MS suggests that it would be possible to determine the actual oxidation state of chromium present in mainstream cigarette smoke using this technique and to determine the concentrations using species-specific isotope dilution. However, at the < 20 ng chromium in the sample boats from the diluted TPM, nearly 100% interconversion between chromium(III) and chromium(VI) was observed, rendering the double spike approach meaningless. Only when total chromium in the sample boats was ≥ 20 ng was the interconversion between chromium(III) and chromium(VI) < 80% (α + β), permitting quantitative analysis of the species.
The natural spiked concentration is more than 20 times greater than the total chromium concentration determined per cigarette in the TPM. The TPM samples were diluted with 30mL t-butanol: water (v/v) and could not be run at lower dilution, because the organic material from the TPM resulted in much higher interferences and caused rapid carbon buildup on the ICP-MS sampler cone, blocking the orifice and preventing ion sampling. Because of the problem with carbon buildup, the samples could not be run at the higher concentrations needed to quantitate the chromium species, and the determination of the actual oxidation state(s) of the chromium in mainstream cigarette smoke was not possible. Cuello et al. also attempted speciation of chromium in mainstream cigarette smoke using HPLC-ICP-MS and synchrotron X-ray absorption spectroscopy.23 They reported that individual chromium oxidation states could not be determined in water leachates of the mainstream smoke TPM using HPLC-ICP-MS. TPM and chromium(III) oxide are poorly water soluble, so this was useful but not unexpected information. Cuello et al. also reported that chromium(VI) was highly unstable when spiked into TPM, confirming that strongly oxidizing chromium(VI) is not stable in a reducing medium such as TPM. The X-ray absorption spectroscopy data for chromium in smoke reported by Cuello et al. added limited support to conclusions on speciation. The glass fiber filter pad (blank) spectrum appeared to be almost identical to the filter with smoke TPM, differing in that the entire spectrum, including baseline, was shifted higher with no presence of chromium (VI) in the spectrum. The spectrum of TPM on kapton tape indicated the possible presence of a chromium(III) edge. They concluded, as did we, that they found no evidence for detectable levels of chromium(VI) in mainstream smoke.
Chromium bioaccumulation from mainstream smoke TPM: Implications on speciation
Chromium bioaccumulates in the lungs as a result of exposure from smoking. The level of accumulation depends on smoking time, number of pack years, and severity of emphysema.7,8 Chromium(VI) oxide as chromate or dichromate is soluble in acidic, neutral, or alkaline aqueous solutions with few exceptions, and is more rapidly absorbed after inhalation exposure than is chromium(III).3 Chromium(III) oxide is poorly soluble in neutral or alkaline aqueous solutions and far more slowly absorbed.3 The bioaccumulation of chromium as a consequence of inhalation exposure from smoking suggests that chromium in TPM is predominantly chromium(III).
Conclusions
This work resulted in an improved method for quantitative analysis of total chromium concentrations in mainstream cigarette smoke. The mainstream smoke yields of total chromium concentrations reported here for selected cigarettes are consistent with the below-LOD ranges previously reported. At the low concentrations reported here, individual chromium species were not determinable due to complete interconversion of the two principle oxidation states of the natural and enriched chromium species.
Acknowledgments
This work was funded by an interagency agreement with the U.S. Food and Drug Administration, Center for Tobacco Products.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. [accessed 10 December 2015];Chromium (VI) compounds. 2012 http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-9.pdf.
- 3.American Toxic Substances and Disease Registry. [accessed 3 October 2016];Toxicological Profile for Chromium. 2012 :11–49. http://www.atsdr.cdc.gov/ToxProfiles/tp7.pdf. [PubMed]
- 4.Pappas RS. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics. 2011;3:1181–1198. doi: 10.1039/c1mt00066g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fresquez MR, Pappas RS, Watson CH. Establishment of Toxic Metal Reference Range in Tobacco from U.S. Cigarettes. J Anal Toxicol. 2013;37:298–304. doi: 10.1093/jat/bkt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas RS, Fresquez MR, Martone N, Watson CH. Toxic Metal Concentrations in Mainstream Smoke from Cigarettes Available in the USA. J Anal Toxicol. 2014;38:204–211. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pääkö P, Kokkonen P, Anttila S, Kalliomäki PL. Cadmium and chromium as markers of smoking in human lung tissue. Environ Res. 1989;49:197–207. doi: 10.1016/s0013-9351(89)80065-9. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiyama F, Hisanaga N, Shibata E, Aoki T, Takagi H, Ando T, Takeuchi Y. Pulmonary metal distribution in urban dwellers. Int Arch Occup Environ Health. 1997;70:77–84. doi: 10.1007/s004200050190. [DOI] [PubMed] [Google Scholar]
- 9.Counts ME, Hsu FS, Tewes FJ. Development of a commercial cigarette “market map” comparison methodology for evaluating new or non-conventional cigarettes. Regul Toxicol Pharmacol. 2006;46:225–242. doi: 10.1016/j.yrtph.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Musharraf SG, Shoaib M, Siddiqui AJ, Najam-ul-Haq M, Ahmed A. Chem Cent J. 2012;6:56. doi: 10.1186/1752-153X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J Anal Toxicol. 2008;32:281–291. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 12.Pappas RS, Martone N, Gonzalez-Jimenez N, Fresquez MR, Watson CH. Determination of toxic metals in little cigar tobacco with ‘Triple Quad’ ICP-MS. J Anal Toxicol. 2015;39:347–352. doi: 10.1093/jat/bkv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter P, Pappas RS, Bravo R, Lisko J, Damian M, Gonzalez-Jimenez N, Gray N, Keong LM, Kimbrell JB, Kuklenyik P, Lawler TS, Lee GE, Mendez M, Perez J, Smith S, Tran H, Tyx R, Watson CH. Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. doi: 10.18001/TRS.2.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Organization for Standardization. Routine analytical cigarette-smoking machine—Definitions and standard conditions. 2000:1–23. ISO 3308. [Google Scholar]
- 15.International Organization for Standardization. Tobacco and tobacco products – atmosphere for conditioning and testing. 1999:1–4. ISO 3402. [Google Scholar]
- 16.Hammond D, Fong GT, Cummings KM, O'Connor RJ, Giovino GA, McNeill A. Cigarette yields and human exposure: A comparison of alternative testing regimens. Cancer Epidemiol Biomar. 2006;15:1495–1501. doi: 10.1158/1055-9965.EPI-06-0047. [DOI] [PubMed] [Google Scholar]
- 17.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul Toxicol Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Pappas RS, Gray N, Gonzalez-Jimenez N, Fresquez M, Watson CH. Triple Quad-ICP-MS measurement of toxic metals in mainstream cigarette smoke from Spectrum research cigarettes. J Anal Toxicol. 2016;40:43–48. doi: 10.1093/jat/bkv109. [DOI] [PubMed] [Google Scholar]
- 20.Pappas RS, Fresquez MR, Watson CH. Cigarette smoke cadmium breakthrough from traditional filters: Implications for exposure. J Anal Toxicol. 2015;39:45–51. doi: 10.1093/jat/bku115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor JK. Quality Assurance of Chemical Measurements. Boca Raton: CRC Press; 1987. p. 79. [Google Scholar]
- 22.Moshier RW, Sievers RE. Gas Chromatography of Metal Chelates. New York: Pergamon Press; 1965. pp. 124–130. [Google Scholar]
- 23.Cuello S, Entwisle J, Benning J, Liu C, Coburn S, McAdam KG, Braybrooka J, Goenaga-Infante H. J Anal At Spectrom. 2016;31:1818–1829. [Google Scholar]


