Abstract
Depletional strategies directed toward achieving tolerance induction in organ transplantation have been associated with an increased incidence and risk of antibody-mediated rejection (AMR) and graft injury. Our clinical data suggest correlation of increased serum B cell activating factor/survival factor (BAFF) with increased risk of antibody-mediated rejection in alemtuzumab treated patients. In the present study, we tested the ability of BAFF blockade (TACI-Ig) in a nonhuman primate AMR model to prevent alloantibody production and prolong allograft survival. Three animals received the AMR inducing regimen (CD3-IT/alefacept/tacrolimus) with TACI-Ig (atacicept), compared to five control animals treated with the AMR inducing regimen only. TACI-Ig treatment lead to decreased levels of DSA in treated animals at 2 and 4 weeks posttransplantation (p < 0.05). In addition, peripheral B cell numbers were significantly lower at 6 weeks posttransplantation. However, it provided only a marginal increase in graft survival (59 ± 22 vs. 102 ± 47 days; p = 0.11). Histological analysis revealed a substantial reduction in findings typically associated with humoral rejection with atacicept treatment. More T cell rejection findings were observed with increased graft T cell infiltration in atacicept treatment, likely secondary to the graft prolongation. We show that BAFF/APRIL blockade using concomitant TACI-Ig treatment reduced the humoral portion of rejection in our depletion-induced preclinical AMR model.
Introduction
Graft rejection mediated by T cells has been successfully controlled by current immunosuppressive regimens. However, long-term efficacy of clinical transplantation is still limited by the development of chronic rejection. Several lines of evidence suggest that B cells also play a crucial role in allograft rejection. Recent data have associated donor specific antibodies (DSA) with chronic rejection (1), increased risk of acute AMR and eventual graft loss (2, 3). The observation of DSA with acute and chronic rejection in transplant recipients implies that current immunosuppression is not adequately targeting the posttransplant humoral response.
BAFF (also known as BLyS) is a homotrimer that has been shown to play a role in B cell survival, maturation, and activation (4, 5). BAFF is elevated in various autoimmune diseases including SLE, rheumatoid arthritis (RA) and Sjögren’s syndrome and BAFF elevation is associated with autoantibody production (6); however, little is known about the role of BAFF and its receptors in transplantation. We have previously observed increased levels of serum B cell activating factor (BAFF) and upregulated humoral responses following depletional induction using alemtuzumab (7). Thompson et al (8) also reported elevated BAFF level in patients with multiple sclerosis (MS) treated with alemtuzumab.
BAFF exerts its activity upon ligation with three receptors: BAFF-R, TACI, and BCMA, all of which promote B cell/ plasma cell survival, T-dependent/independent antibody responses, and T cell co-stimulation (9). Several therapeutics targeting BAFF as treatment for autoimmune diseases have been developed (reviewed in Stohl [10]). Currently, belimumab is approved by the FDA for SLE in 2011. Atacicept, blisibimod, and tabalumab are currently tested in phase III trials for the therapy of SLE.
Atacicept, a recombinant fusion protein containing the soluble TACI receptor, was developed as a potential treatment of autoimmune diseases, such as SLE, rheumatoid arthritis (RA) and multiple sclerosis (MS) and as a treatment of B cell malignancies including multiple myeloma, B cell chronic lymphocytic leukemia and non-Hodgkin’s lymphoma (11). Here, we investigated the effects of BAFF blockade with atacicept on de novo DSA production and resultant antibody-mediated injury to renal allografts in a preclinical model of de novo DSA formation.
Materials and Methods
Animals and transplantation
Eight male, outbred juvenile rhesus macaques (Macaca mulatta) were tested to ascertain their specific pathogen free status and selected for expression of FN18 epitope, the binding site of anti-CD3 immunotoxin (IT). Donor-recipient pairs were selected on the basis of full MHC class I mismatch and maximal MHC class II disparity by DNA typing. Heterotopic renal allotransplantation was performed as previously described in rhesus macaques (12, 13). All medications, procedures, housing, and maintenance were approved by the Emory University Institutional Animal Care and Use Committee, and were conducted in accordance with Yerkes National Primate Research Center and the National Institutes of Health guidelines.
Treatment
All animals received the base regimen of anti-CD3 IT (A-dmDT390-scfbDb[C207], 0.025 mg/kg IV twice daily for 4 days, Massachusetts General Hospital—Dana Farber-Harvard Cancer Center Recombinant Protein Expression and Purification Core Facility, Boston, MA), tacrolimus (Astellas Pharma US, Inc., Deerfield, IL), and alefacept (LFA3-Ig, Astellas Pharma US, Inc.). One monkey, FA5K, received a reduced CD3-IT dose (0.025 mg/kg IV twice daily for 2 days) to avoid significant viremia and weight loss. Methylprednisolone (Solu-Medrol) 125mg was injected intravenously on days the animals received IT to alleviate potential symptoms of cytokine storm. Tacrolimus was started at 0.05 mg/kg intramuscular injection twice daily to maintain a trough of 8–12 ng/mL. Alefacept was administered at 0.3 mg/kg once weekly for 8 weeks, starting on POD −3, 0, then 7, 14, etc., based on the dosage used in Weaver et al (14). Three animals (DN2B, FA4P, DP7A) additionally received 50mg of TACI-Ig (Atacicept, Merck Serono, Geneva, Switzerland) subcutaneously posttransplant twice weekly for the first week and weekly up to 4 weeks. The five control animals have previously been reported (12, 13).
BAFF ELISA
The concentration of serum BAFF level was determined by enzyme linked immunosorbent assay (ELISA) using a Quantikine kit for human BAFF purchased from R&D systems (Minneapolis, MN). The BAFF level for each time point was an average of the measurements of duplicates.
Antibodies and flow cytometry
Peripheral blood was obtained by femoral venipuncture and isolated by Ficoll method using 5 mL of lymphocyte separation medium (Mediatech, Inc., Manassas, VA) per sample. Washed PBMCs were stained for live/dead cell using Aqua dead cell stain kit (Life Technologies, Eugene, OR) and were surface stained with the following antibodies: APC-Cy7 conjugated CD20 (2H7) and Pacific Blue conjugated IgM (MHM-88) (Both from Biolegend, San Diego, CA). FITC conjugated Goat anti-human IgD (δ chain specific, catalog no. 2030-02) was obtained from Southern Biotech (Birmingham, AL). Anti-donor specific IgG was detected and measured by flow cytometry using donor lymphocytes as target cells with recipient serum samples as previously described (12). The DSA production of each time point was expressed as fold increase compared to the pretransplant MFI value. Samples were collected with an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software 9.2. (Tree Star, Ashland, OR).
Histology
Tissue specimens were obtained from histopathological and immunopathological analysis at the time of necropsy. Routine light microscopy on H&E and PAS stained sections were performed on paraffin-embedded tissues. The degree of rejection was evaluated blindly by a pathologist (A.B.F.) according to the Banff -07 criteria (15), taking into consideration recent Banff updates (16). Antibody-mediated rejection score was calculated by combining allograft glomerulitis (g), allograft glomerulopathy (cg), mesangial matrix (mm), and peritubular capillary inflammation (ptc), and C4d staining score. Acute cellular rejection was defined by the presence of focal or diffuse interstitial inflammation (and edema) associated with mononuclear inflammatory cell invasion into the tubular epithelium (tubulitis) and/or intima of blood vessels (vasculitis).
Immunohistochemistry and quantitative image analysis (QIA)
All tissue staining procedures and QIA were performed as described in our previous studies (13). We immunostained human Ki67 (clone MM1, Vector, Burlingame, CA), CD20 (Thermo Scientific, Rockford, IL), and human CD3 (clone CD3-12, AbD Serotec, Raleigh, NC) with antibodies. Appropriate secondary antibodies (Jackson ImmunoResearch, West Grove, PA) and nucleus dye (Hoechst 33342, Invitrogen, Carlsbad, CA) were used. All images were acquired with an Axio Imager Z1 microscope (Carl Zeiss Microscopy, LLC, Peabody, MA) using 20× objectives. Mean fluorescence intensities of Ki67, CD3 and CD20 were analyzed using AxioVs40 V4.8.1.0 program (Zeiss) and Image J1.43u (NIH).
Statistics
Experimental variables were analyzed by Prism statistical analysis program (GraphPad Software 6.0, San Diego, CA) using the log-rank test for differences in graft survival and nonparametric Mann–Whitney or Kolmogorov–Smirnov tests for others. The repeated-measures ANOVA test was used for comparison of serum BAFF level over time. Error bars represent the mean ± SD in all bar graphs. P values less than 0.05 were considered to be statistically significant.
Results
Improved renal graft function and graft survival with atacicept treatment
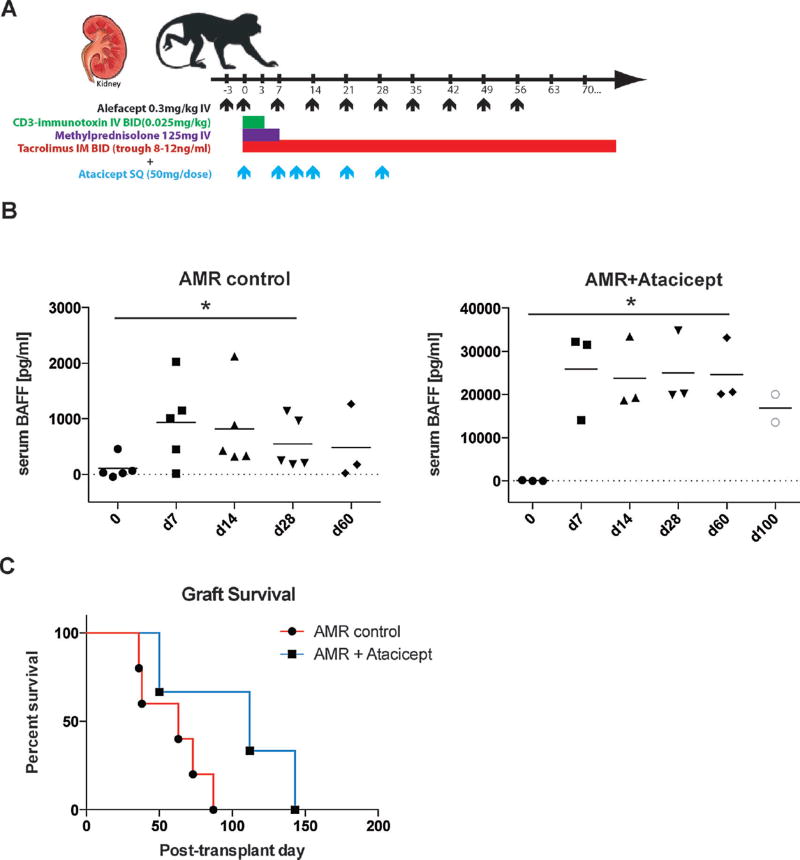
All animals received the AMR regimen of CD3-IT, alefacept, and tacrolimus maintenance, as outlined in the methods (Figure 1A). Three animals that additionally received atacicept (TACI-Ig, Merck Serono) were compared to five animals given the AMR regimen only (13). As shown in Figure 1B, serum BAFF level showed an increasing trend upon T cell depletion. Surprisingly, increased level of BAFF was measured after atacicept treatment (Figure 1B). The nonphysiologic level of serum BAFF suggests a detection of atacicept bound BAFF, which was confirmed by antibody-pull down assay (Supplemental Figure S1). The atacicept treated animals showed a modest prolongation of graft survival (50, 112, 143 days) relative to the AMR control animals (36, 38, 63, 73, 87 days). Mean graft survival of atacicept treated animals was near twice (n=3, 102±47 days) that of the AMR controls (n=5, 59±22 days). However, likely due to the low sample number, this difference did not reach statistical significance (p=0.11; Figure 1C).
Figure 1. Adding atacicept to AMR inducing regimen marginally prolongs graft survival.
(A) AMR dosing strategy and additional atacicept treatment are represented. Atacicept (TACI-Ig) was added to the AMR regimen, administered subcutaneously weekly for a month. Five animals received the AMR regimen, which includes CD3-immunotoxin, alefacept, and tacrolimus (trough 8–12 ng/mL). Three animals received the AMR regimen plus additional atacicept for 4 weeks (duration and frequency indicated by blue arrows along the x-axis). (B) Serum BAFF level from AMR control and additional atacicept treatment are represented. (C) Additional atacicept treated animals have a marginally prolonged graft survival (MST 101 days; p=0.1) compared to AMR controls (MST=56 days).
Decreased de novo alloantibody production with additional atacicept treatment
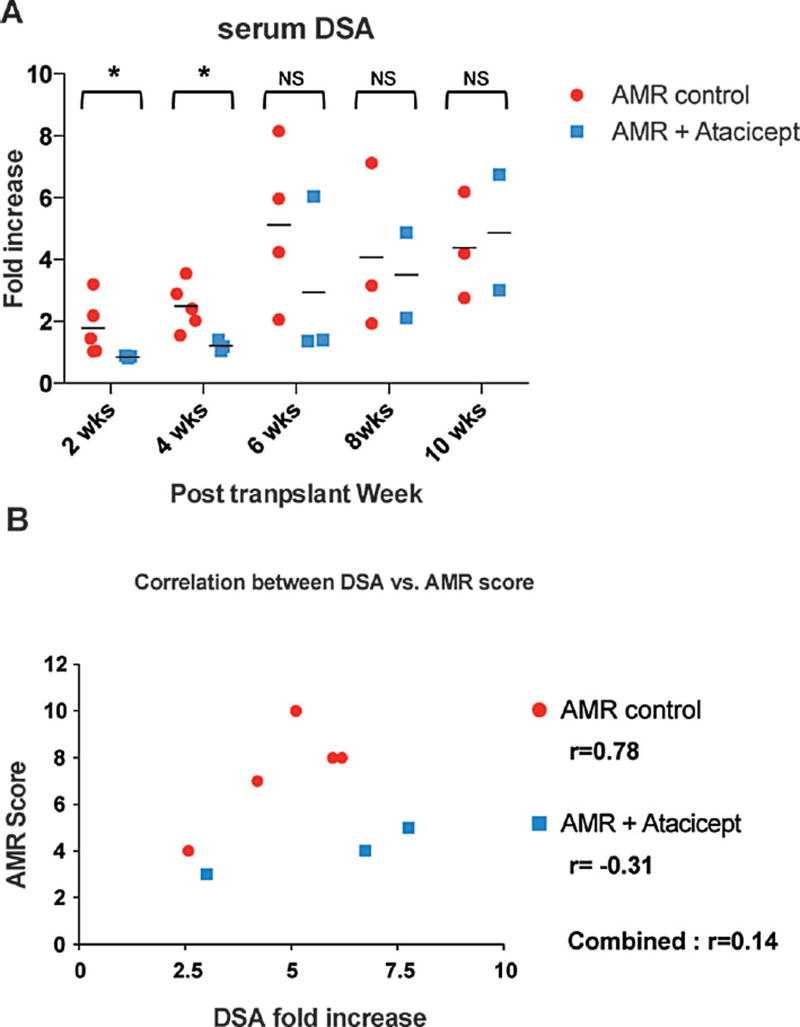
The prolongation of graft survival in the atacicept treated animals could reflect adequate inhibition of the humoral response via BAFF blockade. We therefore examined prospectively collected serum samples for de novo DSA production. The addition of atacicept resulted in significantly decreased de novo DSA compared to AMR controls at 2 and 4 weeks posttransplantation (Figure 2A). The rapid de novo DSA increase seen with AMR controls was blunted with atacicept treatment; however a compensatory production of DSA seemed to follow the discontinuation of atacicept at 4 weeks, as evidenced by increased DSA levels at 8 and 10 weeks posttransplantation. We also calculated a correlation between DSA levels at the time of sacrifice and AMR scores shown in Figure 2B. The correlation coefficient (r) between DSA and AMR score showed a strong positive linear relationship for AMR control animals (r=0.78). However this strong correlation was abrogated (r=0.14) by the inclusion of the atacicept treated animals (Figure 2B). These data suggest that in this depletion-based AMR model, the addition of atacicept suppresses early de novo DSA production, which leads AMR reduction but does not prevent graft rejection nor induce graft tolerance. It appears that the inhibition of DSA was dependent on atacicept treatment but DSA resumed once the atacicept treatment was discontinued. Maintenance of atacicept treatment may have further suppressed DSA production.
Figure 2. Atacicept reduced the level of early de novo DSA.
(A) DSA production after atacicept treatment. The formation of donor specific antibody in serum was monitored by flow cytometry analysis. All five AMR control animals had DSA detected by 2–4 weeks posttransplantation. Reduced level of serum DSA was detected in animals receiving additional atacicept at 2 and 4 weeks (*p < 0.05 vs. AMR control) but not the time points thereafter. (B) Pearson’s correlation coefficients were calculated between DSA level versus AMR score. Serum DSA showed strong correlation (r=0.78) to the calculated AMR score among AMR control animals (circle). Abrogation of correlation between serum DSA and AMR score (r=0.14) was found by adding atacicept treated animals (rectangle).
Peripheral B cell but not T cell repopulation is significantly decreased with additional atacicept treatment
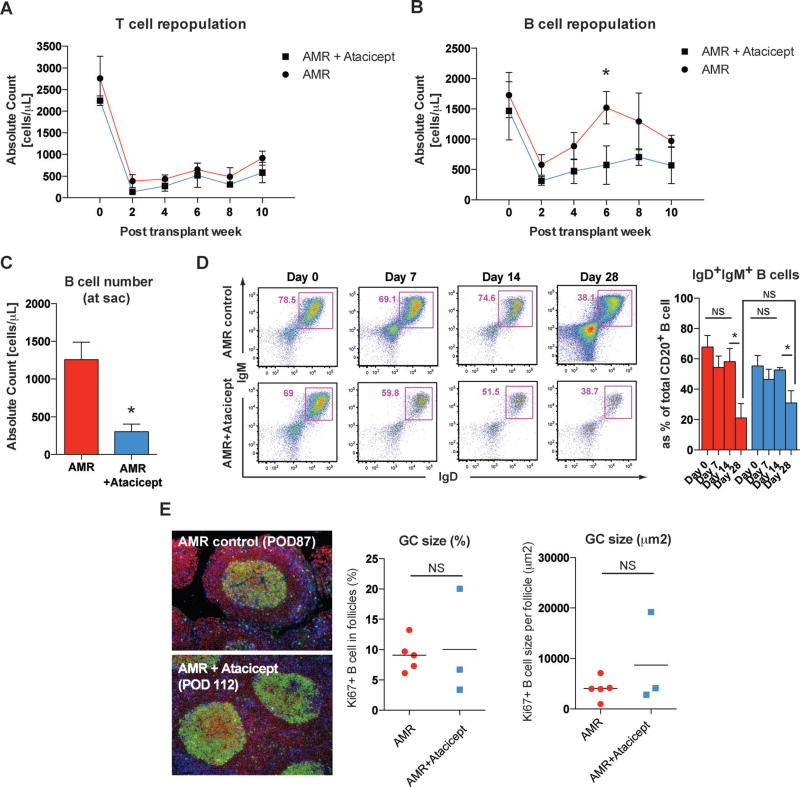
We speculated that this reduction or delayed mode of de novo DSA with additional atacicept treatment could be due to reduced germinal center (GC) responses. Previously, we showed that the early abruption but rapid reconstruction of GC in the AMR model coincided with de novo DSA formation after T cell depletion (13). Interestingly, the number of repopulating T cells in the periphery showed no difference at 2, 4, 6, 8, and 10 weeks posttransplantation between AMR controls and atacicept treated animals (Figure 3A). On the contrary, as shown in Figure 3B, the B cell repopulation was delayed with additional atacicept treatment where as B cell counts in the AMR model returned to baseline level by 6 weeks. Furthermore, B cell counts were significantly decreased at the time of sacrifice in atacicept treated animals (Figure 3C). However, isotype switching in the GC measured by the reduction of IgD+IgM+B cells in the periphery was not affected by atacicept treatment (Figure 3D). In accordance with this, clonal B cells expansion (Ki67+CD20+ cells) in the B cell follicles did not show any difference (Figure 3E), which suggests that atacicept might affect pregerminal center B cell populations rather than directly affecting the GC response.
Figure 3. Atacicept prevents B cell repopulation but not isotype switching or maturation.
(A) Absolute counts of peripheral T cells were decreased after induction immunosuppression, as reported previously, in both treatment groups (12). T and B cell counts were calculated based on the complete blood cell count (CBC). *p < 0.05. (B) In contrast to AMR controls, atacicept treated animals showed significantly lower numbers of peripheral B cells at 6 weeks after the transplantation. (C) Absolute peripheral B cell counts at the time of sacrifice were significantly reduced in atacicept treated animals. (D) Isotype switching (IgM to IgG) was tracked by evaluating peripheral IgD+IgM+ B cells. Immature B population based on IgD/IgM expression in the periphery was assessed by flow cytometry, and representative dot-plots of live B cells were depicted. Numbers in dot plots indicate the percentage of cells in each gate. Significant reduction of immature phenotype (IgD+IgM+CD20+) B cells in the peripheral blood occurred at 4 weeks posttransplantation in both AMR controls and atacicept treated animals. The proportion of IgM+ B cells was not significantly different between two groups. NS > 0.05, *p < 0.05. (E) Representative immunofluorescence image of GC staining with CD3 (blue), CD20 (red), and Ki67 (green) in inguinal lymph node sections from AMR control and additional atacicept treated animals. Lymph nodes were collected at the sacrifice time point. Original magnification 200×. B cell clonal expansion measured by Ki67+CD20+ in B cell follicles was not different in atacicept treated animals compared to AMR controls. NS > 0.05.
Humoral portion of rejection was decreased with atacicept treatment
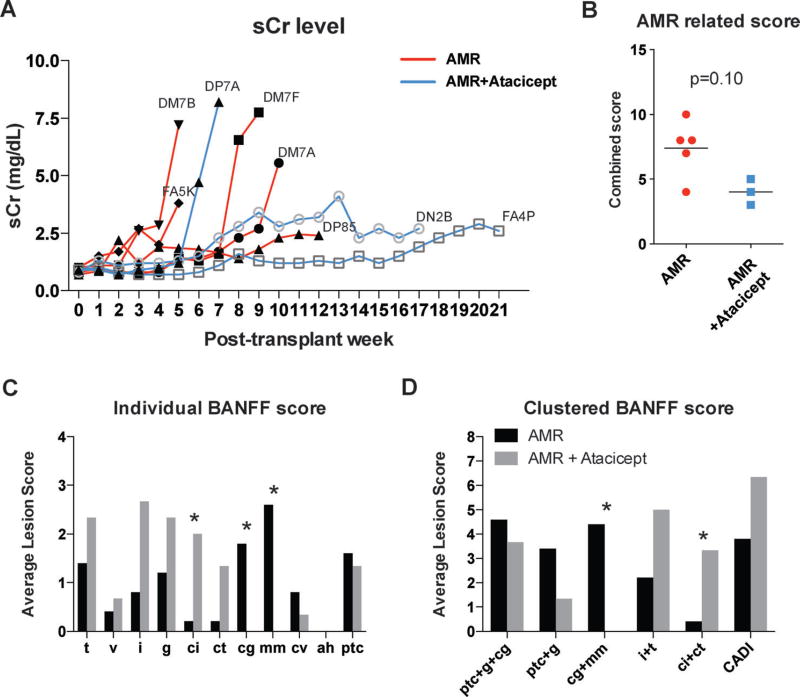
To verify that atacicept prevented AMR-associated injury, renal allografts were analyzed in a blinded fashion (by A.B.F.) according to the Banff 07 classification (15), as well as the Banff 13 report (17). Histological analysis is summarized in the Figure 4. Among the diagnosis, glomerulitis (g), glomerulopathy (cg), mesangial matrix (mm), peritubular capillaritis (ptc), and C4d deposition are commonly identified with AMR injury (18, 19), which we presented the combined scores as an AMR related scoring system (12), akin to the previously described chronic allograft damage index (CADI) score (20). As shown in Figure 4A, the timing of rejection as well as the kinetics of serum creatinine levels are very heterogeneous among AMR controls. Even with a broad spectrum of graft injuries, atacicept treatment does mitigate the humoral portion of graft rejection (Figure 4B). A distinct pattern of rejection was also notable from the Banff individual items (i, t, ptc, g, ci, ct, cg, mm, cv, ah, and v) in that g (p=0.12), ci (p=0.035), and ct (p=0.053) were increased while cg (p=0.035) and mm (p=0.017) were decreased in atacicept treated animals (Figure 4C). Based on the recent diagnostic criteria of AMR injury with renal microcirculation deterioration (21, 22) and transplant glomerulopathy (23), increased cg and mm scores could represent humoral damage. Clustered Banff score also revealed cg + mm representing glomerulopathy was completely diminished (Figure 4D). On the contrary, ci + ct representing tubulointerstitial scarring was increased (p=0.17). Increased ci + ct, a measure of interstitial fibrosis and tubular atrophy, could represent an increased acute cellular rejection (ACR). Furthermore, cortical inflammation (%), cortical tubular fibrosis, and cortical tubular atrophy showed elevated tendencies, which are strongly correlated with cell-mediated rejection and chronic changes. Interestingly, an analysis of a combined score similar to the CADI score (ci + ct + cv + i + gs) showed an increasing trend (p=0.14; Figure 4D). This could contribute to the even less statistically significant correlation between DSA and chronic rejection. Taken together, these data suggest that antibody-mediated-associated injury was reduced by additional atacicept treatment. However, due to the heterogeneous nature of rejection in our controls, atacicept-treated animals also showed more findings associated with cell-mediated rejection and chronic injury in prolonged graft survival.
Figure 4. Atacicept treatment reduced antibody-mediated injuries.
(A) Serum creatinine level of AMR controls and additional atacicept treated animals. (B) AMR related score was calculated by combining points for glomerulitis (g), allograft glomerulopathy (cg), mesangial matrix increase (mm), peritubular capillaritis (ptc) and C4d deposition. (C) The individual Banff histologic score. (D) The Banff histologic score as functional clusters (ptc + g + cg referring to microcirculation lesions, ptc + g to microcirculation inflammation, cg + mm to microcirculation deterioration, i + t to tubulointerstitial inflammation and ci + ct to tubulointerstitial scarring, and i + ci + ct + cg + cv + gs to chronic allograft damage (similar to known as CADI score (20)). Data are presented as mean, with analysis by the Mann–Whitney U-test. *p < 0.05.
Discussion
B cell depletion using rituximab has shown promising outcomes in suppressing DSA in human patients (24, 25). However, in some cases, the depletion of general B cell populations have still resulted in DSA production, which could be supported by high BAFF level after rituximab treatment due to the lack of available B cells to consume BAFF (26). Recently, elevated posttransplant BAFF level showed association with an increased risk of AMR (27). Blocking BAFF could also be particularly beneficial in T cell depletion since elevated BAFF has been reported with lymphocytic depletion (7). Instead of general B cell depletion, preemptive atacicept treatment could limit de novo allo-B cell survival or activation during T cell repopulation.
Previously, we described a T cell depletion-based AMR model with rapid onset of de novo DSA and pathological features of AMR (12). In an attempt to evaluate a therapeutic strategy designed to suppress de novo B cell responses in a nonhuman primate AMR model, we added a 4-week treatment of atacicept to the AMR-inducing regimen (Figure 1A). Counterintuitively, dramatic elevation of BAFF was observed with atacicept treatment; however, antibody pull down assay suggested detection of atacicept bound BAFF by ELISA (supplemental Figure S1). With atacicept supplementation, graft survival was more than doubled relative to controls (Figure 1C); however, this difference was not statistically significant likely due to the low animal numbers in the experimental group. In addition, the nature of AMR, which contains mixed humoral and cell-mediated rejection features, contributed to the heterogeneous outcomes. Considering the above, the graft prolongation is very impressive.
Furthermore, atacicept treated animals showed lower DSA levels during active treatment (Figure 2A). The correlation between DSA and AMR score was clearly lost in the atacicept treated animals, which showed decreased (or at least delayed) DSA kinetics (Figure 2B). Early DSA reduction and decreased DSA kinetics could contribute to lesser antibody-mediated injury. These findings in addition to lower overall B cell numbers (Figure 3) suggest that atacicept reduces early de novo B cell responses compared to AMR controls. Despite the prolonged B cell depletion, the restoration of serum titers of DSA following atacicept treatment may indicate a donor-antigen driven B cell reconstitution in the setting of postdepletional homeostatic pressure.
De novo DSA is produced from plasmablasts generated in germinal centers of secondary lymphoid organs, which constitute the major inductive sites of T-dependent IgG antibodies. However, the kinetics of isotype switching (reduction of IgM B cells in the periphery) was not impaired during atacicept treatment. Furthermore, GC response measured by Ki67+ B cells in the B cell follicles did not reveal any difference between groups (Figure 3) despite the fact that represented GC may not fully reflect early GC response during atacicept therapy since lymphoid organs were collected at the time of sacrifice. This might reflect that atacicept reduces the total numbers of B cells but does not selectively alter allo-B cell formation or GC response in lymph nodes. It may also imply that under the control of canonical isotype switching factors (i.e., IL-21 and CD40/CD40L signal), BAFF is dispensable with regard to isotype switching and GC development.
Even though GC reaction significantly contributes to anti-donor humoral responses, extrafollicular responses also support T-dependent responses to protein antigens. Extrafollicular responses are thought to rapidly generate large numbers of short-lived plasmablasts (28). It is highly possible that atacicept may effectively suppress extrafollicular responses, which would normally occur before the GC response. Alternatively, atacicept could neutralize APRIL, a cytokine documented to be crucial for plasma cell survival, and rapidly reduce DSA in atacicept treated animals (29). Both mechanisms could reduce the early DSA without interfering with isotype switching or GC formation.
Delayed DSA formation (or the reduction of early DSA) had a significant impact on AMR development but not ACR. As shown in Figure 4, our rhesus AMR model contains both AMR and ACR features as is found clinically with AMR. As pointed out in the most recent Banff meeting report, the overlap between AMR and ACR is substantial; and it is likely that both processes work together in many cases (17). Some atacicept-treated animals showed more AMR than ACR while others showed the reverse; all AMR control animals showed evidence of AMR, such as basement membrane duplication and elevated AMR scores with DSA formation, with or without C4d deposition (12). Surprisingly, additional atacicept treatment greatly reduced AMR injury despite the prolonged graft survival, which would have allowed the allograft to sustain prolonged exposure to antibody-mediated damage. Temporizing the humoral portion of rejection may have prolonged the graft survival and made ACR more prominent for when the grafts ultimately rejected (Figure 4). The finding that both study groups had similar DSA by 6 weeks with distinct outcomes further supports that prolonged exposure to DSA in the AMR control group may have led to increased humoral injury and AMR. The heterogeneous nature of AMR in our model was possibly due to a greater genetic disparity between the donors and the recipients, heterologous immunity, and various number of T memory cells in mature outbred large animals, which made it difficult to demonstrate a more dramatic effect of atacicept on graft survival even with great reduction in AMR.
In summary, anti-BAFF/APRIL treatment exerted a profound suppression of early de novo DSA production. The consequence of early DSA is unknown, but we observed an improved graft survival without further serious side effects of additional immunosuppression, including PTLD or CMV reactivation (data not shown). Additionally, a lesser degree of antibody-mediated injury, though more prominent ACR, was found in atacicept treated animals. Here, we have shown that targeting BAFF/APRIL is useful in reducing DSA and prolonging the survival of renal allografts in a nonhuman primate AMR model. While the short course of the treatment failed to induce humoral tolerance, extended therapy with atacicept may improve the graft survival if used in combination with drugs that are more available for the human patient.
Supplementary Material
Acknowledgments
This work was primarily supported by the National Institute of Health (1U01AI074635), awarded to S.J.K. and the NIAID/NIDDK Nonhuman Primate Transplantation Tolerance Cooperative Study Group (NHPCSG) Opportunities Pool (Round 6) via NIH (2U19-AI051731) in collaboration with the Yerkes National Primate Center (Yerkes base grant OD P51POD111). We thank Merck Serono (Geneva, Switzerland) for provision of atacicept. We would like to gratefully acknowledge Dr. Denise Lo for critical review of the manuscript; Frank Leopardi and Kelly Hamby for assisting in animal surgeries; the YNPRC staff and the expert assistance of Dr. Elizabeth Strobert and Dr. Joe Jenkins for animal care; the Emory Transplant Center Biorepository team for their work in weekly and retrospective viral monitoring.
Abbreviations
- ACR
acute cell-mediated rejection
- AMR
antibody-mediated rejection
- BAFF
B cell activating factor
- CADI
chronic allograft damage index
- DSA
donor-specific antibodies
- GC
germinal center
- IT
immunotoxin
- LFA3
lymphocyte function associated antigen 3
- PBMC
peripheral blood mononuclear cell
- QIA
quantitative image analysis
- SLE
systemic lupus erythematosus
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Authors’ Contributions
J.K. designed experiments, performed surgical procedures, cared for experimental macaques, conducted in vitro experiments, interpreted data and prepared the manuscript. E.J.P. designed experiments, performed surgical procedures and cared for experimental macaques, conducted in vitro experiments. J.J.H. performed histology and immunohistochemistry, interpreted data. A.C.G. conducted in vitro experiments. J.Y. conducted BAFF ELISA. A.B.F. interpreted data (pathologist). F.V. interpreted data and prepared the manuscript. S.J.K. conceived of experimental design, performed surgical procedures, cared for experimental macaques, interpreted data and prepared manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: From association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 2.Lefaucheur C, Suberbielle-Boissel C, Hill GS, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 3.Wahrmann M, Exner M, Schillinger M, et al. Pivotal role of complement-fixing HLA alloantibodies in presensitized kidney allograft recipients. Am J Transplant. 2006;6(5 Pt 1):1033–1041. doi: 10.1111/j.1600-6143.2006.01285.x. [DOI] [PubMed] [Google Scholar]
- 4.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 5.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 6.Pers JO, Daridon C, Devauchelle V, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Annals of the New York Academy of Sciences. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 7.Bloom D, Chang Z, Pauly K, et al. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9:1835–1845. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson SA, Jones JL, Cox AL, Compston DA, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30:99–105. doi: 10.1007/s10875-009-9327-3. [DOI] [PubMed] [Google Scholar]
- 9.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 10.Stohl W. Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin Ther Targets. 2014;18:473–489. doi: 10.1517/14728222.2014.888415. [DOI] [PubMed] [Google Scholar]
- 11.Gatto B. Atacicept a homodimeric fusion protein for the potential treatment of diseases triggered by plasma cells. Curr Opin Investig Drugs. 2008;9:1216–1227. [PubMed] [Google Scholar]
- 12.Page EK, Page AJ, Kwun J, et al. Enhanced de novo alloantibody and antibody-mediated injury in rhesus macaques. Am J Transplant. 2012;12:2395–2405. doi: 10.1111/j.1600-6143.2012.04074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EJ, Kwun J, Gibby AC, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. 2014;14:59–69. doi: 10.1111/ajt.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15:746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 16.Sis B, Mengel M, Haas M, et al. Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 18.Smith RN, Kawai T, Boskovic S, et al. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1672. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: Implications for treatment. Am J Transplant. 2008;8:1367–1373. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 20.Colvin RB. CADI, Canti, Cavi. Transplantation. 2007;83:677–678. doi: 10.1097/01.tp.0000262011.05196.a1. [DOI] [PubMed] [Google Scholar]
- 21.Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: New concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosio FG, Lager DJ, Lorenz EC, Amer H, Gloor JM, Stegall MD. Significance and implications of capillaritis during acute rejection of kidney allografts. Transplantation. 2010;89:1088–1094. doi: 10.1097/TP.0b013e3181d368f1. [DOI] [PubMed] [Google Scholar]
- 23.Issa N, Cosio FG, Gloor JM, et al. Transplant glomerulopathy: Risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008;86:681–685. doi: 10.1097/TP.0b013e3181837626. [DOI] [PubMed] [Google Scholar]
- 24.Faguer S, Kamar N, Guilbeaud-Frugier C, et al. Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation. 2007;83:1277–1280. doi: 10.1097/01.tp.0000261113.30757.d1. [DOI] [PubMed] [Google Scholar]
- 25.Smith RN, Malik F, Goes N, et al. Partial therapeutic response to Rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transpl Immunol. 2012;27:107–113. doi: 10.1016/j.trim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarkhin V, Li L, Sarwal MM. BAFF may modulate the rate of B-cell repopulation after rituximab therapy for acute renal transplant rejection. Transplantation. 2009;88:1229–1230. doi: 10.1097/TP.0b013e3181bbba1a. [DOI] [PubMed] [Google Scholar]
- 27.Banham G, Prezzi D, Harford S, et al. Elevated pretransplantation soluble BAFF is associated with an increased risk of acute antibody-mediated rejection. Transplantation. 2013;96:413–420. doi: 10.1097/TP.0b013e318298dd65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLennan IC, Toellner KM, Cunningham AF, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 29.Benson MJ, Dillon SR, Castigli E, et al. Cutting edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.