Abstract
The role of antibodies in chronic injury to organ transplants has been suggested for many years, but recently emphasized by new data. We have observed that when immunosuppressive potency decreases either by intentional weaning of maintenance agents or due to homeostatic repopulation after immune cell depletion, the threshold of B cell activation may be lowered. In human transplant recipients the result may be donor-specific antibody, C4d+ injury, and chronic rejection. This scenario has precise parallels in a rhesus monkey renal allograft model in which T cells are depleted with CD3 immunotoxin, or in a CD52-T cell transgenic mouse model using alemtuzumab to deplete T cells. Such animal models may be useful for the testing of therapeutic strategies to prevent DSA. We agree with others who suggest that weaning of immunosuppression may place transplant recipients at risk of chronic antibody-mediated rejection, and that strategies to prevent this scenario are needed if we are to improve long-term graft and patient outcomes in transplantation. We believe that animal models will play a crucial role in defining the pathophysiology of antibody-mediated rejection and in developing effective therapies to prevent graft injury. Two such animal models are described herein.
Keywords: B cell, Antibody-mediated rejection, Chronic rejection
1. Introduction: Alloantibody in human transplantation
Evidence that alloantibody may develop more often in human renal allograft recipients following alemtuzumab induction was noted by Barth et al. in analyzing the initial results with alemtuzumab at the University of Wisconsin [1]. The kinetics of depletion and repopulation of immune cells in renal transplant patients treated with alemtuzumab were first studied and reported in this communication. Although an uncontrolled trial, of 13 patients with acute rejection during the first 3 years (out of 29 subjects), 7 (54%) included a component of alloantibody-mediated rejection (AMR). This suggested that either lower maintenance immunosuppressive drug therapy used, or the induction therapy itself might be responsible for augmenting AMR.
Given the higher incidence of AMR in the alemtuzumab cohort, we aimed to avoid AMR by modifying the maintenance therapy in subsequent trials. In collaboration with the Immune Tolerance Network, we performed a clinical trial of alemtuzumab induction (three consecutive daily doses of 30 mg) with 60 days of tacrolimus therapy and sirolimus therapy starting on day 1. This carefully monitored cohort of ten patients achieved excellent clinical outcomes at 4 years follow-up [2]. However, the incidence of alloantibody detected by solid phase bead assay for MHC class I and class II was 50% overall. One of the ten patients developed clinical evidence of chronic allograft rejection by two years, and the other patients with alloantibody either had resolution of antibody or no clinical evidence of deteriorating graft function. Nevertheless, this carefully observed population of patients had a remarkably high incidence of detectable donor-specific antibody compared to historical controls, again suggesting that depletion may be followed by alloantibody in the clinical setting of minimal maintenance immunosuppression.
The mechanistic explanation of alloantibody production was explored by Bloom et al. by evaluating B cell cytokines in renal transplant patients following alemtuzumab treatment compared to non-depleting induction with basiliximab (anti-CD25 mAb). It was noted that BAFF levels were significantly elevated compared to control patients tested at early time points, but with return of BAFF levels towards baseline at 2 years following therapy [3]. This was not true of APRIL levels that were similar in both populations. Furthermore, it was shown that B cell activation threshold is substantially altered by BAFF, and that both BAFF protein and RNA were elevated following depletion by alemtuzumab. The reason for BAFF elevation is not clear, and several explanations are possible including depletion of T cells expressing BAFF receptor, homeostatic proliferation of cells that produce BAFF, and enhanced BAFF production by surviving immune cells. BAFF is expressed in both membrane bound and soluble form and is the sole known ligand of BAFF receptor that is preferentially expressed on B cells. The cells types known to produce BAFF include monocytes, neutrophils, and activated lymphocytes in peripheral blood [4–6]. T cells also express BAFF receptor that upon engagement participates in costimulation of T cell activation [6–8].
New data from Iwakoshi et al. suggests that T cell depletion by antibody may not be the only mechanism by which B cell activation is enhanced, but that calcineurin-inhibitors (CNI) may also hold B cells in a transitional state and that weaning of CNI may release B cells to become fully activated and differentiate. We are interested in studying how current immunosuppressive agents used in transplantation may influence the B cell and thus downstream alloantibody. Given the clinical observation that non-adherent patients or patients on minimal immunosuppression are prone to developing DSA leading to acute or chronic rejection, this topic is germane to strategies to promote long-term graft survival.
2. Historical perspective on antibody-mediated rejection in kidney transplantation
The initial BANFF classification established in 1991 did not refer to antibody-mediated rejection (AMR). Subsequently, in 1997 the term “hyperacute rejection” from the first BANFF criteria was renamed “antibody-mediated rejection”. With significant advances and development of novel assays in detection of antibody, there has been an increased recognition of AMR in renal transplant recipients and this concept was incorporated into the BANFF criteria in the 2001 meeting (the modified Banff classification was subsequently published in 2003) [9]. With progress in immunohistochemical techniques, Feucht’s demonstration of C4d, a complement-degradation product in peritubular capillaries of allografts, marked a major breakthrough in diagnosing humoral rejection [10]. C4d staining is evident when the intragraft complement cascade is activated and serves as indirect proof of complement-fixing antibody deposition in renal allografts. When comparing one-year graft survival of patients with C4d vs. without C4d, biopsies which stained positive for C4d had significant lower graft survival (57% vs. 90%). Studies have shown that C4d staining is a strong independent predictor for subsequent graft loss [11–13].
In 1969, Morris et al. reported the first association of HLA antibodies post transplantation with graft failure, and 54% of their reported patients with renal allograft rejection had HLA alloantibodies [14]. At that time, patients were only tested for class I antibodies and it wasn’t until 1978 that class II antibodies were identified [15]. This discovery led to an increased recognition and detection of patients with antibodies (72%) [16]. With further improvements in sensitivity and the introduction of flow cytometry, sensitivities for antibody detection have improved and it became more evident that antibodies are commonly present in the setting of graft dysfunction.
Since then, a series of studies have evaluated DSA production after transplantation. Hourmant et al. screened 1229 kidney transplant recipients between 1972 and 2002 over a prospective time period of 5 years to assess the frequency and clinical implications of the development of donor-specific (DS) and non-donor-specific (NDS) HLA antibodies. Methods used for DSA and NDS antibody detection were complement-dependent crossmatch (CDC), enzyme-linked immunosorbent assay and flow cytometry. A total of 5.5% of the patients had DS, 11.3% had NDS, and 83% had no HLA antibodies after transplantation. NDS antibodies appeared earlier (1 to 5 yr post-transplantation) than DSA (5–10 years). In multivariate analysis, HLA-DR matching, pretransplantation immunization, and acute rejection were significantly associated with the development of both DS and NDS antibodies and also of DS versus NDS antibodies. The presence of either DS or NDS antibodies significantly correlated with lower graft survival, poor transplant function, and proteinuria [17]. Terasaki et al. initiated an international multi-center study including 4763 patients to determine the frequency of HLA antibodies in patients with functional allografts. The study included not only renal transplant recipients but also liver, heart, and lung transplant recipients. The frequency of HLA antibodies among kidney transplant recipients was 20.9%; 19.3% in the liver, 22.8% in the heart, and 14.2% in the lung. Subsequently a second, prospective multi-center trial was performed at 23 kidney transplant centers to determine whether HLA antibodies could forecast graft failure within the first year of transplant. Of 500 patients who had HLA antibodies, graft failure occurred in 6.6%, compared with 3.3% among 1778 patients where HLA-antibodies were not detected. Among 244 patients who made de novo antibodies, 8.6% failed compared with 3.0% failures among 1421 patients who did not make antibodies. This prospective trial showed that 1 year after transplantation, patients with HLA antibodies had significantly higher rates of graft failure compared to those without antibodies [18]. Lee et al. provided further evidence that development of HLA antibodies post-transplantation precedes clinical manifestations of chronic rejection. 139 patients who had undergone renal transplantation were systematically screened for class I and class-II HLA antibodies 3 months, 6 months, and then yearly over a 8 year time period. All 29 patients who were diagnosed with chronic rejection on biopsy had HLA antibodies detected before rejection was evident. 14 out of these 29 patients developed de novo antibodies. Interestingly, there was detection of HLA antibodies post-transplant in 27% of patients with stable function [19]. Although HLA-antibodies were detectable many years before diagnosing clinical chronic rejection, their presence does not always implicate AMR. Observations by other groups have shown that antibody appearance foreshadows the rise in baseline creatinine serum levels and that there is great variability in time between antibody detection and apparent graft damage [20].
3. Antibody-mediated rejection in liver transplantation
The role of donor-directed antibody, more commonly referred to as donor specific antibody (DSA), in kidney transplantation and its effects on kidney allograft outcomes has been well established [21,22]. In fact, reemergence of DSA after kidney transplantation in sensitized patients, despite having undergone clearance of antibody prior to transplant, with concomitant occurrence of antibody mediated rejection serves as a potent example and model of how detrimental the presence of DSA can be to allograft outcomes [23].
By contrast, the role of donor-directed antibody in liver transplantation in the context of early and late graft outcomes has only recently begun to be investigated. This in part stems from numerous early reports that suggested that the presence of alloantibody had no deleterious impact on liver allografts immediately after transplantation [24,25]. However, recent reports suggest that a positive crossmatch prior to liver transplantation has a significant deleterious effect on early and late survival of liver allografts [26–29].
Particularly compelling evidence for the role of donor-directed antibody being detrimental to liver allografts comes from a number of groups. First, Musat et al. recently demonstrated that in patients who received ABO compatible liver allografts, irrespective of crossmatch, the presence of serum DSA and deposition of the complement component C4d in allograft biopsies had a strong correlation with the coexistant finding of acute cellular rejection in these patients. Musat et al. further concluded that the presence of DSA and C4d in these patients’ sera and their biopsies respectively also indicated the simultaneous presence of acute cellular and antibody mediated rejection [30]. Interestingly, positive DSA in sera and C4d in biopsy specimens also appear to have a strong correlation with chronic rejection and late graft failure in liver transplantation as well [31].
Kozlowski et al. found that in patients who underwent liver transplantation with a positive pre-transplant crossmatch and in whom their DSA persisted after transplant were at higher risk for early graft dysfunction [32]. In particular, this early graft dysfunction was mediated by donor-directed antibodies, i.e. antibody mediated rejection. In their reports, similar to the findings of Musat et al., the diagnosis of antibody-mediated rejection was confirmed by the both the presence of DSA in patient sera as well as strong staining for C4d in the liver parenchyma.
At this point, one must begin to wonder how the early findings of successful liver transplantation across a positive crossmatch and more recent reports of poorer outcomes in patients with positive crossmatch and persistent circulating DSA can coexist. They seem to be two mutually exclusive outcomes. One potential model whereby these findings could be explained is that in some patients who have donor-directed antibody prior to transplant, this antibody is cleared after liver transplantation [33–35]. But, clearance of donor-directed antibody is not complete and, in fact, may follow a pattern based on whether it is Class I or Class II DSA [27,35–37].
Unfortunately, further insight into the relationship between alloantibody and liver allografts must remain purely speculative. As of this moment, no good animal or clinical model exists to study the interaction of DSA and allograft in liver transplantation. The one conclusion that can be made is that based on the recent reports cited above, donor-directed antibody in liver transplantation has a role in the outcome of allografts both early and late after liver transplantation and that further investigation into this question is certainly required.
4. ABO-compatibility in antibody mediated rejection
In addition to the HLA system, the ABO-system plays an important role in the success of solid organ transplantation. ABO-incompatible organ transplantation in general carries a high risk of antibody-mediated hyperacute rejection leading to graft loss within minutes to hours. All humans have preformed blood group antibodies presents (except patients with the blood group AB, who do not produce anti-A and anti-B antibodies). In the setting of ABO-incompatible organ transplantation, antigens present on the graft endothelium are recognized by anti-A or anti-B antibodies of the recipient. This can initiate a cascade of events leading to AMR with resulting thrombosis of graft vasculature and graft loss. ABO-identical or compatible transplantation is therefore preferred over ABO-incompatible transplantation because the results are superior [38].
Translating and applying scientific observations to clinical practice is generally first performed in adult cohorts before it is being applied to the pediatric population. The guidelines requiring ABO compatibility for safe transplantation evolved for a population of individuals that did not include infants [39].
Some of the historical ground breaking observations in the field of transplantation, however, were seen and described in immature immune systems. Owen reported in 1945 that mono-chorionic, dizygotic twin calves permanently accepted and expressed blood cells from their twin that were shared in utero [15]. It would follow that they might accept skin grafts from one another. Inter-twin skin grafts however, were rejected by calves who had separate placental circulation [40]. In 1953 Billingham, Brent and Medewar described acquired tolerance in a murine animal model that proved that neonatal mice were sufficiently immature to be susceptible to tolerance induction. Intravenous infusion of donor-splenocytes into mice in utero showed that these animals later accepted skin grafts and other tissues from the donor strain, but not from other mouse strains [41]. Neonatal tolerance also occurs naturally in human mono-chorionic dizygotic twins who have different blood groups, as such twins become tolerant to each other’s blood group in utero [42]. Approximately 50 years after Medewar’s findings were reported, West et al. reported successful ABO-incompatible heart transplantation in 10 infants. All recipients received standard immunosuppression, without aggressive therapies to remove donor-specific antibodies. Eighty percent of the patients survived and no episodes of hyperacute rejections were observed [43]. It has been shown that the mechanism by which ABO-incompatible heart grafts are tolerated in infancy is through development of B-cell tolerance to donor blood group A and B antigen by allospecific B-cell depletion [44]. Tolerance in this setting seems to require some degree of peripheral antigen persistence and persists as long as the incompatible allograft is present. Interestingly, upon re-transplantation, when the ABO-incompatible allograft is removed and replaced by an ABO-compatible heart graft, the previously present ABO epitope reappeared in patients – which gives the impression that the allospecific B-cell depletion is transient [45]. Proving tolerance to ABO-incompatible heart grafts West et al. demonstrated for the first time that ABO tolerance could be acquired in humans. Interestingly, the development of tolerance to donor-HLA could not be demonstrated in these patients.
This successful clinical experience has lead to adaptation of this protocol by various groups around the world, confirming the reliable clinical success of ABO-incompatible heart transplantation in infants. This has contributed significantly to reduction of mortality of listed infants awaiting heart transplantation [46–49]. There have not been any reported problems due to isohemagglutinins late after transplantation, because donor-directed antibody levels generally remain absent or clinically insignificant [45]. Estimated actual patient survival has been identical to that observed in ABO-compatible infant heart transplants [49].
With regard to outcomes from transplantation, ABO-incompatible heart transplantation has been shown to be at least “noninferior” to ABO-compatible transplantation in infants [50].
It is well known that isohemagglutinins are not produced until 5–6 months of age, which presents a window of opportunity during which ABO-incompatible transplantation carries a reduced risk of hyperacute graft rejection [51]. Compared to the adult immune system, the neonatal immune system provides a unique environment for susceptibility to tolerance induction due to relatively smaller immunologic memory and antigen naivety. The infant is limited in his or her ability to mount an effective response to encapsulated pathogens and carbohydrate antigen vaccines since B-cells (in the first few months of life), are largely inept to stimulation by T-independent polysaccharide antigens.
5. The effects of immunosuppressants on B- cells
5.1. Conventional immunosuppressants
Immunosuppressive agents currently used in transplantation are primarily designed to target T-cells and prevent acute cellular rejection. The effect of these conventionally used drugs on B-cell function has not been fully elucidated. B-cell activation is tightly regulated by T-cells, suggesting that suppression of T-cells could have an effect on B-cells as well (Fig. 1). We address below the role of commonly used immunosuppressive drugs on B-cells.
Fig. 1.
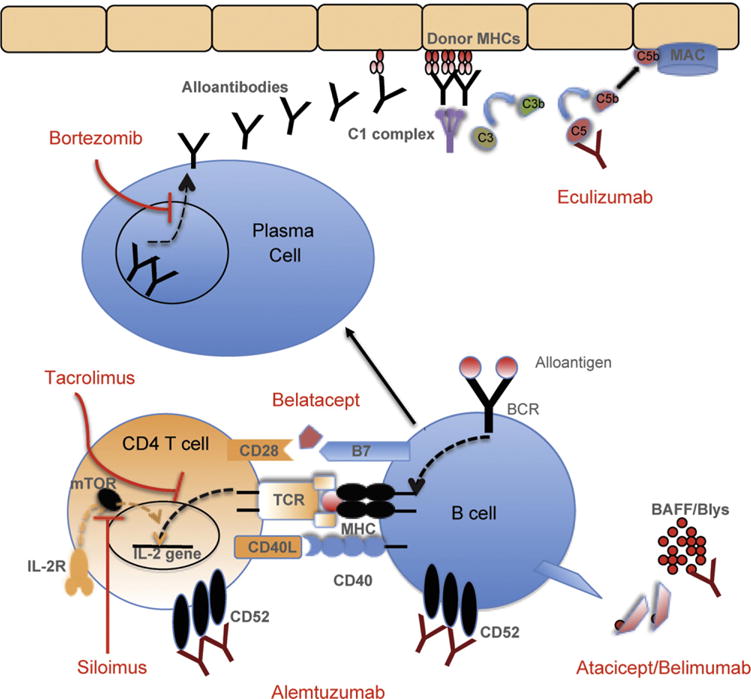
The effect of immunosuppressants currently used in transplantation on B cell. Most of conventional immunosuppressants designed to target T-cells, however, B-cell activation is tightly regulated by T-cells, suggesting that suppression of T-cells could have an effect on B-cells as well. The effect of these conventionally used drugs on B-cell function has not been fully elucidated.
5.1.1. Calcineurin Inhibitors (CNIs)
Tacrolimus and cyclosporine both belong to the drug class of calcineurin inhibitors with their primary effect being the inhibition of T-cell activation. Studies have failed to show any conclusive effects on T cell-independent B cell antibody production [52–54]. Human in vitro studies have shown that neither tacrolimus nor cyclosporine inhibit B-cell proliferation or increase B-cell apoptosis [55]. Interestingly, cyclosporine has shown to cause a minor decrease in B1a and B1b cells but did not decrease anti-ABO antibodies that are produced by B1a cells [56,57]. Tacrolimus has been shown to cause a decrease in B1a cells and an increase in B2 cells [56,57]. Both drugs decrease levels of most major cytokines [52], except for levels of IL-4, -5, and -10 with cyclopsorine and IL-5 levels with tacrolimus, which decreases the alloimmune response and potentially B cell stimulation. This might lead to a decrease in immune responses to allografts and even decreased B cell stimulation. Cyclosporine and Tacrolimus were shown to decrease alloantibody production in mice that were challenged with blood transfusion [58,59].
5.1.2. Rapamycin
Rapamycin is a macrolide antibiotic which binds to FKBP 12 and inihibits the mTOR pathway [60]. This leads to an inhibition of CD28 mediated upregulation of IKBa which blocks transcription of IL-2. Rapamycin decreases T-cell dependent antibody formation [52,53] but unlike CNIs, it decreases T cell independent B cell antibody formation, increases B-cell apoptosis, and inhibits B-cell proliferation [52–55]. It has been shown that heart-transplanted pigs treated with rapamycin alone did not form DSA and had decreased numbers of macrophages in the allograft 30 days post transplantation. In contrast, the untreated group, tacrolimus group, and cyclosporine treated group developed DSA. In human renal transplant recipients, rapamycin was found to inhibit the anti-equine antibody response in patients treated with antithymocyte globulin [61].
5.1.3. Mycophenolate mofetil
Mycophenolate mofetil (MMF) inhibits the enzyme IMPDH used for DNA synthesis, and arrests cells in the G1/S stage [62]. This leads not only to an inhibition of lymphocyte responses but also to an inhibition of induction of cytotoxic T cells. This drug was also found to have an effect on dendritic cells in vitro by suppressing maturation and alloimmune function. Due to decreased GTP formation it also decreases the expression of adhesion molecules as there is lack of GTP to activate fucose and mannose transport needed for glycoprotein synthesis. This results in decreased B cell proliferation and increased B cell apoptosis [52–55]. Among the B cell subtypes, B1b cells are most affected by MMF [56]. Leder et al. observed a decrease in HLA antibody formation and also a decrease in rejection episodes in renal transplant recipients when comparing MMF treated patients vs. non-MMF treated patients [63].
5.1.4. Alemtuzumab
Alemtuzumab is a humanized CD52 specific IgG1 monoclonal antibody, which causes profound peripheral lymphodepletion by complement mediated lysis [64]. CD52 is present on T cells, B cells, monocyte and neutrophils. After administration of alemtuzumab B cells remains suppressed for 3–12 months while T cell levels, in particular T helper cells, can remain suppressed for up to 3 years [65]. Despite its suppressive effects on B cells, alemtuzumab induction has been associated with an increased incidence of humoral rejection [66–69]. This may be attributed to weak CD52 expression on plasma cells compared to normal B cells [70], resistance of memory T cells to alemtuzumab [62], or to lack of adequate maintenance immunosuppression. Protective immunity is not severely compromised, as the innate arm of immunity remains intact with alemtuzumab therapy [71–76] but viral and fungal infections are increased as might be expected in the setting of potent lymphocyte depletion.
5.1.5. Belatacept
Two signals are needed for T cell activation, signal 1 (engagement of the T cell receptor with MHC: protein complex) and signal 2 (engagement of CD28 with CD80 and CD86 on antigen presenting cells). This leads to an increase in the expression of CTLA-4 (CD152) which binds to CD80/86 and down-regulates T cell activation by arresting the cell cycle by inhibition of IL-2 transcription [77]. Two drugs, abatacept and belatacept, are humanized fusion proteins which are comprised of the extracellular domain of CTLA4 and the Fc domain of human IgG. These drugs bind to CD 80/86 and inhibit the interaction with CD 28 and thus preventing signal 2 which is needed for T cell activation. Belatacept is a second generation drug which was has more avid binding to CD80/86 than abatacept [78]. Belatacept has been shown to decrease serum antibodies in primates after islet cell transplantation. Interestingly, B cell levels were normal in these animals [78,79].
In a multicenter prospective study comparing belatacept (high vs. low dose) with cyclosporine, non-sensitized cross-match negative patients were studied and found to have remarkably low incidence of de novo alloantibody production in the belatacept cohorts, suggesting that belatacept may effectively reduce DSA post-transplantation [80].
5.2. New B cell therapeutics in transplantation
5.2.1. Bortezomib
Bortezomib is a proteasome inhibitor that selectively targets the 26s proteasome complex, which prevents the degradation of key proteins and leads to cell apoptosis [81]. This drug has been approved for use in multiple myeloma [82] and holds promise for transplantation due to its inhibitory affects on alloreactive B cells. Bortezomib was also found to inhibit NF-kB which plays an important role in cytokine release, adhesion molecule expression and antigen presentation, thus further lowering the threshold of immune response towards the graft [83]. When given in combination with rapamycin, bortezomib decreases rapamycin-resistant memory T cells without affecting Tregs, potentially due to the inhibition of the down-regulation of p27kip1 which suppresses CD8 T cells [84]. Two patients with acute AMR experienced rejection reversal and durable DSA elimination with bortezomib primary therapy after plasmapheresis [85].
5.2.2. Anti-BAFF agents (atacicept/belimumab)
Atacicept is a recombinant fusion proteins formed by combining the BAFF and APRIL-binding extracellular portion of the Transmembrane and Calcium Modulating Ligand Interactor (TACI) and the Fc portion of human immunoglobulin (IgG1). BAFF/BLyS and APRIL blockade prevents B cell maturation, differentiation, and survival [86]. Xu et al. found a gradual increase in levels of BAFF over time in kidney transplant recipients as well as higher levels of BAFF expression on CD3 T cell in patients with poorer graft function [87]. BAFF has been shown to influence class switching of immunglobulins, enhance survival and effector function of plasmablasts [88–90] as well as memory B cell differentiation into Ig-secreting cells in a T cell dependent manner, while opposite results were seen with respect to T cell independent responses [91]. In kidney transplantation, a positive correlation was seen between levels of BAFF and HLA 1 and 2 antibodies. Some B cells avoid immunosuppressant mediated death in tertiary lymphoid organs in the grafts probably by BAFF dependent paracrine survival signals [92]. Atacicept has an inhibitory role on BAFF and a biphasic response leading to a transient increase in mature B cells. Decreased levels of Ig’s have been noted in rheumatoid arthritis and systemic lupus erythematosus patients treated with atacicept [93,94].
Belimumab is a monoclonal antibody that binds and inhibits soluble BAFF/BlyS. In a phase 3, multicenter study, it was found to induce overall improvement in systemic lupus erythematosus compared to placebo; it also significantly reduced IgG, IgM and IgA concentrations from baseline compared to placebo [95].
5.2.3. Eculizumab
Eculizumab is a humanized antibody that binds to C5, inhibiting its cleavage and generation of the membrane attack complex [96]. This interrupts the complement pathway just before the formation of membrane attack complex. Wang et al. used this antibody in a heart xenotransplantation model (rat to mouse) and showed that hyperacute rejection was prevented; however, the grafts eventually failed [94]. They extended their experiment and used eculizumab in combination with cyclosporine in C3H to BALB/C mouse heart transplanted animals, and the grafts survived for more than 100 days but failed once eculizumab was removed [97]. Later, the same group transplanted hearts into presensitized mice and treated them with a combination of cyclophosphamide and cyclosporine. This led to prolongation of graft survival to over 100 days. When eculizumab was removed from the regimen at day 60, the grafts functioned well despite little deposition of C5, which was absent at day 60, suggesting accomodation [98]. This was thought to be due to a shift of Ig’s, with an increase in IgG1b and decrease in IgG2a, and an increase in antiapoptotic factors Bcl-2 and Bcl-xl.
6. B cells in animal model of chronic rejection
Chronic rejection is the leading cause of graft failure after organ transplants with an unchanged incidence despite a 50% decline in acute rejection over the past decades [99,100]. For instance, despite substantially improved rates of 1- and 3-year graft survival in human deceased-donor kidney transplantation, the 10 year graft survival is almost unchanged over the past 25 years despite many innovations and advancements in the field, remaining at approximately 40% [101]. Yet, chronic rejection is arguably the most poorly understood phenomenon among post-transplant outcomes such as acute rejection, PTLD, post-transplant infection. In large part, this lack of understanding is due to the complexity of etiology of chronic rejection often referred as “multifactorial” [102,103].
Animal models have been of great importance to our understanding of transplant immunology in general. More specifically, animal models are essential to our understanding of the basic mechanisms of chronic rejection and will likely guide development of strategies to prevent it. Due to the substantial evidence of alloantibody-mediated rejection (AMR) contributing to chronic rejection [104,105], the B cell has become an important focus of research on chronic rejection.
We will discuss the differences between current murine experimental models of chronic rejection and the impact of these differences on the interpretation of the data. We will also discuss a model of B cell mediated chronic rejection (or antibody-mediated chronic rejection) that addresses clinically relevant mechanisms of chronic rejection.
6.1. Current rodent models of chronic allograft rejection
The rat aortic transplant model has been used to study the gradual development of vascular lesions that mimic the vascular changes associated with chronic rejection. In this model, the graft undergoes an acute rejection episode that evolves over weeks into a chronic type of inflammation and ultimately leads to sclerosis [106]. Most laboratories have chosen heterotopic heart transplantation or orthotropic kidney transplantation rather than aortic transplantation for this purpose with the rationale that they more closely approximate clinical organ transplantation. Hetero-topically transplanted heart grafts with chronic rejection develop atherosclerosis, interstitial fibrosis, myocytes loss, and hypertrophy of the remaining myocytes [107]. In contrast, the mouse kidney or liver transplant model has been performed by only a few centers, because of its complexity, including additional anastomosis [108,109]. However, the low incidence and intensity of rejection following renal or hepatic grafting in mice may render this models fit to study chronic rejection [110].
As shown in Table 1, several mouse strain combinations have been reported with heart, kidney and other organs transplants to result in chronic rejection. These chronic rejection models were produced with (1) less disparity of histocompatible antigens (such as class I, class II or minor Ag mismatch), (2) various degree (weak vs. strong) of immunosuppressive agents, or (3) more immunologically permissive organs (such as kidney or liver).
Table 1.
Selected chronic rejection model in mouse.
| Organ | Donor | H-2 | Recipient | H-2 | Mismatch | Treatment | MST (d) | Acceptancea (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Heart | Bm12 | bm12 | C57BL/6 | b | IA | N/A | >56 | 70 | [151] |
| C57BL/6 | b | Bm1 | bm1 | K | N/A | >56 | [152] | ||
| B10.A | a | B10.BR | k | D | N/A | >56 | 70% | [153,154] | |
| BALB.B | b | C57BL/6 | b | Multiple minors | N/A | >66 | 50% | [112] | |
| C57BL/6 | b | Balb/c | d | Full | Anti-CD4 | >56 | 50% | [155] | |
| Balb/c | d | C57BL/6 | b | Full | Anti-CD4 | >60 | [156] | ||
| DBA/2 | d | C57BL/6 | b | Full | Anti-CD4 | >60 | 78% | [157] | |
| DBA/2 | d | C57BL/6 | b | Full | Gallium Nitrate | >60 | 75% | [115] | |
| Kidney | DBA/2 | k | C57BL/6 | b | Full | N/A | >60 | 70–80% | [158] |
| C57BL/6 | b | Balb/c × DBA/2 | d | Full | N/A | >42 | 20% | [159] |
Acceptance rate is based on the contraction (beating) of the cardiac allograft at MST for heart transplantation model.
6.1.1. Chronic rejection model without immunosuppression
Models without immunosuppression using a lesser degree of MHC disparity between donor and recipient showed features of chronic rejection (CR) such as intimal hyperplasia [111]. However, CR was often accompanied by rapid medial necrosis with extensive adventitial infiltrate [111]. In these models, acute rejection was felt to be the main contributor to subsequent chronic rejection development. For example, BALB.B to B6 cardiac allograft, recipients showed 50% spontaneous graft acceptance without immunosuppression [112]. On the other hand, the other 50% of grafts surviving long-term experienced some type of acute injury (Fig. 2). However, the number of immunodominent mH-Ag-specific T cell were not different, and untreated recipients clearly showed decreased beating compared to syngeneic allografts. Therefore, CR models with no immunosuppression are more “acute rejection-associated” rather than examples of ischemia/reperfusion injury. Targeting T cells in this model showed a dramatic reduction of chronic rejection development. [113].
Fig. 2.
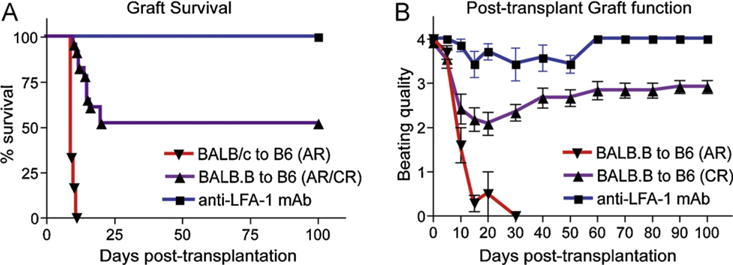
Graft survival and post-transplant graft function in BALB.B to C57BL/6 heart transplantation. Cardiac allograft survival (A) and post-transplant graft function (B) were monitored daily by palpation. 50% of spontaneous graft acceptance was observed from BALB.B to C57BL/6 cardiac allograft model. In these long-term surviving allografts showed decreased beating qualities (cardiac impulses) to <2+ between post-transplant 10–30 days, but beating qualities recovered to 3–4+ and maintained. Anti-LFA-1mAb treated recipients showed no acute rejection and no compromised beating quality.
6.1.2. Chronic rejection model with strong immunosuppression
Mouse models using immunosuppression that results in long-term graft survival (i.e. >100 days) most often showed CR lesions characterized by an intact media and relatively sparse mononuclear cell infiltrates [111]. Morphometric analysis of allografts also reveals gradual increase of hyperplasia and fibrosis in intima and fibrosis in the myocardium [114]. In this model, T cell mediated acute rejection is relatively well controlled by the immunosuppression. However, the model also can show a delayed acute rejection due to gradual immunosuppression weaning or discontinuation. For instance, Galium Nitrate (GN) and anti-CD4mAb resulted in over 60 days graft survival [115]. Based on graft survival and post-transplant graft function, chronic rejection in this model was associated with acute rejection. Among the three patterns (no decline, decline/recovery, and decline only) of cardiac function, any declination of the beating quality might represent T cell mediated acute rejection. As shown in Fig. 2, beating quality of BALB.B to B6 cardiac allografts showed a decline/recovery pattern and continuous infiltration of T cells in the graft [112]. Interestingly, anti-LFA-1mAb treatment resulted in no loss of graft function and no alloreactive T cell activation. The treatment showed significantly reduced chronic rejection (unpublished data). Taken together, chronic rejection models with potent immunosuppression may not completely control the T cell-mediated response.
Costimulation blockade (CTLA-4Ig and MR-1) prolonged graft survival without chronic rejection development, at least within 100 days post-transplant in CBA recipients of full MHC mismatched cardiac allografts [116]. However, B6 recipients with the same treatment regimen showed delayed acute rejection. It is highly likely that different strain combinations produce different cytokine patterns in response to donor antigen even under immunosuppression [117]
6.1.3. Chronic rejection model with permissive organ
As mentioned above, the transplanted kidney in mice is more permissive of graft tolerance than aortic grafts. BALB/c to B6 renal transplantation showed 70% spontaneous graft acceptance while 0% acceptance was found in cardiac allografts using the same strain combination [110]. Liver allografts are still more tolerogenic, and many fully MHC mismatched strain combinations showed indefinite acceptance after orthotropic liver transplantation, while skin or heart grafts are rejected acutely [118]. However, the mechanisms responsible for the tolerance induced by liver allograft are not fully understood. Moreover, the development of spontaneous acceptance of organs could be associated with both an initial cellular and humoral immune response and suggests that tolerance involves an active process to achieve.
6.2. Chronic rejection model studying B cells effector function
The chronic rejection models we mentioned do not specifically show a prominent B cell role in chronic rejection development, perhaps because B cell effector function has not been a main focus of chronic rejection studies until recently. Alloantibody, the effector arm of B cells, was not always considered to be associated with graft rejection. Since alloantibody transfer sometimes enhanced graft survival in animal models [119,120], its role was considered ambiguous. Lack of rejection in the setting of alloantibody is now referred to as accommodation. The mechanisms required for accommodation are currently unknown but may be due to inadequate injury to cause measurable dysfunction. More recently, the association of alloantibody formation and chronic rejection has been postulated [105,121]. Development of graft arteriopathy after passive transfer of polyclonal antibodies to cardiac allograft-bearing SCID mouse recipients showed a causal link between alloantibody and chronic rejection [107]. Interestingly, the clinical observation in humans of chronic rejection associated with DSA is more convincing than experimental models reveal. No clinically relevant animal model to define antibody-mediated chronic rejection are available yet, whereas some acute antibody-mediated rejection models are available (Table 2).
Table 2.
Selected acute antibody-mediated rejection model.
6.2.1. T cell depletion and B cell responses (cellular response vs. humoral response)
Some strain combinations produce long-term graft survival but include features of chronic rejection such as arteriopathy and fibrosis. A single minor antigen (Ag) mismatch alone can induce chronic rejection in heart transplantation. Russell et al. [122] showed that H4 mH-Ag mismatched cardiac allografts (H4 congeneic → wild type B6) induced coronary artery vasculopathy (CAV) when evaluated at 8 weeks post-transplantation. Their result suggests that a single minor Ag mismatch in a fully-MHC matched situation can induce CAV. It is also been shown that chronic rejection is driven by H13-specific CD8 T cells in a H13a to H13b single mH-Ag mismatched cardiac allograft, suggesting that T cells can mediate chronic rejection even with miniscule histoincompatibilty [123].
Based on currently available chronic rejection models, most of which depend on T cell-mediated acute rejection, an antibody-mediated chronic rejection model should meet following conditions:
Stable long-term graft survival: No sudden graft loss due to delayed acute rejection.
Stable allograft function: No sudden decrease of heart beating quality (cardiac) or sCr elevation (kidney).
Alloantibody production.
C4d staining or equivalent.
Gradual development of chronic rejection histologically.
As we mentioned previously, patients receiving alemtuzumab induction showed higher incidence of antibody-mediated rejection [67,69,124,125]. Their acute rejection rate was not increased compared to the control group, but rather the humoral component was increased in patients with acute rejection in this patients group.
We mimicked the human situation by using human CD52 transgenic mice with alemtuzumab to deplete T cells. CD4 T cell depletion using anti-CD4mAb has also been used to promote chronic rejection as shown in Table 1. Bishop et al. showed chronic rejection development after depleting CD4 T cells in an altered cytokine milieu [126]. However, alloantibody production was not reported with this model, and based on Oroze et al. it might be more T cell mediated-chronic rejection [115]. T cell depletion with alemtuzumab using huCD52Tg mice showed rapid T cell repopulation without any acute rejection (acute rejection measured by heart beat quality). Excellent graft function is maintained (+3/4 to +4/4) for more than 200 days. Alloantibody production was consistently observed in this model and neo-intimal hyperplasia/fibrosis was present (Fig. 3). We also used novel and advanced flow cytometric methods to track donor-specific B cells using H-2KbDb or H-2KdDd tetramers. Tetramers are comprised of four identical biotinylated H-2 molecules bound to a fluorescently (APC- Allo-phycocyanin) labeled streptavidin molecule that is able to bind the donor-specific B cell antigen receptor (surface immunoglobulin). Thus, anti-H-2KbDb or H-2KdDd reactivity of the donor-specific B cells in allograft recipients will be identified using fluorescently-labeled H-2KbDb or H-2KdDd tetramers. We have used several tetramers with various peptide specificities and shown that the H-2 reactivity to B cells is peptide independent (Fig. 4). Currently, the effects of several B cell biologics and conventional drugs in conjunction with alemtuzumab on alloantibody production and chronic rejection are under investigation.
Fig. 3.
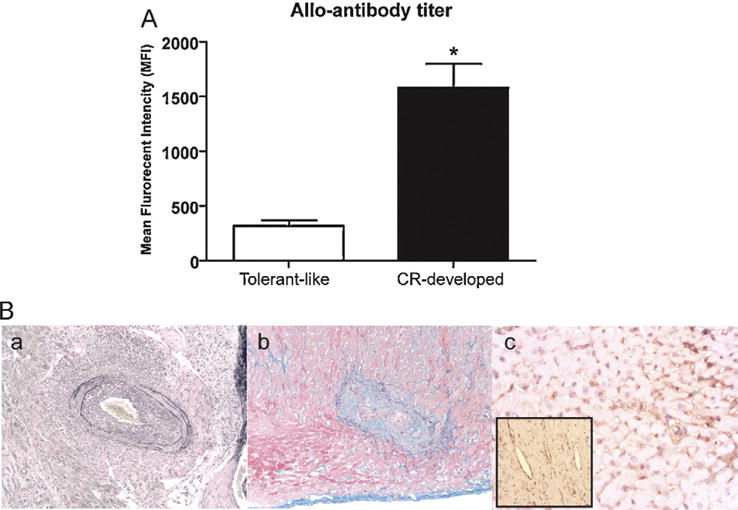
Serum alloantibody production and chronic rejection development in alemtuzumab treated CD52Tg mice. (A) Elevated alloantibody production at 10 weeks post transplantation with CR-developed recipients (*p < 0.05). (B) Histologic and immunohistochemical eveidence of antibody-mediated chronic rejection. Slides prepared of post-transplant day 100 cardiac allograft with Elastic, trichrome, and C4d. (a) Elastic staining shows increased neo-intimal hyperplasia in the coronary artery. (b) Trichrome staining reveals higher pathological grades in fibrosis indicated in blue. (c) Immunoperoxidase demonstrates diffuse deposits of C4d (dark brown) in the capillary and arterial endothelium (inset) in a section from a cardiac allograft from C57BL/6 to CD52Tg recipient with alemtuzumab treatment at day 100.
Fig. 4.
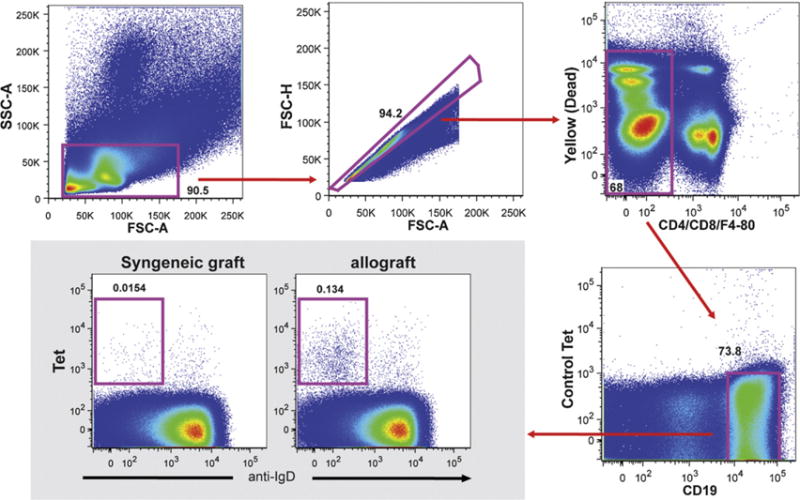
Gating strategy for allospecific B cells. Lymphocytes were selected for CD4/8/F4-80− and far yellow− (dead cells) cells. B cells were separated as CD19+ syngeneic tetramer− and were further gated against allospecific tetramer Tet B (H-2b) and IgD.
6.3. NHP models of antibody mediated rejection
The final step in translating immunomodulatory agents into human clinical trials is to demonstrate their safety and efficacy in the nonhuman primate (NHP) – the next of kin in our phylogenetic family. Conservation of signaling pathways, MHC protein expression, and antibody-binding specificity between macaques and humans makes therapeutic investigation in the nonhuman primate not only the preferred method but also an ethical requirement [127–130].
Long-term graft survival is challenged by chronic rejection, and de novo anti-donor HLA antibodies have been identified as a causative factor in humans [105] and at least predictive in macaques [131]. Accordingly, humoral immunity has been evaluated in nonhuman primates in the context of allo- and xenotransplantation. Experimental xenotransplantation returned in the 1960s and ushered an increased awareness of humoral rejection [132]. With improved understanding of the role of xenoreactive antibodies and complement, hyperacute rejection became preventable by inhibiting complement activation and removing offending antibodies or altering their targets, namely Gal 1,3 Gal in the pig-to-NHP model [133–135]. The resulting “delayed” acute humoral rejection also involved xenoreactive antibodies against Gal as well as other components of the donor xenograft. Richards et al. examined serum samples from 20 animals collected every 3 days post-transplant in an hDAF transgenic pig-to-cynomolgus monkey renal transplantation model and found that assays for IgM antibodies to porcine endothelial cells and leukocytes correlated with conventional anti-Gal assays in monitoring xenoreactive humoral immunity [136]. Furthermore, McCurry et al. discussed that low-level humoral responses (either adaptive or secondary to immunosuppression) could lead to delayed humoral or chronic rejection [137]. Studies in xenotransplantation illuminated the complexity and significance of anti-donor antibodies and implied their role in the development of chronic rejection.
In allotransplantation, chronic and antibody mediated rejection have been increasingly studied, but no nonhuman primate model currently exists exhibiting 100% de novo alloantibody production and antibody mediated rejection. To date, several groups have described the presence of alloantibodies or C4d deposition in their studies, as outlined in Table 3. Wieczorek et al. reported a chronic rejection model induced by suboptimal CsA administration after kidney transplantation in cynomolgus monkeys [138]. They observed low grade transplant endarteritis progressing to chronic sclerosing vasculopathy with collagen and myofibroblast accumulation. Three of the 5 animals in the suboptimal CsA treatment group also demonstrated C4d deposits in the peritubular capillaries. De novo alloantibody production was not reported. Other groups designating CsA monotherapy as control groups detected alloantibodies in 33–100% of animals.
Table 3.
Nonhuman primate organ transplantation: reports of alloantibody, C4d deposition, and humoral rejection.
| Group (reference) | Species (n) | Organ | Treatement regimen | MST (d) | Alloab+ | C4d+ | Comments |
|---|---|---|---|---|---|---|---|
| Haanstra et al. [139] | Rhesus (5) | Kidney | [Control] Anti-CD40 (ch5D12) + anti-CD86 (chFun-1) | 80 | 2/5 | N/A | |
| Kawai et al. [142] | Cynomolgus (5) | Heart | Total body irradiation, thymic irradiation, ATG, splenectomy, BMT + CsA | 235 | 3/5 | N/A | 3/3 animals with mixed chimerism formed DSA upon losing chimerism |
| Aoyagi et al. [140] | Cynomolgus (12) | Kidney | Anti-CD40 (4D11) at low dose induction or maintenance (1–5 mg/kg) | 97 | 7/12 | 5/12 | High dose maintenance therapy inhibited DSA |
| Smith et al. [146] | Cynomolgus (17) | Kidney/BMT | Total body irradiation, bone marrow transplantation, antiCD154, CsA +/− splenectomy (4) or anti-CD8 (4) | 500+ | 9/17 | 9/17 | 9/9 animals with C4d deposition had alloantibodies. 0/8 without C4d deposition had alloantibodies |
| Torrealba et al. [104] | Rhesus (9) | Kidney | CD3 immunotoxin + methylprednisolone ± CsA, IL2R, DSG, MMF, intrathymic donor vs saline injection, Fab2, sirolimus | 614 | N/A | N/A | 9/9 animals had varying grades of chronic rejection at autopsy |
| Azimzadeh et al. [141] | Cynomolgus (6) | Heart | [Control] CsA monotherapy | 29 | 2/6 | N/A | Strong IgM and IgG responses by day 14 |
| Azimzadeh et al. [141] | Cynomolgus (8) | Heart | Anti-CD154 + BMT ± ATG | 28 | 5/8 | N/A | |
| Azimzadeh et al. [141] | Cynomolgus (19) | Heart | Anti-CD154 ± ATG | 74 | 13/19 | N/A | Delayed alloab production compared to +BM |
| Kelishadi et al. [163] | Cynomolgus (7) | Heart | [Control] CsA monotherapy | 71 | 5/7 | 20–50% vessels | |
| Wieczorek et al. [138] | Cynomolgus (6a) | Kidneyb | Suboptimal CsA; rabbit ATG + methylprednisolone upon diagnosis of transplant endarteritis | 65 | N/A | 60%a | |
| Schroder et al. [144] | Cynomolgus (6) | Heart | Anti-CCR5 (CMPD 167) + CsA | ∼46 | 4/6 | N/A | 3/3 CsA monotherapy controls had alloab by 3 weeks |
7 of 12 animals receiving suboptimal CsA developed transplant endarteritis; 6 of these were subsequently treated with ATG and methylprednisolone.
Non-life supporting kidney transplantation.
Several groups have investigated the roles of costimulation blockade, mixed chimerism induction, and chemokine blockade in NHP humoral alloimmunity. Haanstra et al. observed that CD40 and CD86 blockade combined with CsA in a rhesus renal allograft model prolonged graft survival and eliminated alloantibody production, compared to 40% DSA presence with costimulation blockade alone [139]. Aoyagi et al. administered anti-CD40 (4D11) as either induction or maintenance therapy, at 4 varying doses in a cynomolgus renal allograft model. Animals in the low dose treatment groups demonstrated +DSA and C4d deposition (7/12 animals) whereas high dose CD40 blockade abrogated the humoral response [140]. Azimzedeh et al, in a cynomolgus cardiac allograft model, employed CD154 blockade with a subset of animals receiving ATG. Thirteen of 19 treated animals demonstrated alloantibody production, albeit delayed compared to CsA controls and animals that underwent bone marrow transplantation [141]. These data indicate that costimulation blockade will often delay but not prevent alloantibodies. Kawai et al. also utilized bone marrow transplantation in cynomologus cardiac recipients and detected alloantibodies with subsequent rejection in 3/3 monkeys that achieved multilineage chimerism, a strategy used for tolerance induction in renal allograft recipients [142,143]. Finally, chemokine (CCR5) blockade has been widely studied in the murine population and found to attenuate acute and chronic allograft rejection. In the nonhuman primate, two groups have replicated this finding and have also observed a decrease in cardiac allograft vasculopathy as well as delayed alloantibody production [144,145].
As evidenced by Table 3, not all groups have characterized B cell alloimmunity in cardiac and renal transplantation in a uniform fashion. To standardize the diagnostic evaluation of chronic antibody mediated rejection in macaques, Smith et al. reviewed their NHP experience in renal transplantation with mixed chimerism induction in two studies. First, they selected transplant recipients that demonstrated allograft C4d deposition (n = 9) and found a high correlation with the presence of alloantibodies detected by flow cytometric crossmatch of recipient serum with donor PBMCs. Other morphologic changes included glomerular basement membrane duplication (chronic allograft glomerulopathy) and fibrous intimal thickening (chronic allograft arteriopathy) in 89% of these animals [146]. In their follow-up study, they assessed the natural history of chronic alloantibody-mediated rejection in 417 specimens of their 143 long-term renal allograft recipients. A temporal progression was determined, starting with alloantibody production (48%), C4d positivity (29%), transplant glomerulopathy (22%) and finally renal insufficiency/failure [147]. These findings are in concordance with the 2005 Banff meeting concensus, which lists transplant glomerulopathy, C4d deposition in peritubular capillaries, and presence of donor specific antibodies as the diagnostic triad of chronic antibody-mediated rejection [148]. Capillary C4d deposition and alloantibodies are essential in diagnosing antibody mediated rejection in other transplanted organs as well [149,150].
With humoral alloimmunity comprising a significant component of chronic rejection and long term graft failure, the development of a nonhuman primate model of antibody mediated rejection is necessary. The application of various B cell therapeutics to such a model may elucidate mechanisms of humoral immunity and take us one step closer to achieving tolerance.
7. Summary
The importance of B cell immunity in organ transplantation has become increasingly apparent although effective strategies to prevent or reverse DSA remain elusive. It may be that costimulation blockade with Belatacept more effectively prevents DSA despite being a weaker T cell immunosuppressant than CNI agents. We predict that this agent will result in improved long-term graft survival for human kidney transplants due to its effective suppression of the B cell and DSA. Nevertheless, better strategies for controlling late development of DSA will be needed, and animal models that truly reflect the human situation permit a mechanistic understanding of B cells in transplantation and will drive rationale testing of new agents before introducing them into human clinical trials.
Acknowledgments
The work in this paper is supported in part by grants: U01 AI074635 (SJK); ROTRF (Roche transplant research foundation) grant 962141545 (NI); Emory URC (University research committee) grant (JK).
Abbreviations
- AMR
alloantibody-mediated rejection
- MHC
major histocompatibility complex
- BAFF
B-cell activating factor
- APRIL
a proliferation-inducing ligand
- CNI
calcineurin inhibitor
- HLA
human leukocyte antigen
- DS
donor-specific
- NDS
non-donor-specific
- mTOR
mammalian target of rapamycin
- MMF
mycophenolate mofetil
- CTLA-4
cytotoxic T lymphocyte antigen 4
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- CR
chronic rejection
- LFA
lymphocyte function-associated
- CAV
chronic allograft vasculopathy
- DSA
donor specific antibody
- I/R
ischemia/reperfusion
- SCID
severe combined immunodeficiency
- SMC
smooth muscle cells
- CsA
cyclosporine A
References
- 1.Barth RN, Janus CA, Lillesand CA, Radke NA, Pirsch JD, Becker BN, et al. Outcomes at 3 years of a prospective pilot study of Campath-1H and sirolimus immunosuppression for renal transplantation. Transpl Int. 2006;19:885–92. doi: 10.1111/j.1432-2277.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 2.Knechtle SJ, Pascual J, Bloom DD, Torrealba JR, Jankowska-Gan E, Burlingham WJ, et al. Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: clinical results and immune monitoring. Am J Transplant. 2009;9:1087–98. doi: 10.1111/j.1600-6143.2009.02581.x. [DOI] [PubMed] [Google Scholar]
- 3.Bloom D, Chang Z, Pauly K, Kwun J, Fechner J, Hayes C, et al. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9:1835–45. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daridon C, Youinou P, Pers JO. BAFF, APRIL, TWE-PRIL: who’s who? Autoimmun Rev. 2008;7:267–71. doi: 10.1016/j.autrev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–75. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell costimulation by the TNF ligand BAFF. J Immunol. 2001;167:6225–31. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 8.Mackay F, Mackay CR. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 2002;23:113–5. doi: 10.1016/s1471-4906(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 9.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria – an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–14. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 10.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333–8. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 11.Feucht HE. Complement C4d in graft capillaries – the missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003;3:646–52. doi: 10.1034/j.1600-6143.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 12.Feucht HE. Significance of donor-specific antibodies in acute rejection. Transplant Proc. 2005;37:3693–4. doi: 10.1016/j.transproceed.2005.09.114. [DOI] [PubMed] [Google Scholar]
- 13.Feucht HE, Mihatsch MJ. Diagnostic value of C4d in renal biopsies. Curr Opin Nephrol Hypertens. 2005;14:592–8. doi: 10.1097/01.mnh.0000168943.54115.ac. [DOI] [PubMed] [Google Scholar]
- 14.Morris PJ, Mickey MR, Singal DP, Terasaki PI. Serotyping for homotransplantation. XXII. Specificity of cytotoxic antibodies developing after renal transplantation. Br Med J. 1969;1:758–9. doi: 10.1136/bmj.1.5646.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soulillou JP, Peyrat MA, Guenel J. Association between treatment-resistant kidney-allograft rejection and post-transplant appearance of antibodies to donor B-lymphocyte alloantigens. Lancet. 1978;1:354–6. doi: 10.1016/s0140-6736(78)91081-4. [DOI] [PubMed] [Google Scholar]
- 16.Iwaki Y, Terasaki PI, Iwatsuki S, Starzl T, Berne T, Karp R, et al. Posttransplant serum analysis in human kidney allografts. Transplant Proc. 1981;13:178–80. [PubMed] [Google Scholar]
- 17.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16:2804–12. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- 18.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4:438–43. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74:1192–4. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 20.Mao Q, Terasaki PI, Cai J, Briley K, Catrou P, Haisch C, et al. Extremely high association between appearance of HLA antibodies and failure of kidney grafts in a five-year longitudinal study. Am J Transplant. 2007;7:864–71. doi: 10.1111/j.1600-6143.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 21.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Fresnedo G, Pastor JM, Lopez-Hoyos M, Ruiz JC, Zubimendi JA, Gonzalez-Cotorruelo J, et al. Relationship of donor-specific class-I anti-HLA antibodies detected by ELISA after kidney transplantation on the development of acute rejection and graft survival. Nephrol Dial Transplant. 2003;18:990–5. doi: 10.1093/ndt/gfg068. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando) 2009;23:34–46. doi: 10.1016/j.trre.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Doyle HR, Marino IR, Morelli F, Doria C, Aldrighetti L, McMichael J, et al. Assessing risk in liver transplantation. Special reference to the significance of a positive cytotoxic crossmatch. Ann Surg. 1996;224:168–77. doi: 10.1097/00000658-199608000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hathaway M, Gunson BK, Keogh AC, Briggs D, McMaster P, Neuberger JM. A positive crossmatch in liver transplantation – no effect or inappropriate analysis? A prospective study. Transplantation. 1997;64:54–9. doi: 10.1097/00007890-199707150-00011. [DOI] [PubMed] [Google Scholar]
- 26.Opelz G, Dohler B, Susal C. Analysis of positive kidney, heart, and liver transplant crossmatches reported to the Collaborative Transplant Study. Human Immunol. 2009;70:627–30. doi: 10.1016/j.humimm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Watson R, Kozlowski T, Nickeleit V, Woosley JT, Schmitz JL, Zacks SL, et al. Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant. 2006;6:3022–9. doi: 10.1111/j.1600-6143.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 28.Della-Guardia B, Almeida MD, Meira-Filho SP, Torres MA, Venco F, Afonso RC, et al. Antibody-mediated rejection: hyperacute rejection reality in liver transplantation? A case report. Transplant Proc. 2008;40:870–1. doi: 10.1016/j.transproceed.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 29.Hori T, Uemoto S, Takada Y, Oike F, Ogura Y, Ogawa K, et al. Does a positive lymphocyte cross-match contraindicate living-donor liver transplantation? Surgery. 2010;147:840–4. doi: 10.1016/j.surg.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11:500–10. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martelius T, Halme L, Arola J, Hockerstedt K, Lautenschlager I. Vascular deposition of complement C4d is increased in liver allografts with chronic rejection. Transpl Immunol. 2009;21:244–6. doi: 10.1016/j.trim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17:357–68. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 33.Morrissey PE, Gordon F, Shaffer D, Madras PN, Silva P, Sahyoun AI, et al. Combined liver-kidney transplantation in patients with cirrhosis and renal failure: effect of a positive cross-match and benefits of combined transplantation. Liver Transpl Surg. 1998;4:363–9. doi: 10.1002/lt.500040512. [DOI] [PubMed] [Google Scholar]
- 34.Faenza A, Fuga G, Nardo B, Varotti G, Faenza S, Stefoni S, et al. Combined liver-kidney transplantation: the experience of the University of Bologna and the case of preoperative positive cross-match. Transplant Proc. 2006;38:1118–21. doi: 10.1016/j.transproceed.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 35.Senaldi G, Lobo-Yeo A, Mowat AP, Mieli-Vergani G, Vergani D. Class I and class II major histocompatibility complex antigens on hepatocytes: importance of the method of detection and expression in histologically normal and diseased livers. J Clin Pathol. 1991;44:107–14. doi: 10.1136/jcp.44.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Dar W, Agarwal A, Watkins C, Gebel HM, Bray RA, Kokko KE, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–7. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 38.Port FK, Held PJ, Wolfe RA, Garcia JR, Rocher LL. The impact of nonidentical ABO cadaveric renal transplantation on waiting times and graft survival. Am J Kidney Dis. 1991;17:519–23. doi: 10.1016/s0272-6386(12)80492-6. [DOI] [PubMed] [Google Scholar]
- 39.West LJ, Platt JL. And justice for all: consideration of ABO compatibility in allocation of hearts for infant transplantation. Circulation. 2010;121:1884–6. doi: 10.1161/CIR.0b013e3181e0b032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–1. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 41.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–6. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 42.Rieben R, Tucci A, Nydegger UE, Zubler RH. Self tolerance to human A and B histo-blood group antigens exists at the B cell level and cannot be broken by potent polyclonal B cell activation in vitro. Eur J Immunol. 1992;22:2713–7. doi: 10.1002/eji.1830221035. [DOI] [PubMed] [Google Scholar]
- 43.West LJ, Pollock-Barziv SM, Dipchand AI, Lee KJ, Cardella CJ, Benson LN, et al. ABO-incompatible heart transplantation in infants. N Engl J Med. 2001;344:793–800. doi: 10.1056/NEJM200103153441102. [DOI] [PubMed] [Google Scholar]
- 44.Fan X, Ang A, Pollock-Barziv SM, Dipchand AI, Ruiz P, Wilson G, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10:1227–33. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]
- 45.West LJ. Targeting antibody-mediated rejection in the setting of ABO-incompatible infant heart transplantation: graft accommodation vs. B cell tolerance. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:223–32. doi: 10.2174/1568006054064762. [DOI] [PubMed] [Google Scholar]
- 46.Patel ND, Weiss ES, Scheel J, Cameron DE, Vricella LA. ABO-incompatible heart transplantation in infants: analysis of the united network for organ sharing database. J Heart Lung Transplant. 2008;27:1085–9. doi: 10.1016/j.healun.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Roche SL, Burch M, O’Sullivan J, Wallis J, Parry G, Kirk R, et al. Multicenter experience of ABO-incompatible pediatric cardiac transplantation. Am J Transplant. 2008;8:208–15. doi: 10.1111/j.1600-6143.2007.02040.x. [DOI] [PubMed] [Google Scholar]
- 48.Daebritz SH, Schmoeckel M, Mair H, Kozlik-Feldmann R, Wittmann G, Kowalski C, et al. Blood type incompatible cardiac transplantation in young infants. Eur J Cardiothorac Surg. 2007;31:339–43. doi: 10.1016/j.ejcts.2006.11.032. [discussion 343] [DOI] [PubMed] [Google Scholar]
- 49.West LJ, Pollock-Barziv SM, Ang A, Dipchand AI, Kantor P, Mazza B, et al. Outcomes of the world experience in ABO-incompatible infant heart transplantation. Am J Transplant. 2005;5:157. [Google Scholar]
- 50.Dipchand AI, Pollock BarZiv SM, Manlhiot C, West LJ, VanderVliet M, McCrindle BW. Equivalent outcomes for pediatric heart transplantation recipients: ABO-blood group incompatible versus ABO-compatible. Am J Transplant. 2010;10:389–97. doi: 10.1111/j.1600-6143.2009.02934.x. [DOI] [PubMed] [Google Scholar]
- 51.Fong SW, Qaqundah BY, Taylor WF. Developmental patterns of ABO isoagglutinins in normal children correlated with the effects of age, sex, and maternal isoagglutinins. Transfusion. 1974;14:551–9. doi: 10.1111/j.1537-2995.1974.tb04576.x. [DOI] [PubMed] [Google Scholar]
- 52.Heidt S, Roelen DL, Eijsink C, Eikmans M, van Kooten C, Claas FH, et al. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol. 2010;159:199–207. doi: 10.1111/j.1365-2249.2009.04051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki N, Sakane T, Tsunematsu T. Effects of a novel immunosuppressive agent, FK506, on human B cell activation. Clin Exp Immunol. 1990;79:240–5. doi: 10.1111/j.1365-2249.1990.tb05185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HS, Raskova J, Degiannis D, Raska K., Jr Effects of cyclosporine and rapamycin on immunoglobulin production by preactivated human B cells. Clin Exp Immunol. 1994;96:508–12. doi: 10.1111/j.1365-2249.1994.tb06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86:1292–300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
- 56.Salinas-Carmona MC, Perez LI, Galan K, Vazquez AV. Immunosuppressive drugs have different effect on B lymphocyte subsets and IgM antibody production in immunized BALB/c mice. Autoimmunity. 2009;42:537–44. doi: 10.1080/08916930903019119. [DOI] [PubMed] [Google Scholar]
- 57.Zhou W, Ohdan H, Asahara T. Calcineurin inhibitors block B-1 cell differentiation: the relevance to immunosuppressive treatment in ABO-incompatible transplantation. Transplant Proc. 2005;37:1808–11. doi: 10.1016/j.transproceed.2005.03.129. [DOI] [PubMed] [Google Scholar]
- 58.Woo J, Propper DJ, Macleod AM, Thomson AW. Influence of FK506 and cyclosporin A on alloantibody production and lymphocyte activation following blood transfusion. Clin Exp Immunol. 1990;82:462–8. doi: 10.1111/j.1365-2249.1990.tb05472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Propper DJ, Woo J, Thomson AW, Catto GR, Macleod AM. FK506 – its influence on anti-class 1 MHC alloantibody responses to blood transfusions. Transplantation. 1990;50:267–71. doi: 10.1097/00007890-199008000-00020. [DOI] [PubMed] [Google Scholar]
- 60.Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72:1181–93. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- 61.Pescovitz MD, Book BK, Henson S, Leapman SB, Milgrom ML, Kimball J, et al. The addition of sirolimus to cyclosporine and steroids inhibits the anti-equine antibody response in renal transplant recipients treated with antithymocyte globulin. Am J Transplant. 2003;3:497–500. doi: 10.1034/j.1600-6143.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 62.Lipsky JJ. Mycophenolate mofetil. Lancet. 1996;348:1357–9. doi: 10.1016/S0140-6736(96)10310-X. [DOI] [PubMed] [Google Scholar]
- 63.Lederer SR, Friedrich N, Banas B, Welser G, Albert ED, Sitter T. Effects of mycophenolate mofetil on donor-specific antibody formation in renal transplantation. Clin Transplant. 2005;19:168–74. doi: 10.1111/j.1399-0012.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 64.Ciancio G, Burke GW., 3rd Alemtuzumab (Campath-1H) in kidney transplantation. Am J Transplant. 2008;8:15–20. doi: 10.1111/j.1600-6143.2007.02053.x. [DOI] [PubMed] [Google Scholar]
- 65.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81:81–7. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 66.Kirk AD, Mannon RB, Kleiner DE, Swanson JS, Kampen RL, Cendales LK, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–9. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 67.Knechtle SJ, Pirsch JDH, Fechner JJ, Becker BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–30. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 68.Flechner SM, Friend PJ, Brockmann J, Ismail HR, Zilvetti M, Goldfarb D, et al. Alemtuzumab induction and sirolimus plus mycophenolate mofetil maintenance for CNI and steroid-free kidney transplant immunosuppression. Am J Transplant. 2005;5:3009–14. doi: 10.1111/j.1600-6143.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 69.Willicombe M, Roufosse C, Brookes P, Galliford JW, McLean AG, Dorling A, et al. Antibody-mediated rejection after alemtuzumab induction: incidence, risk factors, and predictors of poor outcome. Transplantation. 2011;92:176–82. doi: 10.1097/TP.0b013e318222c9c6. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S, Kimlinger TK, Lust JA, Donovan K, Witzig TE. Expression of CD52 on plasma cells in plasma cell proliferative disorders. Blood. 2003;102:1075–7. doi: 10.1182/blood-2002-12-3784. [DOI] [PubMed] [Google Scholar]
- 71.Simpson BS, Coles AJ. Rationale for cytotoxic monoclonal antibodies in MS. Int MS J. 2007;14:48–56. [PubMed] [Google Scholar]
- 72.Isaacs JD, Greer S, Sharma S, Symmons D, Smith M, Johnston J, et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged and profound therapy-induced lymphopenia. Arthritis Rheum. 2001;44:1998–2008. doi: 10.1002/1529-0131(200109)44:9<1998::AID-ART348>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 73.Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 74.Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 75.Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691–5. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 76.Wing MG, Moreau T, Greenwood J, Smith RM, Hale G, Isaacs J, et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest. 1996;98:2819–26. doi: 10.1172/JCI119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 78.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–53. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 79.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–7. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 80.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 81.Jordan SC, Reinsmoen N, Peng A, Lai CH, Cao K, Villicana R, et al. Advances in diagnosing and managing antibody-mediated rejection. Pediatr Nephrol. 2010;25:2035–45. doi: 10.1007/s00467-009-1386-4. [quiz 2045-2038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–4. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 83.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 84.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–59. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 85.Walsh RC, Everly JJ, Brailey P, Rike AH, Arend LJ, Mogilishetty G, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89:277–84. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 86.Ponce R. Preclinical support for combination therapy in the treatment of autoimmunity with atacicept. Toxicol Pathol. 2009;37:89–99. doi: 10.1177/0192623308329477. [DOI] [PubMed] [Google Scholar]
- 87.Xu H, He X, Liu Q, Chen Y, Zhu Y, Shi D, et al. The abnormal high expression of B cell activating factor belonging to TNF superfamily (BAFF) and its potential role in kidney transplant recipients. Cell Mol Immunol. 2008;5:465–70. doi: 10.1038/cmi.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–97. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J Immunol. 2007;178:5612–22. doi: 10.4049/jimmunol.178.9.5612. [DOI] [PubMed] [Google Scholar]
- 92.Xu H, He X, Liu Q, Shi D, Chen Y, Zhu Y, et al. Abnormal high expression of B-cell activating factor belonging to the TNF superfamily (BAFF) associated with long-term outcome in kidney transplant recipients. Transplant Proc. 2009;41:1552–6. doi: 10.1016/j.transproceed.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 93.Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008;58:61–72. doi: 10.1002/art.23178. [DOI] [PubMed] [Google Scholar]
- 94.Pena-Rossi C, Nasonov E, Stanislav M, Yakusevich V, Ershova O, Lomareva N, et al. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus. 2009;18:547–55. doi: 10.1177/0961203309102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiche L, Jourde N, Mancini J. Belimumab for systemic lupus erythematosus. Lancet. 2011;377:2080. doi: 10.1016/S0140-6736(11)60912-4. [author reply 2080–2081] [DOI] [PubMed] [Google Scholar]
- 96.Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231–5. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 97.Wang H, Jiang J, Liu W, Kubelik D, Chen G, Gies D, et al. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation. 2005;79:1121–7. doi: 10.1097/01.tp.0000161218.58276.9a. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179:4451–63. doi: 10.4049/jimmunol.179.7.4451. [DOI] [PubMed] [Google Scholar]
- 99.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–95. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 100.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–83. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 101.U.http://www.usrds.org/2008/slides/htm/vol2_07_tx_08.swf; 2008 [accessed 01.14.11]
- 102.Kwun J, Knechtle SJ. Overcoming chronic rejection-can it B? Transplantation. 2009;88:955–61. doi: 10.1097/TP.0b013e3181b96646. [DOI] [PubMed] [Google Scholar]
- 103.Terasaki PI. The review by Kwun and Knechtle-“can it B? ”-asks whether B cells are responsible for chronic rejection of transplants. Transplantation. 2009;88:978–9. doi: 10.1097/TP.0b013e3181b998fd. [DOI] [PubMed] [Google Scholar]
- 104.Torrealba JR, Fernandez LA, Kanmaz T, Oberley TD, Schultz JM, Brunner KG, et al. Immunotoxin-treated rhesus monkeys: a model for renal allograft chronic rejection. Transplantation. 2003;76:524–30. doi: 10.1097/01.TP.0000075788.72614.D4. [DOI] [PubMed] [Google Scholar]
- 105.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377–83. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 106.Mennander A, Tiisala S, Halttunen J, Yilmaz S, Paavonen T, Hayry P. Chronic rejection in rat aortic allografts. An experimental model for transplant arteriosclerosis. Arterioscler Thromb. 1991;11:671–80. doi: 10.1161/01.atv.11.3.671. [DOI] [PubMed] [Google Scholar]
- 107.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152:5135–41. [PubMed] [Google Scholar]
- 108.Inoue K, Niesen N, Albini B, Milgrom F. Studies on immunological tolerance induced in mice by kidney allografts. Int Arch Allergy Appl Immunol. 1991;96:358–61. doi: 10.1159/000235522. [DOI] [PubMed] [Google Scholar]
- 109.Qian SG, Fung JJ, Demetris AV, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562–4. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Z, Zhu L, Quan D, Garcia B, Ozcay N, Duff J, et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 1996;62:1267–72. doi: 10.1097/00007890-199611150-00016. [DOI] [PubMed] [Google Scholar]
- 111.George JF, Pinderski LJ, Litovsky S, Kirklin JK. Of mice and men: mouse models and the molecular mechanisms of post-transplant coronary artery disease. J Heart Lung Transplant. 2005;24:2003–14. doi: 10.1016/j.healun.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Kwun J, Hu H, Schadde E, Roenneburg D, Sullivan KA, DeMartino J, et al. Altered distribution of H60 minor H antigen-specific CD8 T cells and attenuated chronic vasculopathy in minor histocompatibility antigen mismatched heart transplantation in Cxcr3−/− mouse recipients. J Immunol. 2007;179:8016–25. doi: 10.4049/jimmunol.179.12.8016. [DOI] [PubMed] [Google Scholar]
- 113.Fischbein MP, Yun J, Laks H, Irie Y, Fishbein MC, Espejo M, et al. CD8+ lymphocytes augment chronic rejection in a MHC class II mismatched model. Transplantation. 2001;71:1146–53. doi: 10.1097/00007890-200104270-00023. [DOI] [PubMed] [Google Scholar]
- 114.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection. Transplantation. 2001;72:348–50. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 115.Orosz CG, Wakely E, Bergese SD, VanBuskirk AM, Ferguson RM, Mullet D, et al. Prevention of murine cardiac allograft rejection with gallium nitrate. Comparison with anti-CD4 monoclonal antibody. Transplantation. 1996;61:783–91. doi: 10.1097/00007890-199603150-00019. [DOI] [PubMed] [Google Scholar]
- 116.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 117.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–24. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carpenter CB, d’Apice AJ, Abbas AK. The role of antibodies in the rejection and enhancement of organ allografts. ?7318. Adv Immunol. 1976;22:1–65. doi: 10.1016/s0065-2776(08)60547-7. [DOI] [PubMed] [Google Scholar]
- 120.Tilney NL, Kupiec-Weglinski JW, Heidecke CD, Lear PA, Strom TB. Mechanisms of rejection and prolongation of vascularized organ allografts. Immunol Rev. 1984;77:185–216. doi: 10.1111/j.1600-065x.1984.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 121.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–26. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 122.Russell PS, Chase CM, Madsen JC, Hirohashi T, Cornell LD, Sproule TJ, et al. Coronary artery disease from isolated non-H2-determined incompatibilities in transplanted mouse hearts. Transplantation. 2011;91:847–52. doi: 10.1097/TP.0b013e3182122f82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang J, Jaramillo A, Liu W, Olack B, Yoshimura Y, Joyce S, et al. Chronic rejection of murine cardiac allografts discordant at the H13 minor histocompatibility antigen correlates with the generation of the H13-specific CD8+ cytotoxic T cells. Transplantation. 2003;76:84–91. doi: 10.1097/01.TP.0000072013.21336.64. [DOI] [PubMed] [Google Scholar]
- 124.Pascual J, Pirsch JD, Odorico JS, Torrealba JR, Djamali A, Becker YT, et al. Alemtuzumab induction and antibody-mediated kidney rejection after simultaneous pancreas-kidney transplantation. Transplantation. 2009;87:125–32. doi: 10.1097/TP.0b013e31818c6db0. [DOI] [PubMed] [Google Scholar]
- 125.Pascual J, Bloom D, Torrealba J, Brahmbhatt R, Chang Z, Sollinger HW, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells. Am J Transplant. 2008;8:1529–36. doi: 10.1111/j.1600-6143.2008.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Piccotti JR, Li K, Chan SY, Eichwald EJ, Bishop DK. Cytokine regulation of chronic cardiac allograft rejection: evidence against a role for Th1 in the disease process. Transplantation. 1999;67:1548–55. doi: 10.1097/00007890-199906270-00008. [DOI] [PubMed] [Google Scholar]
- 127.Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 2003;196:176–96. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 128.Rose SM, Blustein N, Rotrosen D. Recommendations of the expert panel on ethical issues in clinical trials of transplant tolerance. National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Transplantation. 1998;66:1123–5. doi: 10.1097/00007890-199811150-00001. [DOI] [PubMed] [Google Scholar]
- 129.Geluk A, Elferink DG, Slierendregt BL, van Meijgaarden KE, de Vries RR, Ottenhoff TH, et al. Evolutionary conservation of major histocompatibility complex-DR/peptide/T cell interactions in primates. J Exp Med. 1993;177:979–87. doi: 10.1084/jem.177.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slierendregt BL, van Noort JT, Bakas RM, Otting N, Jonker M, Bontrop RE. Evolutionary stability of transspecies major histocompatibility complex class II DRB lineages in humans and rhesus monkeys. Hum Immunol. 1992;35:29–39. doi: 10.1016/0198-8859(92)90092-2. [DOI] [PubMed] [Google Scholar]
- 131.Boskovic S, Kawai T, Smith RN, Wee SL, Nadazdin O, Koyama I, et al. Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long-term observations in nonhuman primates. Transplantation. 2006;82:819–25. doi: 10.1097/01.tp.0000234786.26511.a4. [DOI] [PubMed] [Google Scholar]
- 132.Deschamps JY, Roux FA, Sai P, Gouin E. History of xenotransplantation. Xenotransplantation. 2005;12:91–109. doi: 10.1111/j.1399-3089.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 133.Schuurman HJ, Cheng J, Lam T. Pathology of xenograft rejection: a commentary. Xenotransplantation. 2003;10:293–9. doi: 10.1034/j.1399-3089.2003.02092.x. [DOI] [PubMed] [Google Scholar]
- 134.Cozzi E, Severo M, Bosio E, Besenzon F, Ancona E. Antibody mediated rejection in pig-to-nonhuman primate xenotransplantation models. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:233–53. doi: 10.2174/1568006054064799. [DOI] [PubMed] [Google Scholar]
- 135.Lam TT, Hausen B, Hook L, Lau M, Higgins J, Christians U, et al. The effect of soluble complement receptor type 1 on acute humoral xenograft rejection in hDAF-transgenic pig-to-primate life-supporting kidney xenografts. Xenotransplantation. 2005;12:20–9. doi: 10.1111/j.1399-3089.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 136.McCurry KR, Parker W, Cotterell AH, Weidner BC, Lin SS, Daniels LJ, et al. Humoral responses to pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol. 1997;58:91–105. doi: 10.1016/s0198-8859(97)00229-2. [DOI] [PubMed] [Google Scholar]
- 137.Richards AC, Davies HF, McLaughlin ML, Copeman LS, Holmes BJ, Dos Santos Cruz G, et al. Serum anti-pig antibodies as potential indicators of acute humoral xenograft rejection in pig-to-cynomolgus monkey kidney transplantation. Transplantation. 2002;73:881–9. doi: 10.1097/00007890-200203270-00009. [DOI] [PubMed] [Google Scholar]
- 138.Wieczorek G, Bigaud M, Menninger K, Riesen S, Quesniaux V, Schuurman HJ, et al. Acute and chronic vascular rejection in nonhuman primate kidney transplantation. Am J Transplant. 2006;6:1285–96. doi: 10.1111/j.1600-6143.2006.01307.x. [DOI] [PubMed] [Google Scholar]
- 139.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, Boon L, et al. Costimulation blockade followed by a 12-week period of cyclosporine A facilitates prolonged drug-free survival of rhesus monkey kidney allografts. Transplantation. 2005;79:1623–6. doi: 10.1097/01.tp.0000158426.64631.ed. [DOI] [PubMed] [Google Scholar]
- 140.Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9:1732–41. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 141.Azimzadeh AM, Pfeiffer S, Wu G, Schroder C, Zorn GL, 3rd, Kelishadi SS, et al. Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation. 2006;81:255–64. doi: 10.1097/01.tp.0000190099.62847.e6. [DOI] [PubMed] [Google Scholar]
- 142.Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73:1757–64. doi: 10.1097/00007890-200206150-00011. [DOI] [PubMed] [Google Scholar]
- 143.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–62. [PubMed] [Google Scholar]
- 144.Schroder C, Pierson RN, 3rd, Nguyen BN, Kawka DW, Peterson LB, Wu G, et al. CCR5 blockade modulates inflammation and alloimmunity in primates. J Immunol. 2007;179:2289–99. doi: 10.4049/jimmunol.179.4.2289. [DOI] [PubMed] [Google Scholar]
- 145.Li J, Chen G, Ye P, Wang S, Zhang K, Chen W, et al. CCR5 blockade in combination with cyclosporine increased cardiac graft survival and generated alternatively activated macrophages in primates. J Immunol. 2011;186:3753–61. doi: 10.4049/jimmunol.1002143. [DOI] [PubMed] [Google Scholar]
- 146.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, et al. Chronic antibody mediated rejection of renal allografts: pathological, serological and immunologic features in nonhuman primates. Am J Transplant. 2006;6:1790–8. doi: 10.1111/j.1600-6143.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 147.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, et al. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–72. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, et al. Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (’CAN’) Am J Transplant. 2007;7:518–26. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 149.Drachenberg CB, Odorico J, Demetris AJ, Arend L, Bajema IM, Bruijn JA, et al. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008;8:1237–49. doi: 10.1111/j.1600-6143.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- 150.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–71. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 151.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100:550–7. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang J, Popoola J, Khandwala S, Vadivel N, Vanguri V, Yuan X, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117:660–9. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- 153.Burdick JF, Clow LW. Rejection of murine cardiac allografts. I. Relative roles of major and minor antigens. Transplantation. 1986;42:67–72. doi: 10.1097/00007890-198607000-00015. [DOI] [PubMed] [Google Scholar]
- 154.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy in mice: relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 155.Bishop DK, Shelby J, Eichwald EJ. Mobilization of T lymphocytes following cardiac transplantation, evidence that CD4-positive cells are required for cytotoxic T lymphocyte activation, inflammatory endothelial development, graft infiltration, and acute allograft rejection. Transplantation. 1992;53:849–57. [PubMed] [Google Scholar]
- 156.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–55. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 157.Orosz CG, Wakely E, Sedmak DD, Bergese SD, VanBuskirk AM. Prolonged murine cardiac allograft acceptance: characteristics of persistent active alloimmunity after treatment with gallium nitrate versus anti-CD4 monoclonal antibody. Transplantation. 1997;63:1109–17. doi: 10.1097/00007890-199704270-00010. [DOI] [PubMed] [Google Scholar]
- 158.Russell PS, Chase CM, Colvin RB, Plate JM. Induced immune destruction of long-surviving. H-2 incompatible kidney transplants in mice. J Exp Med. 1978;147:1469–86. doi: 10.1084/jem.147.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mannon RB, Griffiths R, Ruiz P, Platt JL, Coffman TM. Absence of donor MHC antigen expression ameliorates chronic kidney allograft rejection. Kidney Int. 2002;62:290–300. doi: 10.1046/j.1523-1755.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 160.Amano H, Bickerstaff A, Orosz CG, Novick AC, Toma H, Fairchild RL. Absence of recipient CCR5 promotes early and increased allospecific antibody responses to cardiac allografts. J Immunol. 2005;174:6499–508. doi: 10.4049/jimmunol.174.10.6499. [DOI] [PubMed] [Google Scholar]
- 161.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, et al. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–36. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 162.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, et al. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008;8:557–66. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 163.Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. 2010;120:1275–84. doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]


