Abstract
Flexing the knee to isolate the single joint soleus from the biarticular gastrocnemius is a strategy for investigating individual plantarflexor’s post activation potentiation (PAP). We investigated the implications of testing plantarflexor PAP at different knee angles and provided indirect quantification of the contribution of gastrocnemius potentiation to the overall plantarflexor enhancements post conditioning. Plantarflexor supramaximal twitches were measured in ten male power athletes before and after a maximal isometric plantarflexion (MVIC) at both flexed and extended knee angles. Mean torque and soleus (SOLRMS) and medial gastrocnemius (MGRMS) activity were measured during the MVIC. The mean torque and MGRMS of the MVIC were lower (by 33.9 and 42.4%, respectively) in the flexed compared to the extended position, with no significant difference in SOLRMS. After the MVIC, twitch peak torque (PT) and the rate of torque development (RTR) potentiated significantly more (by 17.4 and 14.7% respectively) in the extended as compared to the flexed knee position, but only immediately (5 s) after the MVIC. No significant differences were found in the twitch rate of torque development (RTD) potentiation between positions. It was concluded that knee joint configuration should be taken into consideration when comparing studies of plantarflexor PAP. Furthermore, results reflect a rather brief contribution of the gastrocnemius potentiation to the overall plantarflexor twitch enhancements.
Key words: potentiation, twitch, electrical stimulation, triceps surae, knee angle, acute effects
Introduction
Post activation potentiation (PAP) can be defined as the acute enhancement of muscle contractile properties induced by maximal voluntary isometric (MVIC) or near maximal voluntary muscle contractions (Baudry and Duchateau, 2007; Jubeau et al., 2010; MacIntosh, 2010). In the absence of stiffness and muscle architectural changes (Gago et al., 2014a; Rodriguez-Falces et al., 2015), acute enhancements of twitch parameters such as peak torque (PT) and the rate of torque development (RTD) and relaxation (RTR) after an MVIC might be mainly related to enhanced cross-bridge cycle efficiency (Brito et al., 2011; MacIntosh 2010; Stull et al., 2011). PAP can be affected by fiber type composition of the muscle, but the intensity of the conditioning contraction and the muscle length also greatly affect the degree of PAP (Rassier et al., 1997; Tillin and Bishop, 2009). Different muscle fibre lengths might result in different potentials for developing PAP, since changing the muscle fibre length might also affect the calcium kinetics and/or cross-bridge kinetics via changes in osmotic compression, lattice space and myofilament optimal overlap (Millman, 1998; Rassier et al., 1999; Rassieret al., 1997; Yang et al., 1998). However, assessment of PAP behavior at different muscle lengths in humans can be assumed to involve more complex muscle architectural modulations than shown in studies of single skinned fibres, especially if bi-articular muscles such as the gastrocnemius are involved. A change in the knee angle will affect the length of the gastrocnemius, but not the soleus (Kawakami et al., 1998). Variations in the ankle joint angle were found to affect PT enhancements (i.e. higher PAP at shorter muscle lengths) for the tibialis anterior (Mela et al., 2001; Vandervoort et al., 1983), and PAP levels in the medial gastrocnemius (MG) and soleus (SOL) muscles, measured independently via mechanomyographic amplitude, were also found to be ankle joint dependent (Miyamoto et al., 2010).
Less is known about the impact of knee joint configuration (i.e. extended versus flexed knee) on plantarflexor twitch PAP. While Fukutani et al. (2014) investigated PAP of the plantarflexors in a flexed knee position to isolate the soleus muscle, others have used an extended knee position (Fukutani et al., 2013; Gago et al., 2014b; Vandervoort et al., 1983), but no one has compared PAP in a flexed and an extended position within the same subjects. Previous studies have indicated that flexing the knee changes the gastrocnemius muscle architecture resulting in impaired force transfer and neuromuscular inhibition of the gastrocnemius (Cresswell et al., 1995; Kawakami et al., 1998; Kennedy and Cresswell 2001; Tamaki et al., 1997). The aim of this study was to investigate possible differences in the magnitude and timing of plantarflexor PAP between a flexed and an extended knee joint position and as a consequence, the amplitude and duration of gastrocnemius PAP contribution to overall plantarflexor twitch enhancements.
Material and Methods
Participants
Ten male athletes competing in explosive sports (e.g. sprinting and long jump) participated in the present study (age 22.2 ± 1.9 yrs; body height 187.1 ± 6.0 cm; body mass 80.0 ± 9.4 kg; body mass index 22.8 ± 1.3 kg·m-2). All athletes were free from previous injury of the right foot and ankle. Subjects were informed of the methods of the study and had signed an informed consent document. The experimental procedures of the study were approved by the regional ethical review board in Stockholm at Karolinska Institutet and all procedures adhered to the declaration of Helsinki.
Experimental procedures
After a standardized warm up (10 minutes of submaximal ergometer cycling performed at intensity level 12 on the Borg rate of perceived exertion scale), electrodes were attached to the subjects (see electrical stimulation and electromyography measurements section) who afterwards lay in a prone position with their arms and hands to the side of the body. During resting, their shoulders, hips, legs and right foot were securely fixed to an isokinetic dynamometer (Isomed 2000, D&R Ferstl GmbH, Henau, Germany). The axis of the ankle joint was aligned with the centre of rotation of the dynamometer shaft. After these preparations, the intensity of electric stimulation was set (see electrical stimulation) after which the subjects rested for five minutes. Plantarflexor twitches were induced before and at several occasions during 10 minutes of recovery after a conditioning task consisting of a 6 s MVIC. After five further minutes of rest in the prone lying position (extended knee position), the shoulder and hip straps were released and subjects were carefully placed in a four point kneeling position with the knees directly under the hips and the wrists under the shoulders (flexed knee position). The knee angle was individually adjusted to 90°. When the 90° angle was obtained, a steel bar was fixed in front of the mid lower portion of the thigh to facilitate correct positioning while performing an MVIC plantar flexion. Stimulation intensity was re-assessed to ensure supramaximal stimulation at the new position. Plantar flexor twitches were then induced once more before and then at 7 occasions during 10 minutes of recovery after a 6 s MVIC plantar flexion in the flexed knee position.
Electrical stimulation
Electrical stimulation was applied to the posterior tibial nerve using a single rectangular pulse (1 ms) delivered by a Digitimer stimulator (model DS7A, Hertfordshire, UK). A small cathode electrode (blue sensor, 7-mm diameter, Ag–AgCL Medicotest, Denmark) was positioned in the popliteal fossa at the best stimulation site as determined by manual localisation using a custom-made stimulating pen. The anode (rectangular 100 mm × 50 mm carbon rubber electrode, Cefar Medical, Sweden) was placed and taped on the anterior surface of the knee, immediately proximal to the patella. Each subject was initially familiarized with several submaximal electrical stimuli of progressively increased intensity until the compound muscle action potential (M-wave) and the mechanical twitch reached their maximal values. The stimulation intensity was then increased by further 20% to the supramaximal intensity used in the subsequent protocol. Stimulations were always performed at an ankle angle of 90°.
Electromyography measurements
The surface electromyography (EMG) signals were recorded using 7-mm diameter circular Ag–AgCl electrodes (Ambu Blue Sensor, Medicotest, Denmark). Electrode pairs were positioned over the bellies of the MG and in a belly tendon configuration over the SOL for measuring muscle activation during the MVICs and SOL M-waves associated with the electric stimulation. Two ground electrodes were positioned on the skin covering the head of the fibula and the medial femoral condyle. Low impedance at the skin-electrode interface was obtained by shaving and cleaning with alcohol. The EMG signals were amplified 1000 (MG) or 200 (SOL) times (NL 824, Digitimer, UK), band pass filtered (30 Hz–1 kHz) (NL 125, Digitimer, UK) and converted to digital data at a sample rate of 5 kHz, using a 16 bit Power 1401 and Spike2 data collection system (version 7.0, Cambridge Electronic Design (CED), UK).The root mean square (RMS) was obtained from the SOL (SOLRMS) and MG (MGRMS) electromyographic signals between 10% of the rising and 90% of the declining peak MVIC signal in both positions.
Torque and mechanical measurements
Torque about the ankle was measured by the isokinetic dynamometer Isomed 2000. Torque data were analog to digitally converted and sampled together with the position and EMG signals by power 1401 (CED, Cambridge, UK) in the Spike2 software. The mean torque and duration of the MVIC were measured between the instant when the torque passed 10% of the MVIC and the instant when the torque passed the same level at the end of the MVIC. The twitches recorded in the torque signal were analyzed in the same software to extract the following variables: peak torque (PT) measured as the difference between the maximal twitch torque and the torque value at the time of the proximal peak of the SOL M-wave; maximum rate of torque development (RTD) and relaxation (RTR) were measured as the peak of the first derivate of the development of torque (dF/dt) and as the peak of the first derivate of decline of torque (-dF/dt), respectively.
Statistical Analyses
Statistical analysis was performed in Statistica (Version 12, StatSoft Scandinavia AB, Uppsala, Sweden). Normality tests were performed using Shapiro-Wilk tests. Intraclass correlation coefficients (ICC2, k) were calculated to assess consistency between positions for the duration of the MVICs as well as within control twitches for each position and parameter (i.e. PT, RTD, RTR and SOL M-wave). Furthermore, the coefficient of variation (CV) expressed in percentages, was calculated to assess the typical measurement error (Hopkins, 2000) between the three control trials for the main parameters (PT, RTD and RTR). The mean values of the MVIC torque, SOLRMS and MGRMS in the conditioning contraction were compared between positions (i.e. extended versus flexed knee) using a dependent ttest or a Wilcoxon test in case of normality violation. For each position, a repeated-measures analysis of variance (ANOVA) was performed on the absolute values with the factor time (i.e. before and at different time points after the conditioning MVIC) for PT, RTD, RTR and SOL M-wave. To compare percentage changes between positions, two way repeated measures ANOVAs were performed for normalized PT, RTD and RTR. The normalized values were expressed as percentages of the mean of the three control twitches in the flexed or extended position, respectively. Whenever a significant main effect or an interaction was found, the Tukey post hoc test was applied for further analysis. Differences were considered significant at p < 0.05.
Results
Conditioning contraction
The mean MVIC torque was significantly higher in the extended knee position (227.4 ± 34.1 N·m) than in the flexed knee position (150.4 ± 34.2 N·m) (t(9) = 9.3, p < .001). The contraction duration was 6.2 ± 0.4 s and 6.5 ± 0.6 s for the extended and flexed knee position, respectively. There was no significant difference (z = 1.58 p < 0.11) in MVIC duration between positions. The ICC2, k of the MVIC duration was 0.761 indicating good consistency between positions.
Twitch parameters
The lowest ICC2, k and highest CV for the control twitches among the two positions was 0.982 and 4.1% for PT, 0.979 and 4.9% for RTD, and 0.977 and 4.9% for RTR. These values indicate high consistency between control measurements. The lowest significant twitch enhancements were three to five times greater than the typical measurement of error.
Mean control PT, RTD and RTR absolute values were significantly different between positions. Values from extended and flexed position were 24.6 ± 3.0 and 18.6 ± 3.2 N·m, respectively, for PT, 240.5 ± 26.0 and 173.4 ± 38.0 N·m·s-1 for RTD, and 137.5 ± 14.6 and 102.2 ± 16.2 N·m·s-1 for RTR.
PT was immediately and significantly enhanced for up to three minutes in the extended knee position (F7,63 = 94.7) and for up to five minutes in the flexed knee position (F7,63 = 24.3). For the normalized PT a significant interaction between the position and time was identified (F6,54 = 12.2), with post hoc tests revealing a significant PT difference between positions at 5 s after the MVIC (Figures 1 and 2A). Five seconds after conditioning PT was enhanced by 60.6 ± 19.3% versus 43.2 ± 8.4% for extended and flexed knee positions, respectively (Figures 1 and 2A).
Figure 1.
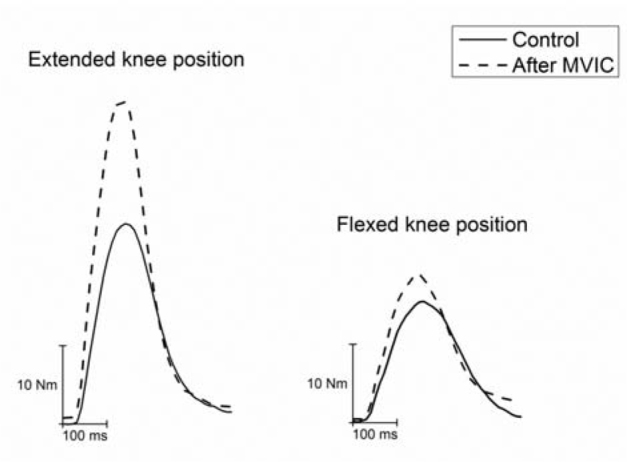
Raw data representation of a supramaximal twitch before (Control) and 5 seconds after
conditioning (After MVIC). Note that potentiation (i.e. difference between Control and
After MVIC peak torque) was substantially greater in the extended (left) than the flexed
(right) knee position.
Figure 2.
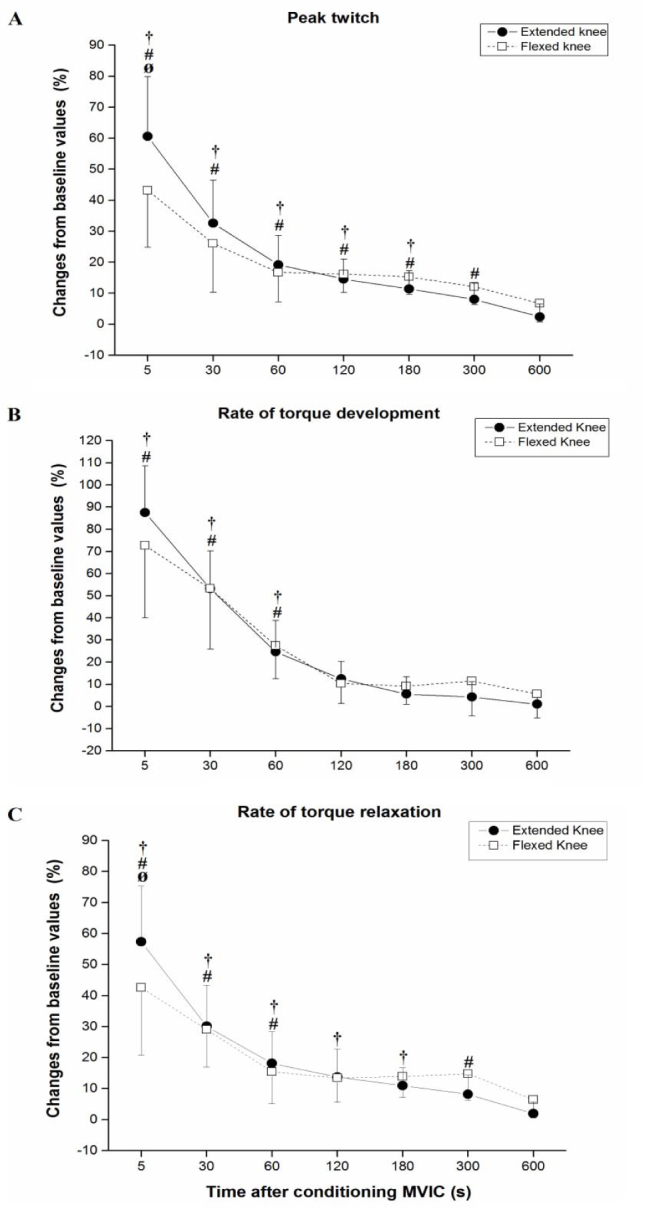
(A) Peak twitch, (B) Rate of torque development and (C) Rate of torque relaxation at
different time points after the conditioning MVIC in extended (black circle) and
flexed (white square) knee position. Significant (p < 0.05) differences from control
baseline values are indicated with a † for extended knee and with # for flexed knee
position. Differences between extended and flexed knee positions are indicated with
Ø. Values expressed as percentages of baseline value are means ± SD.
Immediately after the MVIC, the RTD was significantly enhanced up to one minute in both extended (F7,63 = 121.4) and flexed knee positions (F7,63 = 25.8) (Figure 2B). A significant interaction between the position and time was identified for the normalized RTD (F6,54 = 2.3). The post hoc tests, however, revealed no significant differences at the time points of interest (Figure 2B). For reference, the RTD was maximally enhanced by 87.5 ± 21.0% and 72.7 ± 32.7% 5 s after the MVIC for extended and flexed knee positions, respectively (Figure 2B).
In the extended knee position, RTR was significantly and immediately enhanced for up to three minutes after the MVIC (F7,63 = 97.6) and in the flexed knee position for up to five minutes (F7,63 = 17.3) (Figure 2C). For the normalized RTR, there were significant interactions between the position and time (F6,54 = 5.1), with post hoc tests revealing a significant difference between positions at 5 s after the MVIC (Figure 2C). RTR was maximally enhanced by 57.3 ± 18.0% and 42.6 ± 21.7%, 5 s after the MVIC for extended and flexed knee positions, respectively (Figure 2C).
Electromyographic variables
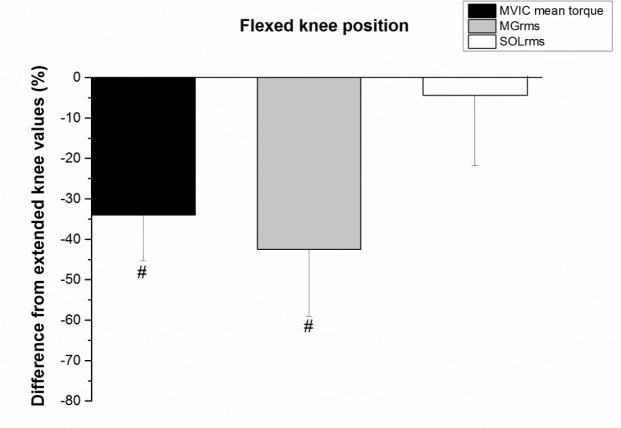
During the MVIC, MGRMS was significantly different between positions (z = 2.8, p = 0.005). MGRMS was 42.4 ± 16.6% lower in the flexed as compared to the extended knee position (Figure 3). The SOLRMS was not significantly different between positions (t(9) = 0.58, p = 0.57) (Figure 3).
Figure 3.
Difference between flexed and extended knee positions for the maximal voluntary
isometric contraction (MVIC) mean torque values and root mean square of the
associated electromyography signals from soleus (SOLRMS) and medial gastrocnemius
(MGRMS). Significant (p < 0.05) differences between extended and flexed knee positions
are indicated with # Values expressed as percent of extended knee value are means ± SD.
Discussion
The aim of this study was to investigate possible differences in the magnitude and timing of plantarflexor PAP using a flexed or an extended knee joint position. The main finding was that in line with our hypothesis, the 6 s MVIC induced more PAP in the extended as compared to the flexed knee position.
MVIC effects on twitch parameters
Twitch parameters (PT, RTD and RTR) were significantly enhanced in both positions following the MVIC conditioning contraction. The greatest changes from baseline values were registered immediately (5 s) after the MVIC and dissipated gradually. Significant enhancements in PT, RTD and RTR lasted for a minimum of one minute to a maximum of five minutes (Figures 2A, B and C). Such behavior is in line with previous findings (Fukutani et al., 2014; Gago et al., 2014b; Vandervoort et al., 1983) and indicates PAP related effects on the muscle contractile properties.
The amplitude and duration of the changes in twitch parameters after a conditioning contraction have previously been shown to be dependent on muscle characteristics (e.g. muscle fibre type) (Hamada et al., 2000, 2003) and subject characteristics (strength levels, training background, training status and age) (Tillin and Bishop, 2009). Furthermore, these changes vary depending on the duration and intensity of the PAP inducing conditioning contraction (Miyamoto et al., 2012; Vandervoort et al., 1983). The results of this study confirm that a brief plantarflexor MVIC is capable of acutely enhancing PT, RTD and RTR for up to five minutes and that this can be achieved in both an extended and flexed knee condition.
MVIC effects on twitch parameters at different knee positions
When assessing contractile properties of muscles, it is often desirable to investigate single joint muscles since the joint torque will then better reflect the contractile behavior of the muscle. In the flexed knee position it would thus be easier to achieve a true measure of the muscle PAP. The results of this study showed, however, that the acute (5 s) PAP effects on twitch parameters were higher in the extended than in the flexed knee position. This indicates that the flexed position may not be representative of the whole plantarflexor muscle group. However, the differences in degree of PAP between positions were only significant for PT and RTR 5 s after the MVIC. No significant differences were found between positions for potentiation in the RTD. The previous studies investigating joint angle dependency of twitch PAP did not report the RTD and RTR (Miyamoto et al., 2010; Rassier 2000; Suter and Herzog 1997; Vandervoort et al., 1983). Contradictory findings have been presented regarding knee angle dependency of knee extensor twitches with Rassier (2000) reporting significantly higher PAP at joint angles implying shorter muscle lengths, while Suter and Herzog (1997) reported similar PAP levels irrespective of the knee joint angle. Vandervoort et al. (1983) reported significantly greater PT enhancements at shorter tibialis anterior lengths. These results were recently replicated for the plantar flexors (Miyamoto et al., 2010). Miyamoto et al. (2010) used mechanomyographic recordings to study PAP of individual muscles at different ankle angles. They reported that, with the exception of the most plantarflexed position, MG potentiated more than SOL. Furthermore, enhancement of overall plantarflexor twitch torque was larger at the most plantar flexed position (i.e. shorter muscle length). The findings of Rassier (2000), Mela et al. (2001) and Miyamoto et al. (2010) indicating that potentiation is more pronounced at shorter muscle lengths, contrast with results presented by Suter and Herzog (1997) and with the lower potentiation 5 s after the MVIC in the flexed knee position observed in this study.
Since both conditioning and testing were performed in the same position, differences between flexed and extended knee positions in the current study could be an effect of either differences in the effects of the conditioning MVICs performed in the two positions, differences related to the testing conditions of the two positions or a combination of the two.
At a 90 ; knee joint angle, MG muscle fibres are at a length close to their active slack length, leading to insignificant contributions to plantarflexion (Kawakami et al., 1998) and possibly neuromuscular inhibition as an efficiency strategy response (Cresswell et al., 1995; Kennedy and Cresswell 2001; Tamaki et al., 1997). These mechanisms may underlie the significant differences between plantarflexion torque (extended knee 227.4 N·m vs flexed knee 150.4 N·m) and MGRMS (42.4%) between the MVICs at extended and flexed knee positions, respectively. The 90º knee angle might thus have caused suboptimal conditioning contraction intensities for PAP induction in the plantarflexors (Vandervoort et al., 1983). However, Vandervoort et al. (1983) specifically addressed the question whether differences in PAP amplitude between positions would be due to effects of the position at which twitch torque is measured or due to the position at which the conditioning was performed. Vandervoort et al. (1983) induced an MVIC at shorter muscle lengths (i.e. complete dorsiflexion) and tested dorsiflexor twitch PAP at longer muscle lengths (i.e. complete plantarflexion) and vice versa. They reported that the degree of potentiation was greater when twitches were elicited in a shortened muscle position independently of the position at which the MVIC was performed. The conclusion of Vandervoort et al. (1983) that the “degree of PAP depended only on the position in which the muscle twitch was elicited” suggests that even if we had have conducted such experiments, the outcomes would have probably been identical to the ones currently reported (i.e. lower twitch PAP in the flexed knee position). Furthermore, the reasons underlying our reports of lower plantarflexor twitch PAP in a position where both MG and LG were in a short muscle length configuration (i.e. flexed knee position) (Kawakami et al., 1998) may be related to limitations imposed by muscle architectural changes that are known to occur due to knee joint angle decreases.
Considering the consequences of inducing the test twitches at different knee positions, the shorter gastrocnemius muscle length in the flexed knee position (Kawakami et al., 1998) should render the muscle more susceptible to PAP (Miyamoto et al., 2010; Rassier 2000; Vandervoort et al., 1983). Interestingly and in line with the results of Suter and Herzog (1997), the present results did not present higher potentiation at shorter muscle lengths. As mentioned above, this is likely due to the fact that at a flexed knee position, the shortened gastrocnemius muscle has a poor force transfer and contribution to total plantarflexor force output, thus counteracting its superior propensity for PAP at shorter muscle lengths. Sarcomere length has previously been associated with muscle length (Rassier et al., 1999). Muscle shortening induced by joint rotations could reflect significant sarcomere shortening and dysfunctional myofilament overlapping, thus hindering force production. At a fascicular level, the gastrocnemius fascicle angle is also known to increase with an increasing knee angle (Kawakami et al., 1998), which impairs the force transfer, since the efficiency of force transmission to the tendon is determined by the cosine of the fascicle angle (Gans and de Vree, 1987; Kawakami et al., 1998). Therefore, even if PAP was greater at shorter muscle lengths as suggested by Miyamoto et al. (2010), the functional transfer of such effects might be limited by architectural sarcomere limitations and fascicle angle alterations. This could partially explain the lower twitch enhancements 5 s after the MVIC at the shorter muscle length in the present study. Some studies have suggested that there is more potentiation in fast twitch muscles (Hamada et al., 2000, 2003). Since gastrocnemius often has a higher proportion of fast twitch muscle fibres than the soleus (Edgerton et al., 1975; Gollnick et al., 1974), this might also explain lower PAP at an angle with a lesser relative gastrocnemius contribution to the plantarflexor twitch. The fact that the degree of potentiation only differed between the extended and flexed knee after 5 s indicates that the SOL was the main contributor to overall potentiation in both positions. This is plausible despite the possibly higher degree of potentiation for the gastrocnemius because the SOL has a substantially larger cross-sectional area than the gastrocnemius with a ratio of 6:2:1 reported for SOL, MG and lateral gastrocnemius, respectively (Out et al., 1996). However, when the gastrocnemius is in a position where potentiation can be efficiently transformed into twitch force (extended knee position), the higher PAP of the gastrocnemius compared to SOL may be sufficient to compensate for the smaller cross sectional area. Based on the current findings, it might further be speculated that the gastrocnemius potentiates more than SOL only for the first few seconds after the MVIC.
Limitations
The present study was conducted in young male power athletes. In that sense, it might be argued that the external validity of our conclusions is limited to that specific population. However, the PAP differences found between positions are presumably associated with impairment caused by architectural changes of the plantarflexor’s MTU dependent upon the knee angle. Such impairment will occur independently of the subject’s characteristics (i.e. age, training status, gender).
Experiments in which the conditioning was performed in a flexed knee position and PAP tested in an extended knee position or vice versa were not conducted. However, we believe that performing these additional experiments would not alter the findings of this study because Vandervoort et al. (1983) reported that the “degree of PAP depended only on the position in which the muscle twitch was elicited”. Regardless of the position at which the conditioning contraction was performed, twitch PAP should therefore still be lower if tested in the flexed knee position.
An ultrasound system could have been used to track the architectural changes of the plantarflexor’s MTU at different knee positions. However, such changes using identical knee angle configurations as in this study and their implications on force production have been previously documented using ultrasound based methods (Kawakami et al., 1998). Using such methods would therefore not have provided any new information not already described in the existing literature.
Conclusions
PAP effects on PT and RTR of the entire plantarflexor group were significantly greater for the extended as compared to the flexed knee for 5 s after a conditioning MVIC. This suggests that the gastrocnemius potentiates more than the soleus, only immediately after the MVIC. Furthermore, the results reflect only a brief contribution of the gastrocnemius potentiation to the overall plantarflexor twitch enhancements.
Acknowledgements
The authors wish to acknowledge the financial support from the Swedish National Centre for Research in Sports (CIF). Paulo Gago also wishes to thank the Fundação para a Ciênda e Tecnologia (FCT), Portugal for the Ph.D. Grant (SFRH/BD/103572/2014).
Authors submitted their contribution to the article to the editorial board.
References
- Baudry S, Duchateau J. Postactivation potentiation in a human muscle: effect on the rate of torque development of tetanic and voluntary isometric contractions. J Appl Physiol (1985) 2007;102:1394–1401. doi: 10.1152/japplphysiol.01254.2006. [DOI] [PubMed] [Google Scholar]
- Brito R, Alamo L, Lundberg U, Guerrero JR, Pinto A, Sulbaran G, Gawinowicz MA, Craig R, Padron R. A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J Mol Biol. 2011;414:44–61. doi: 10.1016/j.jmb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Fukutani A, Hirata K, Miyamoto N, Kanehisa H, Yanai T, Kawakami Y. Effect of conditioning contraction intensity on postactivation potentiation is muscle dependent. J Electromyogr Kinesiol. 2014;24:240–245. doi: 10.1016/j.jelekin.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Fukutani A, Miyamoto N, Kanehisa H, Yanai T, Kawakami Y. Potentiation of isokinetic torque is velocity-dependent following an isometric conditioning contraction. Springerplus. 2013;2:554. doi: 10.1186/2193-1801-2-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago P, Arndt A, Tarassova O, Ekblom MM. Post activation potentiation can be induced without impairing tendon stiffness. Eur J Appl Physiol. 2014a;;114:2299–2308. doi: 10.1007/s00421-014-2945-3. [DOI] [PubMed] [Google Scholar]
- Gago P, Marques MC, Marinho DA, Ekblom MM. Passive muscle length changes affect twitch potentiation in power athletes. Med Sci Sports Exerc. 2014b;46:1334–1342. doi: 10.1249/MSS.0000000000000245. [DOI] [PubMed] [Google Scholar]
- Gans C, de Vree F. Functional bases of fiber length and angulation in muscle. J Morphol. 1987;192:63–85. doi: 10.1002/jmor.1051920106. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Sjodin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch. 1974;348:247–255. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. J Appl Physiol (1985) 2000;88:2131–2137. doi: 10.1152/jappl.2000.88.6.2131. [DOI] [PubMed] [Google Scholar]
- Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiol Scand. 2003;178:165–173. doi: 10.1046/j.1365-201X.2003.01121.x. [DOI] [PubMed] [Google Scholar]
- Hopkins WG. Measures of reliability in sports medicine and science. Sports medicine. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- Jubeau M, Gondin J, Martin A, Van Hoecke J, Maffiuletti NA. Differences in twitch potentiation between voluntary and stimulated quadriceps contractions of equal intensity. Scand J Med Sci Sports. 2010;20:e56–62. doi: 10.1111/j.1600-0838.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol (1985) 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Kennedy PM, Cresswell AG. The effect of muscle length on motor-unit recruitment during isometric plantar flexion in humans. Exp Brain Res. 2001;137:58–64. doi: 10.1007/s002210000623. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR. Rassier DE. Cellular and Whole Muscle Studies of Activity Dependent Potentiation Muscle Biophysics. Springer; New York: 2010. pp. 315–342. [DOI] [PubMed] [Google Scholar]
- Mela P, Veltink PH, Huijing PA. The influence of stimulation frequency and ankle joint angle on the moment exerted by human dorsiflexor muscles. J Electromyogr Kinesiol. 2001;11:53–63. doi: 10.1016/s1050-6411(00)00036-5. [DOI] [PubMed] [Google Scholar]
- Millman BM. The Filament Lattice of Striated Muscle. 1998:359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Kanehisa H, Kawakami Y. Potentiation of maximal voluntary concentric torque in human quadriceps femoris. Med Sci Sports Exerc. 2012;44:1738–1746. doi: 10.1249/MSS.0b013e318256b813. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Mitsukawa N, Sugisaki N, Fukunaga T, Kawakami Y. Joint angle dependence of intermuscle difference in postactivation potentiation. Muscle Nerve. 2010;41:519–523. doi: 10.1002/mus.21529. [DOI] [PubMed] [Google Scholar]
- Out L, Vrijkotte TG, van Soest AJ, Bobbert MF. Influence of the parameters of a human triceps surae muscle model on the isometric torque-angle relationship. J Biomech Eng. 1996;118:17–25. doi: 10.1115/1.2795940. [DOI] [PubMed] [Google Scholar]
- Rassier DE. The effects of length on fatigue and twitch potentiation in human skeletal muscle. Clin Physiol. 2000;20:474–482. doi: 10.1046/j.1365-2281.2000.00283.x. [DOI] [PubMed] [Google Scholar]
- Rassier DE, MacIntosh BR, Herzog W. Length dependence of active force production in skeletal muscle. J Appl Physiol (1985) 1999;86:1445–1457. doi: 10.1152/jappl.1999.86.5.1445. [DOI] [PubMed] [Google Scholar]
- Rassier DE, Tubman LA, MacIntosh BR. Length-dependent potentiation and myosin light chain phosphorylation in rat gastrocnemius muscle. Am J Physiol. 1997;273:C198–204. doi: 10.1152/ajpcell.1997.273.1.C198. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Falces J, Duchateau J, Muraoka Y, Baudry S. M-wave potentiation after voluntary contractions of different durations and intensities in the tibialis anterior. Journal of Applied Physiology. 2015;118:953–964. doi: 10.1152/japplphysiol.01144.2014. [DOI] [PubMed] [Google Scholar]
- Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys. 2011;510:120–128. doi: 10.1016/j.abb.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter E, Herzog W. Extent of muscle inhibition as a function of knee angle. Journal of Electromyography and Kinesiology. 1997;7:123–130. doi: 10.1016/s1050-6411(96)00028-4. [DOI] [PubMed] [Google Scholar]
- Tamaki H, Kitada K, Akamine T, Sakou T, Kurata H. Electromyogram patterns during plantarflexions at various angular velocities and knee angles in human triceps surae muscles. Eur J Appl Physiol Occup Physiol. 1997;75:1–6. doi: 10.1007/s004210050118. [DOI] [PubMed] [Google Scholar]
- Tillin NA, Bishop D. Factors modulating post-activation potentiation and its effect on performance of subsequent explosive activities. Sports Med. 2009;39:147–166. doi: 10.2165/00007256-200939020-00004. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, Quinlan J, McComas AJ. Twitch potentiation after voluntary contraction. Exp Neurol. 1983;81:141–152. doi: 10.1016/0014-4886(83)90163-2. [DOI] [PubMed] [Google Scholar]
- Yang Z, Stull JT, Levine RJ, Sweeney HL. Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers. J Struct Biol. 1998;122:139–148. doi: 10.1006/jsbi.1998.3979. [DOI] [PubMed] [Google Scholar]