Abstract
Background
The aim of this study was to identify the optimal equation that accurately estimates the glomerular filtration rate (GFR) and the chronic kidney disease (CKD) stage in the Chinese population.
Methods
A total of 1296 Chinese patients aged 18–65 years old were enrolled in this study. The estimated GFRs (eGFRs) calculated separately by three Diet in Renal Disease (MDRD) equations and three Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations were compared with the reference GFR (rGFR) measured by the 99Tcm-DTPA renal dynamic imaging method.
Results
By Bland-Altman analysis, eGFRcys and eGFRscr_cys performed similarly, showing the tightest limits of agreement among the six equations. They also achieved the first and second highest 30% and 50% accuracies. Using a combination of the serum creatinine and cystatin C levels (eGFRscr_cys) could improve the bias (−0.3 for eGFRscr_cys) of the equation and achieve the highest diagnostic accuracy for renal insufficiency (AUC60, 0.953; P < 0.05, except for eGFR_MDRD). All equations predicted stage 3 CKD with moderate accuracy (49.7–51.4%) and stage 5 CKD with good accuracy (90.2–96.4%). For stage 1 CKD, eGFRcys showed a higher percentage of misclassification than the other equations. All equations seemed to perform poorly at predicting stage 2 and 4 CKD, as compared to the other CKD stages. eGFRscr_cys was the best-performing equation in terms of accurate classification of the CKD stage based on the overall performance (kappa value, 0.423).
Conclusion
For a Chinese population, the CKD-EPIscr_cys equation seems more suitable for estimating the GFR than the other equations. Each equation had its own advantages in predicting different CKD stages.
Keywords: Serum creatinine, Cystatin C, Glomerular filtration rate, Chronic kidney disease
Background
The prevalence of chronic kidney disease (CKD) is estimated to be 8–16% worldwide, generating a heavy economic impact on society in both developed and undeveloped countries [1]. The glomerular filtration rate (GFR) has generally been considered the vital indicator for predicting overall renal function. Therefore, accurate estimation of the GFR is important for assessing the severity and progression of CKD.
Serum creatinine is an easily measurable and widely available marker of renal function. However, its levels are affected by multiple factors, such as muscle mass, weight, gender, etc. [2]. Cystatin C is a nonglycosylated low-molecular-weight protein that is in the cystatin superfamily of cysteine protease inhibitors. It is produced at a constant rate by all nucleated cells and is freely filtered by the glomerulus. Although the serum cystatin C level serves as a valuable tool for early detection of renal dysfunction, it has been reported to be influenced by age, gender, body mass index, smoking status, the C-reactive protein level, nephritis, and hypertension [3–6].
The estimated GFR (eGFR), calculated by different equations, is commonly used for clinical care and research. The Modification of Diet in Renal Disease (MDRD) equation, initiated in 1999 and based on the serum creatinine level, is still applied clinically after several modifications [7, 8]. Recently, new equations such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations based on cystatin C and/or serum creatinine have been recommended for clinical applications [9, 10]. Some reports showed an improved accuracy of the eGFR using the cystatin C-based eq. [11, 12]. However, it is still controversial whether cystatin C-based GFR-estimating formulae are superior to serum creatinine-based ones [6].
Until now, limited data are available on the comparison of six GFR-estimating equations (CKD-EPIscr, CKD-EPIcys, CKD-EPIscr_cys, abbreviated MDRD, Chinese MDRD, and original MDRD) in Chinese CKD patients. The aim of this study was to identify the equation that is the most accurate and acceptable for predicting the GFR and the CKD stage in a large Chinese population in a single center.
Methods
Subjects
A total of 7676 Chinese participants who underwent GFR measurement using 99Tcm-diathylenetriamine pentaacetic acid (99Tcm-DTPA) scintigraphy from January 2009 to March 2016 in Nanfang Hospital, China, were observed. The following exclusion criteria were used: 1) younger than 18 years old or older than 65 years old (n = 1124); 2) obstructive nephropathy (n = 4060); 3) solitary kidney or a single kidney (n = 6); 4) urinary inflammation (n = 58); 5) acute renal insufficiency or injury (n = 10); 6) any history of malignancy or kidney surgery (n = 918); 7) hyperthyroidism (n = 4); 8) use of antibacterial agents within 2 weeks (n = 145); 9) malignant hypertension (n = 44). A total of 1307 patients were screened in the preliminary study, and 11 out of 1307 patients failed to meet diagnostic criteria of CKD who were excluded from the study. Finally, a total of 1296 eligible patients were enrolled in this study. The diagnostic criteria of CKD are in accordance with the K/DOQI practice guidelines. Written informed consent was obtained from each subject prior to participation. This study was approved by the Ethics Committee of the Nanfang Hospital of Sothern Medical University.
Measurement of reference GFR (rGFR) and CKD classification
The rGFR was measured by nuclear medicine techniques. Participants were well hydrated before examination. 99Tcm-DTPA (radiochemical purity, >95%; percentage of 99Tcm-DTPA bound to plasma protein, <5%) was provided by Guangzhou Atomic Isotope Hi-Tech Pharmaceutical Co., Ltd., China.
CKD was classified into five stages based on the rGFR values as follows [13]: stage 1, rGFR ≥90 mL/min/1.73 m2; stage 2, 60 mL/min/1.73 m2 ≤ rGFR <90 mL/min/1.73 m2; stage 3, 30 mL/min/1.73 m2 ≤ rGFR <60 mL/min/1.73 m2; stage 4, 15 mL/min/1.73 m2 ≤ rGFR <30 mL/min/1.73 m2; stage 5, rGFR <15 mL/min/1.73 m2. Renal insufficiency was defined as rGFR <60 mL/min/1.73 m2.
Measurement of serum creatinine and cystatin C levels
Serum creatinine and cystatin C levels were measured in the fasting state by a sarcosine oxidase assay kit (Sichuan Maker Biotechnology Co., Ltd., China) and an immunoturbidimetric assay kit (Beijing Leadman Biotechnology Co., Ltd., China) on an Olympus AU5421® analyzer, respectively.
The eGFR was calculated based on the serum creatinine and/or cystatin C levels using six estimating equations (Table 1).
Table 1.
Different equations for estimation of GFR
| Subjects | Gender | Scr (mg/dL) | Scys (mg/L) | Equation (mL/min/1.73 m2) |
|---|---|---|---|---|
| CKD-EPIscr (eGFRscr) | female | ≤0.7 | 141 × (Scr/0.7)-0.329 × 0.993age × 1.018 | |
| >0.7 | 141 × (Scr/0.7)-1.209 × 0.993age × 1.018 | |||
| male | ≤0.9 | 141 × (Scr/0.9)-0.411 × 0.993age | ||
| >0.9 | 141 × (Scr/0.9)-1.209 × 0.993age | |||
| CKD-EPIcys (eGFRcys) | female | ≤0.8 | 133 × (Scys/0.8)-0.499 × 0.996age × 0.932 | |
| >0.8 | 133 × (Scys/0.8)-1.328 × 0.996age × 0.932 | |||
| male | ≤0.8 | 133 × (Scys/0.8)-0.499 × 0.996age | ||
| >0.8 | 133 × (Scys/0.8)-1.328 × 0.996age | |||
| CKD-EPIscr_cys (eGFRscr_cys) | female | ≤0.7 | ≤0.8 | 130 × (Scr/0.7)-0.248 × (Scys/0.8)- 0.375 × 0.995age |
| >0.8 | 130 × (Scr/0.7)-0.248 × (Scys/0.8)- 0.711 × 0.995age | |||
| >0.7 | ≤0.8 | 130 × (Scr/0.7)-0.601 × (Scys/0.8)- 0.375 × 0.995age | ||
| >0.8 | 130 × (Scr/0.7)-0.601 × (Scys/0.8)- 0.711 × 0.995age | |||
| male | ≤0.9 | ≤0.8 | 135 × (Scr/0.9)-0.207 × (Scys/0.8)- 0.375 × 0.995age | |
| >0.8 | 135 × (Scr/0.9)-0.207 × (Scys/0.8)- 0.711 × 0.995age | |||
| >0.9 | ≤0.8 | 135 × (Scr/0.9)-0.601 × (Scys/0.8)- 0.375 × 0.995age | ||
| >0.8 | 135 × (Scr/0.9)-0.601 × (Scys/0.8)- 0.711 × 0.995age | |||
| abbreviated _MDRD (eGFRa_MDRD) | 186 × (Scr)-1.154 × (age)-0.203 × (×0.742 if female) | |||
| Chinese _MDRD (eGFRc_MDRD) | 175 × (Scr)-1.234 × (age)-0.179 × (×0.79 if female) | |||
| original MDRD (eGFR_MDRD) | 186 × (Scr)-1.154 × (age)-0.203 × (×0.742 if female) × (×1.233 if Chinese) |
Scr serum creatinine, Scys serum cystatin C
Statistical analysis
Statistical analysis was performed using SPSS20.0 (SPSS Inc., Somers, NY, USA) and MedCalc13.0 (MedCalc, Mariakerke, Belgium). Quantitative data were tested for homogeneity of variance by the Kolmogorov-Smirnov one-sample test. Bland-Altman analysis was used to determine the agreement between the rGFR and eGFR values, which were calculated by different equations. The receiver operating characteristic (ROC) curve was used to determine the diagnostic power at predicting the renal insufficiency (ROC60) by the six different equations, with the results reported as the areas under the ROC curve (AUC60), sensitivity, and specificity. Kappa statistics were used to evaluate the agreement between stage classification from the rGFR values and from the eGFR values calculated by different equations, with the following interpretations: poor agreement (0–0.20), slight agreement (0.21–0.40), moderate agreement (0.41–0.60), good agreement (0.61–0.80), and excellent agreement (0.81–1.0). The paired sample t test was carried out to evaluate inter-group differences. Differences with P < 0.05 were considered statistically significant.
Results
Participant characteristics
A total of 1296 participants aged 45.0 (35.0, 55.0) years old were enrolled, including 814 males and 482 females. The mean rGFR was 46.8 (29.8, 68.3) mL/min/1.73 m2, whereas the mean eGFR varied based on the different calculation formulae and ranged from 40.1 (19.2, 69.3) mL/min/1.73 m2 to 52.0 (21.6, 88.3) mL/min/1.73 m2. The basic characteristics of the participants are shown in Table 2.
Table 2.
Baseline characteristics of the participants
| Variable | Value |
|---|---|
| Age, years | 45.0 (35.0, 55.0) |
| Gender, male, n (%) | 814 (62.8%) |
| Weight, kg | 62.0 (54.0, 70.0) |
| Height, cm | 165.0 (158.0,170.0) |
| Body surface area, m2 | 1.7 ± 0.2 |
| Body mass index, Kg/m2 | 22.9 (20.8, 25.4) |
| Serum creatinine, mg/dL | 1.5 (1.0, 3.2) |
| Serum cystatin C, mg/L | 1.7 (1.1, 2.9) |
| rGFR, mL/min/1.73 m2 | 46.8 (29.8, 68.3) |
| eGFR, mL/min/1.73 m2 | |
| eGFRscr | 49.7 (19.6, 85.8) |
| eGFRcys | 40.1 (19.2, 69.3) |
| eGFRscr_cys | 44.7 (18.6, 76.5) |
| eGFRa_MDRD | 48.9 (20.1, 81.0) |
| eGFRc_MDRD | 49.4 (19.1, 85.2) |
| eGFR_MDRD | 52.0 (21.6, 88.3) |
| rGFR based on CKD stage, mL/min/1.73 m2 | |
| Stage 1 (n = 104) | 101.4 (96.2, 111.2) |
| Stage 2 (n = 331) | 73.2 ± 8.6 |
| Stage 3 (n = 529) | 43.5 (37.0, 51.5) |
| Stage 4 (n = 220) | 23.6 (19.5, 27.0) |
| Stage 5 (n = 112) | 10.8 (8.5, 13.2) |
eGFR estimated glomerular filtration rate, rGFR reference glomerular filtration rate, CKD chronic kidney disease, MDRD modification of diet in renal disease, CKD-EPI chronic kidney disease epidemiology collaboration
Performance of the six equations compared with the rGFR
The agreement or disagreement between the eGFR values and the rGFR values was analyzed by Bland-Altman plots (Fig. 1). According to these plots, the limits of the regression lines varied by each equation and were 73.5 for eGFRscr, 64.3 for eGFRcys, 64.7 for eGFRscr_cys, 90.3 for eGFRa_MDRD, 107.6 for eGFRc_MDRD, and 108.3 for eGFR_MDRD. The eGFRcys and eGFRscr_cys equations performed similarly, showing the tightest limits of agreement among the six equations. The biases of eGFRcys and eGFRscr_cys (2.4 and −0.3, respectively) were much less than those of eGFRscr, eGFRa_MDRD, eGFRc_MDRD, and eGFR_MDRD (−4.8, −6.0, −8.9, and −11.5, respectively). Thus, the equations based on serum creatinine, eGFRscr, eGFRa_MDRD, eGFRc_MDRD, and eGFR_MDRD, had poor agreement with the rGFR (eGFRc_MDRD and eGFR_MDRD in particular). Using a combination of serum creatinine and cystatin C levels could improve the bias (−0.3 for eGFRscr_cys) of the equation.
Fig. 1.
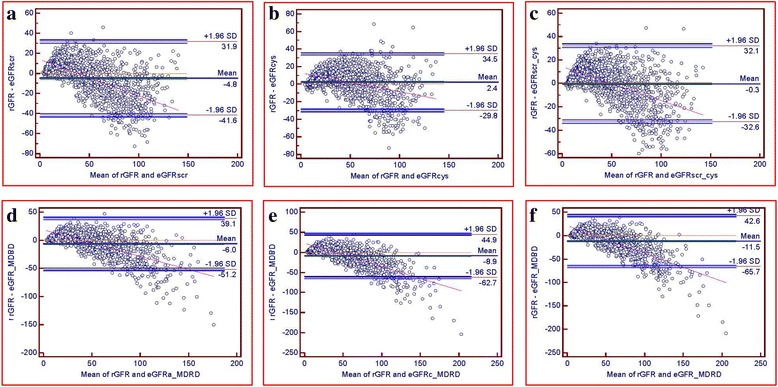
Bland-Altman plots of the rGFR and eGFR (mL/min/1.73 m2). eGFRcys and eGFRscr_cys performed similarly, showing the tightest limits of agreement among the six equations. eGFRscr_cys showed the least bias among the six equations. eGFRscr (a), eGFRcys (b), eGFRscr_cys (c), eGFRa_MDRD (d), eGFRc_MDRD (e), and eGFR_MDRD (f)
Diagnostic performance of the six equations for predicting renal insufficiency
The diagnostic performance for predicting renal insufficiency based on the six equations is summarized in Table 3. The AUC60 at a cutoff point of 59.6 mL/min/1.73 m2 in eGFRscr_cys achieved the highest value (0.953), with a sensitivity of 87.6% and a specificity of 89.1%, suggesting the highest diagnostic accuracy for predicting renal insufficiency (P < 0.05 vs. the others, except for eGFR_MDRD). The optimal cutoff point of eGFRcys for predicting renal insufficiency was 46.8 mL/min/1.73 m2, with a sensitivity of 93.6%, a specificity of 81.0%, and an AUC60 of 0.945. A revised cutoff value of eGFRcys to 60.6 mL/min/1.73 m2 led to an improved specificity of 91.5% and a decreased sensitivity of 77.1%.
Table 3.
Diagnostic performance of the six equations for predicting renal insufficiency (mL/min/1.73 m2)
| Equation | eGFRscr | eGFRcys | eGFRscr_cys | eGFRa_MDRD | eGFRc_MDRD | eGFR_MDRD |
|---|---|---|---|---|---|---|
| Cutoff Value | 62.8 | 46.8 | 59.6 | 59.3 | 63.2 | 69.0 |
| AUC60 | 0.948* | 0.945* | 0.953 | 0.945* | 0.948* | 0.951● |
| 95% CI | 0.934–0.959 | 0.931–0.957 | 0.940–0.964 | 0.933–0.958 | 0.934–0.959 | 0.938–0.962 |
| Youden index J | 0.745 | 0.745 | 0.767 | 0.743 | 0.748 | 0.760 |
| Sensitivity | 89.0 | 93.6 | 87.6 | 89.9 | 88.7 | 86.9 |
| Specificity | 85.5 | 81.0 | 89.1 | 84.5 | 86.1 | 89.1 |
| Adjustment Cutoff Value | 60.4 | 60.6 | 60.2 | 60.0 | 60.3 | 59.8 |
| Sensitivity | 90.3 | 77.1 | 86.7 | 88.1 | 89.9 | 92.2 |
| Specificity | 84.1 | 91.5 | 89.1 | 84.7 | 83.9 | 81.7 |
● P = 0.4206 compared with eGFRscr_cys
*P < 0.05 compared with eGFRscr_cys
Misclassification of CKD stages by the six equations
All equations had a high accuracy (range, 90.2–96.4%) for the diagnosis of stage 5 CKD; whereas all of them exhibited a moderate accuracy for the diagnosis of stage 3 CKD (Table 4). In stage 1 CKD, eGFRcys showed a higher percentage of misclassification than the other equations. Although eGFRscr_cys exhibited the highest accuracy for estimating stage 2 CKD (49.8%), all equations seemed to perform poorly at predicting stage 2 and 4 CKD, as compared to the other CKD stages. In addition, based on the overall performance, eGFRscr_cys had the highest kappa value (0.423), compared to the other five equations, suggesting that eGFRscr_cys might be the best-performing equation in terms of accurate classification of the CKD stage.
Table 4.
CKD stage classification based on eGFR estimation by the six equations (n = 1296)
| Equation | CKD stage based on rGFR | Kappa | ||||
|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | ||
| eGFRscr | 98 (94.2%) | 121 (36.6%) | 263 (49.7%) | 83 (37.7%) | 107 (95.5%) | 0.392 |
| eGFRcys | 78 (75.0%) | 152 (45.9%) | 266 (50.3%) | 101 (45.9%) | 103 (92.0%) | 0.406 |
| eGFRscr_cys | 88 (84.6%) | 165 (49.8%) | 272 (51.4%) | 79 (35.9%) | 108 (96.4%) | 0.423 |
| eGFRa_MDRD | 93 (89.4%) | 137 (41.4%) | 269 (50.9%) | 87 (39.5%) | 107 (95.5%) | 0.407 |
| eGFRc_MDRD | 98 (94.2%) | 116 (35.0%) | 264 (49.9%) | 81 (36.8%) | 108 (96.4%) | 0.387 |
| eGFR_MDRD | 99 (95.2%) | 109 (32.9%) | 264 (49.9%) | 95 (43.2%) | 101 (90.2%) | 0.388 |
Accuracy of the six eGFRs and the rGFR
Among the six equations, eGFRscr_cys had the smallest bias, whereas eGFRcys exhibited the highest 30% accuracy and 50% accuracy (Table 5).
Table 5.
Comparison of bias and accuracy between the eGFR and rGFR
| Equation | Bias (mL/min/1.73 m2) | Bias of 95% CI | 30% accuracy | 50% accuracy |
|---|---|---|---|---|
| eGFRscr* | −4.8 | −5.8, −3.8 | 51.2% | 77.6% |
| eGFRcys* | 2.4 | 1.5, 3.3 | 60.3% | 86.4% |
| eGFRscr_cys ▲ | −0.3 | −1.2, 0.6 | 57.4% | 83.7% |
| eGFRa_MDRD* | −6.0 | −7.3, −4.8 | 52.1% | 76.4% |
| eGFRc_MDRD* | −8.9 | −10.4, −7.4 | 47.1% | 71.3% |
| eGFR_MDRD* | −11.5 | −13.0, −10.0 | 49.0% | 73.5% |
*Compared with rGFR, P = 0.000
▲Compared with rGFR, P = 0.550
Discussion
Each GFR-estimating equation has its own advantages for different stages of impaired renal function. In addition, their performances are affected by various factors. First, the serum creatinine level is determined by different methods. Compared with the Jaffe method, the enzymatic method is less affected by external factors [14–16]. A previous study has shown a significantly higher accuracy for the GFR-estimating equation using the enzymatic method to measure creatinine than that measured by the picric acid method when the rGFR is ≥60 mL/min/1.73 m2 [17]. Second, the patients had different ages. The research performed by Roberts et al. showed that the MDRD equation overestimates the renal function in different age groups, which does not become apparent until after 65 years of age [18]. Thus, the role of age in GFR estimation should be taken into consideration, and the elderly participants (over 65 years old) need to be observed separately. Therefore, this study only included patients aged 18–65 years old in order to minimize the possible bias of the study. Third, racial factors can affect the results. A meta-analysis has revealed that cystatin C has a better diagnostic value for CKD in the West than in Asia [19], suggesting the performance of the equation differs in different racial and ethnic populations.
The inulin clearance rate has been considered as the gold standard, but it is an impractical method for estimating renal function, probably due to its costly, cumbersome features. Thus, radioisotopic methods, such as 99Tcm-DTPA, are commonly used in clinical applications [20, 21]. Serum creatinine and cystatin C represent two other indicators for predicting renal function. Cystatin C seems to perform better at predicting an early decrease in renal function [22–25], particularly in the elderly [6], whereas serum creatinine is insensitive until the impairment is 50% or more [26]. Our previous study revealed that the serum cystatin C measurement is more sensitive than that of serum creatinine for detecting an early decline in the rGFR [22]. In the present study, the Bland-Altman plots showed that eGFRcys and eGFRscr_cys had similar low limits of agreement among the six equations, revealing higher agreement of cystatin C-based equations with the rGFR than the other four serum creatinine-based equations.
Accurate CKD stage classification facilitates the successful management of such patients. The performances of the GFR-estimating equations based on either serum creatinine or serum cystatin C vary with race/ethnicity [7, 19]. Thus, it is extremely important to identify the best-performing equation for CKD stage classification in specific populations. Previously, we found that the CKD-EPI equation based on the serum creatinine level exhibited a better performance than the MDRD equation in estimating the GFR in Chinese diabetics [27]. In the present study, all six equations achieved high classification accuracy for stage 5 CKD (≥90.2%), no matter which serum creatinine or cystain C-based equation was used. However, their diagnostic efficiency differed greatly in CKD at stages 1–4. eGFRcys had a low diagnostic accuracy in stage 1 CKD at a cutoff value of 46.8 mL/min/1.73 m2, which is lower than that used clinically, suggesting that cystatin C might underestimate mild renal dysfunction. Since serum creatinin- and cystatin C-based equations are most applicable in different CKD stages [6], it has been reported that equations based on a combination of creatinine and cystatin C perform better than those equations based on creatinine or cystatin C alone [9, 28]. Among these equations, eGFRscr_cys had the highest AUC60 among the six equations in the ROC60 analysis (P < 0.05 vs. the others, except for eGFR_MDRD), and it also achieved the top accuracy for overall CKD classification (kappa value, 0.423). These findings are consistent with those from Ying Zhu et al. [29] and reveal that GFR-estimating equations based on the combination of serum creatinine and cystatin C levels may improve diagnostic efficiency for renal function.
Conclusions
This study has one particular strength. Considering that racial factors can affect the results, this study focused on data from a Chinese population for the purpose of identifying an appropriate GFR equation for the Chinese population. Also, this study only included Chinese patients aged 18–65 years old to minimize age-related bias of the study effectively.
This study also has some limitations. First, this was a retrospective, single-center study in China. Thus, caution must be used when generalizing the results of this study in a different population. Second, this study included adult patients aged from 18 to 65 years old. Considering the age-related decline in the GFR, we cannot be sure of the relevance of the results among children or elderly patients. Third, the role of some unmeasured factors (diet, muscle mass, etc.) that could have possibly influenced the observed association cannot be entirely ruled out. Forth, although 99Tcm-DTPA renal dynamic imaging has been widely as reference standard for clinical evaluation of renal function, it still has its disadvantages. Some researchers believe that 99Tcm-DTPA renal dynamic imaging may underestimate the true GFR [30] because a very small part of 99Tcm-DTPA bounds to plasma proteins, although this is only speculated theoretically, not on the basis of pathological biopsy, and is usually neglected.
In conclusion, the CKD-EPI equations had higher agreement with the rGFR than the MDRD equations. Our study also found that the CKD-EPIscr_cys equation achieved the top accuracy for overall CKD classification in the Chinese population. Compared with CKD-EPIscr and CKD-EPIcys, the use of the combination of serum creatinine and cystatin C (CKD-EPI scr_cys) levels could improve the bias of the equation and achieve a higher diagnostic accuracy for renal insufficiency. Each equation had its own advantages in predicting different CKD stages and needs further research.
Acknowledgments
We thank Professor An Shengli and Dr. Liu Wenjuan for their help with statistical analysis of the data.
Funding
This work was supported, in part, by grants from the National Natural Science Foundation of China (81570724) and the President Foundation of Nanfang Hospital Southern Medical University (2013C021).
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to the privacy of patients as well as joint ownership of research data in our institution. The data are available from the corresponding author on reasonable request, and the contact way can get through by email to hyxknfyy@163.com or dial the phone number 13926066999.
Abbreviations
- 99Tcm-DTPA
99Tcm-diathylenetriamine pentaacetic acid
- AUC
The areas under the ROC curve
- CKD
Chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFR
Estimated GFR
- GFR
Glomerular filtration rate
- MDRD
Modification of Diet in Renal Disease
- rGFR
Reference GFR
- ROC
Receiver operating characteristic
Authors’ contributions
GPL, QSW, and YMX conceived the study and participated in the design of the study; XHC analyzed the data and drafted the manuscript; YSQ andQZ analyzed the data; KH carried out the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was conducted with the permission of the Ethics Committee of the Nanfang Hospital of Sothern Medical University. Written informed consent was obtained from each subject prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiao-Hua Chi, Email: 546863745@qq.com.
Gui-Ping Li, Email: Ligp62@126.com.
Quan-Shi Wang, Email: wqslph@163.net.
Yong-Shuai Qi, Email: 525430484@qq.com.
Kai Huang, Email: 195816728@qq.com.
Qian Zhang, Email: 195816728@qq.com.
Yao-ming Xue, Phone: +8613926066999, Email: hyxknfyy@163.com.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59:587–592. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 3.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 4.Wei L, Ye X, Pei X, Wu J, Zhao W. Reference intervals for serum cystatin C and factors influencing cystatin C levels other than renal function in the elderly. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groesbeck D, Kottgen A, Parekh R, Selvin E, Schwartz GJ, Coresh J, Furth S. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008;3:1777–1785. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei X, Bao L, Xu Z, Yan C, He J, Zhu B, Wu J, Zhao W. Diagnostic value of cystatin C and glomerular filtration rate formulae in Chinese nonelderly and elderly populations. J Nephrol. 2013;26:476–484. doi: 10.5301/jn.5000181. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 9.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qutb A, Syed G, Tamim HM, Al Jondeby M, Jaradat M, Tamimi W, Al Ghamdi G, Al Qurashi S, Flaiw A, Hejaili F, et al. Cystatin C-based formula is superior to MDRD, Cockcroft-gault and Nankivell formulae in estimating the glomerular filtration rate in renal allografts. Exp Clin Transplant. 2009;7:197–202. [PubMed] [Google Scholar]
- 12.Domingueti CP, Foscolo RB, Simoes ESAC, Dusse LM, Reis JS, Carvalho M, Fernandes AP, Gomes KB. Evaluation of creatinine-based and cystatin C-based equations for estimation of glomerular filtration rate in type 1 diabetic patients. Arch Endocrinol Metab. 2016;60:108–116. doi: 10.1590/2359-3997000000151. [DOI] [PubMed] [Google Scholar]
- 13.Andrassy KM. Comments on 'KDIGO 2012 clinical practice guideline for the evaluation and Management of Chronic Kidney Disease'. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 14.Liu WS, Chung YT, Yang CY, Lin CC, Tsai KH, Yang WC, Chen TW, Lai YT, Li SY, Liu TY. Serum creatinine determined by Jaffe, enzymatic method, and isotope dilution-liquid chromatography-mass spectrometry in patients under hemodialysis. J Clin Lab Anal. 2012;26:206–214. doi: 10.1002/jcla.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg N, Roberts WL, Bachmann LM, Wright EC, Dalton RN, Zakowski JJ, Miller WG. Specificity characteristics of 7 commercial creatinine measurement procedures by enzymatic and Jaffe method principles. Clin Chem. 2012;58:391–401. doi: 10.1373/clinchem.2011.172288. [DOI] [PubMed] [Google Scholar]
- 16.Nah H, Lee SG, Lee KS, Won JH, Kim HO, Kim JH. Evaluation of bilirubin interference and accuracy of six creatinine assays compared with isotope dilution-liquid chromatography mass spectrometry. Clin Biochem. 2016;49:274–281. doi: 10.1016/j.clinbiochem.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Qiu L, Guo X, Zhu Y, Shou W, Gong M, Zhang L, Han H, Quan G, Xu T, Li H, et al. Effect of picric acid and enzymatic creatinine on the efficiency of the glomerular filtration rate predicator formula. Clin Lab. 2013;59:511–522. doi: 10.7754/clin.lab.2012.120524. [DOI] [PubMed] [Google Scholar]
- 18.Roberts GW, Ibsen PM, Schioler CT. Modified diet in renal disease method overestimates renal function in selected elderly patients. Age Ageing. 2009;38:698–703. doi: 10.1093/ageing/afp168. [DOI] [PubMed] [Google Scholar]
- 19.Wei L, Ye X, Pei X, Wu J, Zhao W. Diagnostic accuracy of serum cystatin C in chronic kidney disease: a meta-analysis. Clin Nephrol. 2015;84:86–94. doi: 10.5414/CN108525. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Xia J, Pei G, Zhang Y, Wu S, Qin Y, Deng Y, Guo S, Guo Y, Xu G, et al. A more accurate method acquirement by a comparison of the prediction equations for estimating glomerular filtration rate in Chinese patients with obstructive nephropathy. BMC Nephrol. 2016;17:150. doi: 10.1186/s12882-016-0345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trimarchi H, Muryan A, Martino D, Toscano A, Iriarte R, Campolo-Girard V, Forrester M, Pomeranz V, Fitzsimons C, Lombi F, et al. Creatinine- vs. cystatin C-based equations compared with 99mTcDTPA scintigraphy to assess glomerular filtration rate in chronic kidney disease. J Nephrol. 2012;25:1003–1015. doi: 10.5301/jn.5000083. [DOI] [PubMed] [Google Scholar]
- 22.Chi XH, Li GP, Wang QS, Wu DJ, Huang K. Changes of blood Cys C, creatinine and hemoglobin levels in patients with different degrees of renal function impairment. J of Radioimmunology. 2011;24:6–8. [Google Scholar]
- 23.Christensson A, Ekberg J, Grubb A, Ekberg H, Lindstrom V, Lilja H. Serum cystatin C is a more sensitive and more accurate marker of glomerular filtration rate than enzymatic measurements of creatinine in renal transplantation. Nephron Physiol. 2003;94:p19–p27. doi: 10.1159/000071287. [DOI] [PubMed] [Google Scholar]
- 24.Hari P, Ramakrishnan L, Gupta R, Kumar R, Bagga A. Cystatin C-based glomerular filtration rate estimating equations in early chronic kidney disease. Indian Pediatr. 2014;51:273–277. doi: 10.1007/s13312-014-0400-5. [DOI] [PubMed] [Google Scholar]
- 25.Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H. Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol. 2000;54:203–209. [PubMed] [Google Scholar]
- 26.Tan TZ. Clinical nuclear medicine. People's Medical Publishing House. 2003;755.
- 27.Wang Q, Zhang Q, Qian Y, Gao F, Xie CH. The application of glomerular fitration rate evaluation equation in patients with type 2 diabetes. Clin J Diabetes. 2014;22:799–803. [Google Scholar]
- 28.Liu X, Ma H, Huang H, Wang C, Tang H, Li M, Wang Y, Lou T. Is the chronic kidney disease epidemiology collaboration creatinine-cystatin C equation useful for glomerular filtration rate estimation in the elderly? Clin Interv Aging. 2013;8:1387–1391. doi: 10.2147/CIA.S52774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Ye X, Zhu B, Pei X, Wei L, Wu J, Zhao W. Comparisons between the 2012 new CKD-EPI (chronic kidney disease epidemiology collaboration) equations and other four approved equations. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan TZ. Clinical nuclear medicine. People's Medical Publishing House. 2003:739–40.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to the privacy of patients as well as joint ownership of research data in our institution. The data are available from the corresponding author on reasonable request, and the contact way can get through by email to hyxknfyy@163.com or dial the phone number 13926066999.


