Abstract
The burden of HIV/AIDS and other transmissible diseases is higher in prison and jail settings than in the non-incarcerated communities that surround them. In this comprehensive review, we discuss available literature on the topic of clinical management of people infected with HIV, hepatitis B and C viruses, and tuberculosis in incarcerated settings in addition to co-occurrence of one or more of these infections. Methods such as screening practices and provision of treatment during detainment periods are reviewed to identify the effect of community-based treatment when returning inmates into the general population. Where data are available, we describe differences in the provision of medical care in the prison and jail settings of low-income and middle-income countries compared with high-income countries. Structural barriers impede the optimal delivery of clinical care for prisoners, and substance use, mental illness, and infectious disease further complicate the delivery of care. For prison health care to reach the standards of community-based health care, political will and financial investment are required from governmental, medical, and humanitarian organisations worldwide. In this review, we highlight challenges, gaps in knowledge, and priorities for future research to improve health-care in institutions for prisoners.
Introduction
In 1990, Jonathan Mann, the then first director of WHO's Global Programme on AIDS, stated that if you wanted to find HIV in a country, introduce it, wait a few years, and subsequently look at the disadvantaged populations and you will find it there. Incarcerated populations are among the most disadvantaged in many societies. In nearly all See Online for appendix countries, the burden of HIV and AIDS and many other transmissible diseases is higher in prisons, jails, and other types of detention centres—which will collectively be referred to here as prisons—than in the communities that surround them.1 Partly, the increased prevalence of infectious disease in incarcerated populations can be attributed to structural determinants and social factors: racial and ethnic minorities and people with low income are often over-represented in prisons, and the criminalisation of drug use, sex work, and sexual minorities has resulted in high rates of incarceration among these groups. Mental illness also contributes to incarceration in many settings.2 Before incarceration, members of these marginalised populations tend to have little access to health-care resources, which results in sub-optimal health outcomes.3 During incarceration, few prisoners worldwide have consistent access to internationally recommended strategies4 to reduce the risk of transmission of HIV or other infectious diseases, including voluntary HIV testing, antiretroviral therapy (ART), sterile syringes, condoms, and opioid agonist therapies such as methadone or buprenorphine.5 Because of a culmination of these factors, HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and tuberculosis are particularly concentrated in prisons, and each independently and synergistically contributes to preventable morbidity and mortality in individuals and communities.1
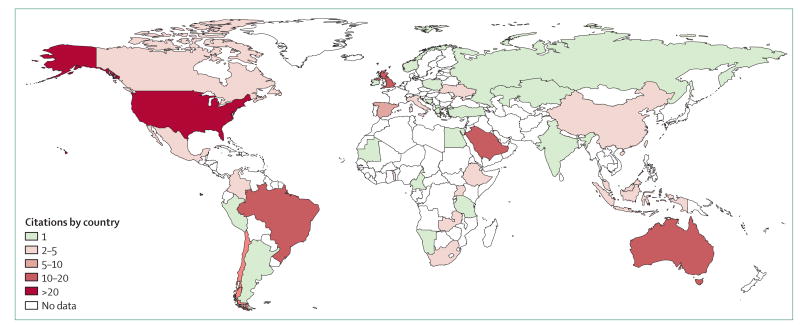
In this paper, we review the clinical management of people with HIV, HBV, HCV, and tuberculosis in prisons. We focus on screening practices, treatment, and strategies applied to link individuals to community-based treatment on their release. We identified 285 articles that were related to clinical care of HIV, viral hepatitis, or tuberculosis in prisons or after release, from 2013 to 2016 (figure). The scarcity of publications describing prison populations in low-income and middle-income countries highlights the need for more information about the overlap of HIV and other infections in these regions.
Figure. Global distribution of publications related to the clinical care and post-release outcomes of incarcerated people with HIV, viral hepatitis, or tuberculosis published 2013–16.
Distribution of the citations acquired as a result of the search strategy used in the systematic review. Search strategy and sources listed in the appendix.
In this paper, HIV care is presented in the context of the HIV treatment criteria that classifies people with HIV as diagnosed, engaged in care, prescribed ART, or achieving viral suppression.6 This cascade has been used as a public health tool to prioritise areas for intervention with the goal of increasing the proportion of people with HIV achieving viral suppression.7 When viral suppression is achieved, HIV transmission is substantially decreased, both by individual and societal standards.8 The related strategy of using HIV treatment as prevention to control the epidemic outlines a multipronged approach to increase identification of people with HIV through testing, improving access to ART, and maintaining long-term treatment. Long-term treatment through retention in care is a priority for HIV care interventions,9 including for prison settings.10,11 This cascade is aligned with UNAIDS 90-90-90 targets by 2020, such that universal treatment of people with HIV would result in 73% of patients achieving viral suppression, which would translate to a 50% reduction in people with HIV by 2030.12 In addition, challenges, gaps in knowledge, and priorities for future research to improve the diagnosis and treatment of HIV, HBV, HCV, and tuberculosis among prisoners are highlighted in this review.
HIV in prisons
Incarcerated people have a disproportionate burden of HIV/AIDS as compared to their non-incarcerated counterparts.13 Thus, initiatives within prison settings for HIV prevention, screening, treatment, and linkage to care after release have been developed; however, incarceration often disrupts HIV care for people who are engaged in HIV/AIDS treatment in the community.14
For people with HIV who are marginalised from care because of sub-optimally treated substance use disorders, psychiatric disorders, and other health disparities, incarceration could enable individuals to access HIV testing, ART, and general health care.15 These benefits, however, require that prisons have health infrastructures that can deliver comprehensive HIV services, including ART.14 High-income countries are better equipped for this purpose than are low-income and middle-income countries. Incarceration can also have negative consequences on HIV treatment. For people receiving ART in the community, arrest and detention often leads to an interruption of ART, which can be brief or long lasting depending on the availability of ART inside prison.16,17 Additionally, people detained in prisons might not acknowledge their HIV status, or might not take their medications in the presence of others because of a real or perceived stigma and discrimination, thus prolonging ART interruptions during detainment.18
Prisons should devise testing strategies that immediately link HIV positive patients to HIV care and ART within prison and ensure continuity of care after release to the community. Such strategies should respect basic human rights and preserve patient autonomy and confidentiality. In 2009, WHO released guidelines recommending that confidential, voluntary HIV testing and counselling be available to all people detained in prison.19 These guidelines also emphasise that screening initiatives should be done ethically, ensuring individual autonomy and access to treatment for people testing positive. In the past 30 years, many prisons have incorporated HIV testing and screening, although not all have ensured that testing is voluntary and confidential. A 2008 review20 of HIV screening guidelines in Europe revealed that 61% of European Union countries have HIV testing standards that emphasise routine testing. In the USA, in 2008, 24 states tested all inmates at some point during custody.21 Adoption and implementation of HIV screening has led to higher identification of HIV while the immune system is preserved, and increased opportunity to provide appropriate treatment.22 Unfortunately, HIV testing is not always delivered in accordance with best practice guidelines within many prison settings; in many jurisdictions, mandatory HIV testing and segregation of people with HIV persists.23
The method of assessing HIV testing within prison settings can vary with respect to the ability of an individual to accept or decline testing. Presently, both mandatory and voluntary testing strategies exist. Mandatory testing requires that all inmates are tested for HIV without the ability to decline; conversely, voluntary testing preserves the ability of the individual to accept or decline an HIV test. Routine testing can include opt-out strategies, which dictate that HIV testing be done as part of routine medical care, unless the inmate declines or opts out of HIV testing. Testing can also be routinely offered, requiring the inmate to opt in. WHO, the European Centre for Disease Prevention and Control (ECDC), and the US Centers for Disease Control and Prevention (CDC) all oppose mandatory HIV testing, stating that it is unethical.24 Testing should be available when requested by the individual and to all people passing through a prison setting. Routine opt-out HIV testing should allow autonomy in decision making, ensuring that inmates are explicitly and suffciently informed and able to decline testing if desired. Routine opt-out testing strategies have historically resulted in the highest proportion of people completing voluntary testing25 and should be implemented when feasible. Furthermore, the way in which opt-out testing is done should be adjusted to maximise testing rates and linkage to care, while preserving informed decision making.
Incarcerated individuals with HIV should be assessed by trained HIV clinicians and offered ART, regardless of whether they are in facilities designed for prison sentences of short or long duration . Nonetheless, ART is only available in prisons in less than a third of countries worldwide.26 Where ART is available, HIV-related mortality in prisons is similar to that of the non-incarcerated community, which supports the need for appropriate diagnosis and treatment.27 In the USA, the rate of AIDS-related deaths in state prisons dropped to less than the rate for the US general population in 2009, suggesting that imprisoned individuals with unrestricted access to ART can have substantial improvements in their HIV disease status.28 Additionally, most prisons and jails in the USA provide ART; however, 16–34% of incarcerated people with HIV do not report taking any prescription medication during incarceration.29 Organisational and institutional barriers, such as a scarcity of specialised care, unavailability of specific ART regimens, and unwillingness to disclose HIV status to prison guards, medical staff, or other inmates can affect HIV treatment inside prisons. Best practices for the clinical care of HIV in prisons should be consistent with national and international guidelines and should include assessment by an HIV specialist; screening for sexually transmitted infections, HBV, HCV, and tuberculosis; and immediate prescription of ART, routine monitoring of HIV treatment, and discharge planning that includes linkage to care and support services to facilitate successful treatment after release.30 Furthermore, addressing co-occurring social and medical comorbidities such as homelessness, unemployment, mental illness, substance use disorders, and trauma are crucial to ensure successful outcomes.
The health benefits from provision of ART during incarceration are often lost following release from prisons, especially for women.31 Although high levels of viral suppression are achieved by many patients during incarceration, community re-entry is associated with loss of viral suppression. Many factors contribute to this loss of viral control after release, including relapse to substance use, unstable housing and unemployment, failure to access ART in the community because of loss of health entitlements, and reduced access to health care.32–34 Similarly, the immediate period after release has been associated with poorer HIV treatment outcomes (such as increased viral load and decreased CD4 cell count),35 high risks of HIV-related mortality, and drug overdose.36 These worsened outcomes among patients with HIV are especially true among those with comorbid alcohol and other substance use disorders.37 Reviews of HIV and incarceration found that there is less viral suppression after release than before incarceration38 and failure to maintain viral suppression leads to increased HIV transmission in the community.39 Therefore, addressing the needs of people with HIV during and following incarceration is imperative and is an essential component of a broader, more comprehensive strategic plan. Indeed, mathematical modelling from Ukraine documents40 show that HIV risk taking among prisoners who inject drugs and who subsequently transition to the community from prison contribute most to HIV transmission in the population. Scaling up opioid agonist treatments in prison and retaining patients on this regimen post-release would be the most effective strategy to reduce new infections in people who inject drugs. These needs are increasing given the continued scale-up of efforts to prevent HIV through treatment in many communities. There is evidence that HIV prevention strategies can be compromised by incarceration, especially among people who use drugs and have HIV infections.
Despite evidence of increased rates of HIV-related complications after release from prison, many studies have identified effective mechanisms for health maintenance, including case management,41 discharge planning,10 transitional care coordination,42 and focusing on combined medical and social needs.43 Findings from a single randomised trial did not find a benefit in the use of case management for released prisoners with HIV; however, these findings may be unique to the particular setting.44 Numerous studies have explored the use of interventions to lower the possibility of interrupted HIV care and ART posed by transfer between prison and community settings. The role of transfers between facilities during incarceration has an unknown effect on care provision, and is under-examined. International recommendations for transitioning prisoners include directly administered ART and opioid agonist therapies for people with HIV and opioid disorders;45 others have suggested that case management, coordinated discharge planning, and peer navigation might also be useful.46 Opioid agonist therapies that are initiated before release and continued into the community have been associated with higher proportions of viral suppression in people with HIV; moreover, randomised trials47 of treatment for alcohol and opioid use disorders with extended-release naltrexone are promising and are ongoing (ClinicalTrials.gov, NCT01077310 and NCT01246401).
The criminalisation of illicit drugs has resulted in a substantial proportion of prisoners in many jurisdictions serving sentences for non-violent drug use. As such, the role of incarceration in the HIV/AIDS pandemic among people who inject drugs deserves special mention. For example, in Vancouver, Canada, epidemiological estimates suggested that one in five HIV infections during an explosive outbreak of HIV/AIDS in the 1990s were associated with incarceration.48 To combat HIV infection associated with incarceration, several evidence-based interventions tailored specifically for those with criminal justice experience who are at risk for HIV have been developed, but these programmes are only available after release and are unavailable in most prisons.49 Prisons should, therefore, consider implementing harm reduction strategies alongside existing prevention programmes to reduce HIV transmission in at-risk prisoners.
HIV transmission within prison settings can occur, but transmission rates vary and are poorly quantified, ranging from zero incidence to well documented outbreaks. HIV transmission within prison is discussed in more detail in accompanying articles in this series.40,50 Ensuring that all people with HIV who are incarcerated are prescribed ART is a key strategy to reduce HIV transmission within prison.
Viral hepatitis in prisons
People who are incarcerated are 9–13 times more likely to be HCV-infected than non-incarcerated members of the community.51 Left untreated, chronic HCV infection leads to cirrhosis and hepatocellular carcinoma and is a leading cause of liver transplantation in high-income countries.52 The staggering rates of HCV infection in prisoners and the substantial risks associated with untreated HCV disease underscore the need for HCV screening and access to HCV treatment in prisons.
WHO recommends that “all prisoners should be tested for hepatitis C”.53 In the USA, risk-based screening is recommended by the CDC and the Federal Bureau of Prisons, meaning that prisoners should be tested only if there are known risk factors, including those with a history of injection drug use (panel 1). People with HIV and those with liver disease should also be tested for HCV. The CDC added one-time HCV testing for all Americans born between 1945 and 1965, as there was an increased prevalence of HCV in this birth cohort, in addition to the risk-based testing recommendations in an effort to diagnose more people with HCV. Some have argued, however, that routine HCV testing for all prisoners is warranted given the high prevalence of disease.54 Prison offcials might be reluctant to expand HCV testing given the potential costs associated with treatment, and this reluctance has led to vast underdiagnosis of the disease. Some prisons in high-income countries are expanding HCV treatment, but uptake remains slow. Internationally, adherence to WHO guidelines for HCV screening in prison settings is inadequate. For instance, in western Europe, only ten (34%) of 29 surveyed countries reported HCV screening programmes for prisoners.24 Where HCV screening is available in prison settings, universal screening methods are recommended55 but are seldom used. There are indications that risk-based screening results in substantial gaps in patient identification.54,56 Universal, opt-out screening for HCV in prison settings is essential to reduce HCV transmission and HCV-associated morbidity and mortality.57
HCV treatment developments have provided therapies that, for the first time, are highly effective (ie, >90% cure rates), tolerable, simple (one pill per day), and of short duration (12 weeks for most patients).58 These medications, however, are very expensive, and treatment for chronic HCV has remained underused in prisons.58,59 In countries where HCV treatment is available to prisoners, treatment remains restricted to individuals who are classified as high-priority candidates for treatment, such as those with cirrhosis.60 This clinical practice—which withholds effective treatment from most incarcerated individuals with chronic HCV infection—could be characterised as a human rights violation.61 Additionally, failure to engage chronically infected prisoners in effective treatment is likely to contribute to viral transmission patterns within groups in prison and community settings. In the near future, the availability of HCV treatment to inmates will probably become an area of increased litigation, policy discussion, and ethical consideration.
Cost frequently restricts HCV treatment, but several studies57,60,62 have documented that early HCV detection and treatment is cost-effective, including for individuals who are incarcerated. Although early-stage treatment of all individuals with chronic HCV infection produces immediate costs, the reduced rates of HCV transmission and liver-related morbidity and mortality reduce costs in the long term.58,63 Indeed, widespread HCV treatment of incarcerated populations (treatment-as-prevention) would help bring about the global elimination of HCV.63 The prevalence of HCV among prisoners is elevated in many countries of eastern Europe and central Asia compared with other regions,64-66 exceeding 60% prevalence in Ukraine,64 where incarceration rates are among the highest in the world and is comprosed of many people who inject drugs.40 Nonetheless, some countries in the region are making progress. Efforts in Georgia are underway to eliminate HCV, including treatment of prisoners.67
As global elimination of HCV through treatment becomes viable, applying lessons learned from increasing access to ART for HIV infections in low-income and middle-income countries could prove to be essential in reducing the burden of HCV globally.68 Mechanisms that have been used to increase the affordability of ART, including generic competition, voluntary licensing, tiered pricing, and the Medicines Patent Pool should be applied to reduce high prices of HCV treatment and improve access to care in low-income and middle-income countries.68 The establishment of intergovernmental agencies and foundations for HCV that are similar to those for HIV (eg, the Global Fund to Fight AIDS, Malaria, and Tuberculosis), which aid in the purchase of diagnostic kits and medications, might also be essential in reducing the burden of HCV in low-income and middle-income countries.68 For maximal effect, future efforts to treat people with HCV should include individuals who are incarcerated. Because of the scarcity of published data, clinical care for HBV infections is explored in panel 2.
Tuberculosis in prisons
The burden of tuberculosis in prison settings remains a substantial public health problem, especially in low-income and middle-income countries.1 Tuberculosis thrives in disadvantaged, impoverished populations including among people in prisons, particularly those that are overcrowded and poorly ventilated. The increased prevalence of tuberculosis in prisons can be partly attributed to the emergence of drug-resistant tuberculosis forms, such as multi-drug resistant and extensively-drug resistant tuberculosis.75 In response to these ongoing challenges, WHO released a global strategy in 201376 with the goal of ending the tuberculosis epidemic by 2035; this strategy includes the expansion of preventive treatment of latent tuberculosis infection for individuals in congregate settings, including prisons.
Tuberculosis screening algorithms, screening procedures, and diagnostic practices differ across the globe. WHO published cost-effective screening algorithms in 2012;77 however, these recommendations are more likely to be adopted by high-income countries that are not as restricted by costs for GeneXpert MTB/RIF (Xpert) and light-emitting diode (LED) fluorescence microscopy, which are the gold-standard in diagnostics.78,79 Nonetheless, it is low-income and middle-income countries that are most profoundly impacted by tuberculosis, especially in prisons, thereby making such settings high priority for tuberculosis elimination efforts. Results from a systematic review79 suggest that in countries of all income levels, symptom questionnaires are the most common screening methods in prison settings, but findings from Malaysia and Brazil suggest that such strategies are insuffcient.23,80 In low-income and middle-income countries, the most common screening procedures outside prison are the presence of cough and chest radiography; in high-income countries the most common screening procedures are chest radiography, tuberculin skin tests, and the observation of at least one tuberculosis symptom.79 Diagnostic practices also vary across income areas. In low-income and middle-income countries, the most common diagnostic practices include sputum smear microscopy and solid culture; but high-income countries also include chest radiography and tuberculin screen tests.79 Interferon-gamma release assays can also play a role in tuberculosis screening in high-income countries.81 A study of screening for tuberculosis by use of several strategies found sputum smear microscopy to be completely ineffective at identifying tuberculosis in prisoners, and Xpert identified only 58% of cases.82
The deffciency of adequate diagnostic tools for tuberculosis in prison settings is a particular challenge in low-income and middle-income countries.79 Diagnostic services are often provided by external laboratories, which can lead to delays in diagnosis.83 Bacteriological services that are run in-house, such as sputum smear microscopy, often lack quality control; the diagnostic microscopes in prisons are often poorly maintained, and staff might have insuffcient training in the use of these diagnostic tools.84,85 In low-income and middle-income countries, improved laboratory services and biosafety measures are crucial to increase detection and treatment of tuberculosis in prisons.
Internationally, data on the clinical management of active and latent tuberculosis infections in prison settings are scarce. A systematic review86 suggests that in countries of all income levels, initiation and completion rates of isoniazid preventive treatment for individuals with latent tuberculosis infection remain low, particularly in short-term detention facilities. In all settings, regardless of income level, the clinical management of active and latent tuberculosis infections in prisons can be complicated by challenges in implementing supervised treatment and the inability of some prisoners to pay for tuberculosis treatment.87,88 In countries at all income levels, the uncontrolled sub-optimal quality of tuberculosis medications pose substantial challenges to tuberculosis control.87,88 Even in high-income countries, tuberculosis treatment completion rates following release from prison remain relatively low, which is likely to be caused by multiple logistical and other challenges to remaining in care.89 Internationally, there is little literature on linkage to care for tuberculosis treatment after release; nonetheless, WHO has prioritised ensuring treatment completion after incarceration for those who are released during treatment.90 Substantial challenges in the clinical management of tuberculosis in prison settings exist in all income areas, and a greater allocation of resources to tuberculosis management is likely to be needed to control the infection in prison settings.
Several factors contribute to tuberculosis transmission in prisons that impede tuberculosis control, including overcrowding, insuffcient ventilation, extended confinement inside cells, generally poorer health, co-infections that exacerbate tuberculosis infection such as HIV, delayed case detection, inadequate treatment of infected patients, high inmate turnover, and poor implementation of tuberculosis infection control measures.79,83,91 Both legislation and international guidelines that address these key areas should be implemented to curb transmission and facilitate tuberculosis control in prisons.
Management of co-occurring disorders
In addition to the amplification of various infectious diseases in prisoners, overlapping psychiatric and substance use disorders are common comorbid conditions, and in the absence of effective screening and treatment in prisons, undermine effective treatment.92 Both psychiatric and substance use disorders are independent correlates of HIV infection, and sub-optimal treatment of these conditions for people with HIV is associated with underdiagnosis and poor continuity of HIV care after release (panel 3). Many of these conditions can be effectively treated with pharmacotherapies, especially opioid and alcohol use disorders and schizophrenia-spectrum disorders. The fifth Edition of the Diagnostic and Statistical Manual now, for the first time, supports treatment for those in “controlled environments” like prisons and jails, even when there is no evidence of ongoing physical or psychological dependence.96 Randomised trials97 support implementing methadone according to patient need during incarceration and continuing treatment after release from prison.
Implications for public health of non-incarcerated communities
Comorbidity of infectious diseases (eg, HIV, hepatitis B, hepatitis C, or tuberculosis), mental illness, and addiction is common among individuals who are incarcerated, and these comorbidities complicate the delivery of care both during incarceration and after release.1 Given the complexity of treatment required during and after incarceration, there is a need for innovation in medical care provision. Prisoners often do not have access to specialists for treatment and those with HIV in particular have poor access to specialty care. Access to care in many places could be diminished because prisoners are required to pay for medical treatment during incarceration, often shifting the financial burden of treatment and medications onto family members.
In many countries, prisons and jails house low-income individuals with many syndemic comorbidities, many of whom do not have access to medical care and social services in the community. In view of this, prisons are important sites to develop and implement public health interventions. The 2012 Geneva Declaration on health-care in prisons provides guidelines based on humanitarian law and is guided by seven principles: having access to a physician, equivalence of care with the community, maintaining patient consent and confidentiality, providing adequately scaled preventive services, offering humanitarian assistance, professional independence (minimising dual loyalty), and professional competence.98 The declaration calls for these principles to be integrated into medical provision across the globe. Prisons should be integral sites of collaboration for screen, test, treat, and retain strategies to reduce community transmission of infectious diseases both in prison and after release. However, other innovative and practical strategies are also necessary. WHO recommends that medical care for prisoners be handled by the same governmental organisation that oversees general health-care provision (eg, the Ministry of Health) rather than the Ministry of Justice or Ministry of the Interior or a separate and unique entity only tasked with prison health, to improve accordance with the legal guarantee of equivalent health care for prison and community populations.99 Oversight of prisoner health care by the Ministry of Health would be able to ensure quality of care in prison that was consistent with the care provided in the community and would enable the improved provision of continued care after release from prison.
Under international law, prisoners retain their human rights and fundamental freedoms, except for such restrictions on their rights required by the fact of incarceration (panel 4).100 States therefore have an obligation to provide medical care to prisoners at least equivalent to that available outside of prisons. The 164 countries that are party to the International Covenant on Economic, Social and Cultural Rights should guarantee minimum core obligations with respect to the right to health, including the provision of essential primary care and medicines.3 States should also pledge non-discriminatory access to health care and the equitable distribution of health facilities and services.105 Infectious disease control is also crucial to protect both prisoners and staff, and to prevent disease outbreaks; a summary of HIV, viral hepatitis, and tuberculosis prevention strategies for prison settings is provided in panel 5.
In addition to immediate reforms aimed at bringing prison health-care into line with the recommendations of the Geneva Declaration, the implementation of innovative strategies to address the challenges inherent in health-care delivery for incarcerated individuals could improve health outcomes. For example, in the USA, the Extension for Community Healthcare Outcomes Project links primary care doctors in prisons with specialty care experts at an academic hub, helping to alleviate the problems caused by a shortage of specialty care physicians in prisons and jails.106,107 Unfortunately, the principle of equivalence of medical care between prison and community settings is too often negated in practice. Furthermore, in many settings the clinical care and health of individuals in susceptible and marginalised groups, such as members of sexual and racial minorities or people who use illicit drugs, would be best served by structural reforms to curb the prevalence of incarceration.
Finally, coordinated general and behavioural health care, or integrative care, is necessary for all prisoners. WHO, UNAIDS, CDC and the International Association of Providers of AIDS Care recommend integrative care for people with HIV. However, the organisational structure of most prison health settings is not set up to abide by these recommendations, especially in low-to-middle-income countries that might not have funding available for community primary care provision. Additionally, clinical staff and social workers should acknowledge the social determinants of health that are also negatively affected by incarceration. Therefore, any treatment initiatives need to recognise the role that social factors play in the health disparities faced by current or former prisoners.
Conclusion
In providing clinical care to incarcerated people with HIV, viral hepatitis, tuberculosis or a combination of infections, prisons should provide confidential screening and treatment, education, linkage to care after release, and address multiple co-occurring issues both during incarceration and once released into the community. Further research on the provision of health care in prisons is needed in all areas of the world—in countries at all income levels. Alternatives to incarceration that preserve public safety and lead to improved health and public health outcomes should be rigorously pursued.
Supplementary Material
Search strategy and selection criteria.
We searched reports related to HIV, hepatitis B virus, hepatitis C virus, and tuberculosis clinical care in adult prison settings. Keywords and MeSH headings related to incarceration (ie, “inmate”, “prisoner”, “felon”, “jail”) were paired with search terms pertaining to each infectious disease of interest. We limited our search to PubMed articles that were published in English between Jan 1, 2013, and March 29, 2015, focusing on systematic reviews and meta-analyses when available. We also reviewed and included key articles that were published before 2013. We retrieved and reviewed over 1122 unique citations, of which 285 were selected for inclusion of this analysis. Most (118 articles, 41%) were focused on populations in the USA, followed by Brazil (18 articles), Australia (14), Iran (13), the UK (ten), and Spain (nine). Further information on our search strategy, inclusion criteria and a bibliography of all included articles are available in the appendix. Nearly a quarter of the global prison population is held in prisons and jails in the USA, and as a result, much of the literature related to infectious diseases in prison settings is from this region. Moreover, there are fewer resources for research and for treatment in low-income and middle-income countries. Wherever data are available, we discuss and present research from around the world to provide a more balanced, international perspective.
Key messages.
Individuals in prison are disproportionately affected by HIV, hepatitis B and C, and tuberculosis compared with the rest of the global community yet often have less access to testing methods and treatments, to care for these infections.
Structural barriers, stigma, and insuffcient resources impede the delivery of optimal clinical care for these diseases in prison settings.
Criminal justice reforms and increased collaboration between prison and public health offcials could lead to substantial positive effect on the health of affected populations in addition to contributing to a general decline in transmission of these infectious diseases in community settings.
Monitoring and accountability of human rights abuses and the availability of treatment for HIV, viral hepatitis, and tuberculosis treatment in prison settings is important to ensure respect for human rights, autonomy, confidentiality, and to maximise public health of the greater public.
The interrelated, negative correlation (ie, syndemic) of incarceration, substance use disorders, mental illness, and infectious disease with disease outcome complicate the optimal delivery of medical care in prison settings and should be addressed.
For prison-based health care to reach the same standards as those provided in the community, financial support in the range of tens of billions of dollars will be needed (appendix), along with support from medical and humanitarian organisations across the globe. This is particularly true in low-income and middle-income countries.
Panel 1: Patient vignette 1.
In the early 1980s, a 24-year-old African American man was incarcerated for the first time for possession of crack cocaine in the USA. During the next 6 years, he was caught in the cycle of arrest for drug-related charges, incarceration, and release back to the community. In 1990, the local Department of Corrections instituted a routine HIV testing programme that provided voluntary HIV testing as part of the intake medical examination to the jail. He tested positive for HIV for the first time that year. Over the next decade, he continued to use and sell crack cocaine, continued to cycle in and out of jail, and served several prison sentences.
When incarcerated, he was assessed by HIV care providers and prescribed antiretroviral therapy (ART), which he took consistently. However, every time he was released, these gains were quickly lost as his environment pulled him back into drug use and dealing. HIV treatment and seeing his HIV care provider in the community were not priorities, replaced by the everyday battle to survive in his disenfranchised, low-income urban environment.
As part of his care within the prison, he tested positive for hepatitis C virus in 1998. He was enrolled in a case management programme that attempted to stabilise his life during transitions between prison and the community. Despite these attempts, the cycle of reoffending continued until 2006 when he was offered early parole from the prison if he entered a community-based residential drug rehabilitation programme. With the support of the prison-based case management programme, he entered and completed drug treatment and subsequently gained temporary employment. He had one relapse with crack cocaine the following year but was able to enter a community-based drug treatment programme that eventually hired him as a maintenance worker.
Since 2007, he has been steadily employed by this community-based organisation and he has maintained his recovery from crack cocaine use. His HIV infection is well controlled by ART. In 2010, he was given treatment for his chronic hepatitis C virus infection but was unable to tolerate interferon-based treatment. In 2013, he was diagnosed with hepatocellular carcinoma that was treated by radiofrequency ablation. His hepatitis C virus infection was cured in 2014 with an antiviral regimen including a new direct-acting antiviral agent. A recurrence of his hepatocellular carcinoma was detected in 2015.
Key points.
Hepatitis C virus infection leads to substantial morbidity and mortality in HIV-infected prisoners.
Prison settings are an important venue for HIV testing to reach people who use drugs.
Treatment of HIV inside of prison settings can be successful.
Community re-entry can destabilise HIVtreatment.
Interventions based on prison-community partnerships can help to mitigate the risks associated with community re-entry.
Employment, housing, health insurance, and other social services can be fundamental stabilising factors for people re-entering the community.
Panel 2: Hepatitis B prevention in prison settings.
People who are incarcerated have a disproportionate burden of hepatitis B virus (HBV) infection.1 Although global HBV immunisation coverage in infants increased from 8% to 82% from 1994 to 2014,69 chronic HBV infection remains high in prison settings, especially in low-income and middle-income countries where HBV vaccine uptake has been lower.70 Prisoners are highly susceptible to HBV because of the low prevalence of vaccine-induced immunity. Data on HBV susceptibility in prison settings are rare, but one study done in New South Wales, Australia, found that more than half of people admitted to prison were susceptible to HBV, and prison entrants were more susceptible to several other vaccine-preventable diseases than members of the surrounding communities.71
To reduce HBV susceptibility in prison settings, some countries have implemented prison-based HBV vaccination programmes. Routine HBV vaccination programmes implemented in prisons in Scotland, England, and Wales have been particularly successful in achieving vaccine coverage and uptake.72,73 Results from one study74 in the USA suggest that a combined hepatitis A and B vaccination programme, with an accelerated schedule among high-risk inmates, can be successfully implemented in a jail setting. These initiatives play an essential role in preventing chronic disease among members of a susceptible population.
Panel 3: Patient vignette 2.
In the mid-2000s, a 16-year-old white American woman was sentenced to a juvenile detention facility for aggravated assault. These charges stemmed from an altercation with her foster mother. In the years before her arrest, she had cycled through countless foster families—some of whom were physically and sexually abusive. During her stay in the juvenile detention facility, she was diagnosed with a generalised anxiety disorder and post-traumatic stress disorder, and after her release she was prescribed a 2-week supply of antidepressants and was referred to a local therapist. Because of the chaos of the post-release environment and insuffcient support from her foster family, she was unable to follow up with therapy.
In her early twenties, she began misusing prescription opioid medications, which later led to heroin use. Her boyfriend at the time encouraged her to engage in sex work to financially support their mutual opioid addiction. At 23 years of age, she was sentenced to an adult prison for charges related to prostitution. Early in her 18-month sentence, she learned that she was HIV positive.
During her stay in prison, she resumed taking antidepressants and initiated antiretroviral therapy. She was also linked with case managers who assessed her medical and social support needs and began to plan for a successful transition into the community after release. She was linked to community HIV and mental health services and many other public assistance programmes. Since her release, she has maintained consistent employment as a cashier and is in recovery from opioid dependence. Her HIV is well controlled on antiretroviral therapy, and symptoms of her generalised anxiety disorder and post-traumatic stress disorder are well managed.
This patient still has many struggles but partly attributes her success after release to the comprehensive discharge planning that enabled her to have continuous HIV-related medical care and her access to employment opportunities provided by a community-based organisation that helps formerly incarcerated women find jobs.
Key points.
While we present a single case here, generally, women who are sex workers and have a history of incarceration have an increased likelihood of becoming HIV positive93, and the number of women incarcerated around the world continues to increase.9395
An abundance of research and individual testimony has provided evidence that providing people with support both before and after release increases successful re-entry and HIV-related outcomes long term.
Panel 4: Seeking access to HIV treatment in prisons through the courts.
In the past two decades, individuals inside and outside prison have sought access to health care, especially HIV treatment, through appeals to courts and claims of human rights. Plaintiffs frequently put forward claims related to right to health care, life, and non-discrimination. One early case, Van Biljon and Others. v. Minister of Correctional Services (1997)101, which was heard in South Africa, addressed the question of whether the state had an obligation to provide ART to prisoners with HIV infection even though access to ART was also unavailable to non-prisoners in public health facilities in the country. The court did not rule on the issue of when prisoners should be eligible to receive treatment, but it did rule in favour of the prisoners that treatment should be provided at the expense of government and that prisoners on antiretroviral therapy at the time of incarceration have a right to continued treatment.101 The Court further found that the state has greater obligations to prisoners than to those outside prisons, as “[u]nlike persons who are free, prisoners have no access to other resources to assist them in gaining access to medical treatment”. It further noted that prisoners with HIV were unique because the Government is “keeping these prisoners in conditions where they are more vulnerable to opportunistic infections than HIV patients outside”.101
A more recent case, Tapela and Others. v. Attorney General (2014), challenged the policy of the government of Botswana that provided prisoners free antiretroviral treatment only if they were citizens of Botswana.102 In their ruling in this case, the Botswana High Court held that the policy of denying antiretroviral treatment to non-citizen prisoners was a violation of the Constitution of Botswana, including the right to life and to non-discrimination. The court found that the denial of antiretroviral treatment increased not only the risk of “premature death” but also HIV and tuberculosis transmission to other inmates. The court noted that although the prisoners' liberty had been curtailed, their other rights remained intact, and that it was impermissible for the State to “extend the limits of punishment by withholding certain services to which inmates are lawfully entitled”.102
Courts have also ruled in favour of prisoners seeking access to treatment for chronic hepatitis C infection (eg, Testa v Croatia, 2007)103 and treatment for tuberculosis (Staykov v Bulgaria, 2006)104.
Panel 5: Summary of HIV, viral hepatitis, and tuberculosis prevention strategies for prisons.
HIV and hepatitis C
Treatment-as-prevention strategies should be used to increase the identification of people with HIV through testing, improving treatment access, and engagement in care
Opioid agonist therapies initiated before release and continued into the community have been associated with reduced engagement in risky behaviour
Sterile needle distribution and condom distribution in prisons can reduce HIV and hepatitis C virus incidence
Tuberculosis
Screening at intake and treatment fortuberculosis and latent tuberculosis infection is crucial to reduce incidence in prisons
Prison administrators should ensure that facilities do not exceed maximum capacity and are suffciently ventilated to prevent tuberculosis transmission
Hepatitis B
Screening and treatment for hepatitis B virus is essential to reduce transmission in prisons
Routine hepatitis B virus vaccination programmes have been successful in achieving vaccine coverage and uptake
Acknowledgments
This paper and The Lancet Series on HIV and Prisoners were supported by: grants to the Center for Public Health and Human Rights at Johns Hopkins Bloomberg School of Public Health from: the National Institute on Drug Abuse; the Open Society Foundations; the United Nations Population Fund; the Johns Hopkins University Center for AIDS Research, a National Institute of Health (NIH)-funded programme (1P30AI094189). This report was also supported by: the National Institute on Drug Abuse (K24DA022112, JDR, AM; R01DA030778, R01DA027211 DP2 DA040236, BDLM, MRFK; T32DA013911, R25DA035692, R25DA037190, LB-R; R01DA025943, R01DA029910, R01DA030768, R01DA033679, R01DA030762, R01AA018944, K24DA017072, FLA), the National Institutes of Health (R01DA021525, M-JM), and by the infrastructure and resources provided by the Lifespan/Tufts/Brown Center for AIDS Research, a National Institutes of Health-funded programme (P30AI42853, CGB). The content is solely the responsibility of the authors and does not necessarily represent the offcial views of any of the funders.
Footnotes
Contributors: JDR, AM, LB-R, CGB, BDLM, and JJA had shared responsibility in developing initial drafts and writing this report. AM did the initial literature search. JDR, M-JM, CGB, BDLM, JJA, LA, JS, MRFK, and FLA revised each draft of this work critically for important intellectual content. All authors made substantial contributions to the design and approach, and approve the final version to be published.
Declaration of interests: The institution of M-JM has received unstructured funding from National Green Biomed Ltd; this funding has partly supported his research. All other authors declare no competing interests.
Contributor Information
Prof Josiah D Rich, Department of Medicine, Brown University, Providence, RI, USA; The Center for Prisoner Health and Human Rights, The Miriam Hospital, Providence, RI, USA; Department of Epidemiology, Brown University School of Public Health, Providence, RI, USA.
Prof Curt G Beckwith, Department of Medicine, Brown University, Providence, RI, USA; The Center for Prisoner Health and Human Rights, The Miriam Hospital, Providence, RI, USA.
Alexandria Macmadu, The Center for Prisoner Health and Human Rights, The Miriam Hospital, Providence, RI, USA.
Prof Brandon D L Marshall, Department of Epidemiology, Brown University School of Public Health, Providence, RI, USA.
Prof Lauren Brinkley-Rubinstein, Department of Social Medicine and Center for Health Equity Research, University of North Carolina, Chapel Hill, NC, USA.
Joseph J Amon, Health and Human Rights Division, Human Rights Watch, New York City, NY, USA.
Prof M-J Milloy, Department of Medicine, University of British Columbia, Vancouver, Canada.
Maximilian R F King, Department of Epidemiology, Brown University School of Public Health, Providence, RI, USA.
Prof Jorge Sanchez, Centro de Investigaciones Tecnológicas, Biomédicasy Medioambientales, Lima, Peru; Department of Global Health, University of Washington, Seattle, WA, USA.
Prof Lukoye Atwoli, Moi University School of Medicine, Eldoret, Kenya.
Prof Frederick L Altice, Section of Infectious Diseases, Yale School of Medicine, New Haven, CT, USA; Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, CT, USA.
References
- 1.Dolan K, Wirtz AL, Moazen B, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016 doi: 10.1016/S0140-6736(16)30466-4. published online July 14. http://dx.doi.org/10.1016/S0140-6736(16)30466-4. [DOI] [PubMed]
- 2.Fazel S, Seewald K. Severe mental illness in 33 588 prisoners worldwide: systematic review and meta-regression analysis. Br J Psychiatry. 2012;200:364–73. doi: 10.1192/bjp.bp.111.096370. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein LS, Amon JJ, McLemore M, et al. HIV, prisoners, and human rights. Lancet. 2016 doi: 10.1016/S0140-6736(16)30663-8. published online July 14. http://dx.doi.org/10.1016/S0140-6736(16)30663-8. [DOI] [PubMed]
- 4.WHO, UNAIDS, & UNOCD. Effectiveness of interventions to address HIV in prisons. Geneva: World Health Organization; 2007. [Google Scholar]
- 5.United Nations Office On Drugs and Crime. HIV prevention, treatment and care in prisons and other closed settings: a comprehensive package of interventions. Vienna: United Nations Office of Drugs and Crime; 2013. [Google Scholar]
- 6.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57:1164–71. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 7.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–39. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull MW, Wu Z, Montaner JS. Optimizing the engagement of care cascade: a critical step to maximize the impact of HIV treatment as prevention. Curr Opin HIV AIDS. 2012;7:579–86. doi: 10.1097/COH.0b013e3283590617. [DOI] [PubMed] [Google Scholar]
- 10.Althof AL, Zelenev A, Meyer JP, et al. Correlates of retention in HIV care after release from jail: results from a multi-site study. AIDS Behav. 2013;17(suppl 2):156–70. doi: 10.1007/s10461-012-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaller ND, Holmes L, Dyl AC, et al. Linkage to treatment and supportive services among HIV-positive ex-offenders in Project Bridge. J Health Care Poor Underserved. 2008;19:522–31. doi: 10.1353/hpu.0.0030. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 13.Dolan K, Kite B, Black E, Aceijas C, Stimson GV. HIV in prison in low-income and middle-income countries. Lancet Infect Dis. 2007;7:32–41. doi: 10.1016/S1473-3099(06)70685-5. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JP, Cepeda J, Wu J, Trestman RL, Altice FL, Springer SA. Optimization of human immunodefficiency virus treatment during incarceration: viral suppression at the prison gate. JAMA Intern Med. 2014;174:721–29. doi: 10.1001/jamainternmed.2014.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanigan TP, Zaller N, Beckwith CG, et al. Testing for HIV, sexually transmitted infections, and viral hepatitis in jails: still a missed opportunity for public health and HIV prevention. J Acquir Immune Defic Syndr. 2010;55(suppl 2):78–83. doi: 10.1097/QAI.0b013e3181fbc94f. [DOI] [PubMed] [Google Scholar]
- 16.Small W, Wood E, Betteridge G, Montaner J, Kerr T. The impact of incarceration upon adherence to HIV treatment among HIV-positive injection drug users: a qualitative study. AIDS Care. 2009;21:708–14. doi: 10.1080/09540120802511869. [DOI] [PubMed] [Google Scholar]
- 17.Belenko S, Dembo R, Copenhaver M, et al. HIV Stigma in Prisons and Jails: Results from a Staff Survey. AIDS Behav. 2016;20:71–84. doi: 10.1007/s10461-015-1098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culbert GJ, Bazazi AR, Waluyo A, et al. The influence of medication attitudes on utilisation of antiretroviral therapy (ART) in Indonesian prisons. AIDS Behav. 2016;20:1026–38. doi: 10.1007/s10461-015-1198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United Nations Office on Drugs and Crime, WHO, UNAIDS. HIV testing and counselling in prisons and other closed settings. New York City: World Health Organization; 2010. [PubMed] [Google Scholar]
- 20.Mounier-Jack S, Nielsen S, Coker R. HIV testing strategies across European countries. HIV Med. 2008;9(suppl 2):13–19. doi: 10.1111/j.1468-1293.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 21.Maruschak LM, Beavers R. HIV in Prisons, 2007–08. Bur Justice Stat Bulletin. 2009:1–12. [Google Scholar]
- 22.De Voux A, Spaulding AC, Beckwith C, et al. Early identification of HIV: empirical support for jail-based screening. PloS One. 2012;7:e37603. doi: 10.1371/journal.pone.0037603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolis B, Al-Darraji HA, Wickersham JA, Kamarulzaman A, Altice FL. Prevalence of tuberculosis symptoms and latent tuberculous infection among prisoners in northeastern Malaysia. Int J Tuberc Lung Dis. 2013;17:1538–44. doi: 10.5588/ijtld.13.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ECDC. Technical report: Surveillance and prevention of hepatitis B and C in Europe. Stockholm: European Centre for Disease Prevention and Control; 2010. [Google Scholar]
- 25.Kavasery R, Maru DSR, Sylla LN, Smith D, Altice FL. A prospective controlled trial of routine opt-out HIV testing in a men's jail. PloS One. 2009;4:e8056. doi: 10.1371/journal.pone.0008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolan K. HIV services in prison: a global systematic review. The 20th International AIDS Conference; Melbourne, Australia. July 20–25, 2014. [Google Scholar]
- 27.Rosen DL, Wohl DA, Schoenbach VJ. All-cause and cause-specific mortality among black and white North Carolina state prisoners, 1995–2005. Ann Epidemiol. 2011;21:719–26. doi: 10.1016/j.annepidem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruschak LM. HIV in prisons, 2001–2010. Bur Justice Stat Bulletin. 2012;20:1–12. [Google Scholar]
- 29.Maruschak LM, Berzofsky M, Unangst J. Medical problems of state and federal prisoners and jail inmates, 2011–12. Bur Justice Stat Spec Rep. 2015:1–23. [Google Scholar]
- 30.WHO. Prisons and health: partnerships for health in the criminal justice system. Geneva: World Health Organization; 2014. [Google Scholar]
- 31.Meyer JP, Cepeda J, Taxman FS, Altice FL. Sex-related disparities in criminal justice and HIV treatment outcomes: a retrospective cohort study of HIV-infected inmates. Am J Public Health. 2015;105:1901–10. doi: 10.2105/AJPH.2015.302687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkley-Rubinstein L, Turner WL. Health impact of incarceration on HIV-positive African American males: a qualitative exploration. AIDS Patient Care STDs. 2013;27:450–58. doi: 10.1089/apc.2012.0457. [DOI] [PubMed] [Google Scholar]
- 33.Milloy M, Kerr T, Buxton J, et al. Dose-response effect of incarceration events on nonadherence to HIV antiretroviral therapy among injection drug users. J Infect Dis. 2011;203:1215–21. doi: 10.1093/infdis/jir032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelenev A, Marcus R, Kopelev A, et al. Patterns of homelessness and implications for HIV health after release from jail. AIDS Behav. 2013;17(suppl 2):181–94. doi: 10.1007/s10461-013-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Diss. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 36.Spaulding AC, Seals RM, McCallum VA, Perez SD, Brzozowski AK, Steenland NK. Prisoner survival inside and outside of the institution: implications for health-care planning. Am J Epidemiol. 2011;173:479–87. doi: 10.1093/aje/kwq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Springer SA, Azar MM, Altice FL. HIV, alcohol dependence, and the criminal justice system: a review and call for evidence-based treatment for released prisoners. Am J Drug Alcohol Abuse. 2011;37:12–21. doi: 10.3109/00952990.2010.540280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iroh PA, Mayo H, Nijhawan AE. The HIV care cascade before, during, and after incarceration: a systematic review and data synthesis. Am J Public Health. 2015;105:e5–16. doi: 10.2105/AJPH.2015.302635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loutfy MR, Wu W, Letchumanan M, et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PloS One. 2013;8:e55747. doi: 10.1371/journal.pone.0055747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and high risk environment perpetuating HIV, HCV and tuberculosis transmission in eastern Europe and central Asia. Lancet. 2016 doi: 10.1016/S0140-6736(16)30856-X. published online July 14. http://dx.doi.org/10.1016/S0140-6736(16)30856-X. [DOI] [PMC free article] [PubMed]
- 41.Ko NY, Liu HY, Lai YY, Pai YH, Ko WC. Case management interventions for HIV-infected individuals. Curr HIV/AIDS Rep. 2013;10:390–97. doi: 10.1007/s11904-013-0183-7. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira PA, Jordan AO, Zaller N, Shah D, Venters H. Health outcomes for HIV-infected persons released from the New York City jail system with a transitional care-coordination plan. Am J Public Health. 2015;105:351–57. doi: 10.2105/AJPH.2014.302234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunn A, Cornwall A, Fu J, Bazerman L, Loewenthal H, Beckwith C. Linking HIV-positive jail inmates to treatment, care, and social services after release: results from a qualitative assessment of the COMPASS program. J Urban Health. 2010;87:954–68. doi: 10.1007/s11524-010-9496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15:356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westergaard RP, Spaulding AC, Flanigan TP. HIV among persons incarcerated in the USA: a review of evolving concepts in testing, treatment, and linkage to community care. Curr Opin Infect Dis. 2013;26:10–16. doi: 10.1097/QCO.0b013e32835c1dd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springer SA, Brown SE, Di Paola A. Correlates of retention on extended-release naltrexone among persons living with HIV infection transitioning to the community from the criminal justice system. Drug Alcohol Depend. 2015;157:158–65. doi: 10.1016/j.drugalcdep.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagan H. The relevance of attributable risk measures to HIV prevention planning. AIDS. 2003;17:911–13. doi: 10.1097/00002030-200304110-00017. [DOI] [PubMed] [Google Scholar]
- 49.Jürgens R, Nowak M, Day M. HIV and incarceration: prisons and detention. J Int AIDS Soc. 2011;14:26. doi: 10.1186/1758-2652-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamarulzaman A, Reid SE, Schwitters A, et al. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016 doi: 10.1016/S0140-6736(16)30769-3. published online July 14. http://dx.doi.org/S0140-6736(16)30769-3. [DOI] [PubMed]
- 51.Larney S, Kopinski H, Beckwith CG, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58:1215–24. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsoulfas G, Goulis I, Giakoustidis D, et al. Hepatitis C and liver transplantation. Hippokratia. 2009;13:211. [PMC free article] [PubMed] [Google Scholar]
- 53.Galea G, Enggist S, Udesen C, Møller L. Prisons and Health. World Health Organization; 2014. pp. 165–70. [Google Scholar]
- 54.Macalino GE, Dhawan D, Rich JD. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95:1739–40. doi: 10.2105/AJPH.2004.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int. 2013;33(suppl 1):68–79. doi: 10.1111/liv.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuncio DE, Newbern EC, Fernandez-Vina MH, Herdman B, Johnson CC, Viner KM. Comparison of risk-based hepatitis C screening and the true seroprevalence in an urban prison system. J Urban Health. 2015;92:379–86. doi: 10.1007/s11524-015-9945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He T, Li K, Roberts MS, et al. Prevention of hepatitis C by Screening and Treatment in U.S. Prisons. Ann Intern Med. 2016;164:84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rich JD, Allen SA, Williams BA. Responding to hepatitis C through the criminal justice system. N Engl J Med. 2014;370:1871–74. doi: 10.1056/NEJMp1311941. [DOI] [PubMed] [Google Scholar]
- 59.Arain A, Robaeys G, Stöver H. Hepatitis C in European prisons: a call for an evidence-informed response. BMC Infect Dis. 2014;14(suppl 6):S17. doi: 10.1186/1471-2334-14-S6-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spaulding AS, Kim AY, Harzke AJ, et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations. Top Antivir Med. 2013;21:27–35. [PMC free article] [PubMed] [Google Scholar]
- 61.Zampino R, Coppola N, Sagnelli C, Di Caprio G, Sagnelli E. Hepatitis C virus infection and prisoners: Epidemiology, outcome and treatment. World J Hepatol. 2015;7:2323–30. doi: 10.4254/wjh.v7.i21.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, Watcha D, Holodniy M, Goldhaber-Fiebert JD. Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in U.S. incarcerated populations: a cost-effectiveness analysis. Ann Intern Med. 2014;161:546–53. doi: 10.7326/M14-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larney S, Beckwith CG, Zaller ND, Montague BT, Rich J. “Seek, test, treat and retain” for hepatitis C in the United States criminal justice system. Int J Prison Health. 2014;10:164–71. doi: 10.1108/IJPH-11-2013-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PloS One. 2013;8:e59643. doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Correlates of HIV infection and being unaware of HIV status among soon-to-be-released Ukrainian prisoners. J Int AIDS Soc. 2014;17:19005. doi: 10.7448/IAS.17.1.19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azbel L, Wickersham JA, Wegman MP, et al. Burden of substance use disorders, mental illness, and correlates of infectious diseases among soon-to-be released prisoners in Azerbaijan. Drug Alcohol Depend. 2015;151:68–75. doi: 10.1016/j.drugalcdep.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a nationwide hepatitis C elimination program—Georgia, April 2015. MMWR Morb Mortal Wkly Rep. 2015;64:753–57. doi: 10.15585/mmwr.mm6428a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham CS, Swan T. A path to eradication of hepatitis C in low-and middle-income countries. Antiviral Res. 2015;119:89–96. doi: 10.1016/j.antiviral.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 69.WHO/ UNICEF coverage estimates 2014 revision. Immunization Vaccines and Biologicals, (IVB) World Health Organization; Jul 24, 2015. [accessed March 30, 2016]. July 2015. http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/HepB_coverage.jpg?ua=1. [Google Scholar]
- 70.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–19. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 71.Larney S, Monkley DL, Indig D, Hampton SE. A cross-sectional study of susceptibility to vaccine-preventable diseases among prison entrants in New South Wales. MedJ Aust. 2013;198:376–79. doi: 10.5694/mja12.11110. [DOI] [PubMed] [Google Scholar]
- 72.Beck CR, Cloke R, O'Moore É, Puleston R. Hepatitis B vaccination coverage and uptake in prisons across England and Wales 2003–2010: A retrospective ecological study. Vaccine. 2012;30:1965–71. doi: 10.1016/j.vaccine.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 73.Hutchinson SJ, Wadd S, Taylor A, et al. Sudden rise in uptake of hepatitis B vaccination among injecting drug users associated with a universal vaccine programme in prisons. Vaccine. 2004;23:210–14. doi: 10.1016/j.vaccine.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Costumbrado J, Stirland A, Cox G, et al. Implementation of a hepatitis A/B vaccination program using an accelerated schedule among high-risk inmates, Los Angeles County Jail, 2007–2010. Vaccine. 2012;30:6878–82. doi: 10.1016/j.vaccine.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Dara M, Chadha SS, Vinkeles Melchers NV, et al. Time to act to prevent and control tuberculosis among inmates. Int J Tuberc Lung Dis. 2013;17:4–5. doi: 10.5588/ijtld.12.0909. [DOI] [PubMed] [Google Scholar]
- 76.Raviglione M, Global TB Director . Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization; 2013. [Google Scholar]
- 77.Rodrigues C, Vadwai V. Tuberculosis: laboratory diagnosis. Clin Lab Med. 2012;32:111–27. doi: 10.1016/j.cll.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PloS One. 2012;7:e36966. doi: 10.1371/journal.pone.0036966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melchers NVV, Van Elsland SL, Lange JM, Borgdorf MW, Van Den Hombergh J. State of affairs of tuberculosis in prison facilities: a systematic review of screening practices and recommendations for best TB control. PloS One. 2013;8:e53644. doi: 10.1371/journal.pone.0053644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carbone Ada S, Paiao DS, Sgarbi RV, et al. Active and latent tuberculosis in Brazilian correctional facilities: a cross-sectional study. BMC Infect Dis. 2015;15:24. doi: 10.1186/s12879-015-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kowada A. Cost-effectiveness of interferon-gamma release assay for entry tuberculosis screening in prisons. Epidemiol Infect. 2013;141:2224–34. doi: 10.1017/S0950268812002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Darraji HA, Abd Razak H, Ng KP, Altice FL, Kamarulzaman A. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PloS One. 2013;8:e73717. doi: 10.1371/journal.pone.0073717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dara M, Acosta CD, Melchers NV, et al. Tuberculosis control in prisons: current situation and research gaps. IntJ Infect Dis. 2015;32:111–17. doi: 10.1016/j.ijid.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 84.Abebe D, Bjune G, Ameni G, Bifa D, Abebe F. Prevalence of pulmonary tuberculosis and associated risk factors in Eastern Ethiopian prisons. Int J Tuberc Lung Dis. 2011;15:668–73. doi: 10.5588/ijtld.10.0363. [DOI] [PubMed] [Google Scholar]
- 85.Banda HT, Gausi F, Harries AD, Salaniponi FM. Prevalence of smear-positive pulmonary tuberculosis among prisoners in Malawi: a national survey. Int J Tuberc Lung Dis. 2009;13:1557–59. [PubMed] [Google Scholar]
- 86.Al-Darraji HA, Kamarulzaman A, Altice FL. Isoniazid preventive therapy in correctional facilities: a systematic review. Int J Tuberc Lung Dis. 2012;16:871–79. doi: 10.5588/ijtld.11.0447. [DOI] [PubMed] [Google Scholar]
- 87.Aerts A, Habouzit M, Mschiladze L, et al. Pulmonary tuberculosis in prisons of the ex-USSR state Georgia: results of a nation-wide prevalence survey among sentenced inmates. Int J Tuberc Lung Dis. 2000;4:1104–10. [PubMed] [Google Scholar]
- 88.Shah SA, Mujeeb SA, Mirza A, Nabi KG, Siddiqui Q. Prevalence of pulmonary tuberculosis in Karachi juvenile jail, Pakistan. East Mediterr Health J. 2003;9:667–74. [PubMed] [Google Scholar]
- 89.Aerts A, Hauer B, Wanlin M, Veen J. Tuberculosis and tuberculosis control in European prisons. Int J Tuberc Lung Dis. 2006;10:1215–23. [PubMed] [Google Scholar]
- 90.Dara M, Grzemska M, Kimerling ME, Reyes H, Zagorskiy A. Guidelines for control of tuberculosis in prisons. Washington, DC: World Health Organization; 2009. [Google Scholar]
- 91.Biadglegne F, Rodlof AC, Sack U. Review of the prevalence and drug resistance of tuberculosis in prisons: a hidden epidemic. Epidemiol Infect. 2015;143:887–900. doi: 10.1017/S095026881400288X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rich JD, Wakeman SE, Dickman SL. Medicine and the epidemic of incarceration in the United States. N Engl J Med. 2011;364:2081–83. doi: 10.1056/NEJMp1102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:538–49. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 94.Walmsley R. World female imprisonment list. London: International Centre for Prison Studies, King's College London; 2006. pp. 1–6. [Google Scholar]
- 95.Youmans E, Burch J, Moran R, Smith L, Dufus WA. Epidemiological characteristics of HIV-infected women with and without a history of criminal justice involvement in South Carolina. J Correct Health Care. 2013;19:15–26. doi: 10.1177/1078345812456376. [DOI] [PubMed] [Google Scholar]
- 96.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5(R)) American Psychiatric Pub. 2013 [Google Scholar]
- 97.Rich JD, McKenzie M, Larney S, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2015;386:350–59. doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geneva Declaration 2012 on health care in prison. 6th European Conference on Health Promotion in Custody; February 1-3, 2012. [Google Scholar]
- 99.World Health Organization. Health in prisons: A WHO guide to the essentials in prison health. Copenhagen: World Health Organization Regional Office for Europe; 2007. [Google Scholar]
- 100.United Nations Economic and Social Council. United Nations standard minimum rules for the treatment of prisoners (the Mandela Rules) Vienna: Commission on Crime Prevention and Criminal Justice; May 18–22, 2015. [Google Scholar]
- 101.Biljon Van, et al. Minister of Correctional Services. Reference: 6 BCLR 789. 1997 [Google Scholar]
- 102.Tapela, et al. Attorney General and others. Reference: MAHGB-000057-14. 2014 [Google Scholar]
- 103.Testa, et al. Croatia. Reference: 20877/04. 2007 [Google Scholar]
- 104.Staykov, et al. Bulgaria. Reference: 49438/99. 2007 [Google Scholar]
- 105.Office of the High Commissioner for Human Rights. CESCR General Comment No. 14: The right to the highest attainable standard of health (Art. 12) 2000 Document E/C.12/2000/4. [Google Scholar]
- 106.Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(suppl 2):74–77. doi: 10.1177/00333549071220S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment—Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124–33. doi: 10.1002/hep.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.