Abstract
Background
We examined frequency of guideline-concordant cancer care in elderly patients, including “older” elderly (age≥80).
Methods
Using the Surveillance, Epidemiology and End Results-Medicare dataset in patients age≥66 years, diagnosed with non-metastatic breast (n=55,094), non-small cell lung (NSCLC) (n=36,203), or prostate (n=86,544) cancer from 2006–2011, chemotherapy, surgery, and radiation (RT) treatments were identified using claims. Pearson chi-square tested associations between age and guideline concordance.
Results
Older patients were less likely to receive guideline-concordant curative treatment: in Stage III breast cancer, receipt of post-mastectomy RT (70%, 46% and 21% in patients age 66–79, 80–89 and ≥90 respectively; P<0.0001); in Stage I NSCLC, RT or surgery (89%, 80%, and 64% in age 66–79, 80–89 and ≥90; P<0.0001); in Stage III NSCLC, RT or surgery plus chemotherapy (79%, 58%, and 27% in age 66–79, 80–89 and ≥90; P<0.0001); and in intermediate/high risk prostate cancer, RT or prostatectomy (projected life expectancy >10 yrs—85% and 82% in age 66–69 and 70–75; and ≤10 years—70%, 42%, and 9% in age 76–79, 80–89, ≥90; P<0.0001). However, older patients were more likely to receive guideline-concordant de-intensified treatment: in Stage I–II node-negative breast cancer, hypofractionated post-lumpectomy RT (9%, 16% and 23% in age 66–79, 80–89 and ≥90; P<0.0001); in stage I ER+ breast cancer, observation after lumpectomy (12%, 42%, and 84% in age 66–79, 80–89 and ≥90; P<0.0001); in stage I NSCLC, stereotactic body RT instead of surgery (7%, 16%, and 25% in age 66–79, 80–89 and ≥90; P<0.0001); and in lower-risk prostate cancer, no active treatment (25%, 54%, and 68% in age 66–79, 80–89 and ≥90; P<0.0001).
Conclusion
Actual treatment of older elderly cancer patients frequently diverged from guidelines, especially in curative treatment of advanced disease. Results suggest a need for better metrics than existing guidelines alone to evaluate quality and appropriateness of care in this population.
INTRODUCTION
Practice guidelines are vital to radiation oncology practice, aiming to promote standardized, evidence-based approaches to care. Guidelines are fundamentally designed to optimize clinical outcomes metrics in cancer patients, by disseminating consensus-based “best” practice. However, guidelines are also increasingly regarded as a valuable source to assess quality metrics of care delivery (1–3), an important consideration, given that creation and reporting of quality metrics have risen as policy priorities in healthcare for the elderly. In particular, Medicare—the primary source of health insurance for elderly patients—has now directly coupled reimbursement to compliance with quality measures through the Medicare Access and CHIP Reauthorization Act (MACRA) (4–6).
With the expectation of quality reporting and MACRA’s future “pay for performance” impact on oncology practice, cancer care quality metrics are currently being formulated, selected, and refined (4,7). Therefore, a thorough understanding of baseline performance—by characterizing contemporary frequencies of concordance with benchmark oncology practice guidelines— is needed in elderly patients, to establish the “current state” of cancer care delivery in this population. The need to understand delivery and quality of cancer care in elderly patients is underscored by cancer’s rising incidence burden in US patients ages 65 and older (8,9).
Prior studies suggest that elderly patients receive less frequent cancer treatment, compared with younger patients (10–14). However, these studies were conducted in cohorts limited to specific disease sites and specific treatment comparisons. How patient age influences overarching patterns in guideline concordance of treatment decision-making is still not clear. We hypothesized that: 1) older age would predict lower rates of guideline-concordant decisions for curative treatment; 2) there is variation by age in guideline-concordant decisions for de-intensified treatment; and 3) there are common patterns of how age modifies treatment decision-making—across disease sites, stages, treatment modalities, and treatment paradigms.
We surmised if deviations from guidelines were detected more frequently with older age in cancer patients, such a finding could help signal barriers to disseminating “best” practices in the elderly (15–18). Alternatively, frequent deviations in older patients might also signal that current guidelines themselves are inherently insufficient for shaping quality and healthcare delivery metrics for the elderly. Such a finding would thereby help establish baseline gaps in cancer guideline and quality metric development for this population. Accordingly, in a nationally representative cohort of elderly cancer patients diagnosed with incident breast, lung, or prostate cancer, we sought to determine the relationship between age and concordance with select cancer care guidelines, with a focus on radiation treatment.
STUDY METHODS
Patient cohort
We used the Surveillance, Epidemiology and End Results (SEER)-Medicare dataset to examine treatment utilization (chemotherapy, surgery, radiation, and hormone therapy), in patients age≥66 with incident breast (AJCC stage I–III), non-small cell lung (NSCLC) (stage I–III), or prostate (clinical stage T1-3, N0M0) cancer from 2006–2011. We excluded patients with prior diagnosis of another malignancy within first year of diagnosis; unknown histology; no pathologic confirmation; and cancer diagnosis at autopsy/by death certificate. Medicare Part A and B coverage and no HMO enrollment 12 months prior to and 12 months after diagnosis was required. Patient and disease factors at diagnosis extracted from SEER included age, diagnosis year, disease stage, tumor grade, and estrogen receptor status (for breast cancer patients). Final samples included 55,094 breast, 36,203 NSCLC, and 86,544 prostate cancer patients. (eTables 1–3)
Age
Age was initially categorized as 66–79, 80–89 and ≥90, especially to highlight patterns of treatment in older elderly-- octogenarians (age 80–89) and nonagenarians (age ≥90). Analyses of patients age≥90 are presented as a unique age category. In certain analyses, when results included n≤11, results are suppressed to prevent compromise of patient identities. Where this was applicable, we state “the number of patients age ≥90 was too low to report”.
Where guidelines specifically reflected age, additional age categories were analyzed (with inflection points at age 70 for early breast cancer patients and age 75 for prostate cancer patients based on use of the National Social Security Index actuarial estimate of life expectancy of at least 10 additional years for US men age≤75) (19,20).
Other covariates
Modified Charlson comorbidity index was derived (21–23) from claims for comorbid diseases occurring between 12 months prior and 1 month after cancer diagnosis. As previously established, to enhance specificity, diagnosis codes identified in Part B files must have occurred in 2 separate claims over >30 days or also in Part A claims (24,25). Performance status score was derived from the Medicare Durable Medical Equipment file, indicating use of home oxygen, cane, commode, wheelchair, or hospital bed (26,27), categorized as 0= none, 1= 1 item, 2= 2 or more items.(26) (eTables 4a–b)
Cancer treatment and outcomes
Treatment through one year from diagnosis was defined by International Classification of Diseases (ICD)-9 procedure and Healthcare Common Procedure Coding System (HCPCS)/Current Procedural (CPT) claims codes for chemotherapy, site-specific surgery (e.g. lumpectomy, mastectomy, radical prostatectomy, sublobar resection, lobar resection, lobectomy, etc.), and radiation treatment (RT). Stereotactic body radiation therapy (SBRT) was based on procedure claims (regardless of number of radiation fractions). Number of radiation fractions was based on radiation delivery claims with unique dates of service, or weekly treatment management codes (representing 5 fractions delivered) when claim dates were unavailable. Chemotherapy codes identified cytotoxic and targeted systemic therapies and androgen deprivation therapy (ADT). (eTables 4a–b) Oral endocrine therapy claims in breast cancer patients were available in patients with Medicare Part D coverage and coded for this subset (28). Overall survival and cause-specific survival were based on Medicare death date and SEER cause of death. Time to event was calculated from diagnosis.
Practice guidelines
Frequency of concordance of treatment with the following select practice guidelines, based upon National Comprehensive Cancer Network (NCCN) guidelines (20) for each disease site was assessed:
Breast
In select early stage breast cancer patients (stage I–II, node negative), treated with lumpectomy plus post-lumpectomy RT: use of hypofractionation, defined as 15–23 RT fractions. This proxy definition of hypofractionation, based on number of fractions, was selected as details of actual radiation dose delivered per fraction are not available in SEER-Medicare (29).
In select early stage breast cancer patients (stage I, ER positive, treated with lumpectomy): use of observation instead of post-lumpectomy RT.
In select advanced stage breast cancer patients (Stage III, treated with mastectomy): use of post-mastectomy RT.
Non-small cell lung cancer (NSCLC)
In stage I NSCLC patients: use of potentially curative treatments including: SBRT, other external beam RT, any surgery (including sublobar resection, lobectomy, and pneumonectomy) vs. no treatment.
In Stage III NSCLC patients; use of potentially curative treatments including: any RT or any surgery (with or without chemotherapy) vs. no treatment
Prostate
In intermediate or high risk prostate cancer patients (Gleason 7–10 or at least clinical T2b stage), use of potentially curative treatments including: RT (external beam RT and/or brachytherapy) or prostatectomy; further stratified by projected life expectancy >10 years (age ≤75) (19).
In lower risk prostate cancer patients (both clinical stage T1 and Gleason 6), use of no active treatment. Prostate-specific antigen (PSA) is not available for patient risk-stratification in SEER-Medicare.
Guidelines were classified into two treatment paradigm groupings for analysis: “Do more”, or curative treatment, including PMRT for stage III breast cancer, surgery or RT for stage I and stage III NSCLC, and definitive RT or surgery for intermediate/high risk prostate cancer; vs. “Do less”, or de-intensified treatment. De-intensification indicated treatments with less invasiveness, time burden, or toxicity compared with other standard treatment options, and in this analysis included hypofractionated RT for early stage breast cancer, observation after lumpectomy for stage I ER+ breast cancer, SBRT for stage I NSCLC, and no active treatment for lower risk prostate cancer.
Statistical analyses
Associations between age and guideline-concordant treatment were tested using the Pearson Chi-square test. Multivariate logistic models examined associations between age and use of guideline-concordant treatments, adjusted for comorbidity and performance status. Because these two covariates demonstrated collinearity, separate models were fitted for each. In multivariate models, age was initially tested as a continuous variable. Because the linearity assumption did not universally show goodness-of-fit in all models by Hosmer and Lemeshow criteria, age was also tested categorically. Final models are presented using the categorical variable, with significance tested using the Wald Chi-square test. Time-to-event analyses evaluated associations between guideline-concordance and OS and CSS using the Kaplan-Meier log-rank test. Analyses were conducted using SAS (Cary, NC). Statistical tests were two-sided. P-value<0.05 was considered statistically significant. This study was exempted by the Institutional Review Board.
RESULTS
Median age was 74 years (interquartile range (IQR) 70–79); for breast patients 75 years (IQR 70, 81), NSCLC 75 years (IQR 71–81), and prostate 73 years (IQR 69–77). A total of 22% of patients were age≥80 and 2% age≥90 years. (eTable 5)
General Treatment Patterns by Age
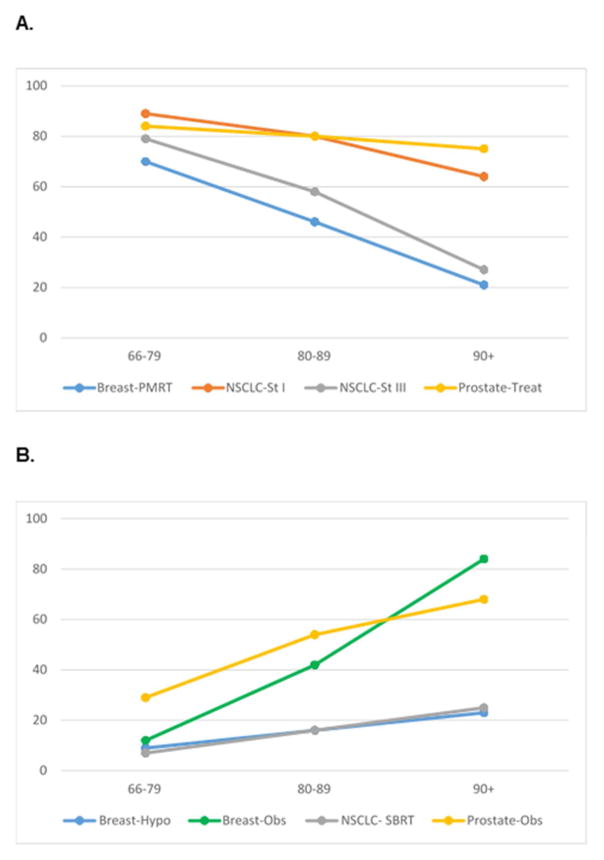
Older patients were less likely to receive guideline-concordant curative treatment, but more likely to receive guideline-concordant de-intensified treatment. (Figure 1a–b) Specific frequencies of treatment use by cancer site are delineated below.
Figure 1.
A. “Do More”: Age and concordance with curative treatment guidelines; B. “Do Less”: Age and concordance with de-intensified treatment guidelines
Specific Treatment Use by Cancer Site
Breast Cancer
Benchmark #1: Use of hypofractionation (15–23 fractions) in stage I–II, node-negative breast cancer patients receiving post-lumpectomy RT
Only 10% (of n=20,631) were treated with hypofractionated RT. Use of hypofractionated RT increased with older age: Utilization was 9% in patients aged 66–79 (of n=16,473), compared with 16% age ≥80 (of n=4,021), and 23% age ≥90 (of n=137); p<0.0001.
Benchmark #2: Use of observation in stage I, ER positive breast cancer patients receiving lumpectomy
A total of 21% (of 18,940) were observed after lumpectomy. Among patients who underwent observation after lumpectomy (with available drug prescription data), 59% (of n=1,688) filled a prescription for endocrine therapy.
Observation increased with older age: 12% in patients aged 66–69 (of 673), compared with 42% age ≥80 (of n=867), and 84% age ≥90 (of n=148); p<0.0001. Because NCCN guidelines specify observation as appropriate in patients age≥70, frequencies of observation (versus RT use) were further delineated using the additional cutpoint of age 70, demonstrating the most dramatic increase in observation after age 75: 6% age 66–67, 8% age 68–69, 10% age 70–74, and 19% age 75–79. Endocrine therapy use in these ER+ patients decreased with older age: 65% aged 66–79 (of n=673), compared with 57% age ≥80 (of n=867), and 41% age ≥90 (of n=148, p<0.0001)
Benchmark #3: Use of post-mastectomy RT in stage III breast cancer patients
Only 60% of this patient group (of n=3,996) underwent RT. Use of post-mastectomy RT decreased with older age: Utilization was 70% in patients aged 66–79 (of n=2,607), compared with 46% age ≥80 (of n=1,196), and 21% age ≥90 (of n=193); p<0.0001. In this group, chemotherapy use was 60% (of n=3,996) and also negatively associated with age: 76% in patients aged 66–79 (of n=2,607), compared with 32% age ≥80 (of n=1,196), and 9% age ≥90 (of n=193); p<0.0001.
Non-Small Cell Lung Cancer
Benchmark #1: Use of curative treatment in Stage I NSCLC patients
A total of 86% of this patient group received any treatment, while 14% received no active treatment (of n=15,777). Use of any treatment decreased with older age: 89% in patients aged 66–79 (of n=11,272), compared with 80% age ≥80 (of n=4,253), and 64% age ≥90 (of n=252); p<0.0001.
Of patients undergoing treatment, 9% received SBRT and 16% received other EBRT, while 72% received surgical resection (lobectomy, sublobar resection, or pneumonectomy) (of n=13,577). SBRT use was associated with older age: 7% in patients aged 66–79 (of n=9,998), compared with 16% age ≥80 (of n=3,416), and 25% age ≥90 (of n=163); p<0.0001.
Benchmark #2: Use of curative treatment in Stage III NSCLC patients
A total of 71% received any treatment, while 29% received no active treatment (of n=17,142). Use of any treatment decreased with older age: 79% in patients aged 66–79 (of n=11,693), compared with 58% age ≥80 (of n=4,958), and 27% age ≥90 (of n=491); p<0.0001.
Specific use of standard curative multi-modality therapy included: chemoradiation in 37% of patients aged 66–79 (of n=11,693), compared with 20% age ≥80 (of n=4,958), and 3% age ≥90 (of n=491); p<0.0001. Use of surgery plus chemotherapy was 12% in patients aged 66–79 (of n=11,693), compared with 3% age ≥80 (of n=4,958); P<0.0001. The number of patients age ≥90 was too low to report.
Prostate cancer
Benchmark #1: Use of curative treatment (definitive RT or prostatectomy) in intermediate or high risk stage and/or grade prostate cancer patients, based on projected life expectancy >10 years (age ≤75) and ≤10 years (age >75)
A total of 84% (of n=31,168) of patients age ≤75 received either up front definitive RT alone (54%, of n=31,168) or prostatectomy (33%, of n=31,168)—both considered curative treatment, and 72% of all patients received curative treatment. Use of curative treatment decreased with older age: Among patients with projected life expectancy >10 years, curative treatment utilization was 85% in patients aged 66–69 (of n=13,397), compared with 82% in age 70–75 (of n=17,771); p<0.0001. Among patients with projected life expectancy ≤10 years, curative treatment utilization was 70% in patients age 76–79 (of n=8,561); 42% age 80–89 (of n=8,914); and 9% age 90+ (of n=617); p<0.0001.
Benchmark #2: Use of no active treatment in lower risk stage and grade prostate cancer patients (cT1 and Gleason 6)
A total of 22% (of 84,272) of these patients received no active treatment. No treatment was used in 18% in patients aged 66–69 (of n=24,262), 20% age 70–75 (of n=31,658), 25% age 76–79 (of n=14,355), 31% age ≥80 (of n=13,198), and 39% age ≥90 (of n=799); p<0.0001.
Almost all remaining patients received definitive treatment (RT or prostatectomy), though a small portion received ADT alone: 4% in patients aged 66–69 (of n=24,262), 6% age 70–75 (of n=31,658), 12% age 76–79 (of n=14,355), 32% age ≥80 (of n=13,198), and 53% age ≥90 (of n=799); p<0.0001.
Comorbidities, performance status, and outcomes
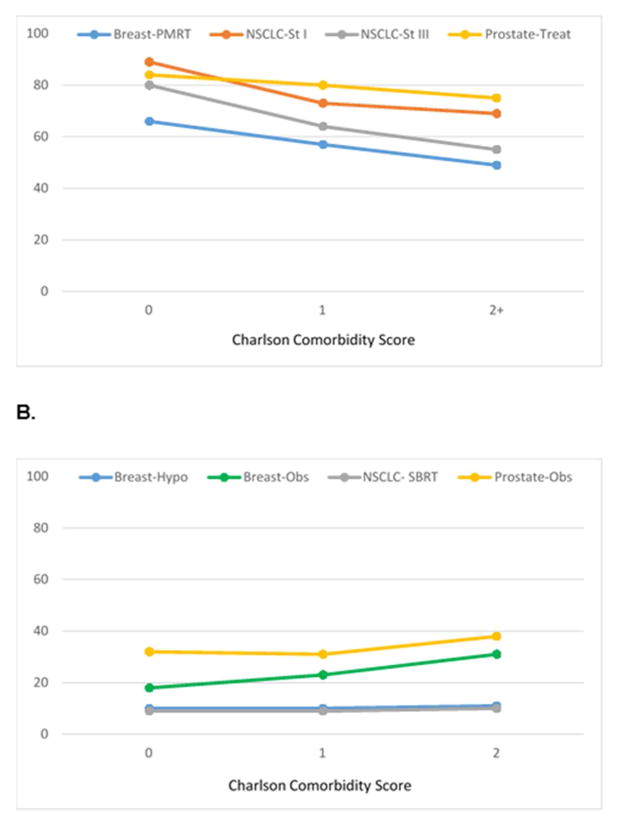
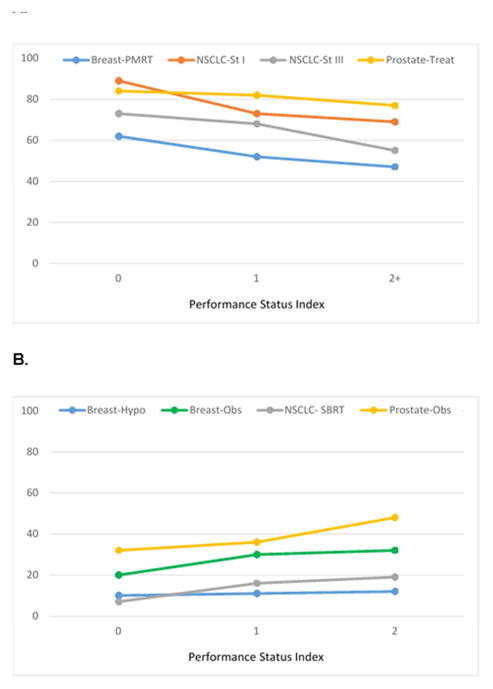
Worse comorbidity and decreased performance status showed similar patterns of association with guideline concordance, demonstrating lower concordance with curative treatments and higher concordance with de-intensified treatment. (Figure 2a–b, Figure 3a–b, eTable 6). However, in multivariable analyses, for every guideline, age remained an independent predictor of guideline concordance (P<0.0001) even after accounting for confounding by comorbidity and performance status. (Table 1) Regarding outcomes, use of curative therapies was associated with increased overall and cause-specific survival. (eTable 7)
Figure 2.
A. “Do More”: Comorbidity and concordance with curative treatment guidelines; B. “Do Less”: Comorbidity and concordance with de-intensified treatment guidelines
Figure 3.
A. “Do More”: Performance status and concordance with curative treatment guidelines; B. “Do Less”: Performance status and concordance with de-intensified treatment guidelines
Table 1.
A.Adjusted odds of achieving guideline-concordance with older age for curative and de-intensified treatment guideline accounting for comorbidity; B. Adjusted odds accounting for performance status
| A. Do More: Adjusted for comorbidity | |||
|---|---|---|---|
|
| |||
| Characteristic | OR | 95% CI | P |
| Breast-PMRT | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.37 | 0.33, 0.43 | |
| Age ≥90 vs. 66–79 | 0.11 | 0.08, 0.16 | |
|
| |||
| NSCLC-St I | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.57 | 0.51, 0.62 | |
| Age ≥90 vs. 66–79 | 0.27 | 0.20, 0.35 | |
|
| |||
| NSCLC-St III | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.40 | 0.38, 0.43 | |
| Age ≥90 vs. 66–79 | 0.11 | 0.09, 0.13 | |
|
| |||
| Prostate-Treat | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.18 | 0.17,0.18 | |
| Age ≥90 vs. 66–79 | 0.02 | 0.02, 0.03 | |
| Do Less-Adjusted for comorbidity | |||
|
| |||
| Characteristic | OR | 95% CI | P |
|
| |||
| Breast-Hypofx | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 2.04 | 1.85, 2.26 | |
| Age ≥90 vs. 66–79 | 3.48 | 2.34, 5.17 | |
|
| |||
| Breast-Obs | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 5.39 | 4.98, 5.83 | |
| Age ≥90 vs. 66–79 | 37.68 | 28.88, 49.16 | |
|
| |||
| NSCLC-SBRT | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 2.53 | 2.24, 2.86 | |
| Age ≥90 vs. 66–79 | 4.50 | 3.12, 6.49 | |
|
| |||
| Prostate-Obs | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 2.81 | 2.59, 3.06 | |
| Age ≥90 vs. 66–79 | 4.96 | 3.33, 7.37 | |
| B. Do More: Adjusted for performance status | |||
|---|---|---|---|
|
| |||
| Characteristic | OR | 95% CI | P |
| Breast-PMRT | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.37 | 0.32, 0.42 | |
| Age ≥90 vs. 66–79 | 0.11 | 0.08, 0.16 | |
|
| |||
| NSCLC-St I | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.52 | 0.47, 0.57 | |
| Age ≥90 vs. 66–79 | 0.24 | 0.18, 0.31 | |
|
| |||
| NSCLC-St III | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.38 | 0.36,0.41 | |
| Age ≥90 vs. 66–79 | 0.10 | 0.08, 0.13 | |
|
| |||
| Prostate-Treat | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 0.18 | 0.17,0.18 | |
| Age ≥90 vs. 66–79 | 0.02 | 0.02, 0.03 | |
| Do Less: Adjusted for performance status | |||
|
| |||
| Characteristic | OR | 95% CI | P |
|
| |||
| Breast-Hypofx | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 2.04 | 1.84, 2.25 | |
| Age ≥90 vs. 66–79 | 3.46 | 2.33, 5.14 | |
|
| |||
| Breast-Obs | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 5.49 | 5.08, 5.94 | |
| Age ≥90 vs. 66–79 | 38.27 | 29.34, 49.91 | |
|
| |||
| NSCLC-SBRT | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 2.59 | 2.29,2.93 | |
| Age ≥90 vs. 66–79 | 4.74 | 3.26, 6.89 | |
|
| |||
| Prostate-Obs | <0.001 | ||
|
| |||
| Age 80–89 vs. 66–79 | 2.83 | 2.60, 3.07 | |
| Age ≥90 vs. 66–79 | 5.03 | 3.38, 7.47 | |
Abbreviations: OR Odds ratio, CI confidence interval, PMRT post-mastectomy radiation treatment (Stage III), NSCLC non-small cell lung cancer, St stage, Hypofx hypofractionation, Obs observation
DISCUSSION
In elderly patients with breast, lung, or prostate cancer, guideline concordant care was significantly associated with age. There were similar patterns of the impact of age on treatment decisions across disease sites, stages, and treatment modalities, though patterns were opposite depending on treatment paradigm. Consistent with our hypothesis, decisions for curative treatment were less frequent as age increased, especially for the older elderly, who comprised almost 20% of this cohort. Concordance with curative therapies were 70% to over 80% in patients age<80 years, compared to approximately 30% to 50% in patients age≥80 years. In contrast, the frequency of decisions for de-intensified treatment increased with age. The relationships between age and guideline concordance could not be fully explained by differences in comorbidity or performance status. This age-associated variation in frequency of guideline-concordant care suggests that current guidelines may insufficiently inform clinical decisions for care, particularly in older elderly patients, and especially in the settings of curative treatment, advanced cancer, and multi-modality therapy (4–6).
In elderly patients, it remains unclear whether lack of guideline-concordant care may reflect appropriate clinical decision-making vs. inappropriate care, undertreatment or overtreatment—the typical quality metrics guideline concordance is thought to reflect. Our results suggested a potential inflection point for age-dependent variations in guideline-concordance, occurring at the age 80 years and older. Under age 80, frequencies of guideline-concordant practice across disease, sites, stages, and treatment modalities was surprisingly consistent, with little “spread” in absolute frequencies of guideline concordance: about 80% for “do more” treatments and about 20% for “do less” treatments. In contrast, variation in frequency of guideline-concordant practices widened for patients age 80 years and older, suggesting that current guidelines may be insufficient for informing quality metrics of cancer care delivery, particularly in older elderly. This insufficiency is notable since older elderly are one of the fastest growing cancer populations (9).
Barriers to guideline-concordant care and quality measures in older elderly cancer patients may be multi-factorial. Particularly for curative treatment, older patients are often underrepresented in randomized trials informing guidelines. For example, only 2% of patients enrolled on CALGB protocols for node-positive breast cancer were >70 years (30), though elder risk stratification has recently been included in select prospective studies, for example in NSCLC treatment trials (31–33).
Where clinical outcomes evidence exists, there remain mixed results for disease benefits vs. toxicity risks for cancer treatments in the elderly. Select prior studies demonstrate undertreatment of elderly patients leading to worse cancer outcomes, and appropriate treatment improving overall survival and quality of life even in patients with comorbidity and poor performance status (10,34–37). Yet other studies demonstrate older patients facing measurably increased toxicity (and mortality) risks associated with a variety of cancer treatment modalities (33,38–40). Still other evidence demonstrates potential benefits of shorter or less intensive regimens (e.g. hypofractionation) as well-tolerated and potentially of special benefit to frail elderly patients (41–44). The need to obtain more age-adapted morbidity and mortality risks associated with interventions (33)—remain key needs for developing age-adapted practice guidelines and quality measures.
Finally, decision-making in elderly patients are driven by additional considerations such as life expectancy, comorbidities (45,46), functional status, biologic aggressiveness of disease (47–48), benefits of palliation, availability of alternatives, and access to treatments are all important considerations in treatment choice (12,49,50), as well as patient preference, including decisions favoring less than curative treatment.
Implications of our results in context are twofold: oversimplified guideline-based quality benchmarking (for example, defining best quality as “100% guideline concordance”) is inadequate. This approach can lead to undiscerning measures of quality cancer care, especially for the older elderly. Optimal quality benchmarking of guideline-based practice may involve, first, stratifying by treatment paradigm and, second, calibrating with age-adapted and case-mix factors, allowing for broader and more nimble definitions of quality benchmarks.
Limitations included proxy variable definitions from claims. Breast hypofractionation was based on number of radiation fractions, as details of radiation dose/fields are unavailable in SEER-Medicare. Endocrine therapy in breast cancer patients may have been underreported, as this variable was derived only from Medicare Part D coverage and not prescriptions filled under secondary insurance. PSA levels are unavailable for risk group-stratification in prostate cancer patients, and our “lower” risk prostate cancer group may have included patients with PSA>10. Validation of age trends may be needed to further understand treatment use for these patient subsets.
CONCLUSION
Actual care for older elderly cancer patients frequently diverges from guidelines, and this divergence was especially marked for curative treatment for advanced disease. Results suggest a need for better metrics than existing guidelines alone to evaluate quality and appropriateness of care in this population.
Supplementary Material
Acknowledgments
This study was supported in part by the Cancer Prevention & Research Institute of Texas (CPRIT RP140020) (GLS, SHG), the Duncan Family Institute (WGH, GLS, BDS, SHG), NIH K07 CA211804-01 (GLS), NIH R01 CA207216 (BDS), the Andrew Sabin Family Fellowship (BDS), the Cancer Prevention & Research Institute of Texas (RP140020 and RP160674) (BDS), the Department of Health and Human Services National Cancer Institute (P30CA016672) (BDS), the Center for Radiation Oncology Research (BDS), and grants from the MD Anderson Cancer Center (BDS).
We examined frequency of guideline-concordant cancer care in elderly patients, including “older” elderly (age≥80) using the Surveillance, Epidemiology and End Results-Medicare dataset. Actual treatment of older elderly patients with breast, lung, and prostate cancer frequently diverged from guidelines, especially in curative treatment of advanced disease. Results suggest a need for better metrics than existing guidelines alone to evaluate quality and appropriateness of care in this population.
Footnotes
Disclosure statement: Dr. B Smith receives research funding from Varian Medical Systems, but this was unrelated to the current research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desch CE, McNiff KK, Schneider EC, Schrag D, McClure J, Lepisto E, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol Off J Am Soc Clin Oncol. 2008 Jul 20;26(21):3631–7. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 2.Holmes JA, Bensen JT, Mohler JL, Song L, Mishel MH, Chen RC. Quality of care received and patient-reported regret in prostate cancer: Analysis of a population-based prospective cohort. Cancer. 2016 Sep 13; doi: 10.1002/cncr.30315. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013 Jun;121(6):1226–34. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 4.Clough JD, McClellan M. Implementing MACRA: Implications for Physicians and for Physician Leadership. JAMA. 2016 Jun 14;315(22):2397–8. doi: 10.1001/jama.2016.7041. [DOI] [PubMed] [Google Scholar]
- 5.Federal Register. Medicare program; merit-based incentive payment system and alternative payment model incentive under the physician fee schedule, and criteria for physician-focused payment models [Internet] [cited 2016 Oct 25]. Available from: http://www.federalregister.gov/a/2016-10032. [PubMed]
- 6.Centers for Medicare & Medicaid Services, Department of Health and Human Services. Physician groups receive upward, neutral, or downward adjustments to their Medicare payments in 2016 based on their performance on quality and cost efficiency measures [Internet] [cited 2016 Oct 25]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/2016-VM-Overview-PDF-Memo.pdf.
- 7.Blayney DW, McNiff K, Hanauer D, Miela G, Markstrom D, Neuss M. Implementation of the Quality Oncology Practice Initiative at a university comprehensive cancer center. J Clin Oncol Off J Am Soc Clin Oncol. 2009 Aug 10;27(23):3802–7. doi: 10.1200/JCO.2008.21.6770. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer Facts and Figures 2016 [Internet] 2016 Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf.
- 9.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol Off J Am Soc Clin Oncol. 2009 Jun 10;27(17):2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 10.Smith BD, Jiang J, McLaughlin SS, Hurria A, Smith GL, Giordano SH, et al. Improvement in breast cancer outcomes over time: are older women missing out? J Clin Oncol Off J Am Soc Clin Oncol. 2011 Dec 10;29(35):4647–53. doi: 10.1200/JCO.2011.35.8408. [DOI] [PubMed] [Google Scholar]
- 11.Rauh-Hain JA, Pepin KJ, Meyer LA, Clemmer JT, Lu KH, Rice LW, et al. Management for Elderly Women With Advanced-Stage, High-Grade Endometrial Cancer. Obstet Gynecol. 2015 Dec;126(6):1198–206. doi: 10.1097/AOG.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 12.King JC, Zenati M, Steve J, Winters SB, Bartlett DL, Zureikat AH, et al. Deviations from Expected Treatment of Pancreatic Cancer in Octogenarians: Analysis of Patient and Surgeon Factors. Ann Surg Oncol. 2016 Jul 26; doi: 10.1245/s10434-016-5456-0. [DOI] [PubMed] [Google Scholar]
- 13.Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015 Jan;193(1):95–102. doi: 10.1016/j.juro.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 14.Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol Off J Am Soc Clin Oncol. 2007 Dec 10;25(35):5570–7. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 15.Lewis CM, Hessel AC, Roberts DB, Guo YZ, Holsinger FC, Ginsberg LE, et al. Prereferral head and neck cancer treatment: compliance with national comprehensive cancer network treatment guidelines. Arch Otolaryngol Head Neck Surg. 2010 Dec;136(12):1205–11. doi: 10.1001/archoto.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland GM, Chang GJ, Haynes AB, Chiang Y-J, Chagpar R, Xing Y, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013 Apr 15;119(8):1593–601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson BK, Martin JY, Shah MM, Straughn JM, Leath CA. Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014 May;133(2):142–6. doi: 10.1016/j.ygyno.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duggan KJ, Descallar J, Vinod SK. Application of Guideline Recommended Treatment in Routine Clinical Practice: A Population-based Study of Stage I-IIIB Non-small Cell Lung Cancer. Clin Oncol R Coll Radiol G B. 2016 Oct;28(10):639–47. doi: 10.1016/j.clon.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Social Security Administration. Actuarial Life Table [Internet] [cited 2016 Oct 25]. Available from: https://www.ssa.gov/OACT/STATS/table4c6.html.
- 20.NCCN Clinical Practice Guidelines in Oncology. Version 2. 2016 [Internet]. National Comprehensive Cancer Network; 2016. Available from: www.nccn.org.
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993 Oct;46(10):1075–1079. 1090. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 24.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 25.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002 Aug;40(8 Suppl):IV-26-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 26.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010 May 1;28(13):2191–7. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 27.Shirvani SM, Jiang J, Chang JY, Welsh J, Likhacheva A, Buchholz TA, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014 Dec;149(12):1244–53. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo J, Giordano SH, Smith BD, Shaitelman SF, Smith GL. Contemporary Toxicity Profile of Breast Brachytherapy Versus External Beam Radiation After Lumpectomy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2016 Mar 15;94(4):709–18. doi: 10.1016/j.ijrobp.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Smith BD, Jiang J, Shih Y-C, Giordano SH, Huo J, Jagsi R, et al. Cost and Complications of Local Therapies for Early-Stage Breast Cancer. J Natl Cancer Inst. 2017 Jan;109(1) doi: 10.1093/jnci/djw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005 Mar 2;293(9):1073–81. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 31.Corre R, Greillier L, Le Caër H, Audigier-Valette C, Baize N, Bérard H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol Off J Am Soc Clin Oncol. 2016 May 1;34(13):1476–83. doi: 10.1200/JCO.2015.63.5839. [DOI] [PubMed] [Google Scholar]
- 32.Presley CJ, Gross CP, Lilenbaum RC. Optimizing Treatment Risk and Benefit for Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Right Treatment for the Right Patient. J Clin Oncol Off J Am Soc Clin Oncol. 2016 May 1;34(13):1438–42. doi: 10.1200/JCO.2015.65.9599. [DOI] [PubMed] [Google Scholar]
- 33.Quoix E, Zalcman G, Oster J-P, Westeel V, Pichon E, Lavolé A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet Lond Engl. 2011 Sep 17;378(9796):1079–88. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 34.Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud-Caspar I, Schäfer P, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol Off J Am Soc Clin Oncol. 2003 Oct 1;21(19):3580–7. doi: 10.1200/JCO.2003.02.046. [DOI] [PubMed] [Google Scholar]
- 35.Yood MU, Owusu C, Buist DSM, Geiger AM, Field TS, Thwin SS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008 Jan;206(1):66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol Off J Am Soc Clin Oncol. 2010 Apr 20;28(12):2038–45. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan JL, Reed MW, Wyld L. Primary endocrine therapy as a treatment for older women with operable breast cancer - a comparison of randomised controlled trial and cohort study findings. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014 Jun;40(6):676–84. doi: 10.1016/j.ejso.2014.02.224. [DOI] [PubMed] [Google Scholar]
- 38.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009 May 14;360(20):2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosaka K, Shibata K, Utsumi F, Yoshida K, Niimi K, Sekiya R, et al. Feasibility and benefit of concurrent chemoradiotherapy for elderly patients with uterine cervical cancer. Tumori. 2016 Jul;19:0. doi: 10.5301/tj.5000530. [DOI] [PubMed] [Google Scholar]
- 40.De Ruysscher D, van Loon J. Radical Radiotherapy for Locally Advanced Non-small Cell Lung Cancer: When Should Concurrent Chemoradiotherapy Not Be Used? Clin Oncol R Coll Radiol G B. 2016 Nov;28(11):708–11. doi: 10.1016/j.clon.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Nanda RH, Liu Y, Gillespie TW, Mikell JL, Ramalingam SS, Fernandez FG, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer. 2015 Dec 1;121(23):4222–30. doi: 10.1002/cncr.29640. [DOI] [PubMed] [Google Scholar]
- 42.Giugliano FM, Falivene S, Esposito E, Di Franco R, D’Aiuto M, Lanza F, et al. Short-course radiotherapy in elderly women with breast cancer: Comparison by age, comorbidity index and toxicity. Int J Surg Lond Engl. 2016 May 30; doi: 10.1016/j.ijsu.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 43.Mancini BR, Park HS, Harder EM, Rutter CE, Corso CD, Decker RH, et al. Elderly patients undergoing SBRT for inoperable early-stage NSCLC achieve similar outcomes to younger patients. Lung Cancer Amst Neth. 2016 Jul;97:22–7. doi: 10.1016/j.lungcan.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Jagsi R, Falchook AD, Hendrix LH, Curry H, Chen RC. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys. 2014 Dec 1;90(5):1001–9. doi: 10.1016/j.ijrobp.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 45.Smith BD, Haffty BG, Buchholz TA, Smith GL, Galusha DH, Bekelman JE, et al. Effectiveness of radiation therapy in older women with ductal carcinoma in situ. J Natl Cancer Inst. 2006 Sep 20;98(18):1302–10. doi: 10.1093/jnci/djj359. [DOI] [PubMed] [Google Scholar]
- 46.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006 May 17;98(10):681–90. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 47.Mamtani A, Gonzalez JJ, Neo D, Slanetz PJ, Houlihan MJ, Herold CI, et al. Early-Stage Breast Cancer in the Octogenarian: Tumor Characteristics, Treatment Choices, and Clinical Outcomes. Ann Surg Oncol. 2016 Jun 30; doi: 10.1245/s10434-016-5368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004 Mar;92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 49.Karuturi M, VanderWalde N, Muss H. Approach and Management of Breast Cancer in the Elderly. Clin Geriatr Med. 2016 Feb;32(1):133–53. doi: 10.1016/j.cger.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Smith GL, Smith BD. Radiation treatment in older patients: a framework for clinical decision making. J Clin Oncol Off J Am Soc Clin Oncol. 2014 Aug 20;32(24):2669–78. doi: 10.1200/JCO.2014.55.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.