Abstract
Background
The connecting peptide in insulin has been associated with cardiovascular risk and overall mortality in the adult population. However, its early determinants are unknown. Assess the association of exposures during pregnancy, delivery, and childhood with C-peptide among 22–23 years old individuals prospectively followed since birth, in a southern Brazilian city.
Methods
In 1982, all hospital births in the city were identified and those livebirths whose families lived in the urban area were evaluated (n = 5914). The 1982 Pelotas Birth Cohort has prospectively followed these subjects at different moments. In this study, we evaluated the association of C-peptide with exposures occurring during pregnancy, delivery and childhood. In the 22–23 years follow-up visit, we tried to follow the whole cohort and the subjects were interviewed, examined and donated a blood sample. C-peptide was measured using the chemiluminescence immunoassay technique (Immulite®–Siemens, Germany).
Results
In the 22–23 years visit, 4297 subjects were interviewed and the C-peptide was measured in 3807. The geometric mean of C-peptide was 0.83 ng/mL and the mean was higher among women. In the adjusted analysis, C-peptide was positively associated with family income at birth, lower among children of non-white mothers (0.90; CI95% 0.84–0.96), higher among females (1.22; CI95% 1.16–1.28), and positively associated with rapid weight gain between two and four years of age (1.18; CI95% 1.05–1.32).
Conclusion
Family income at birth, non-white maternal skin color, and rapid weight gain between two and four years of age were associated with high levels of C-peptide.
Electronic supplementary material
The online version of this article (doi:10.1186/s12872-017-0613-3) contains supplementary material, which is available to authorized users.
Keywords: Pregnancy, Parturition, Growth, C-peptide, Young adults
Backrgound
In 2012, cardiovascular diseases were the main cause of death worldwide [1], accounting for 17.5 million deaths or 46.2% of the deaths due to non-communicable diseases [2, 3]. It is estimated that, by 2020, the higher prevalence of risk factors, such as obesity, diabetes, and dyslipidemia [4] will lead to an increase in ischemic heart disease by up to 30% among women and 60% among men in high-income countries and by 120% and 137% in mid- and low-income countries, respectively [5].
Secreted by β-cells from isles of Langerhans, the connecting peptide, called C-peptide, is released at equimolar amounts as insulin [6]. It was initially considered an inert substance in the pro-insulin molecular chain. But, evidence suggests that C-peptide may play a role at the cell-membrane level, including the endothelium and kidney cells [7–10].
Vasic et al. pointed that C-peptide would have pro-inflammatory effects on different tissues, such as blood vessels and kidney glomeruli [11]. Moreover, Ronnemaa et al. found that coronary artery disease in insulin-dependent diabetics was associated with high C-peptide levels (≥ 0.20 nmol/L) compared with non-diabetics [12]. Donatelli et al. reported higher C-peptide levels among obese hypertensive diabetics compared to obese and obese-hypertensive individuals [13]. Cabrera de Leon et al. reported that among persons with insulin resistance, C-peptide would be positively associated with the risk of myocardial infarction (RR 2.8; CI95% 1.1–6.9) and coronary artery disease (RR 2.4; CI95% 1.3–4.6) [14]. These studies have not been able to evaluate whether C-Peptide is a marker of insulin resistance or an independent cardiovascular risk factor.
Studies on life cycle epidemiology have reported that exposures during gestation or in the first years of life would be associated with human capital and the development of non-communicable chronic diseases in adulthood [15–20]. To our knowledge, the association of early exposures with C-peptide has not been evaluated.
The present study was aimed at assessing the association of exposures during pregnancy, delivery, and childhood with C-peptide among 22–23 years old individuals who have been prospectively followed since birth, in a southern Brazilian city.
Methods
In 1982, the maternity hospitals in Pelotas, RS, Brazil, were visited daily and all births were identified. The live births whose families lived in the urban area of the city (n = 5914) were examined and their mothers interviewed. The 1982 Pelotas Birth Cohort has prospectively followed these individuals at different ages [21, 22]. Between October 2004 and August 2005, an attempt was made to follow all cohort members, who were interviewed at home and invited to visit the laboratory for collection of blood samples. In the present study, we included all subjects who donated a blood sample.
The association of the following exposures related to gestation, delivery, and childhood with the outcome (C-peptide at 22–23 years) was evaluated:
family income at birth in multiples of minimum wage;
maternal education at birth in completed years of schooling;
sex;
maternal age at birth;
maternal skin color;
maternal weight gain during pregnancy (estimated from pregestational weight and weight at childbirth), categorized as adequate or inadequate according to the parameters of the Institute of Medicine [23];
maternal smoking during pregnancy;
maternal morbidity during pregnancy (gestational diabetes and hypertension);
type of delivery (vaginal and cesarean section);
birthweight, measured by the hospital staff with pediatric scales calibrated
weekly by the research team;
breastfeeding duration, assessed in the visits at two and four years of age. The
present study used the information closest to the age at weaning;
weight gain rate between 0 and 2 and 2–4 years old, assessed based on changes in weight for age Z-score. An increase equal to or above 0.67 standard deviations (SD) was employed to define the occurrence of rapid weight gain [24].
C-peptide was measured using the chemiluminescence immunoassay technique.
(Immulite®–Siemens, Germany) [25, 26].
The statistical analysis was performed in the software Stata version 13.1. Since the C-peptide distribution was asymmetrical, the variable was transformed into logarithm and the geometric mean was obtained from the inverse transformation of its logarithm. The multiple linear regression followed a conceptual model with five hierarchical levels. The first level features the sociodemographic variables: family income, maternal education, maternal age, maternal skin color, and sex. The second level included maternal smoking and weight gain during pregnancy, the third level included the occurrence of diabetes and hypertension during pregnancy, and the fourth level included the variables related to delivery and birth conditions, type of delivery, birthweight, and intrauterine growth restriction. The fifth level included weight gain between birth and two years and between two and four years of age. At each hierarchical level, backward selection was carried out and the variables with p < 0.20 were maintained in the model. As the blood samples were collected at random and because the time of fasting is associated with C-peptide, all analyses were adjusted to time of fasting of each participant. Later, residual analyses were performed in order to check for normality, homoscedasticity, and independence of terms.
The study was approved by the Research Ethics Committee of the Medical School of the Federal University of Pelotas (UFPel) under protocol no. OF. 16/12 and the interviews and blood collections were carried out after the participants provided written consent.
Results
In the 2004–5 visit, 4297 individuals were interviewed, which added to the 282 deaths identified among the participants of the cohort, represented a follow-up rate of 77.4% of the original cohort. C-peptide was assessed in 3807 subjects. Table 1 describes the population studied according to its socioeconomic characteristics and conditions related to gestation and childhood, 50.4% of the families earned between 1.1 and 3.0 minimum wage, 43.3% of the mothers had between five and eight years of schooling, 82% reported being white, 35% smoked during pregnancy, 0.3% had diabetes and 5.4% had hypertension during pregnancy. With respect to birthweight, 7% were low birthweight. About one third of the participants had rapid weight gain in the first two years of life and 5%, between two and four years of age.
Table 1.
Participants’ characteristics
| Variables studied | Study participants | |
|---|---|---|
| n | % | |
| Sociodemographic conditions | ||
| Income (multiples of minimum wage) | ||
| <1 | 769 | 20.3 |
| 1.1–3.0 | 1908 | 50.4 |
| 3.1–6.0 | 714 | 18.8 |
| > 6.0 | 39 | 10.5 |
| Mother’s education (years) | ||
| 0–4 | 1266 | 33.3 |
| 5–8 | 1645 | 43.3 |
| 9–11 | 410 | 10.7 |
| ≥ 12 | 481 | 12.7 |
| Gestation conditions | ||
| Mother’s age (years) | ||
| < 20 | 549 | 27.9 |
| 20–29 | 2213 | 48.9 |
| ≥ 30 | 1045 | 23.2 |
| Mother’s skin color | ||
| White | 3114 | 81.8 |
| Non-white | 692 | 18.2 |
| Maternal smoking | ||
| Yes | 1347 | 35.4 |
| No | 2460 | 64.6 |
| Maternal diabetes | ||
| Yes | 13 | 0.3 |
| No | 3794 | 99.7 |
| Maternal hypertension | ||
| Yes | 216 | 5.4 |
| No | 3588 | 94.6 |
| Maternal weight gain | ||
| Insufficient | 960 | 29.8 |
| Adequate | 1188 | 36.9 |
| Excessive | 1070 | 33.3 |
| Childbirth conditions | ||
| Type of childbirth | ||
| Vaginal | 2756 | 72.4 |
| Cesarean section | 1051 | 27.6 |
| Birthweight (grams) | ||
| < 2500 | 267 | 7.0 |
| 2500–2999 | 917 | 24.1 |
| 3000–3499 | 1449 | 38.1 |
| ≥ 3500 | 1173 | 30.8 |
| Intrauterine growth restriction | ||
| AGA | 2590 | 85.2 |
| SGA | 448 | 14.8 |
| Childhood conditions | ||
| Breastfeeding (months) | ||
| < 1 | 801 | 21.8 |
| 1–2.9 | 958 | 26.0 |
| 3–5.9 | 834 | 22.6 |
| ≥ 6 | 1091 | 29.6 |
| Increased weight gain rate (0–2 years old) | ||
| Yes | 973 | 34.5 |
| No | 1845 | 65.5 |
| Increased weight gain rate (2–4 years old) | ||
| Yes | 157 | 4.8 |
| No | 3104 | 95.2 |
SGA Small for the gestational age, AGA Adequate for the gestational age
Table 2 shows the geometric mean of C-peptide according to the exposure variables. Because sex did not modify the associations, the analyses were not stratified by sex. The geometric mean of C-peptide was positively associated with family income (p < 0.001). Regarding maternal education, the mean C-peptide values increased until the group with nine to 11 years of schooling (0.89; CI95% 0.83–0.96) and was lower among the children of non-white mothers (0.73; CI95% 0.69–0.77).
Table 2.
Geometric mean of C-peptide according to the exposure variables
| Variables | C-peptide geometric mean | CI95% | p value |
|---|---|---|---|
| Sex | < 0.001 | ||
| Male | 0.76 | 0.73–0.78 | |
| Female | 1.19 | 1.13–1.25 | |
| Income (multiples of minimum wage) | < 0.001* | ||
| < 1 | 0.76 | 0.72–0.80 | |
| 1.1–3.0 | 0.81 | 0.86–0.84 | |
| 3.1–6.0 | 0.90 | 0.85–0.95 | |
| > 6.0 | 0.88 | 0.81–0.95 | |
| Mother’s education (years) | 0.024 | ||
| 0–4 | 0.79 | 0.76–0.82 | |
| 5–8 | 0.83 | 0.79–0.85 | |
| 9–11 | 0.89 | 0.83–0.96 | |
| ≥ 12 | 0.85 | 0.79–0.91 | |
| Mother’s age (years) | 0.872 | ||
| < 20 | 0.81 | 0.77–0.87 | |
| 20–29 | 0.82 | 0.80–0.85 | |
| ≥ 30 | 0.83 | 0.80–0.87 | |
| Mother’s skin color | < 0.001 | ||
| White | 0.85 | 0.83–0.87 | |
| Non-white | 0.73 | 0.69–0.77 | |
| Maternal smoking | 0.925 | ||
| Yes | 0.83 | 0.79–0.86 | |
| No | 0.83 | 0.80–0.85 | |
| Maternal diabetes | 0.503 | ||
| Yes | 0.72 | 0.40–0.77 | |
| No | 0.83 | 0.81–0.85 | |
| Maternal hypertension | 0.909 | ||
| Yes | 0.83 | 0.75–0.91 | |
| No | 0.82 | 0.80–0.84 | |
| Maternal weight gain during pregnancy | 0.349 | ||
| Insufficient | 0.83 | 0.77–0.84 | |
| Adequate | 0.82 | 0.74–0.87 | |
| Excessive | 0.86 | 0.80–0.88 | |
| Type of childbirth | 0.616 | ||
| Vaginal | 0.82 | 0.80–0.84 | |
| Cesarean section | 0.83 | 0.79–0.87 | |
| Birthweight (grams) | 0.897 | ||
| < 2500 | 0.81 | 0.74–0.89 | |
| 2500–2999 | 0.82 | 0.78–0.86 | |
| 3000–3499 | 0.83 | 0.80–0.86 | |
| ≥ 3500 | 0.82 | 0.78–0.85 | |
| Intrauterine growth restriction | 0.135 | ||
| Yes | 0.80 | 0.74–0.85 | |
| No | 0.84 | 0.81–0.86 | |
| Breastfeeding (months) | 0.557 | ||
| < 1 | 0.81 | 0.76–0.85 | |
| 1–2.9 | 0.84 | 0.80–0.88 | |
| 3–5.9 | 0.81 | 0.77–0.85 | |
| ≥ 6 | 0.83 | 0.79–0.87 | |
| Rapid growth (0–2 years old) | 0.238 | ||
| Yes | 0.82 | 0.79–0.85 | |
| No | 0.85 | 0.81–0.89 | |
| Increased weight gain rate (2–4 years old) | 0.124 | ||
| Yes | 0.83 | 0.81–0.85 | |
| No | 0.91 | 0.80–1.03 | |
*Kruskal-Wallis
Table 3 shows the adjusted analyses. In the first hierarchical level, family income at birth, skin color and sex remained in the model. Family income was positively associated with C-peptide. Children of non-white mothers had lower C-peptide levels (0.90; CI95% 0.84–0.96), while females had higher C-peptide mean (1.22; CI95% 1.16–1.28).
Table 3.
Analysis of the independent variables and C-peptide adjusted for fasting time
| Hierarchical level | Variables | Analysis 1 | Analysis 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | β | CI95% | n | β | CI95% | ||||
| HL I | Income (multiples of minimum wage) | 3660 | 3139 | ||||||
| < 1 | 1.00 | 1.00 | |||||||
| 1.1–3.0 | 1.04 | 0.98 | 1.10 | 1.03 | 0.96 | 1.09 | |||
| 3.1–6.0 | 1.14 | 1.06 | 1.23 | 1.12 | 1.03 | 1.22 | |||
| > 6.0 | 1.12 | 1.03 | 1.23 | 1.07 | 0.98 | 1.19 | |||
| Mother’s education (years) | 3673 | ||||||||
| < 4 | 1.00 | ||||||||
| 5–8 | 1.03 | 0.97 | 1.09 | ||||||
| 9–11 | 1.09 | 1.00 | 1.18 | ||||||
| ≥ 12 | 1.05 | 0.97 | 1.13 | ||||||
| Mother’s age (years) | 3678 | ||||||||
| < 20 | 1.00 | ||||||||
| 20–29 | 1.00 | 0.93 | 1.07 | ||||||
| ≥ 30 | 1.01 | 0.94 | 1.09 | ||||||
| Mother’s skin color | 3677 | ||||||||
| White | 1.00 | 1.00 | |||||||
| Non-white | 0.88 | 0.83 | 0.94 | 0.90 | 0.84 | 0.96 | |||
| Neonate sex | 3678 | ||||||||
| Male | 1.00 | 1.00 | |||||||
| Female | 1.20 | 1.14 | 1.25 | 1.22 | 1.16 | 1.28 | |||
| HL II | Maternal smoking | 3678 | |||||||
| No | 1.00 | ||||||||
| Yes | 1.01 | 0.96 | 1.06 | ||||||
| Maternal weight gain during pregnancy | 3109 | ||||||||
| Adequate | 1.00 | ||||||||
| Insufficient | 0.99 | 0.93 | 1.05 | ||||||
| Excessive | 1.02 | 0.96 | 1.09 | ||||||
| HL III | Maternal diabetes | 3678 | |||||||
| No | 1.00 | ||||||||
| Yes | 0.84 | 0.57 | 1.24 | ||||||
| Maternal hypertension | 3675 | ||||||||
| No | 1.00 | ||||||||
| Yes | 1.00 | 0.91 | 1.11 | ||||||
| HL IV | Type of childbirth | 3678 | |||||||
| Vaginal | 1.00 | ||||||||
| Cesarean section | 1.01 | 0.96 | 1.06 | ||||||
| Intrauterine growth restriction | 3678 | ||||||||
| No | 1.00 | ||||||||
| Yes | 0.98 | 0.91 | 1.06 | ||||||
| Birthweight (grams) | 3677 | ||||||||
| < 2500 | 1.00 | ||||||||
| 2500–2999 | 0.99 | 0.90 | 1.10 | ||||||
| 3000–3499 | 0.99 | 0.91 | 1.10 | ||||||
| ≥ 3500 | 0.97 | 0.88 | 107 | ||||||
| HL V | Breastfeeding (months) | 3560 | |||||||
| < 1 | 1.00 | ||||||||
| 1–2.9 | 1.02 | 0.95 | 109 | ||||||
| 3–5.9 | 0.99 | 0.92 | 1.08 | ||||||
| ≥ 6 | 1.03 | 0.97 | 1.10 | ||||||
| Increased weight gain rate (0–2 years old) | 2718 | ||||||||
| No | 1.00 | ||||||||
| Yes | 1.04 | 0.98 | 1.10 | ||||||
| Increased weight gain rate (2–4 years old) | 3152 | ||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 1.13 | 1.01 | 1.27 | 1.18 | 1.05 | 1.32 | |||
Multiple linear regression. Analysis 1: independent variable; C-peptide and fasting time. Analysis 2: Hierarchical level I (HL I): adjusted for sociodemographic variables and fasting time; HL II to V: hierarchically adjusted for variables selected from the previous level and other variables of subsequent levels and fasting time
The variables in the second (maternal smoking and maternal weight gain during pregnancy), third (maternal diabetes and hypertension during pregnancy), and fourth (type of delivery, intrauterine growth restriction, birthweight) levels did not reach the significance level (p < 0.20) to remain in the model. After adjusting for the variables at a higher hierarchical level, rapid weight gain between two and four years was positively associated with C-peptide (1.18; CI95% 1.05–1.32), while birthweight and weight gain in the first two years of life were not associated with C-peptide Fig. 1.
Fig. 1.
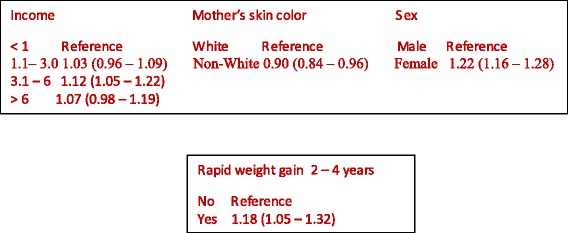
Hierarchical model for C-peptide
Discussion
In a cohort followed since birth in a city in southern Brazil, family income at birth was directly associated with C-peptide at 22-23 years old. Mean C-peptide was higher among women, among those born in families with income between three and six times the minimum wage, and among the children of white mothers. Increased weight gain rate between two and four years old was also positively associated with serum C-peptide levels, while birthweight and weight gain in the first two years of life had no association.
Given the long follow-up period, the low percentages of losses, and the fact there were no great differences in the follow-up rates according to socioeconomic characteristics or gestation and birth, the possibility of selection bias is small (Additional file 1: Table S1). Moreover, the information on the exposures was collected in childhood, close to the events, which lowers the possibility of error in information collection and the possibility of error in the nondifferential classification.
On the other hand, C-peptide was measured in randomly collected blood samples. In order to keep a possible association between fasting time and the exposures of interest from introducing bias into the association measures, the analyses were adjusted for fasting time.
C-peptide levels were positively associated with family income at birth, suggesting that exposure to a higher socioeconomic level during gestation is related to higher C-peptide levels in adulthood. This association can be explained by differences in exposure to contemporary risk factors, such as diet. Another study on this same cohort observed that the consumption of ultra-processed foods was higher among individuals with higher socioeconomic level, which, in turn, would be associated with higher risk of obesity [27].
The children of non-white mothers had lower C-peptide levels. Such findings may derive from the lower socioeconomic level among nonwhite individuals [28]. Aiming to investigate the possibility of confound by income in the association between maternal skin color and C-peptide, the analyses were adjusted for family income. However, even after adjustment, C-peptide levels were lower among the children of non-white mothers (0.90; CI95% 0.85-0.96), which suggests that skin color independently influences family income in the determination of serum C-peptide levels.
In the present study, women had the highest C-peptide means in adulthood, contrasting with the results by Li et al., who reported similar means between the sexes [29].
Increased weight gain rate between two and four years old was associated with higher serum C-peptide levels in adulthood. A study on the same cohort showed that increased weight gain rate in the first years old life reduces morbidity-mortality among small-for-the-gestational-age (SGA) children [30]. On the other hand, other authors reported that rapid recovery of growth may increase the risk of developing cardiometabolic diseases in adulthood [31–33]. However, a study based on data from five cohorts in developing countries observed that the relative weight gain in the first four years of life was associated with higher risk of overweight and high arterial blood pressure in adulthood, but not with plasma glycemia [34]. The present findings, however, indicate that early growth would not be associated with C-peptide in adulthood, while rapid growth between two and four years old would increase C-peptide at 22-23 years old, which suggests that late increased weight gain rate in childhood would impact the glucose-insulin metabolism in adulthood.
Conclusion
The results in the present study suggest that adults from families with income above three times the minimum wage, children of non-white mothers, and those who had increased weight gain rate between the second and fourth years of life had higher serum C-peptide levels at 22-23 years old. Further studies may contribute to confirming.
Acknowledgements
This study was funded by the Wellcome Trust; the International Development Research Center (Canada); the Brazilian National Research Council (CNPq); Rio Grande do Sul State Research Support Foundation (FAPERGS); and the Brazilian Ministry of Health. The study is based on data from the study “Pelotas Birth Cohort, 1982” done by the Postgraduate Program in Epidemiology at Universidade Federal de Pelotas, with the collaboration of the Brazilian Public Health Association (ABRASCO). From 2004 to 2013, the Wellcome Trust supported the 1982 birth cohort study. The International Development Research Center, World Health Organization, Overseas Development Administration, European Union, National Support Program for Centers of Excellence (PRONEX), CNPq, FAPERGS, and the Brazilian Ministry of Health supported previous phases of the study.
Ethics approval and consente to participate
The study complies with the Declaration of Helsinki and was approved by the ethics committee of the Federal University of Pelotas (UFPel), Brazil, of. 16/2012. All individuals gave their consent to participate prior to inclusion.
Funding
The 1982 birth cohort study was funded by the Wellcome Trust initiative titled Major Awards for Latin America on Health Consequences of Population Change. The doctorate was funded by CAPES, an institution of the Brazilian government for the improvement of human resources through DINTER – Inter-institution doctorate between the Federal University of Pelotas (UFPel) and the Federal University of Espírito Santo (UFES).
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Consent for publication
The tables of images published in the article will be freely available on the internet and can be seen by the general public. These images, videos and text may also appear on other websites or printed, may be translated into other languages or used for commercial purposes.
Additional file
Characteristics of the participants in the 1982 cohort and sample of individuals analyzed in the study. (DOC 67 kb)
Authors’ contributions
RLMA participated in the conception and design of the study, data analysis, interpretation of study findings, the drafting and review. DPG participated in the analysis of the data, the interpretation of the study findings. IOOl participated in the processing of laboratory tests and the study’s findings, the drafting and review. BLH participated in the design and study design, data collection, interpretation of the review findings of the study and writing of the article. All authors read and approved the final manuscript.
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12872-017-0613-3) contains supplementary material, which is available to authorized users.
Contributor Information
Romildo Luiz Monteiro Andrade, Email: rlmandrade@hotmail.com.
Denise Petrucci Gigante, Email: denisepgigante@gmail.com.
Isabel Oliveira de Oliveira, Email: isabel.ufpel@gmail.com.
Bernardo Lessa Horta, Email: blhorta@gmail.com.
References
- 1.World Health Organization. Noncommunicable diseases progress monitor, 2015. Geneva: WHO; 2015. ISBN 978 92 4 150945 9. http://apps.who.int/iris/bitstream/10665/184688/1/9789241509459_eng.pdf. Accessed 30 June 2017.
- 2.World Health Organization. Noncommunicable diseases country profiles 2014. Geneva: WHO; 2014. ISBN 978 92 4 150750 9. http://apps.who.int/iris/bitstream/10665/128038/1/9789241507509_eng.pdf?ua=1. Accessed 30 June 2017.
- 3.World Health Organization. Global status report on noncommunicable diseases 2014. Geneva: WHO; 2014. ISBN 978 92 4 156485 4. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf. Accessed 30 June 2017.
- 4.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–53. http://circ.ahajournals.org/content/104/22/2746.long. Accessed 30 June 2017. [DOI] [PubMed]
- 5.Avezum A, Maia LN, Nakazone M. Cenário das doenças cardiovasculares no mundo moderno. In: Timerman A, Bertolami MC, JFM F, editors. Manual de cardiologia. São Paulo: Atheneu; 2012. pp. 1–5. [Google Scholar]
- 6.Steiner DF, Cunningham D, Spigelman L, et al. Insulin biosynthesis: evidence for a precursor. Science. 1967;157(3789):697–700. CMAJ 185(9):E402–408. http://science.sciencemag.org/content/157/3789/697.long. Accessed 30 June 2017. [DOI] [PubMed]
- 7.Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004;29(3):231–8. PMID:15367890. https://www.ncbi.nlm.nih.gov/pubmed/15367890. [DOI] [PubMed]
- 8.Wahren J, Ekberg K, Jornvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50(3):503–9. https://link.springer.com/article/10.1007/s00125-006-0559-y. [DOI] [PubMed]
- 9.Marx N. C-peptide as a mediator of lesion development in early diabetes: a novel hypothesis. Trends Cardiovasc Med. 2007;18(2):67–71. doi: 10.1016/j.tcm.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Walcher D, Nikolaus M. C-peptide in the vessel wall. Rev Diabet Stud. 2009;6(3):180–186. doi: 10.1900/RDS.2009.6.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasic D, Marx N, Sukhova G, et al. C-peptide promotes lesion development in a mouse model of arteriosclerosis. J Cell Mol Med. 2012;16(4):927–935. doi: 10.1111/j.1582-4934.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronnemaa T, Laakso M, Puukka P, et al. Atherosclerotic vascular disease in middle-aged, insulin-treated, diabetic patients. Association with endogenous insulin secretion capacity. Arteriosclerosis. 1988;8(3):237–244. doi: 10.1161/01.ATV.8.3.237. [DOI] [PubMed] [Google Scholar]
- 13.Donatelli M, Scarpinato A, Bucalo ML, et al. Stepwise increase in plasma insulin and c-peptide concentrations in obese, in obese hypertensive, and in obese hypertensive diabetic subjects. Diabetes Res. 1991;17(3):125–9. http://europepmc.org/abstract/med/1841027. Accessed 30 June 2017. [PubMed]
- 14.Cabrera De Leon A, Oliva Garcia JG, Marcelino Rodriguez I, et al. C-peptide as a risk factor of coronary artery disease in the general population. Diab Vasc Dis Res. 2015;12(3):199–207. doi: 10.1177/1479164114564900. [DOI] [PubMed] [Google Scholar]
- 15.Victora CG, Horta BL. Loret de Mola C, Quevedo L, Pinheiro RT, Gigante DP, et al. association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3(4):e199–e205. doi: 10.1016/S2214-109X(15)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Silveira VMF, Horta BL. Birth weight and metabolic syndrome in adults: meta-analysis. Rev Saude Publica. 2008;42(1):10–18. http://www.revistas.usp.br/rsp/article/view/32375/34581. Accessed 30 June 2017. [DOI] [PubMed]
- 17.Restrepo MC, Horta BL, Gigante DP. Perfil lipídico na adolescência: efeito de exposições intra-uterinas. Cadernos Saude Publica. 2009;25(11):2345–2353. doi: 10.1590/S0102-311X2009001100005. [DOI] [PubMed] [Google Scholar]
- 18.Parlee SD, OA MD. Maternal nutrition and risk of obesity in offspring: the trojan horse of developmental plasticity. Biochim Biophys Acta. 2014;1842(3):495–506. doi: 10.1016/j.bbadis.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;8;320(7240):967-71. Erratum in: BMJ 2000 May 6;320(7244):1244. PubMed PMID: 10753147; PubMed Central PMCID: PMC27335. http://www.bmj.com/content/320/7240/967. [DOI] [PMC free article] [PubMed]
- 21.Barros FC, Victora CG, Horta BL, et al. Metodologia do estudo da coorte de nascimentos de 1982 a 2004–5, Pelotas, RS. Rev Saude Publica. 8;42(Suppl2):7–15. http://www.scielo.br/pdf/rsp/v42s2/en_7000.pdf. Accessed 30 June 2017.
- 22.Victora CG, Barros FC. Cohort Profile: The 1982 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 2006;35(2):237–242. https://academic.oup.com/ije/article/35/2/237/694731/Cohort-Profile-The-1982-Pelotas-Brazil-Birth. http://www.ncbi.nlm.nih.gov/pubmed/18420730. Accessed 30 June 2017. [DOI] [PubMed]
- 23.Institute of Medicine. Nutrition during pregnancy. Washington: National Academy Press; 1990. https://www.ncbi.nlm.nih.gov/books/NBK235228/pdf/Bookshelf_NBK235228.pdf. Accessed 30 June 2017.
- 24.de Onis M, Onyango AW. WHO child growth standards. Lancet. 2008;371(9608):204. doi:10.1016/S0140-6736(08)60131-2. PubMed PMID: 18207015. http://www.sciencedirect.com/science/article/pii/S0140673608601312?via%3Dihub. Accessed 12 Sept 2016. [DOI] [PubMed]
- 25.C-peptide (CpS) Assay Summary, ADVIA Centaur and ADVIA Centaur XP Systems 129042 Rev. F, 2009-04 CpS. http://labmed.ucsf.edu/labmanual/db/resource/proc-Centaur_C-Peptide_Rev_F.pdf. Accessed 30 June 2017.
- 26.Little RR, Rohlfing CL, Tennill AL, et al. Standardization of C-peptide measurements. Clin Chem. 2008;54(6):1023–1026. doi: 10.1373/clinchem.2007.101287. [DOI] [PubMed] [Google Scholar]
- 27.Boersma B, Wit JM. Catch-up growth. Endocr Rev. 1997;18(5):646–661. doi: 10.1210/edrv.18.5.0313. [DOI] [PubMed] [Google Scholar]
- 28.Dulloo AG, Jacquet J, Seydoux J, et al. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (London). 2006;30(Suppl4):S23–35. http://www.nature.com/ijo/journal/v30/n4s/full/0803516a.html. [DOI] [PubMed]
- 29.JCK W, Dumith SC, Ekelund U, et al. Associations of intrauterine and postnatal weight and length gains with adolescent body composition: prospective birth cohort study from Brazil. J Adolesc Health. 2012;51(Suppl6):S58–S64. doi: 10.1016/j.jadohealth.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekelund U, Ong K, Linné Y, Neovius M, Brage S, Dunger DB, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am J Clin Nutr. 2006; 83: 324–330. http://ajcn.nutrition.org/content/83/2/324.long. Accessed 12 Sept 2016. [DOI] [PubMed]
- 31.Situação de saúde Indicadores de mortalidade por causas Indicador nº 020208 - Taxa de mortalidade específica por diabete melito na população de 15 anos e mais, por ano, segundo região e escolaridade. http://dssbr.org/site/2013/01/diabetes-e-escolaridade-estudos-revelam-adiferenca-do-numero-de-mortes-causadas-pela-doenca-entre-grupos-com-mais- e-menos-anos-de-estudo/.
- 32.Gomes MB, coordenador. Estudo Multicêntrico de Diabetes Tipo 1 no Brasil. [Acesso em 27 dez 2012]. Disponível em: ttp://www.diabetes.org.br/attachments/congresso2009/Marilia-Brito-Estudo-Multicentrico-2011.pdf.
- 33.Ind020208 – Taxa de mortalidade específica por diabete melito na população de 15 anos e mais, por ano, segundo região e escolaridade [Internet]. Rio de Janeiro: Portal Determinantes Sociais da Saúde. Observatório sobre Iniquidades em Saúde. CEPI-DSS/ENSP/FIOCRUZ; 2012 Jan 30 [acesso em 27 dez 2012]. Disponível em: http://dssbr.org/site/wp-content/uploads/2012/03/Ind020208-20120130.pdf.
- 34.Gerich JE. Is insulin resistance the principal cause of type 2 diabetes? Diabetes Obes Metab. 1999 Sep;1(5):257-63. Review. PubMed PMID: 11225637. http://onlinelibrary.wiley.com/doi/10.1046/j.1463-1326.1999.00027.x/epdf. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


