Abstract
Background:
Acute lymphoblastic leukemia (ALL), accounting for 23% of all malignancies in children, is the most prevalent pediatric malignancy. This study compared dental caries, oral hygiene status, salivary pH, and Streptococcus mutans counts in dental plaques and saliva of children with leukemia with those of healthy controls.
Materials and Methods:
This case–control cross-sectional study assessed 32 children with ALL and 32 healthy children (4–9-year-old) for gingival bleeding index (GBI), decayed, missing, and filled/decayed, missing, and filled surfaces (DMF/dmfs), and plaque index (PI). Sampling was performed to determine salivary pH and S. mutans counts of the participants. The two groups matched in terms of age, gender, and socioeconomic status. The groups were compared using independent t-test, Mann–Whitney test, Chi-square test, and Spearman's and Pearson's correlation analyses.
Results:
The mean DMF/dmfs and GBI were significantly higher in the ALL group (PDMF/dmfs= 0.03; PGBI= 0.04). However, the two groups were not significantly different in the mean PI values (P = 0.47). The mean S. mutans counts in dental plaques and saliva of the children with leukemia were significantly lower than the healthy controls (P < 0.01). Moreover, the mean salivary pH was significantly lower in the ALL group compared to the control group (P < 0.01).
Conclusion:
Higher caries and gingival bleeding rates, higher dental plaque accumulation in children with ALL, decreased salivary pH, and cumulative effects of other risk factors highlight the significance of oral hygiene training programs (for the parents of these children) and regular dental examinations for these children.
Key Words: Acute lymphoblastic leukemia, caries, children, dental plaque, gingival health, Streptococcus mutans
INTRODUCTION
Acute lymphoblastic leukemia (ALL), the most prevalent malignancy in children, accounts for 23% of all malignancies in children younger than 15 years of age worldwide. While advances in the treatment of ALL have increased the 5-year survival rate for all children with the disease to over 85%,[1] various types of infection have been suggested to be responsible for the majority of morbidity and mortality among these patients.[2] Since 25%–45% of cases of septicemia in patients with neutropenic cancer stem from oral bacteria,[3] oral health, both during and after treatment, is critical to the patients' general health.
The nature and treatment of ALL can exert direct and indirect effects on the patients' oral health.[4] As leukemic cells can invade and enter the gums and deeper periodontal tissues, they can cause not only gingivitis but also soreness, bleeding, and infection in various oral tissues. Cytotoxic drugs can also lead to mucositis (atrophy and mucosal lesions) and increase the risk of local and systemic infections.[4]
Despite extensive knowledge about acute oral complications, for example, mucositis and other viral and fungal infections, in children under the treatment for cancer, research has not largely assessed the effects of cancer and anticancer agents on the risk of dental caries. Previous studies have reported the prevalence of dental caries in children with cancer to be equal to or higher than that in healthy children.[1,5,6,7,8] Furthermore, chemotherapy has been found to induce qualitative changes in enamel and dentin structures.[8]
Caries lesion, Streptococcus mutans, and dental plaque counts have long been considered as indicators of caries activity. On the other hand, salivary pH and buffering capacity can also determine the properties of saliva and the risk of caries development. A combination of various indicators can thus enhance the effectiveness of screening programs to identify individuals with high risk of dental caries.[1] In a comparison between children with leukemia and healthy children, Lauritano and Petruzzi[9] found higher risk of dental caries development and more severe dental anomalies in the first group. Similarly, Hegde et al.[5] reported children with ALL to have less favorable oral health status, higher rate of decay, and lower salivary pH, flow, and antioxidant content compared to the control group. Meanwhile, despite the presence of higher gingival bleeding index (GBI) and plaque index (PI) in children (age <13 years) with ALL compared to the control group, Maciel et al.[10] failed to establish significant differences in decayed, missing, and filled teeth (DMFT) score and saliva flow between the two groups. In a study by Ou-Yang et al.,[1] the mean decayed, missing, and filled surfaces (DMFS) score was higher (but not significantly), and salivary pH and buffering capacity were lower in children with ALL than in the control group. On the other hand, while the salivary counts of S. mutans were substantially lower in the patients, the two groups were similar in the number of lactobacilli. Likewise, O'Sullivan et al.[11] detected lower salivary counts of S. mutans in children with ALL.
Since no previous research has focused on oral health status of children with ALL in Iran, the current study sought to compare a group of Iranian children with leukemia with their healthy counterparts in terms of dental caries, salivary pH, dental plaque and salivary S. mutans counts, oral hygiene, and gum health status. We hope that our findings can highlight the significance of preventive measures in the oral health of children with ALL and facilitate the development of a more appropriate treatment protocol for these patients.
MATERIALS AND METHODS
This case–control cross-sectional study recruited 32 children (age: 4–9 years old) with ALL whose diagnosis has been made at least 1 year before the study (ALL group). The patients had been in the maintenance stage for a minimum of 6 months. Convenience sampling was applied to select the participants from the children visiting the Pediatric Hematology Ward of Omid Hospital (Isfahan, Iran). Data were collected during the maintenance stage of the disease when similar medications (mercaptopurine, vincristine, cyclophosphamide, and prednisone) were administered for all patients. None of the children had received prophylactic cranial radiation therapy or radiotherapy as a part of their treatment. The children were not included if they had any other systemic diseases or used medicines other than anticancer drugs.
The control group comprised 32 healthy 4–9-year-old children who visited the Pediatric Dental Clinic of School of Dentistry, Isfahan Azad University (Khorasgan, Iran). These children did not use any medication and matched the ALL group in terms of age, gender, and socioeconomic status (based on the Socioeconomic Classification Criteria in the UK).
Parents of all children were asked to sign a consent form and complete a demographic questionnaire. Oral health of all children was then examined by a person whose intraexaminer reliability had been confirmed. During the examinations, the participants were in a supine position in a dental chair. Dental caries were scored based on the dmfs index for primary teeth and the DMFS index for permanent teeth. DMF and dmfs were also summed up for each child. These indexes were performed according to the World Health Organization criteria, and the collected values were recorded (separately for each tooth) in previously prepared forms. O'Leary's PI and GBI were also evaluated to assess oral hygiene and gum health, respectively.[12]
In the next step, saliva samples were collected from all children at least 2 h after their breakfast. The participants were instructed not to wear lipsticks, brush their teeth during the 2 h before sampling, or use antimicrobial mouth rinses the day before sampling. In addition, none of the participants had received fluoride therapy during the 14-day period before the sampling or consumed antibiotics or any antimicrobial agent over the past 28 days.
A pH indicator strip (Merck, Germany) was placed in each participant's mouth (buccal sulcus) and allowed 10 s any color change to occur. Salivary pH was then determined by matching the strips with the color code chart available in the commercial kit. The obtained values were then recorded in relevant forms.
A chairside microbiological tests (Dentocult SM Strip mutans, Orion Diagnostica, Finland) as used to detect and count dental plaques and salivary S. mutans. The method involved the application of a selective medium and the adhesion and growth of S. mutans on specific strips. To stimulate the secretion of saliva and facilitate the transfer of S. mutans from dental surfaces to saliva, the participants were asked to chew a paraffin pellet (available in the kit) for 1 min. Afterward, strips for saliva and plaque (round- and square-tipped strips, respectively) were used according to the manufacturer's instructions to collect samples from the children's saliva and dental plaques. The strips were vertically placed in vials containing the selective medium. The vials were placed in an incubator and incubated at 37°C for 48 h. After this period, S. mutans manifested as pointed blue colonies. S. mutans counts in saliva and dental plaques (colonies per milliliter) were determined by comparing the density of the observed colonies with the standard colony chart provided by the kit [Figure 1]. The extracted values were recorded and used to calculate caries risk for each child.
Figure 1.
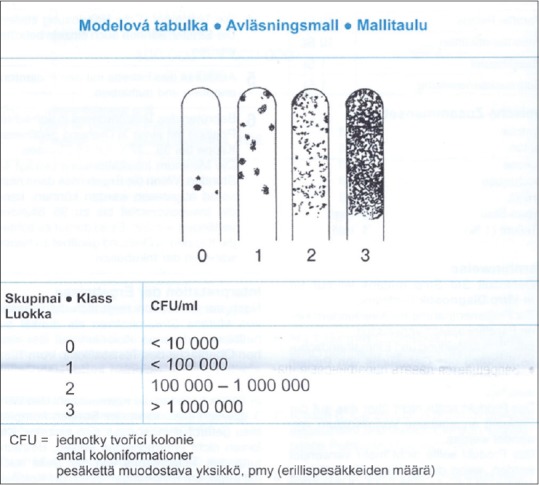
The standard chart for determination of Streptococcus mutans counts.
Independent t-test, Mann–Whitney test, and Chi-square test along with Spearman's and Pearson's correlation analyses were conducted to compare the mean values of quantitative variables in the two groups. All analyses were performed with SPSS for Windows 18.0 and 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The mean age of the ALL and control groups was 7.0 ± 1.8 and 6.8 ± 1.8 years, respectively (P = 0.65). Girls and boys constituted 43.8% (n = 14) and 56.2% (n = 18) of the ALL group, respectively. There were 16 boys and 16 girls (50% each) in the control group. According to Chi-square test results, the two groups were not significantly different in terms of sex distribution (P = 0.61).
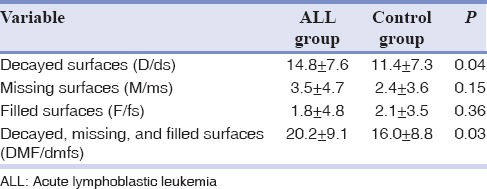
Table 1 shows the mean of DMF/dmfs in the two groups. The frequency of all indices, except for filled surfaces (F/fs), was higher in children with ALL than in the control group. Although the mean frequencies of decayed surfaces (D/ds) and DMF/dmfs were significantly higher in the ALL group than in controls, no significant difference was observed between the two groups in terms of missing surfaces (M/ms) and F/fs.
Table 1.
The mean frequency of decayed, missing, and filled surfaces in 4-9-year-old children with and without acute lymphoblastic leukemia
Oral examinations revealed the mean GBI to be significantly higher in children with ALL compared to the control group (27.4% ± 12.4% vs. 22.1% ± 12.5%; P = 0.04). However, the two groups were not significantly different in the mean PI (42.7% ± 9.2% vs. 42.5% ± 11.8%; P = 0.47). Moreover, while salivary pH ranged between 5 and 7 in both groups, the mean values were significantly lower in the ALL group than in the control group (5.7 ± 0.58 vs. 6.19 ± 0.56; P = 0/001).
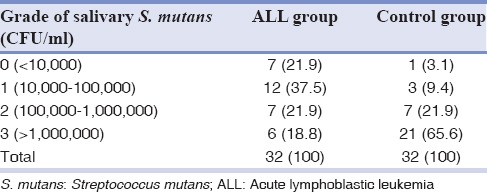
Tables 2 and 3 show the frequency distribution of S. mutans in the saliva and dental plaques of the ALL and control groups. Comparisons between the two groups using Mann–Whitney tests suggested the frequency of S. mutans to be significantly lower in the saliva and dental plaques of the ALL group (P < 0.001). There were direct correlations between salivary and dental plaque S. mutans counts in both the ALL and control groups (r = 0.56; P < 0.001 and r = 0.51; P = 0.001, respectively). In contrast, based on the calculated Spearman's correlation coefficients, salivary pH in either the ALL or the control group was not significantly correlated with salivary or dental plaque S. mutans counts (P > 0.05).
Table 2.
Frequency distribution of Streptococcus mutans in the saliva of children with acute lymphoblastic leukemia and the control group
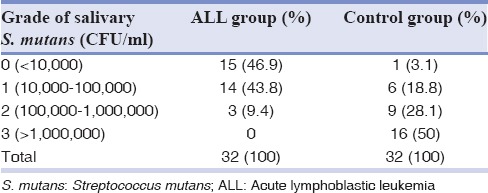
Table 3.
Frequency distribution of Streptococcus mutans in dental plaques of children with acute lymphoblastic leukemia and the control group
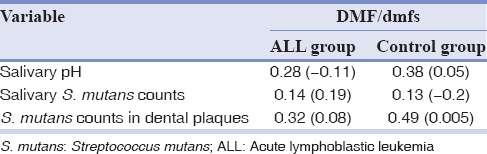
Pearson's correlation coefficients confirmed the absence of significant correlations between salivary pH and DMF/dmfs in the two groups. Moreover, according to the obtained Spearman's correlation coefficients, DMF/dmfs was not significantly correlated with S. mutans counts in the saliva and dental plaques of either group [Table 4].
Table 4.
Correlation coefficients between decayed, missing, and filled surfaces and salivary pH and Streptococcus mutans counts in the saliva and dental plaques of children with acute lymphoblastic leukemia and the control group
DISCUSSION
The severe complications of ALL and chemotherapy on the oral health of affected children, for example, gingivitis, mucositis, candidiasis, and other opportunistic infections get worse by passing time.[8,13] In an attempt to assess the oral health of ALL children, the current study evaluated their dental plaques, degree of caries, and presence/absence of gingival bleeding. It also sought to determine caries risk by measuring salivary pH and S. mutans counts in the dental plaques and saliva of the mentioned patients. To prevent variations in anticancer treatment modalities, all recruited patients were in the maintenance stage of the treatment. On the other hand, the effects of various confounding factors such as health and dietary habits, pH status, and previous microflora were minimized by selecting a control group which matched the ALL group in terms of socioeconomic status (both groups were from low socioeconomic classes).
In the present research, the mean DMF/dmfs was significantly higher in the ALL group than in the control group. Likewise, Lauritano and Petruzzi reported higher caries risk in children with ALL than in their healthy counterparts.[9] In a comparison between children under chemotherapy and a healthy control group, Pajari et al. also found higher DMFT and DMFS in the first group.[14] Ou-Yang et al.,[1] Hegde et al.,[5] and Nasim et al.[8] obtained similar results.
We also examined D/ds, M/ms, and F/fs separately in both groups. While the mean D/ds and M/ms were higher in the ALL group compared to the control group, the mean F/fs in children with ALL was lower (although not significantly) that in the control group. The lower F/fs in the ALL group highlighted their parents' neglect of the significance of periodic dental examinations and dental treatment (if necessary) for these children. In contrast to our findings, some studies have failed to detect any significant difference between the DMFT of ALL and healthy children.[7,10] This inconsistency can be justified by the fact that the mentioned studies have considered DMFT instead of DMFS (which is a more accurate measure). Differences in the participants' age and treatment phase, recommendations of the medical team regarding oral health, and parents' attention to their children's regular dental examinations might have also been responsible for the observed discrepancy.
GBI, first introduced by Barnes and Carter, assesses bleeding in proximal surfaces and reflects the presence or absence of gingivitis.[12] Similar to Maciel et al.,[10] we found the mean GBI to be significantly higher in the ALL group than in the control group (P = 0.04). The mean GBI in ALL children in the present study (27.4%) was also close to the rate reported by Maciel et al.[10](26.5%). On the other hand, Nasim et al.[8] detected moderate gingivitis in children with ALL. Ponce-Torres et al.[15] reported the presence of gingivitis in 91.84% of children with leukemia. In fact, the nature of ALL and the adverse effects of cytotoxic drugs can cause petechiae or ecchymosis in the oral mucosa and create a tendency toward gingival bleeding. As plaque and debris accumulation is considered as a major local stimulus, such a tendency toward bleeding would be more apparent in children with poor oral hygiene.[9,13]
In the assessment of the participants' oral health status, we did not observe a significant difference in O'Leary's PI between the ALL and control groups (42.7% vs. 42.5%). Poor oral hygiene of both groups (due to their low socioeconomic status) and failure to perform regular and correct toothbrushing and flossing can justify the absence of a significant difference between sick and healthy participants. In contrast, Maciel et al.[10] found significantly higher visible PI in children with ALL than in controls. They identified hypoplastic enamel (caused by radiotherapy) and the consequent increase in surface roughness and plaque adhesion to be responsible for the observed difference. Likewise, Ponce-Torres et al.[15] suggested poor oral hygiene to be accountable for dental caries, gingivitis, and periodontitis in children with ALL.
We used a chairside microbiological tests (Dentocult SM Strip mutans) to calculate S. mutans counts. This kit produces reliable results and can be easily used at physician offices to identify high-risk individuals.[1,16,17,18] S. mutans are believed to play a critical role in the initiation of dental caries. Long-term studies have shown substantially higher caries activity in individuals with higher levels of S. mutans.[19,20,21] We detected significantly lower S. mutans counts in the dental plaques and saliva of children undergoing chemotherapy compared to the control group. In fact, 60% and 90% of children with ALL had S. mutans counts lower than 105 CFU/ml in their dental plaques and saliva, respectively. Similarly, Ou-Yang et al.[1] found significantly lower salivary S. mutans counts in children under the treatment for ALL. In addition to this finding, O'Sullivan et al.[11] indicated that S. mutans counts during chemotherapy were lower than the values measured before and after the treatment. However, Pajari et al.[22] found contradicting results as they recruited a large sample of patients with various malignancies (in addition to leukemia) who were in different phases of treatment (either in the course of treatment or years later) and receiving dissimilar treatment modalities. Reduced S. mutans counts during chemotherapy might be caused by decreased volume of saliva and immune system disturbances. Moreover, the inhibitory effects of anticancer drugs such as daunorubicin and methotrexate on S. mutans should also be taken into account in this regard.[1,11,23,24] Using sensitivity tests, O'Sullivan et al.[11] showed that daunorubicin, a chemotherapeutic agent commonly administered during induction and consolidation phases of treatment, resulted in considerable reductions in S. mutans counts. They affirmed that such effects could last throughout the maintenance phase. Decreased S. mutans counts in the dental plaques and saliva of patients in the current research can also be attributed to the administration of antibiotics (e.g., cotrimoxazole) following various infections during treatment. Despite our efforts to select patients who had not received antibiotics for at least 4 weeks before the study, the ongoing impact of such agents might have affected the presence of bacteria in the oral cavity. Furthermore, considering the low socioeconomic status of the participants, their families might have provided inaccurate information about their history of antibiotic consumption.
Under natural circumstances, human saliva contains a wide range of organic and inorganic buffering agents which can neutralize the acidic products of oral bacteria. Salivary pH is a major determinant of an individual's susceptibility to dental caries.[1,25,26] Similar to the findings of Ou-Yang et al.,[1] Hegde et al.,[5] and Sepet et al.,[27] we detected significantly lower salivary pH in the ALL group than in the healthy control group. The inhibitory (and probably irreversible) effects of cytotoxic drugs on salivary glands might have contributed to this difference.[1] Pajari et al.[22] concluded that the reduction in pH remained stable even years after the children's recovery.
Despite the significantly lower S. mutans counts in ALL children (compared to the control group), their DMF/dmfs was significantly higher than the healthy controls. Dental caries is a complicated, multifactorial condition whose development depends on numerous risk factors. Although S. mutans is thought to initiate caries formation, their presence is not sufficient for caries development. In other words, several criteria such as the presence of lactobacilli are required for caries to progress and affect other dental surfaces.[28] Ou-Yang et al.[1] did not establish a significant difference in lactobacilli counts between ALL and healthy children.
Other factors such as reduced salivary pH and flow following chemotherapy and the resultant increase in the consumption of sweet drinks and sweet and soft foods (due to oral lesions and mucositis caused by the treatment and difficulty swallowing) can also be responsible for higher caries rates in ALL children despite their lower oral S. mutans counts. Poor oral hygiene, ignoring the significance of dental examinations and specific dental care routines as a result of parents' inadequate attention to underlying hematologic diseases, and failure to receive fluoride are also accepted to contribute to the mentioned condition.[15,29,30] On the other hand, some dentists refuse to provide children with leukemia with appropriate dental care as they are worried about infections or severe bleeding during the treatment process.
According to the above-mentioned facts, children with hematologic diseases require not only antineoplastic treatments but also special oral care. Therefore, to prevent and treat the probable leukemia-induced dental-mucosal diseases, pediatric dentists are recommended to regularly examine these children until at least 24 months after their recovery. They should also instruct the children to maintain perfect oral hygiene, drink lots of water, chewing gums and use artificial saliva to prevent dry mouth, and use fluoride regularly.[8] Proper oral hygiene and health of patients with leukemia can be ensured by close cooperation between pediatric hematologists and dentists, general dentists, and dental hygienists. Finally, since the complications of ALL and the adverse effects of antineoplastic treatments are inevitable, improving leukemic children's quality of life can play a critical role in preventing harmful effects on their oral health.
CONCLUSION
Dental plaque accumulation and the frequency of gingivitis and gingival bleeding were significantly higher in the studied children with ALL than in their healthy counterparts. Due to the lower salivary pH in the ALL group and the cumulative effects of other risk factors, timely treatment of dental problems and promoting oral and general health in children with ALL will require educating both children and their parents about oral hygiene and planning regular dental monitoring and examination programs for the children.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgments
The authors are grateful to Dr. Alireza Moafi (hematologist, Associate Professor), Omid Hospital's personnel, and the esteemed families of children with ALL who kindly cooperated in the current research.
REFERENCES
- 1.Ou-Yang LW, Chang PC, Tsai AI, Jaing TH, Lin SY. Salivary microbial counts and buffer capacity in children with acute lymphoblastic leukemia. Pediatr Dent. 2010;32:218–22. [PubMed] [Google Scholar]
- 2.Ravindranath Y. Recent advances in pediatric acute lymphoblastic and myeloid leukemia. Curr Opin Oncol. 2003;15:23–35. doi: 10.1097/00001622-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wingard JR. Oral complications of cancer therapies. Infectious and noninfectious systemic consequences. NCI Monogr. 1990;9:21–6. [PubMed] [Google Scholar]
- 4.Ivanovic M, Jovcic O, Mandic J, Bogetic D, Maddalone M. Oral manifestations of acute leukaemia. Srp Arh Celok Lek. 2011;139:103–6. doi: 10.2298/sarh1102103i. [DOI] [PubMed] [Google Scholar]
- 5.Hegde AM, Joshi S, Rai K, Shetty S. Evaluation of oral hygiene status, salivary characteristics and dental caries experience in acute lymphoblastic leukemic (ALL) children. J Clin Pediatr Dent. 2011;35:319–23. doi: 10.17796/jcpd.35.3.u5kx28q33m760834. [DOI] [PubMed] [Google Scholar]
- 6.Maguire A, Craft AW, Evans RG, Amineddine H, Kernahan J, Macleod RI, et al. The long-term effects of treatment on the dental condition of children surviving malignant disease. Cancer. 1987;60:2570–5. doi: 10.1002/1097-0142(19871115)60:10<2570::aid-cncr2820601037>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Pajari U, Larmas M, Lanning M. Caries incidence and prevalence in children receiving antineoplastic therapy. Caries Res. 1988;22:318–20. doi: 10.1159/000261129. [DOI] [PubMed] [Google Scholar]
- 8.Nasim VS, Shetty YR, Hegde AM. Dental health status in children with acute lymphoblastic leukemia. J Clin Pediatr Dent. 2007;31:210–3. doi: 10.17796/jcpd.31.3.73mu542187l75700. [DOI] [PubMed] [Google Scholar]
- 9.Lauritano D, Petruzzi M. Decayed, missing and filled teeth index and dental anomalies in long-term survivors leukaemic children: A prospective controlled study. Med Oral Patol Oral Cir Bucal. 2012;17:e977–80. doi: 10.4317/medoral.17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maciel JC, de Castro CG, Jr, Brunetto AL, Di Leone LP, da Silveira HE. Oral health and dental anomalies in patients treated for leukemia in childhood and adolescence. Pediatr Blood Cancer. 2009;53:361–5. doi: 10.1002/pbc.22108. [DOI] [PubMed] [Google Scholar]
- 11.O'Sullivan EA, Duggal MS, Bailey CC, Curzon ME, Hart P. Changes in the oral microflora during cytotoxic chemotherapy in children being treated for acute leukemia. Oral Surg Oral Med Oral Pathol. 1993;76:161–8. doi: 10.1016/0030-4220(93)90198-d. [DOI] [PubMed] [Google Scholar]
- 12.Carter HG, Barnes GP. The Gingival bleeding index. J Periodontol. 1974;45:801–5. doi: 10.1902/jop.1974.45.11.801. [DOI] [PubMed] [Google Scholar]
- 13.McDonald RE, Avery DR, Dean JA. Dentistry for the Child and Adolescent. 9th ed. Maryland: Mosby; 2011. pp. 498–509. [Google Scholar]
- 14.Pajari U, Ollila P, Lanning M. Incidence of dental caries in children with acute lymphoblastic leukemia is related to the therapy used. ASDC J Dent Child. 1995;62:349–52. [PubMed] [Google Scholar]
- 15.Ponce-Torres E, Ruíz-Rodríguez Mdel S, Alejo-González F, Hernández-Sierra JF, Pozos-Guillén Ade J. Oral manifestations in pediatric patients receiving chemotherapy for acute lymphoblastic leukemia. J Clin Pediatr Dent. 2010;34:275–9. doi: 10.17796/jcpd.34.3.y060151580h301t7. [DOI] [PubMed] [Google Scholar]
- 16.Crossner CG, Hagberg C. A clinical and microbiological evaluation of the Dentocult dip-slide test. Swed Dent J. 1977;1:85–94. [PubMed] [Google Scholar]
- 17.Tanabe Y, Park JH, Tinanoff N, Turng BF, Lilli H, Minah GE. Comparison of chairside microbiological screening systems and conventional selective media in children with and without visible dental caries. Pediatr Dent. 2006;28:363–8. [PubMed] [Google Scholar]
- 18.Emilson CG, Krasse B. Comparison between a dip-slide test and plate count for determination of Streptococcus mutans infection. Scand J Dent Res. 1986;94:500–6. doi: 10.1111/j.1600-0722.1986.tb01792.x. [DOI] [PubMed] [Google Scholar]
- 19.Seki M, Karakama F, Terajima T, Ichikawa Y, Ozaki T, Yoshida S, et al. Evaluation of mutans streptococci in plaque and saliva: Correlation with caries development in preschool children. J Dent. 2003;31:283–90. doi: 10.1016/s0300-5712(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 20.Thibodeau EA, O'Sullivan DM. Salivary mutans streptococci and caries development in the primary and mixed dentitions of children. Community Dent Oral Epidemiol. 1999;27:406–12. doi: 10.1111/j.1600-0528.1999.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 21.Wendt LK, Hallonsten AL, Koch G, Birkhed D. Analysis of caries-related factors in infants and toddlers living in Sweden. Acta Odontol Scand. 1996;54:131–7. doi: 10.3109/00016359609006019. [DOI] [PubMed] [Google Scholar]
- 22.Pajari U, Poikonen K, Larmas M, Lanning M. Salivary immunoglobulins, lysozyme, pH, and microbial counts in children receiving anti-neoplastic therapy. Scand J Dent Res. 1989;97:171–7. doi: 10.1111/j.1600-0722.1989.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 23.Meurman JH, Torkko H, Pyrhönen S. Antineoplastic agents inhibit the growth of Streptococcus mutans and Streptococcus sanguis in vitro. Oral Microbiol Immunol. 1991;6:177–81. doi: 10.1111/j.1399-302x.1991.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 24.Meurman JH, Laine P, Lindqvist C, Teerenhovi L, Pyrhönen S. Five-year follow-up study of saliva, mutans streptococci, lactobacilli and yeast counts in lymphoma patients. Oral Oncol. 1997;33:439–43. doi: 10.1016/s0964-1955(97)00037-7. [DOI] [PubMed] [Google Scholar]
- 25.Sreebny LM. Xerostimia: Diagnosis, management and clinical complications. Br Dent Assoc. 1996;14:43–66. [Google Scholar]
- 26.Larmas M. Saliva and dental caries: Diagnostic tests for normal dental practice. Int Dent J. 1992;42:199–208. [PubMed] [Google Scholar]
- 27.Sepet E, Aytepe Z, Ozerkan AG, Yalman N, Guven Y, Anak S, et al. Acute lymphoblastic leukemia: Dental health of children in maintenance therapy. J Clin Pediatr Dent. 1998;22:257–60. [PubMed] [Google Scholar]
- 28.Pinkham JR. Pediatric Dentistry: Infancy through Adolescence. 4th ed. St. Louis: Elsevier; 2005. pp. 177–83. [Google Scholar]
- 29.Tschoppe P, Wolgin M, Pischon N, Kielbassa AM. Etiologic factors of hyposalivation and consequences for oral health. Quintessence Int. 2010;41:321–33. [PubMed] [Google Scholar]
- 30.Sepúlveda E, Brethauer U, Rojas J, Fernández E, Le Fort P. Oral ulcers in children under chemotherapy: Clinical characteristics and their relation with herpes simplex virus type 1 and Candida albicans. Med Oral Patol Oral Cir Bucal. 2005;10(Suppl 1):E1–8. [PubMed] [Google Scholar]


