Abstract
Background:
This study investigated the impact of different surface treatments, including fractional carbon dioxide (CO2) laser on shear bond strength (SBS) of resin cement to lithium disilicate ceramic.
Materials and Methods:
In this in vitro study, 72 blocks of IPS e.max CAD ceramic were randomly divided into six groups in terms of treatment (n = 12). Group 1 underwent etching with 9.6% hydrofluoric (HF) acid, whereas group 2 was subjected to air abrasion with aluminum oxide particles. Groups 3 and 4 were treated with a fractional CO2 laser for 10 s using 10 W/14 mJ (group 3) or 20 W/10 mJ (group 4). In groups 5 and 6, the CO2 laser was applied similar to that in groups 3 and 4, respectively; then, the specimens were etched by HF acid. After silane application, luting cement was bonded to the specimens. The SBS was assessed with a universal testing machine, and the type of bond failure was determined. Data were analyzed by ANOVA, Duncan, and Fisher's exact tests.
Results:
Surface conditioning with fractional CO2 laser alone resulted in significantly lower SBS than HF acid treatment (P < 0.05). Bond strengths of the specimens treated with a combination of laser irradiation and acid etching were significantly greater than all the other groups (P < 0.05). No significant difference was found in the distribution of failure modes among the groups (P = 0.337).
Conclusion:
The combination of fractional CO2 laser irradiation and HF acid etching could be recommended when extra retention is required for lithium disilicate-based restorations, whereas laser treatment alone cannot produce sufficient SBS.
Key Words: Bond strength, carbon dioxide laser, ceramics, laser treatment, lithium disilicate
INTRODUCTION
All-ceramic restorations have become the point of focus of patients and practitioners in modern dentistry not only due to their high esthetic appearance, but also for favorable mechanical properties, chemical inertness, wear resistance, structural durability and biocompatibility.[1,2] However, the two main limitations of all-ceramic restorations, i.e., low resistance to fracture and brittleness, have remained a challenge to face.[3] Recently, high-strength ceramics have been introduced to overcome the low fracture resistance and brittleness of all-ceramic restorations. Lithium disilicate is a moderately strong glass ceramic with a high crystalline content. It combines esthetic and strength and can be used for metal-free crowns and ultra-thin veneers. IPS e.max CAD is a machinable block of lithium disilicate ceramic developed to fabricate inlays and onlays or other esthetic restorations through computer-aided design/computer-aided manufacturing (CAD/CAM) systems.[4] Lithium disilicate ceramic has little porosity and roughness and thus additional surface treatment is required to create sufficient micromechanical and chemical retention between cement and ceramic and thus increasing the clinical longevity of restoration.[5]
Several attempts have been made to increase the bond strength of cements to lithium disilicate ceramic. Acid etching with fluorine-containing agents such as hydrofluoric (HF) acid, ammonium bifluoride, and acidulated phosphate fluoride is a traditional preparation technique to create micromechanical bonding.[6] Air abrasion with aluminum oxide particles has also been used for surface preparation of lithium disilicate ceramic. It has been suggested that this technique not only promotes surface area and micromechanical interlocking but also increases surface energy and decreases surface tension on ceramic, thereby enabling optimal wetting of silanes, bonding agents, or self-adhesive cements.[3]
Lasers have been used for various applications in dentistry, including conditioning dental and restorative surfaces.[7,8,9,10] Previous studies have focused on the use of neodymium-doped yttrium aluminum garnet (Nd:YAG),[11,12] erbium-doped yttrium aluminum garnet (Er:YAG)[11,12,13,14] and carbon dioxide (CO2)[13] lasers for surface preparation of different types of ceramic. However, there are a few studies that employed laser radiation to prepare lithium disilicate ceramic for the bonding process.[3,15,16,17,18] According to the authors' knowledge, no study has investigated the efficacy of fractional CO2 laser for enhancing the adhesion of bonding cement to IPS e.max CAD ceramic.
The concept of fractional photothermolysis (FP) was introduced in 2003[19] to reduce the side effects of the skin resurfacing with ablative CO2 and Er:YAG lasers, such as prolonged downtime, long-lasting erythema, edema, burning and scarring.[20,21] Instead of producing layers of thermal heating, FP generates multiple columns of microscopic thermal wounds called microscopic treatment zones, while the surrounding tissues remain healthy and untreated, thus supporting the wound healing process. The use of fractional CO2 laser in dentistry could be associated with several advantages. It allows the clinician to predetermine the radiation area where the laser irradiates multiple zones with predefined space between them. In this way, the need for manual movement of the laser handpiece by the clinician is eliminated, and a more homogenous etching pattern is attained on the surface while the risk of thermal damage to the underlying tissues is minimized.[20]
This study was conducted to compare the effect of the fractional CO2 laser with other methods of surface treatment on shear bond strength (SBS) and mode of bond failure of resin cement to lithium disilicate ceramic. The null hypothesis was that fractional CO2 laser conditioning could not increase bond strength to IPS e.max CAD ceramic more than the other surface treatment modalities.
MATERIALS AND METHODS
Specimen preparation
In this in vitro study, 72 lithium disilicate-based all-ceramic cubes (3 × 3 × 2 mm in thickness) were fabricated using CAD/CAM milling process (IPS e.max CAD®; Ivoclar Vivadent, Schaan, Liechtenstein) according to the manufacturer's instructions. No glaze was applied on the ceramic surface of the cubes. The specimens were immersed in distilled water to remove surface residues and dried; then they were examined under a magnifier to discard those with any visible flaws, cracks or other surface defects. The ceramic specimens were mounted horizontally in self-cured acrylic resin blocks (Acropars, Marlic Co., Tehran, Iran), ensuring that the ceramic surface remained intact for the bonding procedure. The surfaces were cleaned with ethyl alcohol and dried carefully in air before treatments. The ceramic specimens were randomly divided into six groups (n = 12) in terms of the surface treatments applied as follows:
Group 1 (acid etching)
The surfaces of ceramic blocks were etched with 9.6% HF acid (Porcelain Etch Gel, Pulpdent Corp., Watertown, MA, USA) for 2 min. The gel was rinsed off with a copious amount of water, and the surface was air-dried.
Group 2 (air abrasion)
Lithium disilicate surfaces were subjected to air abrasion with 50-μm aluminum oxide (Al2O3) particles using an intraoral sandblasting unit (Kolo Multi Functional Micro blaster, Sun Ring Dental Medical Instrument Co., Japan). Sandblasting was performed under 2.5-bar pressure for 15 s, and the tip of the device was held at a distance of 10 mm perpendicular to the ceramic surface. The specimens were then rinsed thoroughly under running tap water to remove remaining particles and air-dried.
Group 3 (carbon dioxide laser, 10 W/14 mJ)
The surfaces of the specimens in group 3 were etched with a fractional CO2 laser (a wavelength of 10.6 μm; Lutronic Inc., Princeton Junction, NJ, USA). The laser device was run in the dynamic mode, and the setting was so that a square area of 4 × 4 mm was irradiated at the middle of the specimen. The laser tip was held manually perpendicular to the ceramic surface at a distance of 3 cm. The frequency of 200 Hz (pulse per se cond) and irradiation time of 10 s were selected. The power and pulse energy were 10 W and 14 mJ, respectively. The pulse duration was 1.75 ms, and the energy delivered to each surface was approximately 28 J, as calculated by the apparatus.
Group 4 (carbon dioxide laser, 20 W/10 mJ)
The surface treatment employed was similar to that in group 3, but the power of 20 W and pulse energy of 10 mJ were selected. The pulse duration was 0.58 ms, and the energy delivered to each surface was approximately 24 J, as calculated by the device.
Group 5 (carbon dioxide laser, 10 W/14 mJ + acid etching)
The ceramic surfaces in group 5 were treated by a fractional CO2 laser at the same parameters as applied in group 3. However, a 2-min period of etching with 9.6% HF acid was then employed in laser-treated specimens, similar to the control group.
Group 6 (carbon dioxide laser, 20 W/10 mJ + acid etching)
The ceramic surfaces in group 6 were treated with a fractional CO2 laser using the same parameters as described in Group 4. A 2-min period of etching with 9.6% HF acid was then applied in laser-treated specimens similar to the control group.
The bonding process
Following surface preparation, a silane coupling agent (Silane Bond Enhancer, Pulpdent Corp., Watertown, MA, USA) was applied on the ceramic surface and allowed to penetrate for 1 min, according to the manufacturer's recommendations. The surface was then dried with an air spray. A dual-cured self-adhesive resin luting cement (Clearfil SA Luting; Kuraray Noritake Dental Inc., Kurashiki, Okayama, Japan) was applied to the ceramic surfaces in all the groups. An adequate and equal length of base and catalyst pastes of luting cement was dispensed and mixed with a plastic mixing spatula. The resin cement was then poured into plastic molds measuring 2 mm in height and 1.5 mm in diameter, held perpendicular over the ceramic substrates. The excess cement was removed with a sharp explorer from the periphery of the mold, and the luting agent was then polymerized for 40 s by putting a light guide (Bluephase C8, Ivoclar Vivadent, Schaan, Liechtenstein) at four opposite directions (10 s each) using a power density of 650 mW/cm2. All the procedures were carried out by one operator.
After 30 min, the plastic molds were separated and carefully removed, and the specimens were stored in distilled water at room temperature for 24 h. Each sample was mounted in a holding device in a Universal Testing Machine (Santam, Model STM-20, Tehran, Iran) to measure the shear bond strength of the adhesive interface at fracture. The cross-head was placed perpendicular to the cement-ceramic interface, and the specimens were loaded at a speed of 1 mm/min. The SBS was calculated in megapascals (MPa) by dividing the load at failure point (Newton) by the surface area of the cement cylinder (mm2).
Fracture analysis
After the specimens were debonded and removed from the testing apparatus, the fracture sites were observed under a stereomicroscope (Dino Lite Pro, AnMo Electronics Corp., Taiwan, ROC) at ×20 magnification to identify the type of bond failure. The fracture modes were classified as follows: (1) adhesive failure at the interface of the resin cement and ceramic substrate; (2) cohesive failure within the resin cement or the ceramic bulk; (3) mixed, a combination of adhesive and cohesive failures.
Statistical analysis
The normal distribution of data was confirmed by Kolmogorov–Smirnov test (P > 0.05). One-way analysis of variance (ANOVA) was run to detect any significant difference in SBS among the study groups followed by post hoc Duncan test for pairwise comparisons. The difference in failure modes between the study groups was analyzed with Fisher's exact test. Statistical analyses were performed with SPSS (Statistical Package for Social Sciences, version 16.0, SPSS Inc., Chicago, IL, USA) software, and the significance level for all the tests was predetermined at P < 0.05.
RESULTS
Table 1 presents the means, standard deviations and ranges of SBS (MPa) of resin cement to lithium disilicate specimens in the study groups. The greatest bond strength values were observed when the ceramic surfaces were treated with a combination of fractional CO2 laser irradiation and HF acid etching (22.4 and 24.3 MPa in groups 5 and 6, respectively), whereas the air-abraded specimens exhibited the lowest SBS (12.2 MPa) among the study groups. Figure 1 compares SBS values of the study groups.
Table 1.
The mean (MPa), standard deviation, range, and the results of statistical analysis for comparing shear bond strength of resin cement to lithium disilicate specimens in the study groups
Figure 1.
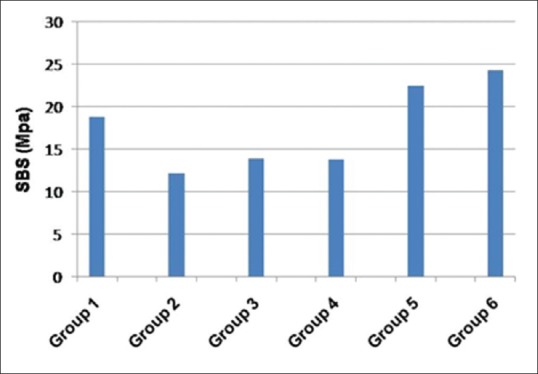
Shear bond strength (MPa) of the study groups.
ANOVA detected a statistically significant difference in bond strength between the study groups (P < 0.001; Table 1). Pairwise comparisons with Duncan test revealed that the air-abraded specimens (group 2) as well as those treated with fractional CO2 laser alone (groups 3 and 4) had bond strength values comparable to each other and significantly lower than those in the other study groups (P < 0.05; Table 1). Bond strengths of the specimens prepared by a combination of CO2 laser irradiation and HF acid etching (groups 5 and 6) were significantly higher than those in all the other groups (P < 0.05; Table 1). The HF acid-treated group exhibited higher bond strength than the air-abraded and CO2 laser-treated specimens, but lower SBS than those subjected to combined CO2 laser and HF acid treatment (P < 0.05; Table 1).
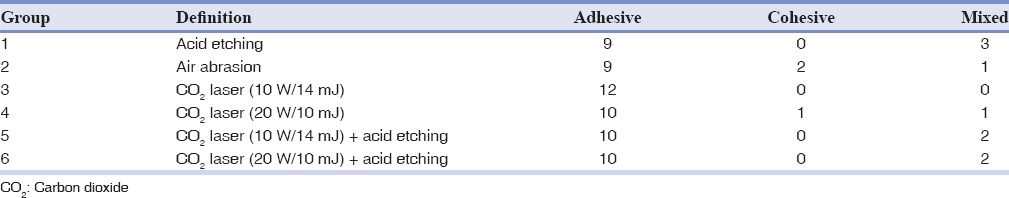
Table 2 indicates the frequency of failure modes in the study groups. The adhesive failure was the main type of fracture in all the study groups, followed by mixed fracture. Fisher's exact test revealed no significant differences in the distribution of failure modes between the study groups (P = 0.337).
Table 2.
The frequency of failure modes in the study groups
DISCUSSION
The present study investigated the effect of different surface treatments, including fractional CO2 laser, on SBS of resin cement to a lithium disilicate-based ceramic. The unglazed ceramic surfaces were used in this study because in the clinical situation the internal surface of the restoration remains unglazed. Theoretically, unglazed surfaces exhibit greater irregularities and micromechanical interlocking and thus higher bond strength compared to glazed surfaces.[20] The luting agent used in this study (Clearfil SA Luting) is a self-adhesive dual-cured resin cement. The use of resin cements is associated with several advantages such as additional retention, enhanced optical properties, low solubility, better marginal adaptation and most importantly enhanced fracture resistance of ceramic restorations.[3]
Chemical bonding through silane application plays an important role in ceramic-resin adhesion. The use of silane improves wettability of the ceramic surface and promotes adhesion between adhesives and ceramic/metal. However, the long-term stability of chemical bonding using silane coupling agents is still under debate.[6] A previous article on bonding to glass ceramics revealed no significant differences in bond strength values between different treatment groups (sandblasting, polishing, HF acid, or phosphoric acid etching) when a silane coupling agent was applied after surface treatment, whereas in the absence of silane application, HF-etched and sandblasted groups exhibited the highest microshear bond strength.[22] In the present study, silane was applied in all the groups because it is a simple clinical procedure that promotes bond strength regardless of the type of surface treatment employed.
HF acid was applied as a surface treatment strategy in the present investigation and produced bond strength value (18.08 MPa) that was significantly higher than that in air-abraded specimens and those treated by fractional CO2 laser alone. Ceramics with a high glassy phase and a large amount of silica are called acid-sensitive or glass ceramics, generally classified into feldspathic, leucite-reinforced, fluormica glass or lithium disilicate. Etching with HF acid could be considered an effective modality for glass ceramics as it dissolves the glassy phase of acid-susceptible ceramics, producing suitable surface texture for bonding and facilitating the penetration of resin into ceramic.[23] The content of silica in lithium disilicate ceramic is approximately 60 wt%, as mentioned by the manufacturer, and could be considered sufficient to obtain reliable bond strength after dissolving in acid solutions.[24] Several studies demonstrated that the use of HF acid on glass ceramics creates higher surface roughness and greater bond strength compared with other acid etchants, surface grinding, or sandblasting techniques.[5,23,25,26,27,28] The combination of HF acid etching and silane application has been recommended by several authors as a method of choice for surface preparation of feldspathic, lithium disilicate or other types of glass ceramics.[5,23,25,26,27,29,30,31,32]
Roughening the surface with 25–50-μ aluminum oxide particles is a traditional way to achieve micromechanical retention on ceramic substrates. Surface roughening increases both the bonding area and the wettability of the ceramic surface.[1] Recent studies indicated higher surface roughness in sandblasted lithium disilicate ceramic in comparison to Er:YAG laser-irradiated or HF acid-treated specimens.[3,18,33] However, the ceramic specimens subjected to air abrasion exhibited the lowest SBS in the present investigation. It is possible that the irregularities created by the air abrasion process were not effective in enhancing the bond strength to lithium disilicate ceramic.[1] The deposition of aluminum oxide particles on the surface, which could interfere with the cement-ceramic adhesion,[5] might be another reason for the lowest bond strength in air-abraded specimens. Other authors believe that air abrasion creates surface damage and removes a significant amount of substances from the surface, thus weakening the ceramic and leading to premature fracture.[34] The outcomes of this study are consistent with the results of Colares et al.,[30] who reported significantly lower bond strength in lithium-disilicate ceramic blocks treated with sandblasting compared to those prepared by HF acid and silane application.
In the present study, the use of fractional CO2 laser at either 10 W/14 mJ or 20 W/10 mJ was not capable of improving the bond strength to IPS e.max CAD ceramic. However, when the CO2 laser irradiation was followed by HF acid etching, the resultant bond strength was significantly higher than all the other modalities employed, indicating a synergistic effect between CO2 laser and HF acid for conditioning lithium disilicate substrate. No significant differences were found between SBS values of 10 W/14 mJ and 20 W/10 mJ groups, either followed or not followed by acid conditioning. It has been assumed that the fractional CO2 laser is capable of roughening the ceramic surface through the process of thermomechanical ablation, increasing micromechanical retention and enhancing bond strength at the cement-ceramic interface. There is also the possibility of chemical alteration of the irradiated ceramic surface favoring enhanced adhesion; however, further studies are warranted to verify this assumption.
To date, no study has employed fractional CO2 laser for conditioning lithium disilicate ceramic; therefore, comparison of the results of this study with those of previous studies is limited. The results of this study are consistent with those of Kursoglu et al.,[17] who reported that the highest bond strength was achieved in lithium disilicate samples treated by HF acid compared to those prepared by erbium, chromium:yttrium-scandium-gallium-garnet (Er, Cr:YSGG) laser (1.5, 2.5, and 6 W). Shiu et al.[35] showed that feldspathic surfaces conditioned with HF acid exhibited significantly higher bond strength than Er:YAG laser-irradiated specimens. Zarif Najafi et al.[36] investigated the effect of superpulse CO2 laser irradiation on SBS of metal orthodontic brackets to porcelain and found that HF etching produced the highest bond strength, but CO2 laser irradiation also provided adequate SBS, while allowing for elimination of the HF acid step. The results of this study contradict those of previous authors[37] who observed the greatest bond strength in zirconia specimens treated with a CO2 laser at parameters similar to those employed in this study. Another study revealed that the use of fractional CO2 laser at either 10 W/10 mJ or 15 W/10 mJ resulted in a significant increase in bond strength of orthodontic brackets to feldspathic porcelain without producing surface damage.[20] The differences between the results of this study and those of previous studies[20,37] might be attributed to the different types of ceramic employed as they have dissimilar compositions and can absorb the laser light differently.
In the present study, the highest bond strength was obtained when the combination of fractional CO2 laser irradiation and HF acid etching was employed for surface treatment of lithium disilicate substrate. Therefore, this method could be recommended in situations when extra retention is required for lithium disilicate restorations. Akyil et al.[12] suggested the use of combined Er:YAG or Nd:YAG laser irradiation and acid application to improve resin adhesion to feldspathic ceramic. They observed in scanning electron microscopy analysis that when HF acid was applied after laser irradiation, the resultant fissures and cracks were larger than those created by laser irradiation alone.
The quality of the bond should not be just assessed through the bond strength data as the mode of failure also provides valuable information for predicting the clinical performance of bonded restorations.[6] The results of the present study showed that adhesive fracture between the luting cement and porcelain occurred with the highest frequency in all the experimental groups. Despite the significant difference in bond strength between the study groups, the mode of bond failure was comparable among groups, indicating the lack of relationship between SBS and the type of bond fracture. Therefore, factors other than bond strength could affect the mode of failure at the bonding interface.
Some authors believe that values ≥20 MPa could provide sufficient bond strength, whereas others considered the higher limit of 25 MPa to represent sufficient bond strength in the clinical setting.[38] Keeping this in mind, the results of the present study suggested that treatment of lithium disilicate ceramic with HF acid produced SBS that was lower but close to the threshold limit required for clinical applications. On the other hand, the use of fractional CO2 laser followed by HF acid etching provided bond strength value that well exceeded the threshold limit required for successful bonding in the clinical situation. This technique might be extremely useful in lithium disilicate restorations with limited or no mechanical retention in the preparation design, requiring a high bond strength at the bonding interface. In addition to the routine benefits of laser etching (less time and elimination of the rinsing step),[10,39] the use of fractional CO2 laser for the preparation of restorative materials is associated with other advantages, as scanning the surface is made by the laser apparatus itself and thus a more homogeneous etching pattern would be created on the ceramic specimen.[20]
A limitation of this study was that it used SBS test for evaluating the effectiveness of surface conditioning procedures. This test can produce fracture at a distance from the resin-ceramic interface, leading to misconception of the bond quality. In spite of such limitations, SBS remains a simple and appropriate test to evaluate bonding performance.[6] The bond between ceramic and resin cement is influenced by several factors such as temperature changes, saliva, diet, chewing force and other habits in a complex oral environment. We did not employ thermacycling or any other fatigue cycle in this study because of the diversity of the treatment groups and the controversial results of previous studies regarding the effect of these procedures on SBS.[40] Further investigations are warranted to evaluate the durability of the bond in CO2 laser-treated ceramic specimens subjected to long-term water storage or fatigue cycling procedures.
CONCLUSION
Under the conditions used in this study:
Etching with 9.6% HF acid resulted in acceptable bond strength between resin cement and lithium disilicate ceramic.
Air abrasion produced the lowest SBS in lithium disilicate ceramic and could not be recommended in the clinical situation.
The application of fractional CO2 laser either at 10 W/14 mJ or at 20 W/10 mJ was less effective than HF acid for enhancing the bond strength of resin cement to lithium disilicate ceramic.
The combination of fractional CO2 laser irradiation and HF acid etching produced the highest SBS among the study groups, and thus it could be recommended in situations when extra retention is required for lithium disilicate restorations.
There were no significant differences in failure modes between the study groups, indicating a lack of relationship between bond strength and type of bond fracture.
Financial support and sponsorship
The authors would like to thank Mashhad University of Medical Sciences for the financial support of this project (grant number: 910845).
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial, in this article.
Acknowledgments
The authors would like to thank the research chancellor of Mashhad University of Medical Sciences for the financial support of this project (grant number: 910845). The results presented in this paper have been taken from a student thesis (thesis number: 2641).
REFERENCES
- 1.Agarwal A. The effect of surface conditioning methods on shear bond strength of reisn luting cement to all ceramic coping material, an in-vitro study. Int J Prosthet Dent. 2012;3:1–10. [Google Scholar]
- 2.Kara HB, Ozturk AN, Aykent F, Koc O, Ozturk B. The effect of different surface treatments on roughness and bond strength in low fusing ceramics. Lasers Med Sci. 2011;26:599–604. doi: 10.1007/s10103-010-0806-9. [DOI] [PubMed] [Google Scholar]
- 3.Kara HB, Dilber E, Koc O, Ozturk AN, Bulbul M. Effect of different surface treatments on roughness of IPS Empress 2 ceramic. Lasers Med Sci. 2012;27:267–72. doi: 10.1007/s10103-010-0860-3. [DOI] [PubMed] [Google Scholar]
- 4.Heymann HO, Swift EJ, Jr, Ritter AV. Sturdevant's Art and Science of Operative Dentistry. St. Louis: Elsevier/Mosby; 2014. [Google Scholar]
- 5.Pollington S, Fabianelli A, van Noort R. Microtensile bond strength of a resin cement to a novel fluorcanasite glass-ceramic following different surface treatments. Dent Mater. 2010;26:864–72. doi: 10.1016/j.dental.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Della-Bona A. Characterizing ceramics and the interfacial adhesion to resin: II. the relationship of surface treatment, bond strength, interfacial toughness and fractography. J Appl Oral Sci. 2005;13:101–9. doi: 10.1590/s1678-77572005000200002. [DOI] [PubMed] [Google Scholar]
- 7.Ahrari F, Akbari M, Akbari J, Dabiri G. Enamel surface roughness after debonding of orthodontic brackets and various clean-up techniques. J Dent (Tehran) 2013;10:82–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Ahrari F, Basafa M, Fekrazad R, Mokarram M, Akbari M. The efficacy of Er, Cr:YSGG laser in reconditioning of metallic orthodontic brackets. Photomed Laser Surg. 2012;30:41–6. doi: 10.1089/pho.2011.3088. [DOI] [PubMed] [Google Scholar]
- 9.Ahrari F, Fekrazad R, Kalhori KA, Ramtin M. Reconditioning of ceramic orthodontic brackets with an Er, Cr:YSGG laser. Lasers Med Sci. 2013;28:223–8. doi: 10.1007/s10103-012-1093-4. [DOI] [PubMed] [Google Scholar]
- 10.Ahrari F, Poosti M, Motahari P. Enamel resistance to demineralization following Er:YAG laser etching for bonding orthodontic brackets. Dent Res J (Isfahan) 2012;9:472–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Poosti M, Jahanbin A, Mahdavi P, Mehrnoush S. Porcelain conditioning with Nd:YAG and Er:YAG laser for bracket bonding in orthodontics. Lasers Med Sci. 2012;27:321–4. doi: 10.1007/s10103-010-0878-6. [DOI] [PubMed] [Google Scholar]
- 12.Akyil MS, Yilmaz A, Karaalioglu OF, Duymus ZY. Shear bond strength of repair composite resin to an acid-etched and a laser-irradiated feldspathic ceramic surface. Photomed Laser Surg. 2010;28:539–45. doi: 10.1089/pho.2009.2586. [DOI] [PubMed] [Google Scholar]
- 13.Kasraei S, Rezaei-Soufi L, Heidari B, Vafaee F. Bond strength of resin cement to CO2 and Er:YAG laser-treated zirconia ceramic. Restor Dent Endod. 2014;39:296–302. doi: 10.5395/rde.2014.39.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehghani M, Ahrari F. The effect of surface treatment with Er:YAG laser on shear bond strength of orthodontic brackets to fiber-reinforced composite. J Clin Exp Dent. 2014;6:e379–83. doi: 10.4317/jced.51613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gökçe B, Ozpinar B, Dündar M, Cömlekoglu E, Sen BH, Güngör MA. Bond strengths of all-ceramics: Acid vs. laser etching. Oper Dent. 2007;32:173–8. doi: 10.2341/06-52. [DOI] [PubMed] [Google Scholar]
- 16.Usumez A, Aykent F. Bond strengths of porcelain laminate veneers to tooth surfaces prepared with acid and Er, Cr:YSGG laser etching. J Prosthet Dent. 2003;90:24–30. doi: 10.1016/s0022-3913(03)00235-x. [DOI] [PubMed] [Google Scholar]
- 17.Kursoglu P, Motro PF, Yurdaguven H. Shear bond strength of resin cement to an acid etched and a laser irradiated ceramic surface. J Adv Prosthodont. 2013;5:98–103. doi: 10.4047/jap.2013.5.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yavuz T, Dilber E, Kara HB, Tuncdemir AR, Ozturk AN. Effects of different surface treatments on shear bond strength in two different ceramic systems. Lasers Med Sci. 2013;28:1233–9. doi: 10.1007/s10103-012-1201-5. [DOI] [PubMed] [Google Scholar]
- 19.Huzaira M, Anderson R, Sink K, Manstein D. Intradermal focusing of near-infrared optical pulses: A new approach for non-ablative laser therapy. Lasers Surg Med. 2003;15:66. [Google Scholar]
- 20.Ahrari F, Heravi F, Hosseini M. CO2 laser conditioning of porcelain surfaces for bonding metal orthodontic brackets. Lasers Med Sci. 2013;28:1091–7. doi: 10.1007/s10103-012-1152-x. [DOI] [PubMed] [Google Scholar]
- 21.Hantash BM, Bedi VP, Chan KF, Zachary CB. Ex vivo histological characterization of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39:87–95. doi: 10.1002/lsm.20405. [DOI] [PubMed] [Google Scholar]
- 22.Shimada Y, Yamaguchi S, Tagami J. Micro-shear bond strength of dual-cured resin cement to glass ceramics. Dent Mater. 2002;18:380–8. doi: 10.1016/s0109-5641(01)00054-9. [DOI] [PubMed] [Google Scholar]
- 23.Tian T, Tsoi JK, Matinlinna JP, Burrow MF. Aspects of bonding between resin luting cements and glass ceramic materials. Dent Mater. 2014;30:e147–62. doi: 10.1016/j.dental.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Kim BK, Bae HE, Shim JS, Lee KW. The influence of ceramic surface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. J Prosthet Dent. 2005;94:357–62. doi: 10.1016/j.prosdent.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Barghi N, Fischer DE, Vatani L. Effects of porcelain leucite content, types of etchants, and etching time on porcelain-composite bond. J Esthet Restor Dent. 2006;18:47–52. doi: 10.2310/6130.2006.00001. [DOI] [PubMed] [Google Scholar]
- 26.Estafan D, Dussetschleger F, Estafan A, Jia W. Effect of prebonding procedures on shear bond strength of resin composite to pressable ceramic. Gen Dent. 2000;48:412–6. [PubMed] [Google Scholar]
- 27.Heravi F, Moazzami SM, Dehghani M. Effects of different surface preparations on shear bond strength of orthodontic brackets to porcelain. J Calif Dent Assoc. 2010;38:794–9. [PubMed] [Google Scholar]
- 28.Pattanaik S, Wadkar AP. Effect of etchant variability on shear bond strength of all ceramic restorations – An in vitro study. J Indian Prosthodont Soc. 2011;11:55–62. doi: 10.1007/s13191-011-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalavacharla VK, Lawson NC, Ramp LC, Burgess JO. Influence of etching protocol and silane treatment with a universal adhesive on lithium disilicate bond strength. Oper Dent. 2015;40:372–8. doi: 10.2341/14-116-L. [DOI] [PubMed] [Google Scholar]
- 30.Colares RC, Neri JR, Souza AM, Pontes KM, Mendonça JS, Santiago SL. Effect of surface pretreatments on the microtensile bond strength of lithium-disilicate ceramic repaired with composite resin. Braz Dent J. 2013;24:349–52. doi: 10.1590/0103-6440201301960. [DOI] [PubMed] [Google Scholar]
- 31.Duzyol M, Sagsoz O, Sagsoz NP, Akgul N, Yildiz M. The effect of surface treatments on the bond strength between CAD/CAM blocks and composite resin. Dent Mater. 2013;29:e71–2. doi: 10.1111/jopr.12322. [DOI] [PubMed] [Google Scholar]
- 32.Neis CA, Albuquerque NL, Albuquerque Ide S, Gomes EA, Souza-Filho CB, Feitosa VP, et al. Surface treatments for repair of feldspathic, leucite – And lithium disilicate-reinforced glass ceramics using composite resin. Braz Dent J. 2015;26:152–5. doi: 10.1590/0103-6440201302447. [DOI] [PubMed] [Google Scholar]
- 33.Dilber E, Yavuz T, Kara HB, Ozturk AN. Comparison of the effects of surface treatments on roughness of two ceramic systems. Photomed Laser Surg. 2012;30:308–14. doi: 10.1089/pho.2011.3153. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Aboushelib MN, Feilzer AJ. Strength influencing variables on CAD/CAM zirconia frameworks. Dent Mater. 2008;24:633–8. doi: 10.1016/j.dental.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Shiu P, De Souza-Zaroni WC, Eduardo Cde P, Youssef MN. Effect of feldspathic ceramic surface treatments on bond strength to resin cement. Photomed Laser Surg. 2007;25:291–6. doi: 10.1089/pho.2007.2018. [DOI] [PubMed] [Google Scholar]
- 36.Zarif Najafi H, Oshagh M, Torkan S, Yousefipour B, Salehi R. Evaluation of the effect of four surface conditioning methods on the shear bond strength of metal bracket to porcelain surface. Photomed Laser Surg. 2014;32:694–9. doi: 10.1089/pho.2014.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahrari F, Boruziniat A, Alirezaei M. Surface treatment with a fractional CO2 laser enhances shear bond strength of resin cement to zirconia. Laser Ther. 2016;25:19–26. doi: 10.5978/islsm.16-OR-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papia E, Larsson C, du Toit M, Vult von Steyern P. Bonding between oxide ceramics and adhesive cement systems: A systematic review. J Biomed Mater Res B Appl Biomater. 2014;102:395–413. doi: 10.1002/jbm.b.33013. [DOI] [PubMed] [Google Scholar]
- 39.Shahabi M, Ahrari F, Mohamadipour H, Moosavi H. Microleakage and shear bond strength of orthodontc brackets bonded to hypomineralized enamel following different surface preparations. J Clin Exp Dent. 2014;6:e110–5. doi: 10.4317/jced.51254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano C, Selaj D, Manzon E, Pasqualini D, Berutti E, Scotti N. Bonding to zirconia and lithium-disilicate: Analysis of different chemical treatments. Dent Mater. 2014;30:e22. [Google Scholar]


