Abstract
Background:
Airway obstruction or tracheoesophageal fistula (TEF) near the tracheal carina requires placement of Y-shaped stents. Herein, we describe our multicenter experience with the placement of Dumon silicone Y-stents. We also conduct a systematic review for studies describing the deployment of airway silicone Y-stents.
Methods:
This was a retrospective analysis of consecutive subjects who underwent placement of silicone Y-stents. The clinical details including the underlying diagnosis, indication for the placement of silicone Y-stents, success of stent placement, and follow-up are presented. The PubMed and EMBASE databases were also reviewed for studies describing the placement of silicone Y-stents.
Results:
During the study, 27 silicone Y-stents were placed. The mean (standard deviation) age of the study population (85.2% males) was 57.7 (13.5) years. The stents were placed for airway obstruction in 77.8% and TEF in 29.6% of the patients. The most common underlying disease was carcinoma of the esophagus. The degree of airway obstruction was grade 3–4 in 18 subjects, and respiratory failure was encountered in 18 subjects. The stent was deployed successfully in all the subjects. No deaths were encountered during stent placement. Most subjects had rapid relief of symptoms following the procedure. Excessive secretions and mucostasis were the most common stent-related complications followed by the development of granulation tissue. The systematic review yielded nine studies (338 subjects with airway obstruction and/or TEF). The most common indication for silicone Y-stent placement was tracheobronchial obstruction and TEF due to malignancy. Benign disorders that necessitated stent placement included postintubation tracheal stenosis, airway malacia, and others. The stent was successfully placed in 98% with only one periprocedural death. Granulation tissue formation and mucostasis were the most common stent-related complications.
Conclusion:
Placement of silicone Y-stent is a safe and effective procedure that provides quick relief of symptoms in subjects presenting with airway obstruction and TEF at or near the tracheal carina.
KEY WORDS: Airway stent, central airway obstruction, esophageal cancer, lung cancer, tracheal stenosis
INTRODUCTION
Airway stents are indicated in various benign or malignant conditions either to restore luminal patency in central airway obstruction (CAO) or to maintain luminal integrity in cases of tracheoesophageal fistula (TEF).[1,2] Broadly, airway stents can be classified into two types, namely, metallic and silicone. The first use of silicone (tube) stents was described by Trendelenburg, who designed a prosthesis that could avoid aspiration during tracheostomy.[3] Subsequently, Montgomery described a special T-tube made of silicone rubber that was used primarily for tracheal stenosis in the subglottic region.[4] However, it was Dumon, who revolutionized the design of silicone airway stents with the description of a dedicated tracheobronchial silicone stent.[5] Airway silicone stents can either be straight or Y-shaped. The straight stent is deployed for conditions involving the upper or mid trachea or the main-stem bronchi. On the other hand, the Y-stent is best suited for lesions involving the lower trachea, tracheal carina, the main-stem bronchi, and the secondary carina.[2,6,7,8] Herein, we describe our multicenter experience with the placement of Dumon silicone Y-stent in the management of benign and malignant diseases involving the lower end of the trachea or the tracheal carina. We also perform a systematic review of literature for studies describing the use of silicone Y-stent for the management of CAO and TEF.
METHODS
This is a retrospective analysis of data collected between January 2012 and May 2016 at seven centers across India (Apollo Hospital, Bengaluru; Kovai Medical Center, Coimbatore; Jaipur Golden Hospital and Rajiv Gandhi Cancer Institute and Research Center, New Delhi; Century Hospital, Hyderabad; Department of Pulmonary Medicine and Sleep Disorders, All India Institute of Medical Sciences, New Delhi; and Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh). The study protocol was approved by the Institute Ethics Committee of all the participating centers. A consent waiver was allowed as this was a retrospective study describing the use of anonymized patient data. However, a procedural consent was obtained from all subjects.
Subjects
The bronchoscopy database of each participating center was searched for records of subjects who underwent placement of silicone Y-stents. The following information was retrieved from the database: demographic details, clinical diagnosis, presence of respiratory failure (defined as a PaO2 <60 mmHg or a PaCO2 >45 mmHg), indication for the placement of silicone Y-stent, details of the Y-stent, site of airway obstruction, degree of airway obstruction, presence of TEF, duration of procedure, success of stent placement, procedure- and stent-related complications, duration of follow-up, and the final outcome.
Study procedure
Flexible bronchoscopy was performed for airway assessment before Y-stent placement, wherever feasible, to ascertain the airway anatomy and the site of obstruction (or TEF). The severity of obstruction was assessed by maneuvering the flexible bronchoscope across the area of obstruction and estimating the lumen size in comparison to the outer diameter of the respective bronchoscope. The degree of luminal obstruction was graded as grade 1: <50%, Grade 2: 50%–74%, Grade 3: 75%–89%, and Grade 4: 90%–100%.[9] The size of the stent was decided on the basis of the airway measurements performed on computed tomography of the thorax and flexible bronchoscopy.
Y-stent placement
All the subjects received a silicone Dumon Y-stent (Novatech, France) with a tracheal limb of 16–18 mm diameter and 4–6 cm length, and bronchial limbs of 13–14 mm diameter (length for the left and right main bronchi being 2.5–3.5 cm and 1.5–2 cm, respectively). The stent was deployed during rigid bronchoscopy performed under general anesthesia as previously described.[6,10]
Briefly, the trachea was intubated with a large lumen rigid bronchoscope (internal diameter, 14 mm). The stent was folded in a stent folding assembly (Tonn Tracheobronchial Stent Applicator, Novatech, France) with the right bronchial limb facing upward, and then loaded in the introducer tube with the help of a loading rod. The stent was placed using the “pull technique” wherein the distal end of the rigid bronchoscope is positioned in the left main bronchus. The introducer tube with the stent-in-situ was introduced into the rigid barrel, and the stent was then pushed gently with a pusher rod while simultaneously retracting the rigid bronchoscope barrel, until the entire stent was deployed. The proper placement of the stent was confirmed using flexible bronchoscopy. If required, the stent was gently manipulated with a rigid forceps.
Systematic review
This review was conducted in accordance with guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[11]
Search strategy
We searched the PubMed and the EMBASE databases (till June 15, 2016) using the following free text terms: (“silicone y stent” OR “tracheobronchial y stent” OR “Dumon y stent” OR “bifurcation tracheobronchial stent” OR “airway silicone stents” OR “airway silicon stents”). We reviewed the reference list of all the included articles and previous review articles. In addition, we sifted through our personal files.
Inclusion criteria
We included studies that had described the use of silicone Y-stent in at least ten subjects. We excluded the following type of studies: (a) case reports, abstracts, comments, editorials, and reviews; (b) studies that did not provide details about silicone Y-stent separately; and (c) studies published in language other than English.
Initial review of studies
The electronic searches were assimilated in a reference manager package and all duplicate citations were discarded. Two authors (ISS, RA) screened these citations by review of the title and abstract to identify the relevant studies. Any disagreement was resolved by consensus between the authors. The database was then scrutinized again to include only primary articles. The full text of each of these studies was obtained and reviewed in detail.
Study selection and data abstraction
The data were extracted into a standard data extraction form, and the following information was catalogued: (i) publication details (authors, year of publication, country where the study was conducted); (ii) study design (randomized controlled trial or observational); (iii) number of subjects and inclusion criteria; (iv) demographic profile; (v) underlying disease and the indication for placement of silicone Y-stent; (vi) symptomatology; (vii) details of stent including the type of stent, diameter of tracheal, and bronchial limbs; (viii) outcome of stent placement; (ix) complications (procedure- and stent-related); and (x) final outcome of subjects during follow-up.
Data analysis
Data are presented in a descriptive fashion as mean with standard deviation (SD) or number with percentage.
RESULTS
During the study, 27 silicone Y-stents were placed. The mean (SD) age of the study population (85.2% males) was 57.7 (13.5) years. The indications for stent placement were CAO (77.8%) and TEF (29.6%). The most common underlying disease causing CAO was carcinoma of the esophagus (33.3%), followed by adenoid cystic carcinoma, and lung carcinoma [Table 1]. The other causes of CAO included extrinsic compression (n = 2), carcinoma breast with paratracheal mass (n = 1), tracheobronchial amyloidosis (n = 1), large cell neuroendocrine tumor of the trachea (n = 1), tracheal leiomyoma (n = 1), postintubation tracheal stenosis (n = 1), and tracheal sarcoma (n = 1). TEF was most commonly caused by tracheal/tracheobronchial invasion by carcinoma of the esophagus (n = 6). Benign causes of TEF included traumatic injury to tracheal mucosa after road traffic accident (n = 1) and endobronchial tuberculosis (n = 1).
Table 1.
Demographic, clinical, and procedural details of the study population (n=27)
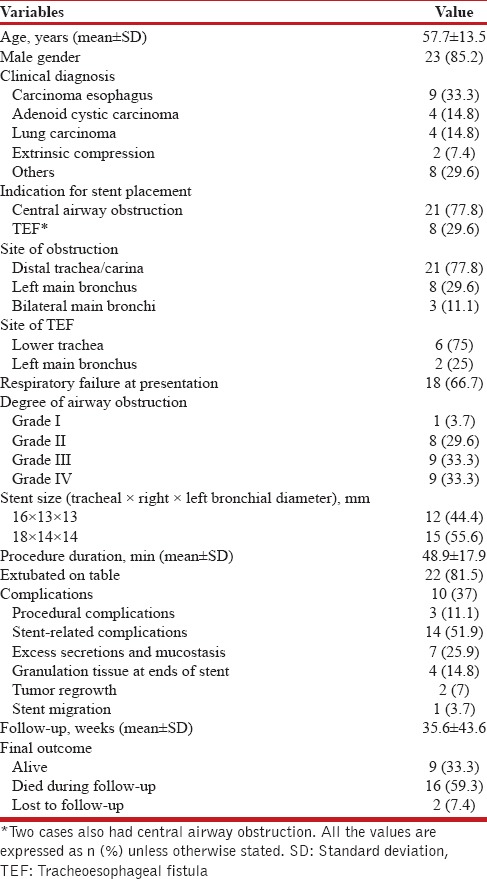
The site of obstruction was located at the lower end of the trachea or carina in 21 (77.8%) subjects and involved origin of the left main bronchus in eight subjects. In three subjects, both the main bronchi were involved by malignant infiltration causing luminal obstruction of approximately 80%–90%. The degree of airway obstruction was grade 3–4 in 18 of the 27 (66.7%) subjects with CAO. Respiratory failure was encountered in 18 (66.7%) subjects at presentation. The mean (SD) duration of the procedure was 48.9 (17.9) min. The stent was successfully deployed in all subjects. Twenty-two (81.6%) subjects were extubated on the operating room table while five subjects required endotracheal intubation after the procedure for a mean duration of 8.9 (9.5) h. All subjects experienced relief of symptoms after the procedure, and there was rapid resolution of respiratory failure following stent deployment.
Three subjects had procedure-related complications that included trauma to teeth, increase in the size of the fistula due to inadvertent deployment of stent in the fistulous tract, and myocardial infarction. Excess secretions and mucostasis were seen in seven subjects and were managed with bronchoscopic toileting. All subjects were also advised twice daily nebulization of ambroxol (Inhalex, Cipla, India, 15 mg) and normal saline. Subjects were also encouraged to use a cough-assist device (Acapella, Smiths Medical, Kent, UK). Development of granulation tissue at either end of the stent was another common complication encountered during follow-up. Granulation tissue was treated with argon plasma coagulation (APC) and systemic steroids. One subject also developed bronchoesophageal fistula at the lower end of the left bronchial limb. The stent was removed, and the subject underwent surgical repair of the fistula. In two subjects with adenoid cystic tracheal tumor, there was recurrence of the tumor with ingrowth of the tumor at the lower end of the stent that was managed successfully using APC. In one subject, the stent migrated proximally 2 days after the procedure. The stent was then removed and redeployed successfully with no subsequent stent migration. There was no procedure-related mortality. The mean duration of follow-up was 35.6 weeks. Two subjects were lost to follow-up. Sixteen subjects died during follow-up due to progression of the underlying malignancy. The stent was removed in three subjects. In one subject, this was because of intractable cough while in two other subjects (one each with traumatic TEF and endobronchial tuberculosis), the stent was successfully removed after healing of the fistula.
Systematic review
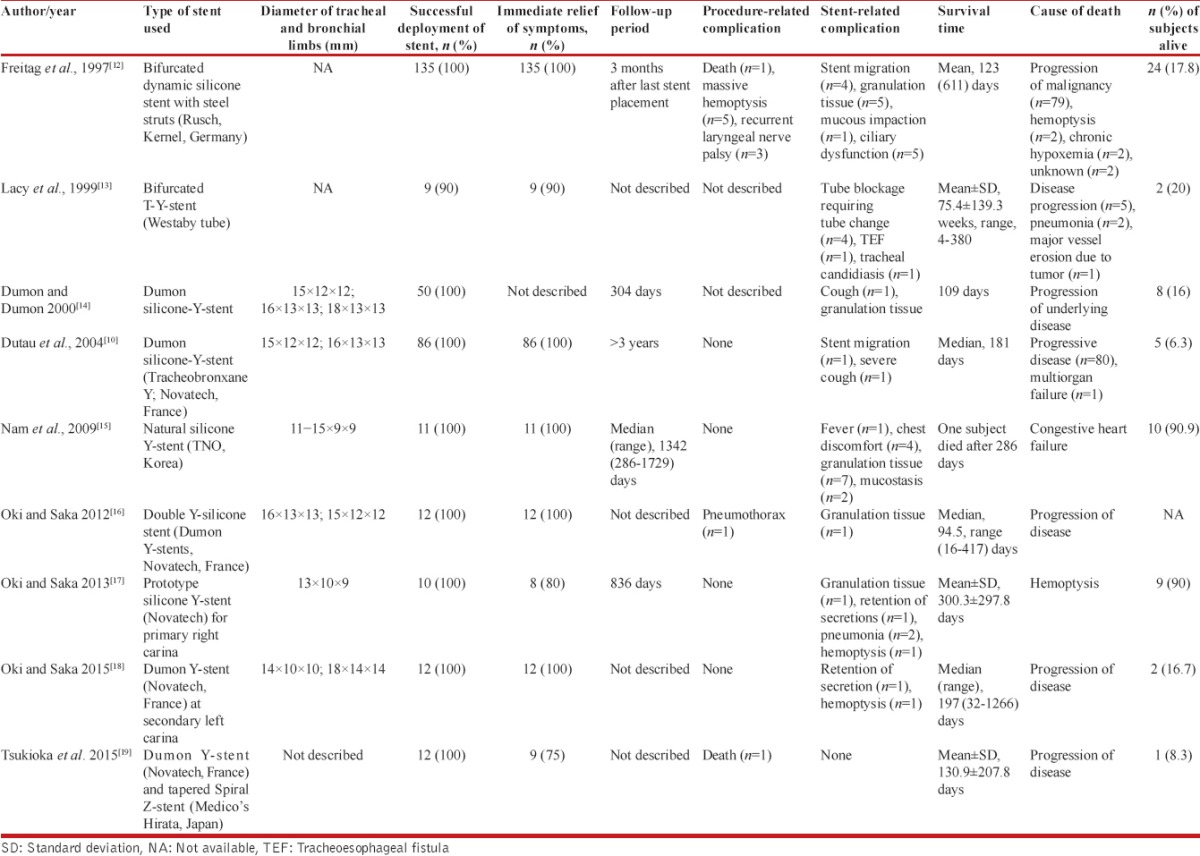
Our search yielded 1006 citations, of which nine studies (338 subjects [346 silicone Y-stents] with CAO and/or TEF) have described the use of the silicone Y-stent for the treatment of tracheobronchial obstruction or TEF involving the tracheal carina or the main stem bronchi [Figure 1]. All the studies had a retrospective study design and were single-center studies. The studies have described the use of different types of silicone Y-stent [Tables 2 and 3].[10,12,13,14,15,16,17,18,19] The most common indication for deploying silicone Y-stent was tracheobronchial obstruction and TEF due to malignancy.[10,17,18,20] Postintubation tracheal stenosis, airway malacia, and others were the benign disorders that necessitated stent placement [Table 2].[12,13,15] Malignancy (285, 84.3%) was the most common underlying disease that caused CAO and/or TEF. Severe dyspnea and respiratory distress were the most common presenting symptoms. Stents could be successfully deployed in 98.3% of the cases. The placement of stent resulted in an immediate relief of symptoms in 97.8% (286/292) subjects. The deployment of Y-stent was associated with complications in 16.6% (56/338) of the subjects. There were ten procedure-related complications (including one mortality) and 46 stent-related complications. The most common stent-related complication was granulation tissue formation followed by mucostasis [Table 3]. In five patients, there was migration of the stent. The mean duration of survival after stent placement ranged from 109 days to 528 days. Progression of underlying malignancy was the most common cause of death during follow-up.
Figure 1.
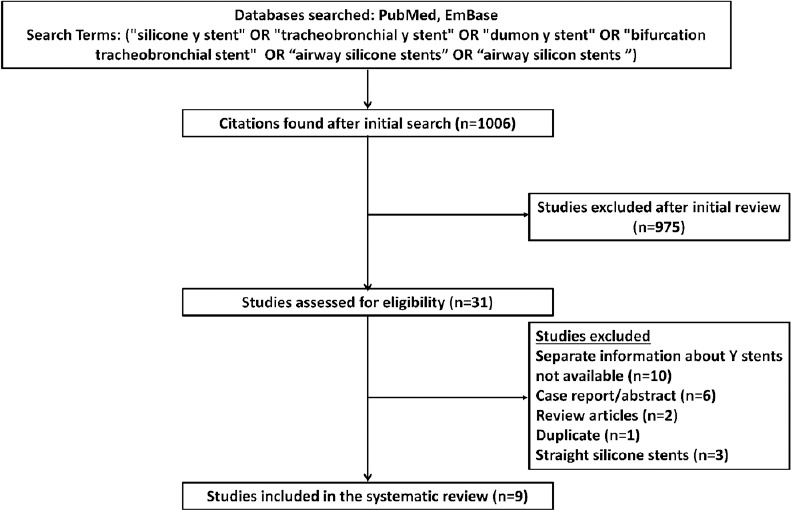
Study selection process for the systematic review
Table 2.
Baseline characteristics of the study participants in studies describing the use of silicone Y-stent
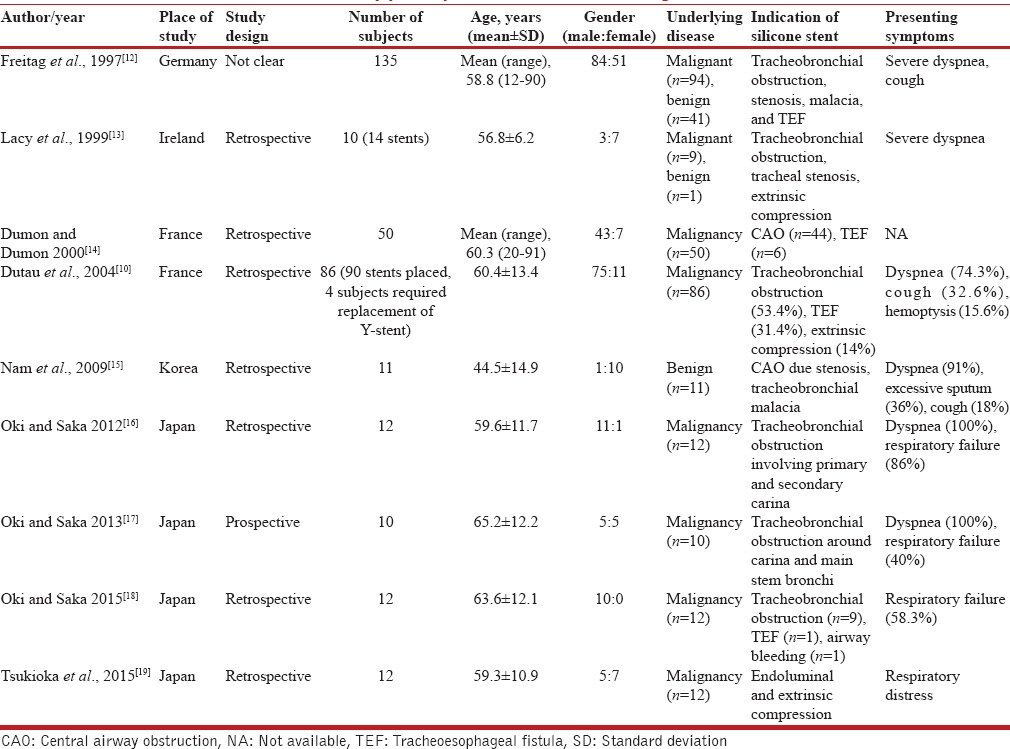
Table 3.
Technical aspects of stents and outcomes of subjects in studies describing the use of silicone Y-stent
DISCUSSION
The results of the current study and the systematic review suggest that placement of silicone Y-stents is a safe and effective treatment option in the management of benign and malignant disorders involving the lower trachea and the tracheal carina. Placement of the silicone Y-stent resulted in rapid relief of patients’ symptoms including the resolution of respiratory failure. The silicone Y-stents were easily deployed with few procedure-related and stent-related complications.
The Y-stents, similar to other airway stents, are available either as metallic or silicone. The metallic Y-stents being self-expanding are easy to deploy and can be inserted either during flexible or rigid bronchoscopy.[21] They uncommonly migrate and generate considerable force to distend the airway such that airway dilatation is not always required before stent placement. Because of the aforementioned benefits, there has been a substantial reduction in the use of silicone Y-stents at all the authors’ institutes.[21] The metallic stent, however, has a few limitations including difficulty in removal or repositioning of stent after epithelialization, which usually occurs at about 8 weeks.[22] In addition, the metallic stent can fracture, and the broken filaments can damage the mucosa.[23] Thus, they are primarily indicated for conditions where the survival of patient is limited as in malignant disorders. On the contrary, silicone Y-stents always require rigid bronchoscopy for deployment but are easy to remove. Hence, they can be used for both benign and malignant conditions.[6] In addition, the internal surface of the stent is varnished with silicone to reduce the porous nature of the stent. This makes the stent smooth thus minimizing the chance of retention of secretions. In the authors’ opinion, silicone Y-stents are the preferred stents in any benign condition or in patients where the survival is judged to be more than four to 6 months. In all the previous studies and even in the present study, the stent could be easily placed, and a majority of the patients were relieved of their symptoms immediately after the procedure. In fact, one-third of the patients in the current study faced an imminent threat of death due to severe airway obstruction (>90%). This suggests that silicone Y-stents can be used as a palliative measure in subjects with critical airway obstruction.[14,17,18,19,20]
The first description of silicone Y-stent in a large series of patients was that of the dynamic Y-stent.[12] The stent is primarily made of silicone but is reinforced by metallic rings along the anterior three-fourths. The stent is placed under direct laryngoscopic vision by folding the stent with special long forceps. The stent is then negotiated through the vocal cords and placed over the carina with or without fluoroscopic guidance. The position of the stent is subsequently confirmed using the rigid bronchoscope. In the aforementioned study, the authors could successfully place the stent in all but two subjects.[12] The Dumon silicone Y-stent was first described in fifty subjects with malignant airway condition [Tables 2 and 3].[14] Subsequently, in the largest series of 86 subjects, Dutau et al. described the successful use of Dumon silicone Y stents in CAO and TEF due to underlying malignancy.[10] The other types of modified silicone Y-stents described are the T-Y-stents (tracheostomy tubes with distal Y-limbs for bronchi),[13] natural silicone-T stent,[15] double Y-stents, and hood silicone Y-stents. Oki and Saka described a novel technique wherein they placed two silicone Y-stents to treat CAO involving tracheal carina and right secondary carina.[16] In a later study, the same authors used a prototype silicone Y-stent to manage CAO involving the right secondary carina.[17] They also later described the use of smaller silicone Y-stents for the management of CAO or TEF at the left secondary carina in twelve subjects.[18] Nam et al. described a prototype natural Y-stent in eleven subjects that had horizontal c-shaped threads and an interposing flexible posterior wall to mimic the posterior membranous trachea.[15] Most of the study subjects had undergone a prior surgical procedure (carinal resection and anastomosis, tracheostomy or external stent placement using a vascular graft) before undergoing placement of silicone Y-stent. The stent was correctly placed in all the subjects.
The placement of silicone Y-stent was found to be safe. In fact, there was only one periprocedural death reported in the studies included in the systematic review.[12] Granulation tissue formation and mucostasis were the most common stent-related complications. This was also highlighted in the current study where mucostasis and granulation tissue formation were the most common stent-related complications. The overall survival of subjects in the current study and in the previous studies involving the silicone Y-stent is rather poor.[10,14,17,18,20] This is because most of the study subjects had advanced malignancy as the underlying cause. In the study that included cases with benign causes only, there was no death, and the patients tolerated stents for prolonged periods of time.[15] This is similar to the subjects in our study where all the patients with benign causes (traumatic TEF, amyloidosis, and post-intubation tracheal stenosis) were alive at the last follow-up whereas most of the subjects with malignant disease had died due to the progression of the underlying malignancy.
Finally, our study has a few limitations. It is a retrospective study with a small sample size. In addition, we do not have information on the proportion of patients presenting with CAO to the participating centers that was selected for the placement of silicone Y-stent. However, the current study is the first multicenter study describing the perspective on outcomes of silicone Y-stent deployment from a developing country, which makes the study unique. In fact, most of the previous studies are single-center studies from authors who had actually developed the stents and the complication rates and outcomes may not be a true reflection of the actual practice elsewhere. In contrast, the current multicenter study provides perspective from several centers and is likely to reflect a real world scenario.
CONCLUSION
Placement of the silicone Y-stent is a safe procedure that provides rapid relief of symptoms in subjects presenting with critical CAO and/or TEF at or near the tracheal carina.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mehta AC, Dasgupta A. Airway stents. Clin Chest Med. 1999;20:139–51. doi: 10.1016/s0272-5231(05)70132-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee P, Kupeli E, Mehta AC. Airway stents. Clin Chest Med. 2010;31:141–50. doi: 10.1016/j.ccm.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Trendelenburg F. Beitrage zu den Operationen an den Luftwegen. Langenbecks Arch Chir. 1872;13:335. [Google Scholar]
- 4.Montgomery WW. T-tube tracheal stent. Arch Otolaryngol. 1965;82:320–1. doi: 10.1001/archotol.1965.00760010322023. [DOI] [PubMed] [Google Scholar]
- 5.Dumon JF. A dedicated tracheobronchial stent. Chest. 1990;97:328–32. doi: 10.1378/chest.97.2.328. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Khan A, Aggarwal AN, Singh N, Bhagat H, Kumar B, et al. Initial experience of endobronchial silicon stents from a tertiary care centre in North India. Indian J Chest Dis Allied Sci. 2011;53:93–8. [PubMed] [Google Scholar]
- 7.Gildea TR, Murthy SC, Sahoo D, Mason DP, Mehta AC. Performance of a self-expanding silicone stent in palliation of benign airway conditions. Chest. 2006;130:1419–23. doi: 10.1378/chest.130.5.1419. [DOI] [PubMed] [Google Scholar]
- 8.Santacruz JF, Folch E, Mehta AC. Silicone and metallic stents in interventional pulmonology. Minerva Pneumol. 2009;48:243–59. [Google Scholar]
- 9.Myer CM, 3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994;103(4 Pt 1):319–23. doi: 10.1177/000348949410300410. [DOI] [PubMed] [Google Scholar]
- 10.Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: A retrospective review of 86 patients. Chest. 2004;126:951–8. doi: 10.1378/chest.126.3.951. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 12.Freitag L, Tekolf E, Stamatis G, Greschuchna D. Clinical evaluation of a new bifurcated dynamic airway stent: A 5-year experience with 135 patients. Thorac Cardiovasc Surg. 1997;45:6–12. doi: 10.1055/s-2007-1013675. [DOI] [PubMed] [Google Scholar]
- 13.Lacy PD, Fenton JE, Smyth DA, Colreavy MP, Walsh MA, O’Dwyer TP, et al. The Westaby T-Y tracheobronchial stent in otolaryngology. J Laryngol Otol. 1999;113:652–6. doi: 10.1017/s0022215100144755. [DOI] [PubMed] [Google Scholar]
- 14.Dumon JF, Dumon MC. Dumon-novatech Y-stents: A four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7:26–32. [Google Scholar]
- 15.Nam HS, Um SW, Koh WJ, Suh GY, Chung MP, Kwon OJ, et al. Clinical application of the natural Y stent in the management of benign carinal stenosis. Ann Thorac Surg. 2009;88:432–9. doi: 10.1016/j.athoracsur.2009.04.083. [DOI] [PubMed] [Google Scholar]
- 16.Oki M, Saka H. Double Y-stenting for tracheobronchial stenosis. Eur Respir J. 2012;40:1483–8. doi: 10.1183/09031936.00015012. [DOI] [PubMed] [Google Scholar]
- 17.Oki M, Saka H. New dedicated bifurcated silicone stent placement for stenosis around the primary right carina. Chest. 2013;144:450–5. doi: 10.1378/chest.12-2834. [DOI] [PubMed] [Google Scholar]
- 18.Oki M, Saka H. Silicone Y-stent placement on the secondary left carina. Respiration. 2015;90:493–8. doi: 10.1159/000441305. [DOI] [PubMed] [Google Scholar]
- 19.Tsukioka T, Takahama M, Nakajima R, Kimura M, Tei K, Yamamoto R. Sequential stenting for extensive malignant airway stenosis. Ann Thorac Cardiovasc Surg. 2015;21:114–8. doi: 10.5761/atcs.oa.14-00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oki M, Saka H, Kitagawa C, Kogure Y, Mori K, Kajikawa S, et al. Double Y-stent placement for tracheobronchial stenosis. Respiration. 2010;79:245–9. doi: 10.1159/000216830. [DOI] [PubMed] [Google Scholar]
- 21.Madan K, Dhooria S, Sehgal IS, Mohan A, Mehta R, Pattabhiraman V, et al. A multicenter experience with the placement of self-expanding metallic tracheobronchial Y stents. J Bronchology Interv Pulmonol. 2016;23:29–38. doi: 10.1097/LBR.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire A, Burfeind WR, Toloza E, Balderson S, Petersen RP, Harpole DH, Jr, et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg. 2005;80:434–7. doi: 10.1016/j.athoracsur.2005.02.071. [DOI] [PubMed] [Google Scholar]
- 23.Chung FT, Chen HC, Chou CL, Yu CT, Kuo CH, Kuo HP, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: A cohort study. J Cardiothorac Surg. 2011;6:46. doi: 10.1186/1749-8090-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]


