Abstract
Background:
Oxidant-antioxidant imbalance forms a prime component in pathogenesis of chronic obstructive pulmonary disease (COPD). Studies of oxidative stress markers in South Asians were sparse.
Methods:
One hundred and eighty COPD patients and eighty healthy nonsmokers were enrolled in the study. Serum malondialdehyde (MDA) and iron levels were estimated for oxidative stress. Three antioxidant markers evaluated-catalase, superoxide dismutase (SOD), and serum copper. Patients on antioxidant therapy and with sepsis and chronic illness were excluded from the study.
Results:
The mean age of COPD patients was 59.29 ± 10.3 years. Serum levels of MDA and iron were significantly higher in COPD patients compared to controls (5.21 ± 1.9 vs. 0.71 ± 0.29 nmol MDA/ml, P = 0.0001 and 69.85 ± 85.49 vs. 79.32 ± 24.39 μg/dl, P = 0.0001, respectively). Mean level of all antioxidant enzymes catalase, SOD, and copper were significantly diminished in cases when compared to control population (P = 0.001). Levels of MDA and iron were found to be significantly elevated in higher Global Initiative for Chronic Obstructive Lung Disease (GOLD) classes (III, IV) when compared to lower GOLD Classes (I, II). The levels of serum antioxidants were significantly depleted in higher GOLD grades too. COPD patients who were male and smoked had significantly higher levels of oxidants and depleted antioxidant levels compared to female and nonsmoking compatriots. Serum MDA levels negatively correlated with forced expiratory volume 1 s and forced vital capacity (r = −0.19 and r = −0.21, P ≤ 0.01). The presence of a cough significantly correlated with higher levels of MDA and iron (P = 0.001). The levels of MDA negatively correlated with SOD and catalase levels.
Conclusion:
Oxidative markers (MDA and iron) are higher whereas antioxidants (catalase, copper, and SOD) are significantly reduced in patients of COPD. Serum MDA levels correlate with lung functions and disease severity.
KEY WORDS: Chronic obstructive pulmonary disease, malondialdehyde smoking, oxidative stress
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality worldwide.[1,2] The disease is characterized as chronic irreversible inflammatory damage, predominantly in small airways, and lung parenchyma.[3] The pathogenic triad of COPD consists of oxidative stress, protease-antiprotease imbalance, and inflammation, of which oxidative stress forms prime component.[4] Oxidative stress affects airways by myriad mechanisms including mucus hypersecretion, damage to airway epithelium, neutrophil influx, airway inflammation, and increased apoptosis.[5] Several processes lead to oxidative stress-related tissue damage, primary among them is lipid peroxidation which leads to the formation of various lipid hydroperoxides and aldehydic products.[6,7,8] Many markers of oxidative stress have been shown to have direct correlation among themselves and severity of airway obstruction represented by forced expiratory volume 1 s (FEV1).[9] Simultaneously, other studies have highlighted the deficiency of native antioxidant defense mechanisms of lungs in COPD. The important naturally occurring antioxidants in body include glutathione system, catalase, and superoxide dismutase (SOD) system.[10,11] Trace elements such as iron, zinc, and copper have also been proposed to involved in oxidant-antioxidant cycle in COPD.[12] Markers of oxidant–antioxidant imbalance can be studied from blood, exhaled breath, sputum, and bronchoalveolar lavage.[13,14] However, large studies regarding oxidative stress in COPD patients in Asian Indians are sparse. The present study was designed to study the serum markers oxidant–antioxidant imbalance in COPD patients and their association with disease progression or severity.
METHODS
Study subjects
Patients of COPD presenting to outpatient, indoor, and intensive care unit in the Department of Respiratory Medicine were studied from August 2014 to August 2015. Healthy nonsmoker subjects with no pulmonary, cardiovascular, or oncological disease, inflammation, infection, and neurological dysfunction that could influence the oxidative status were enrolled as controls. COPD was defined according to the Global initiative for Chronic Obstructive Lung Disease (GOLD) criteria.[1] The lung functions were analyzed using Mir Spirolab II spirometer and the patients with COPD were selected and grouped into mild, moderate, severe, and very severe severity group according to the GOLD criteria. Subjects with history of tuberculosis, bronchial asthma, diabetes mellitus, hypertension, lung cancer, cardiovascular, renal diseases, chronic hepatic failure, and prior antioxidant intake were excluded from the study. The study was approved by local ethics board and performed in accordance with ethical standards outlined in the Declaration of Helsinki. Detailed written and informed consent was taken from all subjects.
Markers of oxidative stress
Malondialdehyde (MDA) was estimated according to the method of Stocks and Dormandy.[15] The levels were estimated as nmol MDA/ml.
Estimation of catalase was done by the method described by Aebi.[16] Activity of catalase was estimated as U/ml.
Estimation of SOD was done by the method of McCord and Fridovich[17] and measured in U/ml.
Statistical analysis
The results were presented in mean ± standard deviation and percentages. The Chi-square test was used to compare the categorical variables. The unpaired t-test was used to compare two discrete variables. The one-way analysis of variance was used to compare more than two discrete variables. Pearson correlation coefficient was calculated to find the direction of association between two discrete variables. The P < 0.05 was considered statistically significant. All the analyses were carried out using SPSS 16.0 version (SPSS Inc., Chicago, IL, USA).
RESULTS
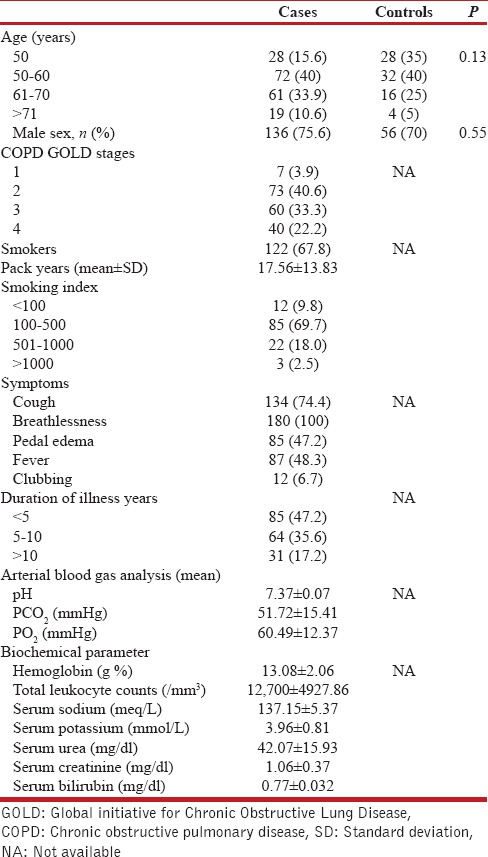
A total of 180 patients of COPD were enrolled in the study. Eighty age- and sex-matched healthy nonsmoker controls were taken for comparison. The mean age of our study population was 59.29 + 10.3 years. Baseline demographic, clinical, and biochemical features are enumerated in Table 1. Majority of the subjects in our study population were male. Out of 180 COPD patients about two third (67.8%) were smokers and majority of smokers (69.7%) had smoking index in between range of 100–500. majority of the patients were in GOLD Class 2 (40.6%) and almost half of subjects had a duration of illness <5 years. Cough and breathlessness were predominant symptoms in study patients. Mean PCO2 levels were elevated as was mean total leukocyte count.
Table 1.
Baseline demographic, clinical, and biochemical parameters of study subjects
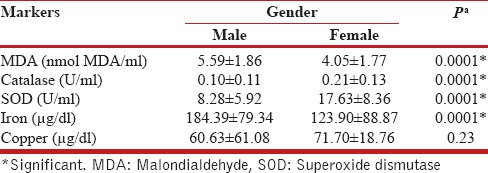
Results of biomarker analysis of the study are presented in Table 2. MDA levels, which is a marker of oxidative stress, were significantly higher in COPD patients than controls (5.21 ± 1.9 vs. 0.71 ± 0.29 nmol MDA/ml, P = 0.0001) [Table 2 and Figure 1b] and serum iron levels were also significantly elevated in patients vis-a-vis controls (169.85 ± 85.49 vs. 79.32 ± 24.39 μg/ml, P = 0.0001). On the contrary, mean level of antioxidant enzymes catalase (0.13 ± 0.12 U/ml), and SOD (10.57 ± 7.71 U/ml) were significantly diminished in cases compared to control population and the levels of serum copper were also significantly diminished in cases (63.33 ± 54.04 vs. 102.02 ± 10.88 μg/ml, P = 0.0002); [Table 2 and Figure 1a, c and d]. Among patients of COPD, the serum level of all biomarkers of oxidative stress (MDA and serum iron) was significantly higher in males compared to females [P = 0.0001, Table 3]. Consequently, the antioxidant levels (catalase and SOD) were correspondingly, significantly lower in male population vis-à-vis female cohort (P = 0.0001). All these data point toward a greater oxidative and anti-oxidative imbalance in males when compared to their female COPD counterparts.
Table 2.
Comparison of biomarkers between cases and controls
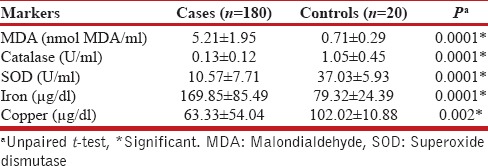
Figure 1.
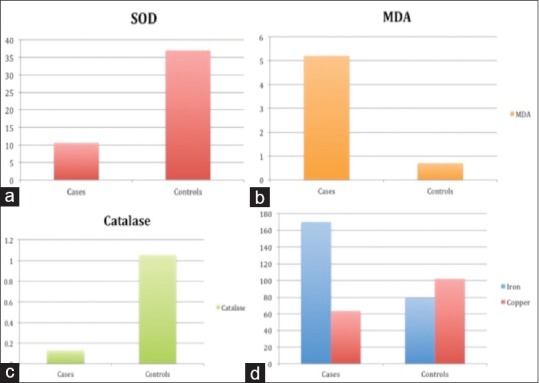
Distribution of biomarkers among chronic obstructive pulmonary disease patients (cases) and controls, (a) superoxide dismutase levels (in U/ml) were significantly decreased in cases (P = 0.0001), (b) serum malondialdehyde levels (in nmol MDA/ml) were significantly higher in cases (P = 0.0001), (c) serum catalase levels (U/ml) were significantly diminished in cases (P = 0.0001), (d) serum iron levels (μ gm/dl, blue bars) were significantly higher (P = 0.0001) in cases and serum copper levels (μ gm/dl, red bars) were significantly lower in cases (P = 0.002)
Table 3.
Comparison of markers between male and female among the chronic obstructive pulmonary disease subjects
Among patients with COPD, the mean levels of serum biomarkers of oxidative stress (MDA and serum iron) were significantly higher in smokers compared to nonsmokers (P = 0.0001), Consequently, the antioxidant levels (catalase, SOD, and copper) were significantly lower in smoking population vis-à-vis nonsmoking cohort (P = 0.0001 for former two and 0.01 for latter). All these data corroborate with a greater oxidative and anti-oxidative pathway imbalance in smokers. However, nonsmoking COPD patients still have significantly higher values of oxidative markers compared to their non-COPD counterparts.
The serum levels of oxidative stress marker MDA were found to be significantly elevated in higher GOLD Classes (III, IV) when compared to lower GOLD Classes (I, II). There was a similar significant pattern of prevalence higher serum iron levels in higher GOLD class and vice versa. The levels of serum antioxidants (catalase and SOD) diminished significantly in higher GOLD grades when compared with lower grades.
There was no difference in oxidative stress markers according to site of enrolment – outpatient, inpatient, or intensive care setting. This implies that oxidative stress is prevalent at all stages of COPD. Similarly, there was no significant difference in distribution of antioxidant enzymes the different care setting from which the patients were enrolled. However, serum copper levels tended to be lower in an outpatient setting compared with other two setting with difference reaching borderline significance (P = 0.08).
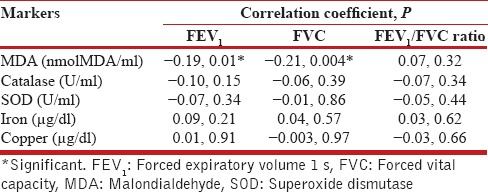
Of the various symptoms, the presence of cough significantly correlated with higher levels of MDA and iron (P =0.001). On the contrary, it predicted lower levels of catalase, SOD, and copper. Cough also more frequent in high COPD severity grades than lower grades (P = 0.04). This could explain the relationship of cough and higher levels of oxidative stress in the study. The levels of MDA correlated positively with number of pack years and increasing age (r = 0.24, P = 0.02 and r = 0.15, P = 0.01). The levels of catalase and SOD negatively correlated with age (r = −0.14, P = 0.05 and r = −0.23, P = 0.001). MDA levels had also negative correlation with FEV1 and forced vital capacity (FVC) (r = −0.19, P = 0.01 and r = −0.21, P = 0.004; [Table 4]). Levels of other markers did not significantly correlate with pack-years, duration of illness, and age.
Table 4.
Correlation of biomarkers with forced expiratory volume 1 s and forced vital capacity among chronic obstructive pulmonary disease subjects
The levels of MDA had negative correlation (r > 0.50, P < 0.01) with catalase, SOD, copper [Figure 2a and b]. Catalase also had negative correlation with iron and copper and positive correlation with SOD. SOD levels positively correlated with copper and inversely with iron.
Figure 2.
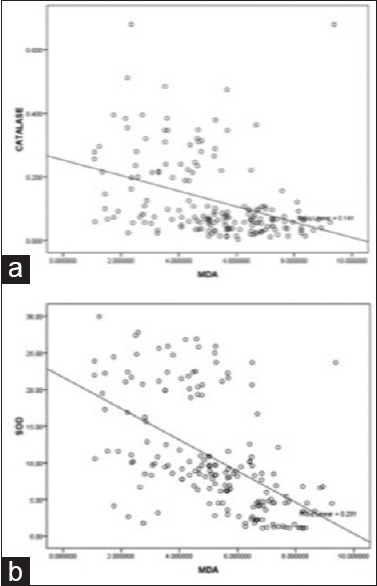
Scatter diagram showing negative linear correlation of malonidialdehyde with catalase (a) and superoxide dismutase (b), respectively
DISCUSSION
The study results support the oxidant and antioxidant imbalance theory of COPD. Lungs are exposed to high levels of free radicals. Production of reactive oxygen species has been found directly linked to oxidation of proteins, DNA, and lipids, which may cause direct lung injury or may induce a variety of cellular responses through the generation of secondary metabolic reactive species. Membrane lipids are highly susceptible to free radical damage which is found to be highly detrimental to the functioning of the cell. MDA is a product of lipid peroxidation and an indirect measure of free radical activity in body. As free radical injury increases lung function decreases. Oxidative stress is reported to play an important role in the pathophysiology of COPD. The aim of the present study was to evaluate the oxidant-antioxidant imbalance in healthy nonsmoker controls and COPD group. The levels of oxidative markers such as MDA were significantly higher in COPD patients when compared with controls. Similarly, the levels of antioxidants – catalase and SOD were significantly reduced in patients of COPD. Various studies have similarly found significantly raised levels of MDA and reduced levels of catalase and SOD in patients of COPD when compared to healthy controls.[18,19] However, very few studies have evaluated role of metals in COPD. We found significantly higher levels of iron (as a marker of oxidative stress) and low copper in COPD patients as compared to controls. The part played by copper in oxidant-antioxidant mechanism is controversial. Some studies have labeled it as an oxidant and demonstrated higher copper levels in COPD patients.[20] Other studies have highlighted role of copper as an integral part of copper-zinc SOD system, and hence, as an antioxidant.[21] In the present study, copper levels were hand in glove with antioxidant enzymes catalase and SOD and inversely correlated with MDA and iron. In a nutshell, copper behaved similar to conventional antioxidants in our study.
Gender and smoking had discriminating effects on oxidative and antioxidant imbalance. In our study, males had higher serum level of all biomarkers of oxidative stress (MDA and serum iron) compared to females. The level of antioxidant enzymes was depleted in females when compared to males. Very few studies have elucidated gender differences in levels of oxidative markers. Our study would be first of its kind to show signal of gender differences of oxidative markers. The higher prevalence of smoking and greater external exposure would be key factors for this difference. Role of sex hormones may have some role. Smoking patients with COPD in our study had higher levels of oxidative stress markers and diminished levels of antioxidant enzymes when compared to nonsmokers. The results are not surprising as cigarette smoke is the prime generator of oxidative free radicals.[22] However, nonsmoking COPD patient do continue to have oxidative stress from other sources such as respiratory infections, inflammation, dust, and air pollution. Depletion of naturally occurring antioxidants also plays a major role in perpetuation of oxidative stress.
With increasing grades of GOLD class for COPD severity, the levels of MDA and iron were progressively increased. Similarly, catalase and SOD were depleted significantly with advancing GOLD stages. Copper levels followed similar trend but failed to show significance.
The elevated oxidative stress biomarkers in stable COPD patients (those attending outpatient department) indicate its predominant role in pathogenesis and not a second fiddle to exacerbations or infections. Surprisingly, the presence of cough indicated high-oxidative stress and diminished antioxidants.
The levels of MDA correlated positively with number of pack years and increasing age. MDA levels had also negative correlation with FEV1 and FVC. The levels of MDA had negative correlation with catalase, SOD, copper. Catalase levels had also negative correlation with iron and positive correlation with SOD and copper.
Our study and various studies[23,24] mentioned clearly prove the point that MDA is the marker which has consistently shown correlation with lung functions, namely, FEV1 and hence disease severity.
The understanding of COPD has undergone a paradigm shift in recent years. It is now considered rather a systemic disease associated with extrapulmonary manifestations such as cardiovascular disease, diabetes, obstructive sleep apnea, and metabolic syndrome.[25] Even subclinical atherosclerosis is common in COPD patients contributing to overall COPD morbidity.[26] Oxidative stress can be the only plausible common pathogenetic link between COPD and other systemic manifestations.
COPD is a chronic disease without any disease modifying therapy till date. Oxidative stress is potential mechanism which can be altered to halt its progression. A biomarker-based (preferably MDA) study can be utilized to assess the efficacy of novel antioxidant or other agents in modifying the course of this disease.
The primary limitations of the study include smaller sample size and lesser number of healthy volunteers compared to diseased subjects. Lack of follow-up of these patients over time to see the temporal trends of these biomarkers values is also one.
CONCLUSION
Oxidative stress markers (MDA and iron) are significantly elevated in North Indian patients of COPD, whereas antioxidant (catalase, SOD, and copper) levels are depleted. Male and smoking COPD patients demonstrate this disparity further. Of these markers, MDA levels correlate with decline in lung functions (FEV1 and FVC).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. A. K. Pradhan, DM Assistant Professor, Department of Cardiology, King George's Medical University, Lucknow, India, for persistent support and motivation.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD): GOLD 2017 global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2017 report. [Last Accessed on 2016 Nov 27]. November 17, 2016. http://goldcopd.org/gold-2017-global-strategy-diagnosismanagement-preve .
- 2.Gupta D, Agarwal R, Aggarwal AN, Maturu VN, Dhooria S, Prasad KT, et al. Guidelines for diagnosis and management of chronic obstructive pulmonary disease: Joint ICS/NCCP (I) recommendations. Lung India. 2013;30:228–67. doi: 10.4103/0970-2113.116248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute (NHLBI) Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases. NHLBI Website. [Last accessed 2015 Dec]. Available from: http://www.nhlbi.nih.gov/research/reports/2012.mortality.chart.book .
- 4.Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–21. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekhuijzen PN, Aben KK, Dekker I, Aarts LP, Wielders PL, van Herwaarden CL, et al. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154(3 Pt 1):813–6. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 6.Adler KB, Holden-Stauffer WJ, Repine JE. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism. J Clin Invest. 1990;85:75–85. doi: 10.1172/JCI114436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusznak C, Mills PR, Devalia JL, Sapsford RJ, Davies RJ, Lozewicz S. Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2000;23:530–6. doi: 10.1165/ajrcmb.23.4.3959. [DOI] [PubMed] [Google Scholar]
- 8.Drost EM, Selby C, Lannan S, Lowe GD, MacNee W. Changes in neutrophil deformability following in vitro smoke exposure: Mechanism and protection. Am J Respir Cell Mol Biol. 1992;6:287–95. doi: 10.1165/ajrcmb/6.3.287. [DOI] [PubMed] [Google Scholar]
- 9.Rogers DF. Mucus hypersecretion in chronic obstructive pulmonary disease. In: Chadwick D, Goode JA, editors. Chronic Obstructive Pulmonary Disease: Pathogenesis to Treatment. UK: John Wiley & Sons; 2001. pp. 65–83. [Google Scholar]
- 10.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 11.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159:473–9. doi: 10.1164/ajrccm.159.2.9804080. [DOI] [PubMed] [Google Scholar]
- 12.Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: State of the art. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad A, Shameem M, Husain Q. Altered oxidant-antioxidant levels in the disease prognosis of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2013;17:1104–9. doi: 10.5588/ijtld.12.0512. [DOI] [PubMed] [Google Scholar]
- 14.Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol. 2003;284:L533–40. doi: 10.1152/ajplung.00277.2002. [DOI] [PubMed] [Google Scholar]
- 15.Stocks J, Dormandy TL. Antioxidant of human red cell induced by hydrogen peroxide. Br J Hematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase In vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 18.Tavilani H, Nadi E, Karimi J, Goodarzi MT. Oxidative stress in COPD patients, smokers, and non-smokers. Respir Care. 2012;57:2090–4. doi: 10.4187/respcare.01809. [DOI] [PubMed] [Google Scholar]
- 19.Tsukagoshi H, Shimizu Y, Iwamae S, Hisada T, Ishizuka T, Iizuka K, et al. Evidence of oxidative stress in asthma and COPD: Potential inhibitory effect of theophylline. Respir Med. 2000;94:584–8. doi: 10.1053/rmed.2000.0785. [DOI] [PubMed] [Google Scholar]
- 20.Kirkil G, Hamdi Muz M, Seçkin D, Sahin K, Küçük O. Antioxidant effect of zinc picolinate in patients with chronic obstructive pulmonary disease. Respir Med. 2008;102:840–4. doi: 10.1016/j.rmed.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Karadag F, Cildag O, Altinisik M, Kozaci LD, Kiter G, Altun C. Trace elements as a component of oxidative stress in COPD. Respirology. 2004;9:33–7. doi: 10.1111/j.1440-1843.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- 22.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115:195–205. doi: 10.1164/arrd.1977.115.2.195. [DOI] [PubMed] [Google Scholar]
- 23.Montaño M, Cisneros J, Ramírez-Venegas A, Pedraza-Chaverri J, Mercado D, Ramos C, et al. Malondialdehyde and superoxide dismutase correlate with FEV (1) in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal Toxicol. 2010;22:868–74. doi: 10.3109/08958378.2010.491840. [DOI] [PubMed] [Google Scholar]
- 24.Waseem SM, Hussain MM, Ahmad Z, Islam N. A study of pulmonary functions and lipid peroxidation biomarker in COPD correlation between malondialdehyde and lung functions. Biomed Res. 2012;23:66–71. [Google Scholar]
- 25.Koul PA. Metabolic syndrome and chronic obstructive pulmonary disease. Lung India. 2016;33:359–61. doi: 10.4103/0970-2113.184865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chindhi S, Thakur S, Sarkar M, Negi PC. Subclinical atherosclerotic vascular disease in chronic obstructive pulmonary disease: Prospective hospital-based case control study. Lung India. 2015;32:137–41. doi: 10.4103/0970-2113.152624. [DOI] [PMC free article] [PubMed] [Google Scholar]


