Abstract
Gastric sarcoidosis is a very rare manifestation of sarcoidosis. Only few case reports have been described in the literature. We present a case of coexisting gastric and pulmonary sarcoidosis in a 56-year-old female, who presented with gastrointestinal symptoms. Gastric mucosal biopsies, transbronchial needle aspiration, and endobronchial mucosal biopsies revealed noncaseating granulomas. This case report emphasizes on early evaluation of other organ system and initiation of treatment.
KEY WORDS: Gastrointestinal symptoms, noncaseating epithelioid granulomas, pulmonary evaluation, sarcoidosis
INTRODUCTION
Sarcoidosis is a multisystem disorder characterized by noncaseating granulomas. Sarcoidosis can involve all organ systems to a varying extent and degree. Lung is the mostly commonly affected organ approximately 90%.[1] Up to 10% of patients present with extrapulmonary sarcoidosis. The most common sites include lymph node, skin, eyes, heart, kidney, and liver. Gastrointestinal (GI) disease occurs in 0.1%–0.9% of patients with sarcoidosis.[1] The stomach is the most commonly involved organ in GI tract.[1] Only one case has been reported from the Indian literature.[2] Pulmonary sarcoidosis may coexist or precede the development of gastric sarcoidosis. This case demonstrates the importance of evaluation of other organ systems in case of gastric sarcoidosis.
Clinical data
A 56-year-old female patient was admitted to our hospital with dry mouth, dysphagia, severe recurrent nausea and vomiting for 2 months. The patient did not smoke or use alcohol. Her temperature was 36.8°C, blood pressure 130/80, and respiratory rate of 22/min. Systemic examination was normal.
A chest radiography revealed bilateral hilar prominence, no calcification was seen. Blood cell counts, liver function test, renal function test, and blood sugar were normal. The serum calcium was 10 mg/dL (normal 6–8 mg/dL), angiotensin converting enzyme (ACE) level was 174 U/L, and antinuclear antibody (ANA) blot was negative. Computed tomography (CT) abdomen showed an incisional hernia, no other significant abnormality was seen. CT Chest showed small subpleural nodules, confluent, and discrete interstitial opacity in both lower lobes and mediastinal lymphadenopathy [Figure 1]. Pulmonary function test showed mild restriction.
Figure 1.
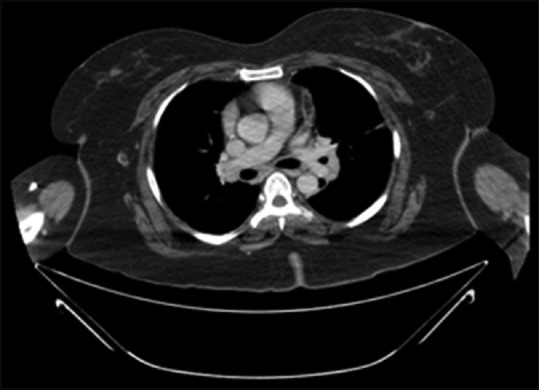
Computer tomography showing the mediastinal adenopathy
The patient underwent gastroduodenoscopy which showed nodular mucosa [Figure 2], lax lower esophageal sphincter, and antrum-predominant gastritis. Esophageal and gastric biopsies were taken. Stomach biopsy revealed noncaseating epithelioid granulomas [Figure 3]. Esophageal biopsy showed gastroesophageal reflux disease changes.
Figure 2.
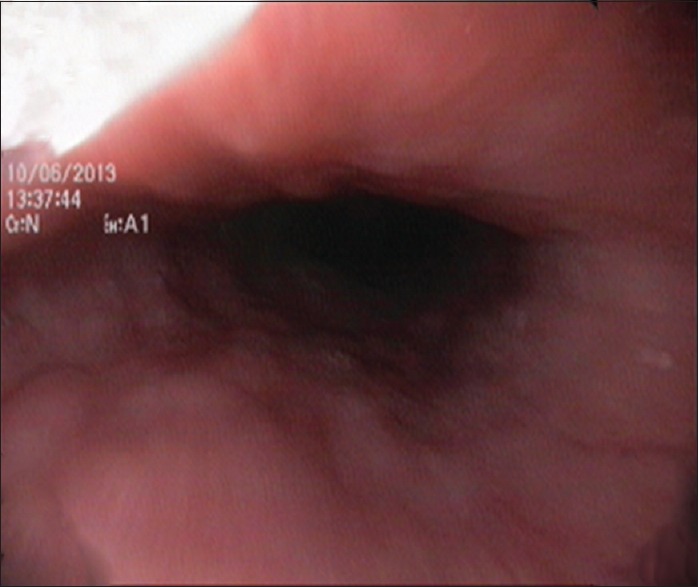
Gastroduodenoscopy revealing nodular mucosa
Figure 3.
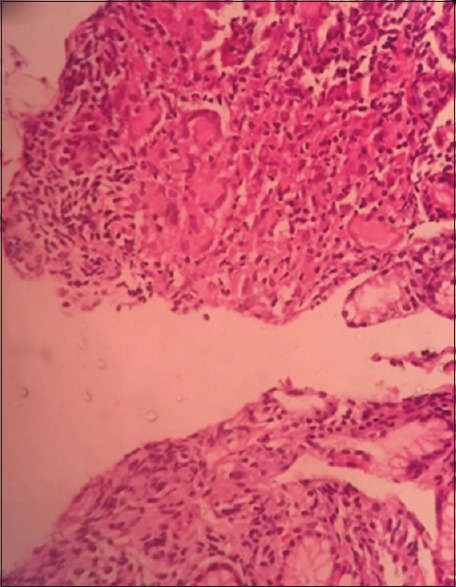
Histopathology of stomach biopsy revealing noncaseating granulomas
Bronchoscopy showed mucosal granularity in tracheobronchial tree; endobronchial biopsy was performed, endobronchial ultrasound-guided transbronchial needle aspiration of the right paratracheal and subcarinal nodes revealed noncaseating epithelioid granulomas [Figure 4]. Endobronchial biopsy showed noncaseating granulomas. Gastric biopsy and esophageal biopsies stained for helicobacter pylori, fungal organism, and acid-fast bacillus were all negative. Gastric and endobronchial biopsies cultures for Mycobacterium tuberculosis and gene Xpert for tuberculosis (TB) were negative.
Figure 4.
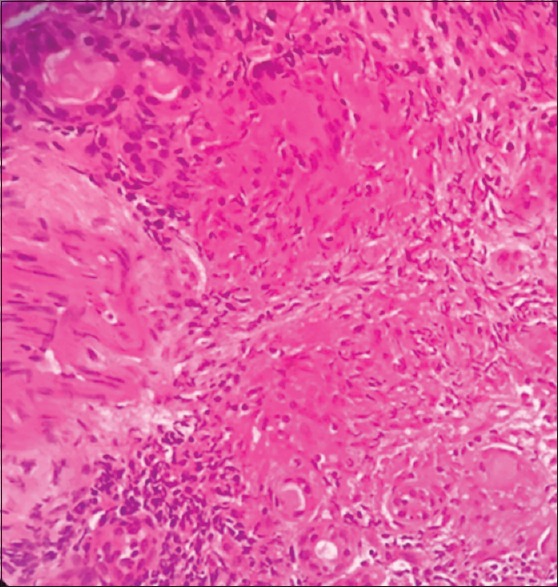
Histopathology of endobronchial biopsy revealing noncaseating granulomas
The patient was treated with steroids for about 6 months with regression of lymph nodes and decreased gastrointestinal symptoms.
The patient was subsequently lost to follow-up and had stopped her medications for 5 months. She presented again with fatigue and recurrent nausea. Serum calcium was found to be 16 mg/dL, and she was found to be in acute renal failure. Complete blood count, serum electrolytes, urine analysis with microscopy, urine electrolytes, ultrasonography with Doppler, ANA, serology for HIV, hepatits B and C was done for the evaluation of acute renal failure. All investigations were normal except serum calcium. The patient required hemodialysis for hypercalcemia and renal failure. Repeat ACE level was 95 U/L. Steroids were resumed with a resolution of hypercalcemia [Figures 1–4].
DISCUSSION
The review of the literature using the keywords “gastric sarcoidosis” and “gastric and pulmonary sarcoidosis” was done on PubMed. Our search yielded twenty-seven abstracts and eight full articles. Among these eight cases, five were males and three were females. Age varied from 27 to 66 years with the median age being 45 years.[3,4,5,6,7,8,9,10,11] In India, it predominantly occurs during fourth to the fifth decade of life.[12]
Dyspepsia has been the most common complaint followed by epigastric pain and weight loss. One patient presented with upper GI bleed.[6] Among eight cases, five had the previous history of pulmonary sarcoidosis. Remaining three patients, presented with GI symptoms had normal chest X-ray but CT showed hilar and mediastinal adenopathy in two cases.[4,7] To the best of our knowledge, this is the second case of gastric sarcoidois reported in the Indian literature. This is the first case report in western literature and Indian literature presenting with hypercalcemia and acute renal failure in sarcoidosis.
Among eight cases, six were treated with steroid, one with proton pump inhibitor and details were not available in one case. Six patients showed good resolution of symptoms with steroid.
GI sarcoidosis may occur in the context of generalized disease or as an isolated finding. Isolated gastric sarcoidosis is very rare. It should be remembered that with the diagnosis of gastric sarcoidosis, there is a need to evaluate other organ system especially lungs, eye, and skin.
Most common presentation is an epigastric pain, weight loss, and GI bleed. Gastric sarcoidosis can mimic other GI diseases in presentation, hence, other granulomatous gastritis including Crohn's disease, Menetrier's disease, localized hypertrophic gastritis, TB, syphilis, and tuberculous sarcoidosis need to be excluded.[13] Our patient also underwent colonoscopy which was a normal study.
Establishing a diagnosis of gastric sarcoidosis depends on three components: (1) biopsy evidence of noncaseating granulomas, (2) exclusion of other causes of granulomatous disease, and (3) clinical, radiological, and histopathological evidence of sarcoidosis in at least one other organ system. Over the last decade, fluorodeoxyglucose positron emission tomography scanning has emerged as a very useful modality in assessing disease activity in sarcoidosis.[14]
Treatment is based on the activity and extent of disease. Asymptomatic patients generally do not require treatment. For patients who are symptomatic and have a substantial amount of granulomatous inflammation on tissue biopsy, glucocorticoids are the treatment of choice.[7] The antisecretory therapy or promotility agents may be useful in managing dyspepsia or symptoms related to delayed gastric emptying.
CONCLUSION
We describe a rare case of biopsy-proven gastric sarcoidosis and pulmonary sarcoidosis. Solitary gastric sarcoidosis is very rare. This case emphasizes the need for evaluation of other organ system in the diagnosis of gastric sarcoidosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ceylan E, Sen S, Coskun A, Meteoglu I, Demirtas N, Çildag O. Gastric involvement of sarcoidosis in a patient with multiple lung nodules. J Res Med Sci. 2015;20:525–8. doi: 10.4103/1735-1995.163981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SK, Soneja M, Sharma A, Sharma MC, Hari S. Rare manifestations of sarcoidosis in modern era of new diagnostic tools. Indian J Med Res. 2012;135:621–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Corda L, Benerecetti D, Ungari M, Facchetti F, Radaeli E. Kaposi's disease and sarcoidosis. Eur Respir J. 1996;9:383–5. doi: 10.1183/09031936.96.09020383. [DOI] [PubMed] [Google Scholar]
- 4.Dugas M, Wallaert B, Tonnel AB, Voisin C. From subclinical alveolitis to granulomatosis. Sequential evaluation of pulmonary involvement in extrathoracic sarcoidosis. Chest. 1989;96:931–3. doi: 10.1378/chest.96.4.931. [DOI] [PubMed] [Google Scholar]
- 5.Tokala H, Polsani K, Kalavakunta JK. Gastric sarcoidosis: A rare clinical presentation. Case Rep Gastrointest Med 2013. 2013:260704. doi: 10.1155/2013/260704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinyemi E, Rohewal U, Tangorra M, Matin A, Abdullah M. Gastric sarcoidosis. J Natl Med Assoc. 2006:948–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Ungprasert P, Kue-A-Pai P, Srivali N, Cheungpasitporn W, Griger DT. A rare case of symptomatic gastric sarcoidosis. QJM. 2013;106:569–70. doi: 10.1093/qjmed/hct067. [DOI] [PubMed] [Google Scholar]
- 8.Ziora D, Trzepiora B, Kozielski J. Gastric sarcoidosis – A case report. Pneumonol Alergol Pol. 2010;78:374–8. [PubMed] [Google Scholar]
- 9.Leeds JS, McAlindon ME, Lorenz E, Dube AK, Sanders DS. Gastric sarcoidosis mimicking irritable bowel syndrome – Cause not association? World J Gastroenterol. 2006;12:4754–6. doi: 10.3748/wjg.v12.i29.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103:3184–92. doi: 10.1111/j.1572-0241.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 11.Hernández CJ, González BS, Alvarez LM, Lisboa BC. Pulmonary and gastric sarcoidosis. Report of one case. Rev Med Chil. 2009;137:923–7. [PubMed] [Google Scholar]
- 12.Mahapatra QS, Sahai K, Rathi KR, Singh S, Sharma S. Pulmonary sarcoidosis: An important differential diagnosis in transbronchial lung biopsies. Lung India. 2014;31:139–41. doi: 10.4103/0970-2113.129839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah JR, Hede J, Mathur RS. Diagnostic criteria of tuberculous sarcoidosis. Lung India. 2009;26:86–8. doi: 10.4103/0970-2113.53232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guleria R, Jyothidasan A, Madan K, Mohan A, Kumar R, Bhalla AS, et al. Utility of FDG-PET-CT scanning in assessing the extent of disease activity and response to treatment in sarcoidosis. Lung India. 2014;31:323–30. doi: 10.4103/0970-2113.142092. [DOI] [PMC free article] [PubMed] [Google Scholar]


