Abstract
There are currently several in vitro strategies to differentiate human CD14+ monocytes isolated from peripheral blood mononuclear cells into M1 or M2 macrophage cell types. Thereafter, each cell type is then verified using flow cytometric analysis of cell surface markers. Human CD14+ monocytes have the potential to differentiate into M1 and M2 macrophages, both of which demonstrate varying degrees of cell surface antigen overlap. Using multiple surface markers with current macrophage polarization protocols, our data reveal several limitations with current methods, such as highly ambiguous cell types that possess cell surface marker overlap and functional similarities. By utilizing interleukin-6 and two phases of cytokine exposure, we have developed a protocol to differentiate human monocytes into M1, M2, or dendritic cells with a greater efficiently and fidelity relative to macrophages and dendritic cells that are produced from commonly used methods. This is achieved via alterations in cytokine composition, dosing, incubation times, and improvements in verification methodology. This report provides a reliable method to reproduce human in vitro monocyte derived dendritic cells and macrophage models that will aid in defining and understanding innate and adaptive immunity better as well as several pathologic states.
Keywords: Phased strategy, M1 Macrophages, M2 Macrophages, monocyte differentiation, monocyte-derived dendritic cells, cytokine, Macrophage polarization, monocyte differentiation, Peripheral Blood Mononuclear Cells, Multi-nucleated Giant Cells
Introduction
The myeloid compartment of the innate immune system consists of a number of cell types whose function is to clear damaged and apoptotic cells, antigen presentation, immunosuppression, and to protect the host from foreign microorganisms. Monocytes represent a highly plastic subset of cells that comprise the innate immune system. These cells are capable of traversing the vasculature and, when exposed to specific cytokines, undergo differentiation into different cell types. Circulating monocytes are classified as either non-classical or classical and can be distinguished by their pattern of cell surface receptor expression. The classical monocytes within the circulation display CD14+++Hi/CD16−/lo and are present especially at the location of infection or disease [1, 2]. Human non-classical monocytes within the circulation display CD16+++Hi/CD14+/lo surface expression [1]. After activation, monocytes undergo several morphological and biochemical functional changes, resulting in monocyte differentiation into new cell types such as macrophages [3].
In a paradigm that has been termed “classical activation”, human monocytes display phenotypic plasticity that can be initiated by exposure to Th1 response-promoting cytokine interferon-γ (IFN-γ), or tumor necrosis factor alpha (TNF-α) as well as the endotoxin lipopolysaccharide (LPS) [4, 5]. This mechanism of classical activation leads to M1 macrophages which function to produce pro-inflammatory mediators that provide host protection against bacteria and viruses [6]. Human monocytes can also be differentiated to M2 macrophages by encountering Th2 response-promoting cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [4, 7]. M2 macrophages express high levels of CD206 (mannose receptor) and CD163, produce low levels of pro-inflammatory cytokines, and promote wound healing and matrix remodeling.
Dendritic cells (DCs) are another set of monocyte derived immune cells, whose function is to present antigens to T Cells to initiate an adaptive T Cell based immune response. DCs can be categorized largely into three subgroups: classical (cDCs), plasmacytoid (pDCs), and monocyte-derived DCs (moDCs). The cDCs are typically found in the lymphoid tissue, spleen, lymph nodes and bone marrow. The pDCs resemble plasma cells and are typically found in the blood circulation [8]. Monocytes can become differentiated into DCs, often termed monocyte-derived dendritic cells (moDCs), following exposure to granulocyte macrophage colony stimulation factor (GM-CSF) and IL-4 [9, 10]. Within in vitro settings, moDCs have been reported to express cell surface markers CD80, CD83, CD86, and CD1a [11–13]. While moDCs differ from macrophages in cell shape and function, they share expression of several cell surface antigens, including CD11c, CD206, and CD1a [14].
Several groups have reported successful differentiation of human CD14+ monocytes into macrophages however; many of these published protocols are dissimilar. A common variance among these published protocols is the inclusion [15] or omission [16] of interleukin-6 (IL-6). Another is variance is the treatment timing. Both of these are a problem. To address this, our studies examined and compared the inclusion and omission of IL-6 in cytokine milieu in common human monocyte differentiation methodologies. What we found was that in the absence of IL-6, these strategies produce cells with low homogeneity, which can yield ambiguous experimental results. Secondly and to further address this problem, we developed a novel phased in vitro strategy with the inclusion of IL-6. We found that this strategy greatly improved cellular homogeneity of M1, M2 macrophages, and moDCs that were differentiated from human CD14+ monocytes. This improvement in methodology will give researchers better models to utilize while investigating the immune system and disease states.
Materials and Methods
Cell Preparations
Buffy coats were collected from whole blood of five or more healthy male donors. PBMCs were prepared from buffy coats by Ficoll-Paque (1.077 g/mL density) (GE Healthcare Bio-sciences, Piscataway, NJ; catalog number 17-1440-02) density gradient centrifugation at 400 xg at 18°C for 35 minutes to separate blood constituents. Cells were washed several times using 1X PBS and counted using a Nexcelom Cellometer Auto T4 plus cell counter (Nexcelom Bioscience, Lawrence, MA). Once counted, CD14+ monocytes were isolated from PBMCs by magnetic labeling using mAb CD14 conjugated microbeads (Miltenyl Biotec, San Diego, CA; catalog number 130-050-201) followed by separation using magnetic columns (Miltenyl Biotec, San Diego, CA; catalog numbers 130-042-401 and 130-042-302) according to the manufacturer’s instructions, with one exception: when collecting CD14+ cells from the magnetic column, cells were flushed out initially using 5 mL of buffer relying only on gravity (without use of the supplied plunger). Following the initial 5 mL rinse, an additional 5 mL of buffer was added and gently flushed using the provided plunger.
Cell Culture
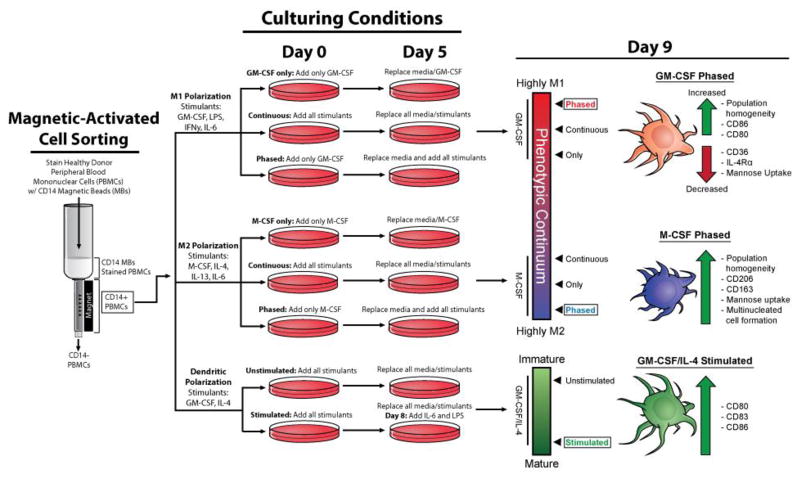
Human CD14+ monocytes were seeded at a cell density of 2.0 to 3.0 x 105 cells/ml in RPMI 1640 medium with 2 mM/L L-glutamine (Life Technologies, Frederick, MD) supplemented with 10% heat inactivated FBS (Sigma, Carlsbad, CA), 100 U/ml penicillin (Life Technologies, Frederick, MD), and 100 mg/ml streptomycin (Life Technologies, Frederick, MD) at 37°C in a humidified 5% CO2 incubator. To differentiate cells into M1 macrophages, cytokines used were GM-CSF (20 ng/ml) (PeproTech, Rocky Hill, NJ), interferon-γ (IFN-γ) (20 ng/ml), interleukin 6 (IL-6) (20 ng/ml), and endotoxin LPS (20 ng/ml). For M2 macrophage differentiation, cytokines used were M-CSF, IL-4, IL-6, and IL-13 at a concentration of 20 ng/ml (PeproTech, Rocky Hill, NJ). moDCs were generated by the addition of GM-CSF (100 ng/ml) and IL-4 (20 ng/ml). The ztiming of each strategy is further discussed in (Figure 1).
Figure 1. Schematic of Treatment strategies tested to drive monocyte differentiation into homogenous and functional myeloid cell types.
Several polarization strategies were used (GM-CSF only, M-CSF only, continuous or phased). For polarization to the M1 phenotype, CD14+ PBMCs were given GM-CSF (20 ng/ml) for six days and then on day six cells were spiked with GM-CSF in concert with LPS, IL-6, and IFN-γ (20 ng/mL) for an additional four days. For M2 polarization, cells were treated with M-CSF (20 ng/mL) for six days and then on the sixth day, cells were then spiked with M-CSF in concert with IL-4, IL-6 and IL-13 (20 ng/mL) for an additional four days. Isolating human CD14+ monocytes and differentiating them into multiple myeloid cell types for 9 days, we demonstrate that timing, cytokine composition and dosage yields homogenous populations of macrophages and dendritic cells. CD14+ PBMCs under the phased cytokine treatment strategy were driven further than other strategies within the phenotypic continuum to produce homogenous populations that expressed canonical markers of their respective cell types. Under the phased strategy, M2 macrophages also displayed high mannose uptake and cellular fusion. Specific cytokine treatment on day 9 also increased moDC maturation as well as an increase in cell surface markers CD80, CD86, and CD86. We anticipate that the usage of the phased polarization method will be helpful for many immunology research groups as well as cancer researchers seeking to model the tumor microenvironment by incorporation human macrophages.
Flow Cytometry
Polarized macrophages and dendritic cells were cultured for nine days on 10 cm2 dishes (BD Falcon, Billerica, MA). On day nine, media/non-adherent cells were collected, adherent cells were washed using 1X PBS and removed using cell dissociation buffer (Life Technologies, Frederick, MD), then added to collected media/adherent cells to be washed. Suspended cells were centrifuged and washed (1X PBS, 0.5% BSA, 2mM EDTA) twice, counted, then incubated with fluorophore-conjugated primary antibodies against CD206(FITC), CD36(PE), CD163(PE-Cy7), E-Cadherin(AlexaFluor-488), CD86(PE), CD1a(FITC), CD83(PE), CD80(PE), CD124(PE) and CD64(FITC) in the dark for 45 min at 4°C. Fluorescence was detected by a S3TM Cell Sorter (Bio-RAD, Hercules, CA).
Endocytosis Assay
Polarized human macrophages were plated into 4-well chamber glass slides (Thermo Scientific, Nunc Lab-Tek II) at a density of 3.0 x 105 cell/ml at day 7 and continued to be cultured with appropriate cytokines for the remaining 2 days. Perylene bisimide (PBI-12-Man) [17] was a kind gift from Dr. Ke-Rang Wang from Hebei University. Cells were then cultured with 10 μg/mL PBI-12-Man for 30 minutes at 37°C then washed with 1X PBS. Cells were fixed using 70% ethanol, mounted using Prolong Diamond antifade mountant with DAPI (Life Technologies, cat. no. P36962) and excited and emitted at 585nm (RFP). Red fluorescent images were quantitated using Metafer slide scanning software (MetaSystems, Boston, MA). Statistical testing was performed using MATLAB R2015a software (The Mathworks, Inc. Nathick, MA). Rank-sum tests were performed to test for univariate statistical significance between samples.
Immunofluorescence
Macrophages were differentiated using previously described methods for 7 days then transferred to 4-well glass chamber slides (Thermo Scientific, Nunc Lab-Tek II) with appropriate cytokines. Cells were left to grow for the next 7 days, then 14 days after initial plating cells were fixed using 70% ethanol, mounted using Prolong Diamond anti-fade mountant with DAPI (Life Technologies). Cells were visualized using phase contrast and fluorescence microscopy on the EVOS FL Auto (Life Technologies).
Results and Discussion
Strategies and conditions for Human CD14+ monocyte differentiation
We began by assessing which strategies were most effective in producing homogenous populations of human M1 and M2 macrophages, as well as moDCs. Human CD14+ monocytes were isolated and seeded onto tissue culture plates to be differentiated into macrophages or moDCs in vitro. We then tested three specific treatment conditions to determine the method that yielded the most uniform macrophage and moDC populations. For the first condition, we cultured cells with the addition of only GM-CSF or M-CSF for nine days (Figure 1). This condition is termed “only” (Figure 1). The second treatment strategy was to continuously expose cells to applicable cytokine milieu at day zero, replenish the media, cytokines and LPS on day five and, analyze the cells on day nine. This condition is termed “continuous” (Figure 1). The third strategy was to stimulate CD14+ monocytes with either GM-CSF or M-CSF for five days, followed by the addition of fresh media containing LPS for human M1 macrophages and all applicable cytokines for a given treatment group (GM-CSF, IFN-γ, and IL-6 for M1 macrophages or M-CSF, IL-4, IL-13, and IL-6 for M2 macrophages). This treatment strategy was termed “phased” (Figure 1). Treating CD14+ monocytes with 100 ng/ml of GM-CSF and IL-4 for five days and replacing media and all cytokines on day five generated the moDCs. moDCs were then stimulated with IL-6 and LPS for 24 hours before analysis (day 8) to promote DC maturation and activation. This culturing method was termed “stimulated” (Figure 1). The moDCs that never received IL-6 and LPS exposure were “un-stimulated” (Figure 1). After nine days of culturing under specific conditions, cell surface receptor expression and cell functionality were analyzed.
IL-6 exposure increases M2 polarization efficiency in vitro
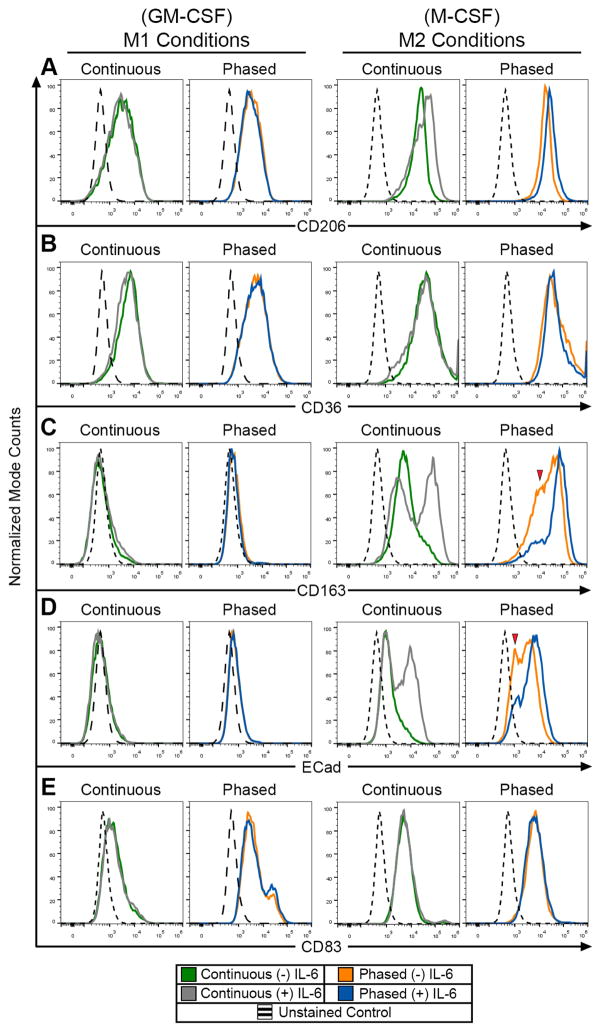
Previous studies have shown that macrophage exposure to cytokine IL-6 not only skews monocyte differentiation from a dendritic cell to a macrophage phenotype, but IL-6 exposure also increases the mRNA expression profile associated with M2 macrophages [15, 18]. To confirm these findings and to test the effect of IL-6 on M1 and M2 polarization, human CD14+ monocytes under the continuous and phased culturing conditions were treated in the presence or absence IL-6. Examination of M2 macrophage surface marker expression in M1 differentiated cells showed no significant changes when cultured with IL-6 (Figure 2), suggesting that IL-6 treatment does not shunt M1 polarized towards a M2 phenotype. Conversely, analysis of the continuous and phased M2 macrophage cultured groups revealed that IL-6 treatment increased expression of M2 macrophage surface markers. In M2 macrophage populations, both CD206 and CD36 expression remained high, and appeared unaltered when exposed to IL-6 (Figure 2A–B). CD163 and E-Cadherin, however, showed significant increases in surface expression levels and population homogeneity when treated with IL-6 (Figure 2C–D). This is demonstrated by a decrease in the low expressing CD163 and E-Cadherin peaks (red arrows in Figure 2C–D) and the resulting shift towards a homogeneously high expressing population. Furthermore, these same population changes in cell surface antigen expression were observed when comparing M2 macrophages in phased and continuous culturing groups. Additionally, IL-6 is often used to induce moDC maturation [18, 19]. In order to determine if IL-6 treatment could reveal undesired moDCs within the macrophage-polarized populations, mature DC surface marker (CD83) expression was examined. Flow cytometric analysis demonstrated that none of the M1 or M2 culture conditions promoted any significant increases in CD83 expression (Figure 2E). This suggests that there was no moDC contamination within these macrophage populations, and if so, these moDCs were IL-6 insensitive.
Figure 2. Changes in cell surface marker expression, cell size, and complexity of DCs, M1 and M2 macrophages after phased or constant cytokine treatments.
Flow cytometric analysis of M1 and M2 macrophage cell surface markers expression for CD206 (A), CD36 (B), CD163 (C), E-Cadherin (Ecad) (D) and CD83 (E) under continuous or phased treatment regimens.
Phased polarization leads to increased expression of canonical M1, M2, and moDC cell surface markers
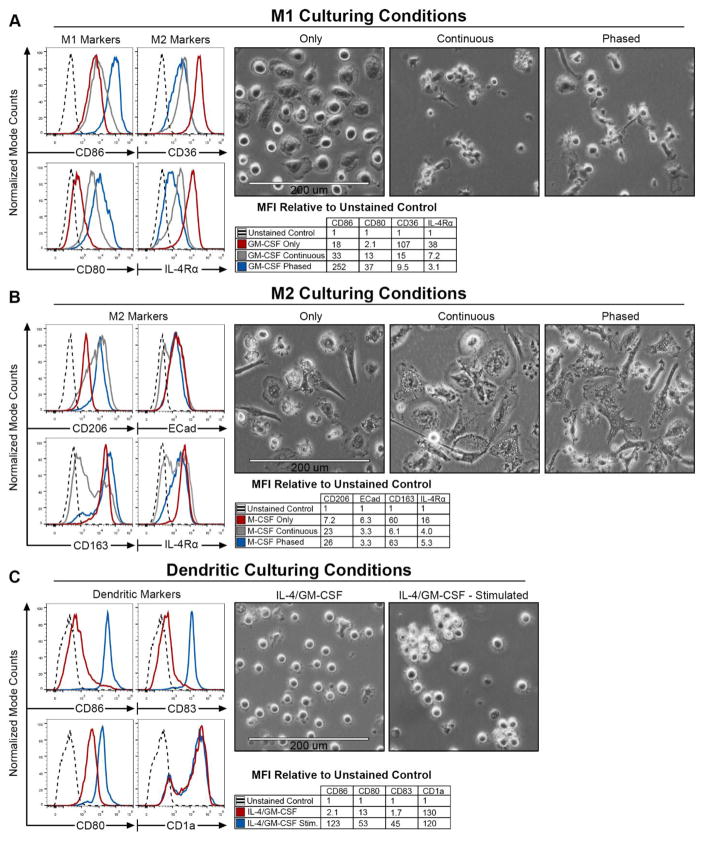
To confirm that human CD14+ monocytes were fully differentiated into either macrophages or moDCs by day nine, flow cytometry was utilized to analyze cell surface markers and assess morphological differences (Figure 3A–C). Human CD14+ monocytes were differentiated (Figure 1) to assess which strategy would yield high expression of cell type appropriate surface markers. A panel of cell surface markers was tested for each of the cell types. Our M1 cell surface marker panel consisted of canonical markers CD86, and CD80 (Figure 3A–C). For the M2 macrophage panel, we used CD206, CD163, CD124 (IL-4 Receptor-α), E-Cadherin, and CD36 (Class B Scavenger Receptor) (Figure 3A–C). For moDCs, CD83, CD86, and CD1a were utilized. Surface expression levels for all of the tested markers above were determined for all cell types (Supplemental Figure 1). Median fluorescence intensity (MFI) was calculated and shown as fold change relative to corresponding unstained control (tables in Figure 3).
Figure 3. Successful polarization leads to increased surface expression of canonical M1, M2, and moDC cell surface markers.
Panels (A–C) are separated into three sections. Histograms depict flow cytometric analysis of labeled surface markers with dashed lines depicting a representative unstained control. Accompanying tables represent the median fluorescent intensities (MFI) associated with the histograms. Values are shown as MFI relative to the unstained controls for that sample. Phase contrast images were taken of the cells prior to flow cytometric analysis. M1 surface markers analysis examining M1 markers, CD86 and CD80 are shown on the left, next to M2 markers, CD36 and IL-4Rα, on the right (A). M2 surface marker expression was determined for M2 markers CD206, CD163, E-Cadherin, and IL-4Rα (B). Expression levels for dendritic markers CD86, CD80, CD83 and CD1a were determined for moDCs (C).
Interestingly, analysis of the M2 cell surface markers, CD36 and IL-4Rα, in the M1 GM-CSF only polarized macrophages revealed dramatic increases (Figure 3A). When comparing GM-CSF only to all M2 treatment groups, the GM-CSF only had markedly higher CD36 expression. These GM-CSF only cells also expressed low levels of the M1 associated markers CD86 and CD80. Similarly, the continuous condition yielded cells that were not only low in CD86 and CD80, but also high in CD36 and IL-4Rα expression. Conversely, when human CD14+ monocytes were differentiated under the M1 phased condition, CD86 and CD80 were noticeably up regulated, while CD36 and IL-4Rα remained low. Further flow cytometric analysis of surface marker expression revealed that M1 macrophages had lower expression of CD206, CD36, and CD163 relative to M2 macrophages under both phased and continuous exposure (Supplemental Figure 2, 4). Additionally, these varied culturing conditions generate cells with distinct morphological features and structure (Figure 3A). While the GM-CSF only treated cells appear to be larger, FSC data revealed minimal differences in size between the groups (Supplemental Figure 2, 3). Comparing internal complexity between treatment conditions did reveal an increase in granularity in the GM-CSF and phased groups (Supplemental Figure 2). Together, these results show that GM-CSF only and continuous treatments yields cells that express low levels of M1 markers and high levels of certain
M2 markers as well as morphological changes. M2 macrophage culturing conditions yielded slightly more ambiguous results than those found in the M1 macrophage culturing conditions (Figure 3B). Relative to the M-CSF only group, the phased and continuous groups showed increased expression levels of CD206. Conversely, IL-4Rα surface levels were markedly higher in M-CSF only conditions, relative to both continuous and phased. E-Cadherin expression remained uniform across groups, while CD163 was significantly higher in both the M-CSF only and phased conditions. One observation of note is that the continuous exposure group was consistently far less homogeneous in regards to surface marker expression. This is illustrated by the bimodal distribution and large variance in expression levels found on the histogram. Additionally, dramatic morphological differences can be seen between the treatment groups. Based on the phase contrast images, the M-CSF only group produced cells that appear to be smaller and highly granular (Figure 3B). Flow cytometric analysis demonstrates that these cells have similar size (FSC), however, there is an increase in cellular granularity (SSC) in the phased and continuous treatment conditions (Supplemental Figure 2). Lastly, glutamine deprivation altered M2 polarization demonstrating that metabolic products are necessary under this strategy (Supplemental Figure 5). Altogether, these data suggest the phased conditions yield the greatest efficiency of M2 macrophage polarization.
We then examined surface marker expression of moDCs that had been un-stimulated or stimulated with IL-6, and LPS 24 hours prior to flow cytometric analysis. Stimulation with IL-6 and LPS resulted in an increase in CD80, CD83, and CD86 expression (Figure 3C). Mature DC marker CD83 cell surface expression dramatically increased upon activation using IL-6 and LPS on day eight relative to the un-stimulated group. This confirmed that IL-6 and LPS were able to promote dendritic cell maturation (Supplemental Figure 1L). CD1a expression remained uniform between un-stimulated and stimulated groups. Interestingly, the stimulated DCs remained in suspension, however, they did appear to begin to aggregate and adhere to one another (Figure 3C). These results confirm previous findings in moDC polarization and give us an additional control to compare surface marker analysis to identify potential undesired moDC populations within the various macrophage culturing conditions.
M2 Macrophages generated by phased cytokine exposure possess M2-associated functionality
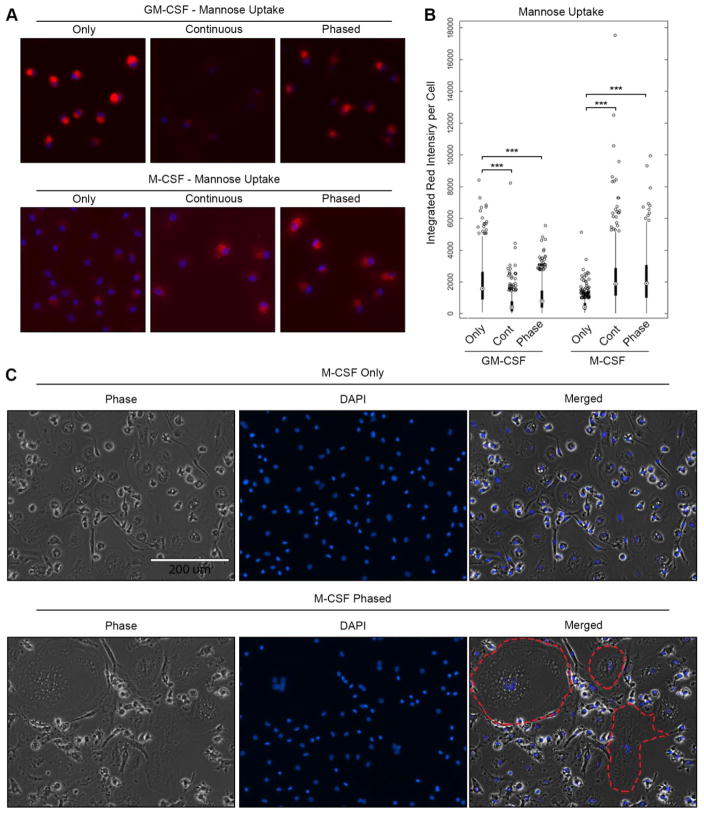
The mannose receptor (CD206) is an endocytic and recycling receptor that is highly expressed in our M2 macrophage populations under phased treatment conditions. We next wanted to assess CD206-mediated endocytic uptake in our macrophages. This was determined using PBI-12-Man, a biocompatible agent that displays fluorescence following binding to mannose receptor [17]. After a sixty-minute treatment of both M1 and M2 macrophages with PBI-12-Man, the red fluorescence of PBI-12-Man was predominantly intracellular in M2 macrophages under phased and continuous treatment conditions (Figure 4A–B). This finding suggests that cell surface binding to CD206 and endocytosis results in vesicular localization. This aligns with the previous data from flow cytometry, which demonstrated that under phased treatment conditions, the M2 macrophages displayed a higher cell surface expression of CD206 relative to the M1 macrophage population. Interestingly, we found that that GM-CSF only cells displayed high levels of PBI-12-Man endocytosis (Figure 4A–B). This correlates with our flow cytometric data (Figure 2), which demonstrated that GM-CSF only culturing conditions produce M1 cells with M2, associated surface marker expression. Altogether, we demonstrate that these cells possessed normal characteristics of macrophages and that CD206 was functional in the M2 macrophage population.
Figure 4. Polarized Macrophages are functional in vitro.
M1 and M2 cells were treated with PBI-12-Man (10 μg/mL), a fluorescently tagged mannose for 30 min at 37°C to determine endocytosis. After time point was reached, both M1 and M2 cells were rinsed with 1X PBS, fixed, DAPI stained, mounted, and then visualized (A). Red fluorescence intensity per cell was quantified using the Metafer slide scanning software and presented as a box and whisker plot with *** representing p-values < 0.01. (B). M2 only and phased culturing conditions were grown for 14 days in culture, fixed, DAPI stained, mounted, and then visualized using phase contrast and fluorescence imaging (C). Individual multinucleated cells are outlined in the merged image with a red dashed line.
After fourteen days in culture, M2 macrophages also displayed the fusion capabilities reaching over 200 μm in size and contained multiple nuclei per cell (Figure 4C). These phenomena resembled multi-nucleated giant cells (MNGCs) and have been reported to possess phagocytic and other abilities [20]. Additionally, MNGCs have been associated with foreign materials in the body, tuberculosis, and cancers [20–23]. This cellular fusion was found exclusively in our M2 macrophage populations and was far more significant in the M2 phased condition. While the M-CSF only groups did have some multinucleated cells (Figure 4C), their size and numbers were much lower than the phased group. Given the ability of these cells to perform this macrophage associated function, these results further support that the phased culturing conditions produces highly M2-like macrophages.
Here we described our newly developed phased protocol for macrophage polarization. What we found was the inclusion of IL-6 and phased polarization treatment timing is absolutely critical in generating the most M1 and M2 like macrophages in vitro. This is the major finding of this study and was tested rigorously when compared to other polarization methods. From this, we believe that the phased polarization method provides a substantial step forward based on recommendations made which gives experimenters unifying experimental standards, homogenous populations of macrophages to use in vitro, and the ability to compare their results. While this protocol offers a number of improvements on current polarization strategies, we believe that additional research and improvements regarding exposure times and cytokine cocktail should continue. This will help to further understand the complex roles of M1 and M2 macrophages in various forms of disease progression and advance the development of novel therapeutics.
Supplementary Material
Acknowledgments
Research for this study was supported by the National Cancer Institute grants U54CA143803, CA163124, CA093900, CA143055, the UNCF/Merck Postdoctoral Science Research Fellowship award (J.C.Z) and by a collaborative award from Medimmune, LLC. We thank Drs. Amanda Brown, Dionna W. Williams, J. James Frost, Kris Sachsenmeier (MedImmune, LLC.), Robert Hollingsworth (Medimmune, LLC.), David M. Mosser (University of Maryland), Suzanne Ostrand-Rosenberg (University of Maryland-Baltimore County), Cherie Butts (Biogen Idec) and Donald S. Coffey for their fruitful discussions of this work.
Footnotes
Competing interests statement: The authors declare no competing interest.
Author contributions: Conceptualization, J.R.H*, J.C.Z*, J.E.V, C.G.D, K.J.P; Methodology, J.R.H*, J.C.Z*, S.P.C and C.G.D; Investigation, J.R.H.*, and J.C.Z*; Validation, J.R.H* and J.C.Z*; Formal Analysis, J.E.V; Writing – Original Draft, J.C.Z* J.R.H*; Writing – Review & Editing, J.C.Z*, J.R.H*, J.E.V., K.J.P., and C.G.D.; Funding Acquisition, K.J.P. J.C.Z*; Supervision, J.C.Z*, and K.J.P (*Authors contributed equally to this work)
References Cited
- 1.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 2.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 3.Jha AK, Huang SC, Sergushichev A, Lamproupoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Sozzani S, Locati M, Allavena P, Siva A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79(1):17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mildner A, Yona S, Jung S. A close encounter of the third kind: monocyte-derived cells. Adv Immunol. 2013;120:69–103. doi: 10.1016/B978-0-12-417028-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 11.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996;93(6):2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stokinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol. 1993;150(2):579–84. [PubMed] [Google Scholar]
- 13.Kiertscher SM, Roth MD. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J Leukoc Biol. 1996;59(2):208–18. doi: 10.1002/jlb.59.2.208. [DOI] [PubMed] [Google Scholar]
- 14.Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Gunther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol. 2002;118(2):327–34. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9(4):e94188. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel DY, Glim JE, Stauvenuiter AW, Breur M, Heijen P, Amor S, Dijkstra CD, Beelen RH. Human Macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219(9):695–703. doi: 10.1016/j.imbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang KR, An HW, Rong RX, Cao ZR, Li XL, et al. Fluorescence turn-on sensing of protein based on mannose functionalized perylene bisimides and its fluorescence imaging. Biosens Bioelectron. 2014;58:27–32. doi: 10.1016/j.bios.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 19.Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 20.Quinn MT, I, Schepetkin A. Role of NADPH oxidase in formation and function of multinucleated giant cells. J Innate Immun. 2009;1(6):509–26. doi: 10.1159/000228158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forkner CE. The Origin and Fate of Two Types of Multi-Nucleated Giant Cells in the Circulating Blood. J Exp Med. 1930;52(2):279–97. doi: 10.1084/jem.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans HM, Bowman FB, Winternitz MC. An Experimental Study of the Histogenesis of the Miliary Tubercle in Vitally Stained Rabbits. J Exp Med. 1914;19(3):283–302. doi: 10.1084/jem.19.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC, Ogden IM, Catalona W, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A. 2014;111(9):3514–9. doi: 10.1073/pnas.1320198111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.