Abstract
Purpose
As adjuvant therapy for enhancing the effects of stimulants and thereby minimizing medication doses, we hypothesized that aerobic exercise might be an effective adjunctive therapy for enhancing the effects of methylphenidate on the clinical symptoms, cognitive function, and brain activity of adolescents with attention deficit hyperactivity disorder (ADHD).
Methods
Thirty-five adolescents with ADHD were randomly assigned to one of two groups in a 1/1 ratio; methylphenidate treatment + 6-wk exercise (sports-ADHD) or methylphenidate treatment + 6-wk education (edu-ADHD). At baseline and after 6 wk of treatment, symptoms of ADHD, cognitive function, and brain activity were evaluated using the Dupaul attention deficit hyperactivity disorder rating scale–Korean version (K-ARS), the Wisconsin Card Sorting Test, and 3-T functional magnetic resonance imaging, respectively.
Results
The K-ARS total score and perseverative errors in the sports-ADHD group decreased compared with those in the edu-ADHD group. After the 6-wk treatment period, the mean β value of the right frontal lobe in the sports-ADHD group increased compared with that in the edu-ADHD group. The mean β value of the right temporal lobe in the sports-ADHD group decreased. However, the mean β value of the right temporal lobe in the edu-ADHD group did not change. The change in activity within the right prefrontal cortex in all adolescents with ADHD was negatively correlated with the change in K-ARS scores and perseverative errors.
Conclusions
The current results indicate that aerobic exercise increased the effectiveness of methylphenidate on clinical symptoms, perseverative errors, and brain activity within the right frontal and temporal cortices in response to the Wisconsin card sorting test stimulation.
Keywords: Attention Deficit Hyperactivity Disorder, Exercise, Prefrontal Cortex, Temporal Cortex
Attention deficit hyperactivity disorder (ADHD) has a worldwide prevalence ranging from 2% to 10% in children and frequently continues into the period of adolescence (28). The primary symptoms of ADHD are inattention, hyperactivity, difficulty in organizing tasks, including school work and workplace responsibilities, and impulsive behaviors (28). The dorsolateral prefrontal cortex (DLPFC) is thought to regulate human motor, cognitive, and emotional responses through extensive connections with other brain regions (2). In addition, many arousal neurotransmitter pathways within the brain (involving norepinephrine, do-pamine, acetylcholine, and serotonin) project to the DLPFC (31). Taken together, these observations suggest that the clinical symptoms of ADHD are associated with decreased function of the DLPFC (3).
Many studies have reported that pharmacotherapies for ADHD, including dextroamphetamine, methylphenidate, and atomoxetine, are affordable, and clinicians routinely use these agents for the treatment of ADHD (4). However, it has been reported that 10%–30% of children with ADHD have inadequate clinical responses to methylphenidate treatment (38). In addition, treated individuals may experience short-term side effects, including insomnia, loss of appetite, and headaches, and rare side effects including mania, psychosis, and increased risk of sudden death (27). Therefore, adjuvant therapies for enhancing the effects of stimulants and thereby minimizing medication doses have recently been studied (22,26). Mongia and Hechtman (22) have suggested that cognitive behavior therapy combined with medication is an effective method for improving clinical symptoms and comorbid problems in adult patients with ADHD. Philipsen (26) reported that psychotherapy targeted at improving low self-esteem improved ADHD symptoms and associated problems in adult patients with ADHD.
As one adjuvant therapy for ADHD, exercise has been shown to improve clinical, cognitive, and scholastic performance (16,29). Several studies on healthy children have suggested that participation in physical activity and exercise is beneficial for improving concentration, reading and mathematics achievement, as well as inhibitory control (13). Pontifex et al. (29) reported that a 20-min aerobic exercise improved inhibitory control and scholastic performance in children with ADHD age 8–10 yr. Our previous study suggested that 6 wk of athletic activity improved attention, cognitive symptoms, and social skills of children with ADHD (16). Verret et al. (37) have also reported that 10 consecutive weeks (45 min·d−1, three times a week) of physical training improved muscular capacity, motor skills, and attention (sustained and divided) of children with ADHD. In a functional magnetic resonance imaging (fMRI) study with a cognitive control flanker paradigm, Chaddock et al. (5) have suggested that aerobic fitness increased prefrontal and parietal lobe activity in response to cognitive control.
By grouping subjects into two treatment groups, methylphenidate + exercise (sports-ADHD group) and methylphenidate + education (edu-ADHD group), our previous study assessed the adjunctive efficacy of exercise on methylphenidate treatment response in adolescents with ADHD. In that study, 6 wk of athletic activity improved attention, cognitive symptoms, and social skills in children with ADHD (16). In the current research, the study was conducted in two phases. Initially, we identified areas of brain activation that differed between adolescents with ADHD and healthy adolescents in response to the Wisconsin card sorting test (WCST) using fMRI. Subsequently, adolescents with ADHD were divided into two treatment groups: sports-ADHD group and edu-ADHD group. We then evaluated changes in brain activity within those clusters that were found in the first analysis in both the sports-ADHD and the edu-ADHD groups. We hypothesized that aerobic exercise would improve clinical symptoms and cognitive function as well as increase brain activity in adolescents with ADHD compared with those in adolescents with ADHD who did not participate in exercise.
Methods
Subjects
Thirty-five male adolescents (13–18 yr old) with ADHD and 15 age-matched healthy control subjects were recruited from the outpatient department, Chung-Ang University Hospital. All adolescents were assessed using the Korean Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version, and a diagnostic interview using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria. The inclusion criteria for research subjects included the following: 1) diagnosed as having ADHD, 2) drug naive or drug free during the previous 6 months, and 3) intelligence quotient (IQ) ≥ 80. The exclusion criteria for research subjects included the following: 1) other axis I disorders, including depression and tic disorder, 2) history of head trauma with loss of consciousness, seizure disorder, multiple sclerosis, brain tumor, claustrophobia, metal implants, or cerebrovascular accident, 3) IQ < 80, and 4) a history of substance abuse. The inclusion criteria for healthy control subjects included the following: 1) age 13–18 yr, 2) having no psychiatric diseases or medical illness, and 3) IQ ≥ 80. The exclusion criteria for healthy control subjects were the same as those for research subjects. The research protocol was approved by the Chung-Ang University Hospital institutional review board. A written informed assent was provided by adolescents, and a written informed consent was provided by parents.
Study procedure
Thirty-five adolescents with ADHD were randomly assigned to one of the two groups in a 1/1 ratio: sports-ADHD or edu-ADHD for behavior control. All adolescents with ADHD were asked to complete baseline clinical scales, cognitive function tests, and brain imaging and then started treatment with either sports-ADHD or edu-ADHD. All of the adolescents were started on methylphenidate (Metadate CD™, Whanin Pharmaceutical Co. Ltd., Korea) at a dosage of 10 mg·d−1 . In response to changes in clinical symptoms, the dosage of methylphenidate was increased within a range of 10–40 mg·d−1 during the first 4 wk of treatment. For the subsequent 6-wk research period, stable dosages of methylphenidate were maintained. Three adolescents (one in sports-ADHD and two in edu-ADHD) maintained treatment with 10 mg·d−1 of methylphenidate without a dosage increase. During the research period, adolescents were asked to maintain the dosage of methylphenidate. One subject in the sports-ADHD group and one subject in the edu-ADHD group discontinued treatment because of nausea and headache. Three subjects in the sports-ADHD group were excluded because of five or more absences from sports therapy. Finally, 30 subjects (13 in sports-ADHD (76.5% of the initial cohort) and 17 in edu-ADHD (94.4% of the initial cohort)) completed the respective intervention program. At baseline and after 6 wk of treatment, the clinical symptoms of ADHD and cognitive function were evaluated using the Dupaul ADHD rating scale (10) and the WCST (20), respectively. At baseline, clinical characteristics and cognitive functioning in the control subjects were assessed in a manner similar to the assessment of the subjects with ADHD.
Exercise
The study team for sports therapy consisted of one psychiatrist, two sports psychologists, and four graduate teaching assistants majoring in sports psychology. Three times per week, 90-min sessions of sports therapy were scheduled as follows: 10 min for stretching and warming up, 60 min for aerobic exercise, and 10 min for feedback and cooling down. Aerobic exercises consisted of running (shuttle run, zigzag run), jumping rope (individual and group jumps), and basketball (dribble, pass, shoot, and game) to achieve a target HR (THR) of 60% HRmax intensity. Assessments of HR before and during exercise have been recommended as a method for establishing exercise intensity (1). HRrest and HRmax were measured once at the beginning of the study. HRmax was calculated using the “220 – age” formula. THR was calculated by the Karvonen formula, as follows: THR = (HRmax – HRrest) × % intensity desired + HRrest (17). We used 60% as the “intensity desired” in this formula on the basis of previous studies conducted in Thailand on pre-adolescents (9.5 ± 0.5 yr old) (13) and youths (18–25 yr old) (14). During the sports therapy period, if adolescents reported excessive exertion, they were allowed to rest until their HR went to down to HRrest level (12). The mean rest time in our research was 7.1 ± 2.7
Education for behavior control
As an exercise control, 50 min·d −1 of educational sessions for behavior control were provided to the edu-ADHD group but not to the sports-ADHD group. These sessions are described in detail in our previous study (16). Briefly, the edu-ADHD subjects attended 12 sessions (S): S1, self-introduction; S2, good behavior and bad behavior; S3, a review of self-behavior; S4, a comparison of self and others; S5, interaction with family; S6, making friends I; S7, making friends II; S8, how to pay attention; S9, how to control hyperactivity; S10, anger control; S11, guest speakers who have recovered from ADHD; and S12, summary of ADHD education. All sessions were presented by one psychiatrist and one social worker.
Brain activity
In subjects with ADHD, at baseline and after 6 wk of treatment, brain activity in response to the modified WCST was assessed using 3-T fMRI. In healthy control subjects, brain activity was assessed in the same manner as that in the subjects with ADHD but only at baseline. Two versions of the WCST were presented to the adolescents with ADHD. Before scanning, all adolescents with ADHD were asked to complete the WCST-computerized version. Inside the scanner, adolescents with ADHD watched a black screen presenting four reference cards on the upper part of the screen and one stimulus card in the middle of the lower part of screen. Adolescents matched the stimulus card to one of the four reference cards using a keypad with two buttons when each stimulus card was presented for 4000 ms. In response to the subjects' selection, a feedback (correct or incorrect) screen was presented for 500 ms after a blank screen (500 ms). The neutral control block consisted of reference cards only in the upper section of the screen without a stimulus card. During the neutral period, adolescents were asked to push the keypad buttons randomly.
The task consisted of a 450-s stimulus with five continuous 90-s segments. The 90-s segment consisted of three sub-segments (30 s each): a white cross on a black background (BG), a neutral control (N, reference cards + pushing button), and the task (T, WCST). The order of the five segments was as follows: BG-N-T, BG-T-N, T-BG-N, N-BG-T, and T-N-BG This scenario was presented using IFIS-SA™ (MRI Device Corporation, Waukesha, WI) during a single fMRI scanning session. For the fMRI session, 180 echo planar images (33 transverse slices; 4.0-mm thickness; voxel size, 1.8 × 1.8 × 4.0 mm; echo time, 30 ms; repetition time, 3000 ms; flip angle, 90°; in-plane resolution, 128 × 128 pixels; field of view, 230 × 230 mm) were recorded at 3-s intervals. For anatomical imaging, three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient echo data were collected with the following parameters: repetition time, 2000 ms; echo time, 4.00 ms; field of view, 256 × 256 mm; 340 slices; voxel size, 0.9 × 0.9 × 1.0 mm; flip angle, 30°.
FMRI data analysis
The Brain Voyager software package (BVQX 1.9; Brain Innovation, Maastricht, The Netherlands) was used for analyzing fMRI data. The data in fMRI time series were coregistered to the anatomical 3D data sets using the multiscale algorithm provided by BVQX. Individual 3D structural images were spatially normalized to standard Talairach space. Talairach space is derived from an atlas of the human brain, composing a 3D coordinate system (36). It is commonly used in functional brain imaging studies and in targeting transcranial stimulation of brain regions (36). An identical nonlinear transformation was subsequently applied to the T2*-weighted fMRI time series data. After the preprocessing steps for slice scan time correction and 3D motion correction, the functional data were spatially smoothed using a Gaussian kernel with a full width at half maximum of 6 mm and temporally smoothed using a Gaussian kernel of 4s using algorithms provided by BVQX. The detection (trilinear interpolation) and correction (sinc interpolation) of 3D motion were performed using the first volume as reference. A head motion of less than 3.5 mm (translation) or 3.5° (rotation) relative to the target volume was deemed acceptable (30). No data were excluded because of excessive head movement in the present study.
Statistical analysis
The demographic characteristics including age, education year, IQ, K-ARS scores, and perseverative responses/errors between adolescents with ADHD and healthy comparison adolescents were analyzed with Mann–Whitney U tests. The demographic characteristics (same categories in the comparisons between ADHD and healthy subjects) between the sports-ADHD and edu-ADHD groups were also analyzed with Mann–Whitney U tests.
For analyzing the fMRI signal time courses on a voxel-by-voxel basis and generating individual and group statistical parametric maps of brain activation, general linear model and random effects analysis were used. For all analyses, we regarded the associations as significant when the false discovery rate (FDR) correction was equal to or less than 0.05 in 40 adjacent voxels.
As a first level analysis, the brain regions that showed different patterns of activity in response to the WCST-computerized version were identified between all adolescents with ADHD and healthy control adolescents. In an F test, interactions within factor (task scene vs neutral scene) × between factor (adolescents with ADHD vs healthy comparison subjects), six clusters were identified. As a second-level analysis, the changes in brain activity within these six clusters in the sports-ADHD group were compared with those in the edu-ADHD group after the 6-wk treatment period. Repeated-measures ANOVA was used to evaluate differences in changes of brain activity (mean β values) in clusters between the sports-ADHD and edu-ADHD treatment cohorts. The correlation between changes of K-ARS scores and the changes in brain activity (β value) was analyzed with Pearson correlations. In all analysis, we set significant P values < 0.05, with P value at a trend value from 0.05 < P < 0.1.
Results
Demographic characteristics
There were no significant differences in age or education between the ADHD group and the healthy comparison group (Table 1). The K-ARS scores (z = 5.3, P < 0.01), perseverative responses (z = 2.95, P < 0.01), and perseverative errors (z = 3.29, P < 0.01) in the ADHD group were higher than those in the healthy comparison subjects (Table 1). The IQ of the subjects with ADHD was slightly lower than that of the healthy comparison subjects (z = 2.08, P < 0.03) (Table 1). However, there were no significant differences in age, school grade, K-ARS scores at baseline, subitems of WCST at baseline, and IQ between the sports-ADHD and edu-ADHD groups.
Table 1.
Demographic characteristics.
| ADHD (n = 30) | Healthy Comparison (n = 15) | z, P | |||
|---|---|---|---|---|---|
|
| |||||
| Sports-ADHD (n = 13) | Edu-ADHD (n = 17) | ||||
| Age | 15.9 ± 1.2 | 16.0 ± 1.9 | 0.31, 0.76 | ||
| 15.8 ± 1.7 | 16.0 ± 1.2 | 0.45, 0.65 | |||
| School year | 8.7 ± 1.3 | 8.9 ± 1.9 | 0.25, 0.97 | ||
| 8.6 ± 1.4 | 8.8 ± 1.2 | 0.56, 0.58 | |||
| K-ARS | 25.8 ± 7.5 | 4.07 ± 3.9 | 5.3, <0.01* | ||
| 25.3 ± 8.1 | 26.1 ± 7.3 | 0.63, 0.53 | |||
| MTX (mg d−1) | 26.9 ± 9.5 | 26.5 ± 10.0 | — | 0.11, 0.91 | |
| WCST | |||||
| TCC | 46.0 ± 26.4 | 33.6 ± 19.3 | 1.94. 0.06 | ||
| 45.8 ± 25.8 | 46.3 ± 28.2 | 0.06, 0.94 | |||
| Total errors | 22.4 ± 12.3 | 17.6 ± 9.4 | 1.23, 0.21 | ||
| 20.7 ± 10.7 | 24.6 ± 14.3 | 0.48, 0.63 | |||
| Perseverative responses | 21.0 ± 9.2 | 12.5 ± 5.2 | 2.95, <0.01* | ||
| 21.2 ± 8.2 | 20.8 ± 10.7 | 0.21, 0.83 | |||
| Perseverative errors | 13.3 ± 5.6 | 8.1 ± 3.1 | 3.29, <0.01* | ||
| 14.2 ± 6.4 | 12.6 ± 4.9 | 0.69, 0.49 | |||
| NPE | 4.7 ± 3.7 | 4.3 ± 3.5 | 0.29, 0.77 | ||
| 5.4 ± 4.8 | 4.2 ± 2.7 | 0.27, 0.78 | |||
| IQ | 95.4 ± 13.2 | 104.3 ± 10.5 | 2.08, 0.03* | ||
| 94.9 ± 11.8 | 95.9 ± 15.2 | 0.10, 0.91 | |||
WCST was performed before brain imaging.
The value is statistically significant.
K-ARS, Dupaul attention deficit hyperactivity disorder rating scale–Korean version; MTX, stabilized methylphenidate dosage; NPE, nonperseverative errors; TCC, total trials to complete categories.
Differences in the changes of the K-ARS scores and WCST between the sports-ADHD and edu-ADHD groups during the 6-wk treatment period
The K-ARS total score in the sports-ADHD group (baseline (B), 25.3 ± 8.1; 6 wk (6w), 9.4 ± 4.8) significantly decreased compared with that in the edu-ADHD group (B, 26.1 ± 7.3; 6w, 14.9 ± 3.6) (F = 4.53, P = 0.04). Perseverative errors in the sports-ADHD group (B, 14.2 ± 6.4; 6w, 7.8 ± 8.7) also decreased compared with those in the edu-ADHD group (B, 12.6 ± 4.9; 6w, 11.1 ± 4.0) (F = 5.61, P = 0.03).
Brain activity in response to WCST
At baseline, in response to WCST stimulation, adolescents with ADHD showed decreased activation in the right occipital lobe, both middle temporal gyri, right cerebellum, right frontal lobe, and both parietal lobes compared with those in healthy comparison adolescents (FDR < 0.05, P = 0.003). At baseline, in response to WCST stimulation, adolescents with ADHD showed increased activation in the right temporal lobe (limbic lobe) compared with that in the healthy adolescents (FDR < 0.05, P = 0.003) (Table 2 and Fig. 1).
Table 2.
Areas of different brain activation between the ADHD group and the healthy control group in response to the WCST.
| Talairach Code | Voxels | Brain Regions | |||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Adolescents with ADHD < healthy adolescents | |||||
| 15 | −82 | 5 | FDR < 0.05, P = 0.002925 | 60 | CL1, right occipital lobe, cuneus |
| 62 | −43 | −11 | FDR < 0.05, P = 0.002925 | 60 | CL2, right middle temporal gyrus |
| −62 | −42 | −15 | FDR < 0.05, P = 0.002925 | 60 | CL3, left middle temporal gyrus |
| 34 | −54 | −14 | FDR < 0.05, P = 0.002925 | 60 | CL4, right cerebellum, posterior lobe |
| 36 | 7 | 35 | FDR < 0.05, P = 0.002925 | 60 | CL5, right frontal lobe, precentral gyrus |
| 19 | −62 | 40 | FDR < 0.05, P = 0.002925 | 60 | CL6, right parietal lobe, precuneus |
| −18 | −60 | 39 | FDR < 0.05, P = 0.002925 | 60 | CL7, left parietal lobe, precuneus |
| Adolescents with ADHD > healthy adolescents | |||||
| 31 | 2 | −26 | FDR < 0.05, P = 0.002925 | 60 | CL8, right limbic lobe, uncus |
Figure 1.
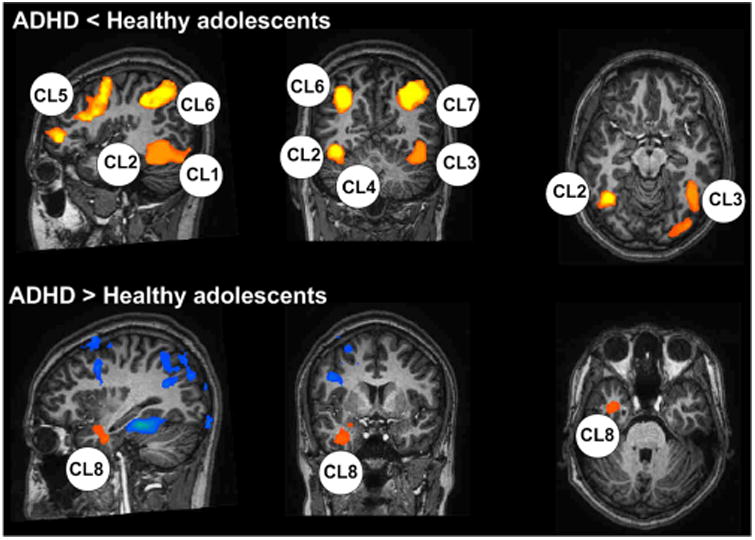
Areas of different brain activation between the ADHD group and the healthy control group in response to the WCST. CL, clusters (see Table 2).
At a second-level analysis (pre–post comparisons), three clusters (right frontal lobe, left parietal lobe, and right limbic lobe) showed changes in brain activity between the sports-ADHD and edu-ADHD groups. The mean β values of the right frontal lobe in both the sports-ADHD (z = 2.83, P = 0.01) and the edu-ADHD (z = 2.21, P = 0.03) groups increased (Table 3). In a group comparison, the mean β value for activation in the right frontal lobe in the sports-ADHD group increased significantly compared with that in the edu-ADHD group (F = 4.3, P = 0.04). The mean A value for the left parietal lobe in the sports-ADHD group increased (z = 2.4, P = 0.02). However, the mean β value of the left parietal lobe in the edu-ADHD only increased at a trend level (z = 1.8, P = 0.06). In a group comparison, the mean β value for the left parietal lobe in the sports-ADHD group marginally increased compared with that in the edu-ADHD group (F = 2.9, P = 0.09) (Table 3). The mean β value for the right temporal lobe in the sports-ADHD group decreased (z = 2.4, P = 0.02). However, the mean β value of the right temporal lobe in the edu-ADHD group did not change (z = 1.1, P = 0.25). In a group comparison, the mean β value of the right limbic lobe in the sports-ADHD group marginally decreased compared with that in the edu-ADHD group (F = 3.7, P = 0.06). There were no significant changes in β values for the right occipital lobe, left middle temporal lobe, right cerebellum, and right parietal lobe (Table 3).
Table 3.
The changes in brain activity in both sports-ADHD group and edu-ADHD group.
| β Value | Sports-ADHD (A) | Edu-ADHD (B) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| B | 6w | z, Pa | B | 6w | z, Pa | |
| Rt-PF | 0.51 ± 0.44 | 1.22 ± 0.47 | 2.8, 0.01* | 0.56 ± 0.50 | 0.80 ± 0.65 | 2.2, 0.03* |
| A vs B: F = 4.3, P = 0.04* | ||||||
| Lt-PAL | 0.43 ± 0.40 | 0.90 ± 0.51 | 2.4, 0.02* | 0.38 ± 0.41 | 0.57 ± 0.48 | 1.8, 0.06** |
| A vs B: F = 2.9, P = 0.09** | ||||||
| Rt-Temp | 0.28 ± 0.19 | –0.16 ± 0.48 | 2.4, 0.02* | 0.11 ± 0.21 | 0.03 ± 0.31 | 1.1, 0.25 |
| A vs B: F = 3.7, P = 0.06** | ||||||
| Rt-Occ | 0.33 ± 0.39 | 0.39 ± 0.41 | 0.55, 0.57 | 0.11 ± 0.39 | 0.15 ± 0.29 | 0.58, 0.52 |
| A vs B: F = 0.1, P = 0.76 | ||||||
| Rt-PAL | 0.34 ± 0.42 | 0.38 ± 0.33 | 0.9, 0.72 | 0.25 ± 0.24 | 0.27 ± 0.21 | 0.8, 0.68 |
| A vs B: F = 1.2, P = 0.29 | ||||||
| Rt-Cerbell | 0.57 ± 0.46 | 0.51 ± 0.44 | 1.1, 0.36 | 0.52 ± 0.39 | 0.48 ± 0.35 | 1.0, 0.37 |
| A vs B: F = 0.1, P = 0.82 | ||||||
| Lt-Temp | 0.37 ± 0.27 | 0.32 ± 0.19 | 0.9, 0.42 | 0.33 ± 0.36 | 0.29 ± 0.27 | 0.9, 0.37 |
| A vs B: F = 1.2, P = 0.29 | ||||||
Paired t test between baseline and 6 wk.
Statistically significant changes (P < 0.05).
Statistically significant changes at a trend level (0.05 < P < 0.1).
A vs B, groups A (sports-ADHD) and B (edu-ADHD) have been compared in terms of the change in brain activity by repeated-measures ANOVA; Lt-PAL, left parietal lobe; Lt-Temp, left middle temporal gyrus; Rt-Cerbell, right cerebellum; Rt-FP, right frontal lobe; Rt-Occ, right occipital lobe; Rt-PAL, right parietal lobe; Rt-Temp, right middle temporal gyrus.
Correlation between the changes in brain activity, K-ARS s, and perseverative response
The change in activity within the right prefrontal cortex in all adolescents with ADHD was negatively correlated with the change in K-ARS scores (r = −0.57, P < 0.01). The change in the activity within the right prefrontal cortex in all adolescents with ADHD was also negatively correlated with the change in perseverative errors (r = −0.53, P < 0.01). There was no significant correlation between K-ARS scores, perseverative errors, and the brain activity within other clusters.
Discussion
The current results indicate that aerobic exercise increased the effectiveness of methylphenidate in reducing clinical symptoms and perseverative errors as well as in increasing brain activity within the right frontal cortex in response to WCST stimulation relative to education for behavioral control. The brain activity of the left parietal cortex increased at a trend level in response to WCST stimulation. However, there were no significant changes within the right middle temporal, right occipital, right parietal, right cerebellum, and left middle temporal cortices.
At baseline, the ADHD group demonstrated higher K-ARS scores, perseverative responses, and perseverative errors on the WCST compared with those in the healthy comparison subjects. After 6 wk of treatment, the K-ARS scores in the sports-ADHD improved compared with those in the edu-ADHD group. The number of perseverative errors in the sports-ADHD group decreased compared with those in the edu-ADHD group. The improvement in clinical symptoms and cognitive performance is consistent with the results of our previous study. In that study, 6 wk of aerobic exercise in children with ADHD improved K-ARS scores and cognitive functions, which were assessed with the digit symbol and trail making test B, compared with those in the edu-ADHD group (16). In the cognitive and executive research on WCST in children with ADHD conducted by Yáñez-Téllez et al. (40), the scores of total errors, perseverative errors, and perseverative responses were higher than those in healthy comparison children. Verret et al (37) reported that 10 wk of sports activity enhanced physical abilities and attention.
In response to WCST stimulation, brain activity within the right frontal and parietal cortices in the sports-ADHD group increased compared with that in the edu-ADHD group. In addition, changes in the activity of the right prefrontal cortex were negatively correlated with changes of K-ARS scores and perseverative errors. Attention shifting, which includes the evaluative process of determining whether or not to shift a response, is thought to be associated with DLPFC activity (3). The DLPFC is a critical area for mediating perseverative errors (25).
FMRI techniques have demonstrated altered functional activation within the frontal, temporal, parietal, and cerebellar regions in children with ADHD (8,32). Cubillo et al. (8) reported reduced activation in the bilateral inferior prefrontal cortices, left parietal lobe, caudate, and thalamus during a stop-and-switch task in subjects with ADHD relative to healthy controls. Rubia et al (32) reported that the activation of the DLPFC, ventrolateral prefrontal cortex, inferior frontal cortex, and superior parietal cortex was decreased in response to multiple cognitive performance tasks. In a resting-state fMRI study, Cocchi et al. (6) reported abnormal interregional connectivity within a frontal–amygdala–occipital network and a frontal–temporal–occipital network in young adults with ADHD. The present study demonstrates an influence of aerobic exercise on brain activity in response to attention shifting in adolescents with ADHD.
The present results also indicate that right temporal lobe activity in response to WCST decreased after 6 wk of aerobic exercises in adolescents with ADHD. In the fMRI study on ADHD conducted by Kobel et al. (19), the brain activity within the temporal lobe was negatively correlated with stimulation on a working memory task (2N, 3N back task). As an extension of the frontostriatal deficit hypothesis of ADHD, Rubia et al (33) have suggested that the middle temporal gyrus may mediate attention processing. The temporal lobe as a part of the limbic system (amygdala and hippocampus) is thought to have a role in focusing on a task in the presence of distracting stimuli and to execute quickly in response to the task (35). We suggest that aerobic exercise may improve the speed of attention processing in the temporal lobe in response to working memory task (WCST) in adolescents with ADHD.
Several medications and cognitive behavior therapy improve clinical symptoms and increase brain activity in response to inhibition on a working memory task (15,39). Wong and Stevens (39) have reported that psychostimulants (methylphenidate and dextroamphetamine) increased fronto-parietal brain connectivity and improved working memory reaction times. After 10 d of cognitive training, children with ADHD demonstrated increased activity within the orbito-frontal, superior frontal, middle temporal, and inferior frontal cortices in response to an inhibition paradigm (15).
The clinical symptoms and set shifting of individuals with ADHD have been closely associated with the regulation of dopamine release within the prefrontal, striatal, and basal ganglia areas (9). Several studies have suggested that endurance exercise and sports activity induce dopamine release in patients with Parkinson disease (24). Exercises consisting of complex movement sequences with visual and spatial cognition are known to be influenced by the modulation of dopamine within the prefrontal, striatal, and basal ganglia regions in patients with Parkinson disease (23). Increased brain activity due to aerobic exercise may be attributed to at least two factors: angiogenesis and dopamine release under hypoxic conditions.
In a review of pharmacological MRI studies, Knutson and Gibbs (18) suggested that dopamine release in the nucleus accumbens might be positively associated with blood oxygenation. Several studies have suggested that the effectiveness of aerobic exercise on brain activity may be related to increased perfusion secondary to angiogenesis (7,34). Three months of endurance training improved cerebral oxygenation and metabolism in healthy overweight males (34). Six months of aerobic exercise increased global brain volume of 59 healthy older adults age 60–79 yr compared with that of nonparticipants in exercise (7). Recent studies have reported that aerobic and anaerobic exercise increased brain oxygenation and perfusion in healthy individuals (11,34). Fisher et al (11) found increased cerebral perfusion after cycling exercise with the calculation of O2 saturation between the internal jugular vein and brachial artery. Angiogenesis can occur via a splitting process, known as intussusception, or a sprouting process, in which new branches sprout from one capillary and merge onto another (21).
There were several limitations in the current study. First, the number of subjects was modest and the short period of exercise may not have been long enough to generalize the current findings. In addition, time differences between education (50 min·d−1 × 12 sessions = 600 min) and sports (90 min·d−1 × 18 sessions = 1620 min) could affect the present results. Second, the present results do not show isolated aerobic exercise effects on clinical symptoms and brain activity because of the combined use of methylphenidate. Third, only single assessments of clinical symptoms and cognitive functions in adolescents were performed.
The findings of decreased brain activity within the DLPFC, parietal cortex, and temporal lobe relative to healthy control subjects are consistent with previous fMRI studies on ADHD. In addition, aerobic exercise can be an adjunctive therapy with medication treatment for improving clinical symptoms, perseverative errors, and brain activity within the right frontal and temporal cortices in response to WCST stimulation in adolescents with ADHD. Future studies may focus on the development of customized exercises for improvement of attentional capacity.
Acknowledgments
This work was supported by the Korean Game Culture Foundation and a grant from the Korean Health Technology Research and Development Project, Ministry of Health and Welfare, Republic of Korea (A120013).
Footnotes
All authors have no conflict of interest in the article, including financial, consultant, institutional, and other relationships that might lead to bias or a conflict of interest. The results of the study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. New York (NY): Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 2.Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry. 2011;69(12):1133–9. doi: 10.1016/j.biopsych.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Barch DM, Braver TS, Sabb FW, et al. Anterior cingulate and the monitoring of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12(2):298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- 4.Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529–34. doi: 10.1097/YCO.0b013e328356f87f. [DOI] [PubMed] [Google Scholar]
- 5.Chaddock L, Erickson KI, Prakash RS, et al. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89(1):260–8. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi L, Bramati IE, Zalesky A, et al. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci. 2012;32(49):17753–61. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 8.Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 2010;44(10):629–39. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145–57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 10.DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties of a community sample. J Clin Child Psychol. 1991;20:245–53. [Google Scholar]
- 11.Fisher JP, Hartwich D, Seifert T, et al. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol. 2013;591(7):1859–70. doi: 10.1113/jphysiol.2012.244905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehan M, Smaiha M. Effect of regular aerobic exercises on behavioral, cognitive and psychological response in patients with attention deficit-hyperactivity disorder. Life Sci J. 2011;8(2):392–7. [Google Scholar]
- 13.Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159(3):1044–54. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiruntrakul A, Nanagara R, Emasithi A, Borer KT. Effect of once a week endurance exercise on fitness status in sedentary subjects. J Med Assoc Thai. 2010;93(9):1070–4. [PubMed] [Google Scholar]
- 15.Hoekzema E, Carmona S, Tremols V, et al. Enhanced neural activity in frontal and cerebellar circuits after cognitive training in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2010;31(12):1942–50. doi: 10.1002/hbm.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang KD, Choi JW, Kang SG, Han DH. Sports therapy for attention, cognitions and sociality. Int J Sports Med. 2011;32(12):953–9. doi: 10.1055/s-0031-1283175. [DOI] [PubMed] [Google Scholar]
- 17.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. 1988;5(5):303–11. doi: 10.2165/00007256-198805050-00002. [DOI] [PubMed] [Google Scholar]
- 18.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191(3):813–22. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 19.Kobel M, Bechtel N, Specht K, et al. Structural and functional imaging approaches in attention deficit/hyperactivity disorder: does the temporal lobe play a key role? Psychiatry Res. 2010;183(3):230–6. doi: 10.1016/j.pscychresns.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Lee JA, Shin DJ, Lee CU, et al. Executive function in psychiatric patients groups through Wisconsin Card Sorting Test Computer Version (WCST) J Korean Neuropsychiatr Assoc. 2002;41:322–34. [Google Scholar]
- 21.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angio-genesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12(2):113–23. doi: 10.1007/s10456-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 22.Mongia M, Hechtman L. Cognitive behavior therapy for adults with attention-deficit/hyperactivity disorder: a review of recent randomized controlled trials. Curr Psychiatry Rep. 2012;14(5):561–7. doi: 10.1007/s11920-012-0303-x. [DOI] [PubMed] [Google Scholar]
- 23.Muller T, Benz S. Quantification of the dopaminergic response in Parkinson's disease. Parkinsonism Relat Disord. 2002;8(3):181–6. doi: 10.1016/s1353-8020(01)00010-4. [DOI] [PubMed] [Google Scholar]
- 24.Muller T, Muhlack S. Effect of exercise on reactivity and motor behaviour in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81(7):747–53. doi: 10.1136/jnnp.2009.174987. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen A, Wilmsmeier A, Wiedl KH, et al. Anterior cingulate cortex activation is related to learning potential on the WCST in schizophrenia patients. Brain Cogn. 2012;79(3):245–51. doi: 10.1016/j.bandc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Philipsen A. Psychotherapy in adult attention deficit hyperactivity disorder: implications for treatment and research. Expert Rev Neurother. 2012;12(10):1217–25. doi: 10.1586/ern.12.91. [DOI] [PubMed] [Google Scholar]
- 27.Pliszka SR. Pharmacologic treatment of attention-deficit/ hyperactivity disorder: efficacy, safety and mechanisms of action. Neuropsychol Rev. 2007;17(1):61–72. doi: 10.1007/s11065-006-9017-3. [DOI] [PubMed] [Google Scholar]
- 28.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 29.Pontifex MB, Saliba BJ, Raine LB, Pichietti DL, Hillman CH. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J Pediatr. 2013;162(3):543–51. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reithler J, van Mier HI, Goebel R. Continuous motor sequence learning: cortical efficiency gains accompanied by striatal functional reorganization. Neuroimage. 2010;52(1):263–76. doi: 10.1016/j.neuroimage.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AC. The importance of serotonin for orbitofrontal function. Biol Psychiatry. 2011;69(12):1185–91. doi: 10.1016/j.biopsych.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Rubia K, Halari R, Cubillo A, Mohammad AM, Scott S, Brammer M. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Hum Brain Mapp. 2010;31(12):1823–33. doi: 10.1002/hbm.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry. 2007;62(9):999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Seifert T, Rasmussen P, Brassard P, et al. Cerebral oxygenation and metabolism during exercise following three months of endurance training in healthy overweight males. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R867–76. doi: 10.1152/ajpregu.00277.2009. [DOI] [PubMed] [Google Scholar]
- 35.Sterzer P, Stadler C, Krebs A, et al. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57(1):7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. New York (NY): Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- 37.Verret C, Guay MC, Berthiaume C, Gardiner P, Be´liveau L. A physical activity program improves behavior and cognitive functions in children with ADHD: an exploratory study. J Atten Disord. 2012;16(1):71–80. doi: 10.1177/1087054710379735. [DOI] [PubMed] [Google Scholar]
- 38.Wigal SB. Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults. CNS Drugs. 2009;23(Suppl 1):21–31. doi: 10.2165/00023210-200923000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Wong CG, Stevens MC. The effects of stimulant medication on working memory functional connectivity in attention-deficit/ hyperactivity disorder. Biol Psychiatry. 2012;71(5):458–66. doi: 10.1016/j.biopsych.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yán˜ez-Teéllez G, Romero-Romero H, Rivera-Garcıía L, et al. Cognitive and executive functions in ADHD. Actas Esp Psiquiatr. 2012;40(6):293–8. [PubMed] [Google Scholar]


