Abstract
Metabolic consequences of obesity including insulin resistance, type 2 diabetes mellitus, hyperlipidemia, hypertension, polycystic ovarian syndrome, and nonalcoholic fatty liver infiltration are rapidly emerging in the pediatric population. Identifying effective strategies for identifying and treating these obesity related co-morbidities in children are crucial to the prevention of future cardiovascular disease and poor health outcomes. This review discusses the pathophysiologic connections between obesity, metabolic disease and cardiovascular risk. Current evidence and recommendations for screening and treatment for the metabolic consequences of pediatric obesity are reviewed.
Keywords: Insulin resistance, Fatty liver disease, Type 2 diabetes mellitus, Polycystic ovary disease, Metabolic syndrome
1 Introduction
Rates of pediatric obesity (BMI ≥ 95th percentile) have almost tripled over the past 25 years, with current estimates showing a prevalence rate of 16% for girls and 18% for boys. When one includes children who are at risk of overweight, the prevalence increases to 32% for girls and 35% for boys [1]. As rates of obesity have increased, more children have been identified with obesity-associated metabolic problems that were previously only encountered in the adult population. It has become increasingly evident that these metabolic complications stem from obesity-associated inflammation and insulin resistance (IR). Although IR may persist for years without clinical symptoms, progression to type 2 diabetes mellitus in the pediatric population is now more frequently recognized and is becoming the most common form of diabetes in certain ethnic backgrounds [2]. Equally concerning are the associated long term health risks of IR and its associated metabolic derangements including dyslipidemia and hypertension. The clustering of these abnormalities comprises the metabolic syndrome, and is strongly associated with premature cardiovascular disease. Additional disorders associated with IR such as polycystic ovarian disease and fatty infiltration of the liver, each carry their own additional health risks in addition to links to future cardiovascular disease. Identifying and treating the metabolic consequences of pediatric obesity are not only crucial for the immediate health of the child, but for future prevention of early heart disease. In this review, the premise for development of metabolic complications in pediatric obesity is discussed. Current data regarding prevalence patterns, appropriate screening, and best available evidence on how to effectively treat these complications is presented.
2 Obesity, inflammation and metabolic disease
2.1 Introduction
In recent years, there has been increasing appreciation of obesity as a state of chronic, low-level inflammation in adipose tissue. In adults as well as children, obesity-related inflammation has been implicated in the pathogenesis of insulin-resistance and atherosclerosis (Fig. 1). Furthermore, the level of inflammation correlates with multiple other obesity-related conditions including non-alcoholic fatty liver disease [3], polycystic ovarian syndrome [4]hypertension [5], dyslipidemia [6] and obstructive sleep apnea [7].
Fig. 1.
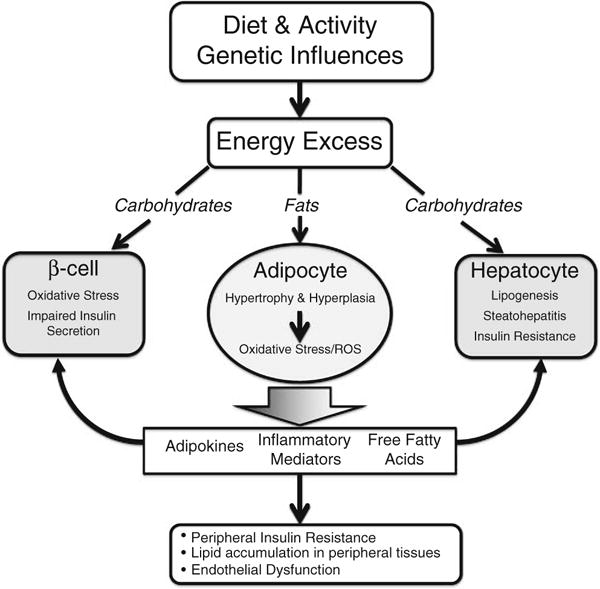
Energy excess, systemic inflammation and metabolic consequences. Excess dietary carbohydrates cause glucotoxicity and impaired insulin secretion in the pancreatic beta cell as well as steatohepatitis and insulin resistance in the hepatocyte. Excess dietary fats cause adipocyte hypertrophy and adipose hyperplasia, which leads to oxidative stress and altered production of adipokines and inflammatory mediators as well as increased circulating free fatty acids. This leads to a generalized inflammatory state and lipid accumulation in muscles, liver, vascular tissue, heart and beta cells. Adapted from reference [204]
The exact mechanism of the interactions between obesity-related inflammation and various disease states is a focus of active investigation. An increasing number of adipose tissue-derived mediators (adipokines) and obesity-associated inflammatory markers (cytokines and chemokines) have been associated with these disease states. Functions of several adipokines implicated in obesity related metabolic disease are found in Table 1. Adipose tissue is made up of both adipocytes as well as macrophages, and both cell types contribute to the generation of these inflammatory markers which have both paracrine as well as systemic effects. These markers may serve a critical role not only as indicators of disease progression but as potential therapeutic targets.
Table 1.
Adipokines and their potential metabolic roles in influencing development of metabolic syndrome
| Adipokine | Site | Role |
|---|---|---|
| Adiponectin | Muscle | Stimulates glucose uptake |
| Pancreas | Stimulates insulin secretion | |
| Immune | Anti inflammatory | |
| Liver | Suppresses gluconeogenesis Stimulates fatty acid oxidation | |
| Vascular | Antiatherogenic | |
| Leptin | Muscle | Stimulates fatty acid oxidation |
| Adipose | Inhibits lipogenesis | |
| Pancreas | Stimulates fatty acid oxidation | |
| Immune | Pro-inflammatory | |
| Liver | Stimulates fatty acid oxidation Indirect modulation of gluconeogenesis | |
| Vascular | Associated with atherosclerosis | |
| CNS | Reduces appetite/promotes energy expenditure | |
| Elevates sympathetic activity (hypertension) | ||
| RBP4 | Muscle | Impairs insulin signaling (in mice) |
| Adipose | May play a role in fuel sensing | |
| Liver | Induces insulin resistance | |
| Resistin | Muscle | (insulin resistance in mice) |
| Immune | Increases cytokine production in macrophages | |
| Vascular | Possible endothelial dysfunction | |
| Visfatin | Muscle | Possibly insulin sensitizing |
| Adipose | Stimulates TNF-α and IL-6 production Promotes adipogenesis | |
| Pancreas | Stimulates insulin secretion (mice) | |
| Liver | Reduces hepatic gluconeogenesis |
2.2 Cytokines, chemokines, and other inflammatory markers
The classical inflammatory pathway is triggered in macro-phages by both infectious stimuli as well as disordered metabolic states. This generates a cascade of pro-inflammatory cytokines, including IL-6, TNF-α, IL-1 and IL-10, as well as reactive oxygen species and cell adhesion molecules. Of these cytokines, TNF-α appears to have the most prominent and ubiquitous role in metabolic disease, acting through multiple pathways, including the NF- кB pathway. Not only is expression of TNF-α increased in insulin resistant states, but it directly results in insulin resistance in both human and mouse models [8]. IL-6 is a primary mediator of the acute phase response, similar to TNF-α. IL-6 is present at higher levels in obese children [9]. It is released in response to increased levels of IL-1 β and TNF-α. Monocyte chemoattractant protein-1 (MCP-1) is secreted by adipocytes and functions to increase monocyte infiltration into adipose tissue, which is an important step in increased inflammation in adipose tissue [8]. While C-reactive protein (CRP) is not produced in adipose tissue, it is generated in the liver in response to obesity-associated rise in IL-6 levels [10]. It plays a role in amplifying the inflammatory response and activates NF- кB [11]. As a marker of obesity-related inflammation, CRP is a robust predictor of cardiovascular events, hypertension and diabetes in adults [12].
2.3 Adipokines
Factors synthesized and secreted by adipocytes, called adipokines, have been increasingly studied as potential mediators of the association between obesity and the development of metabolic disease.
Leptin was the first such adipocyte-derived factor to be identified and its circulating levels are strongly associated with BMI and total fat mass in both lean and obese children and adolescents [13]. While leptin’s primary roles are in controlling energy balance at the level of the hypothalamus and as a permissive factor in regulating the onset of puberty, other more acute effects on metabolism in the liver, skeletal muscle, gastrointestinal tract, vascular smooth muscle cells and sympathetic nerves have been identified [14]. There has also been evidence that leptin potentiates the production of TNF-α and IL-6 by macrophages; however, the mechanisms underlying this relationship are not clearly understood [15].
Adiponectin is a highly-expressed adipokine which has robust and pervasive relationships to both obesity and multiple components of metabolic disease. Unlike the other adipokines, its circulating levels are inversely related to obesity and abdominal obesity in particular [16]. Adiponectin has multiple beneficial effects, including a strong correlation with insulin sensitivity and a protective role in the development of atherosclerosis and hypertension [8]. The potentially beneficial metabolic role of adiponectin may be explained in part by its anti-inflammatory effects. Adiponectin reduces the production of TNF-α by macrophages and its inhibition of NF- кB signaling. Conversely, TNF-α and IL-6 act to reduce the production of adiponectin by adipocytes [16]. Interestingly, adiponectin levels are not only affected by adiposity, but also by gender and by pubertal stage. Women have significantly higher adiponectin levels than men [17]. While boys have decreasing adiponectin levels with advancing pubertal stage, girls do not have a significant correlation with pubertal stage. This pubertal decline in boys was strongly associated with androgen levels, implying that this may be an important factor in the differences seen in adults as well [18].
Resistin was first identified in animal models as an adipocyte-derived factor whose expression was reduced after treatment with thiazolidinediones. In rodent models, resistin induced insulin resistance and contributed to fasting hyperglycemia. In humans, however, it appears that the majority of resistin is produced by circulating macrophages and monocytes, rather than adipocytes [16]. Further, there has been no clear correlation in most human studies between resistin levels and insulin resistance when adjusting for BMI [8].
Retinol binding protein 4 (RBP4) was identified as a highly expressed adipokine which caused insulin resistance when overexpressed in mice [19]. As in the case of resistin, the relationship between insulin resistance and RBP4 levels in humans is less clear. While some studies have not found a relationship, others have found strong positive correlations [8]. There is a suggestion that this relationship may be age-related, as it was present only in younger subjects [20]. Similarly, there is discordance in response of RBP4 levels to thiazolidinedione treatment, with some studies suggesting a decrease and others showing no effect [8].
Visfatin is expressed in many tissues; however, it is predominantly expressed in visceral adipocytes [21]. It was initially reported to have insulin-like function, but further research demonstrated that it has an important role in β -cell function [22]. Human studies have shown that with increasing BMI, visfatin expression increases in visceral adipose tissue, but decreases in subcutaneous tissue. This may, therefore, be an important link between cardiovascular disease and metabolic syndrome as visfatin levels are associated with coronary artery disease and acute coronary syndromes in adults, independent of other known risk factors [23]. The relationship between visfatin and insulin resistance is more variable. There is some evidence that visfatin has proinflammatory properties [24], further signifying this hormone’s potential role in the inflammation associated with increased visceral adiposity.
2.4 Connection of inflammation to organ dysfunction and disease
It is believed that the various inflammatory mediators released by the adipose tissue of obese individuals contribute to dysfunction and disease in a variety of other tissues [25]. Adipose specific and non-specific inflammatory markers have been shown to predict the presence and severity of atherosclerosis, type 2 diabetes mellitus, steatohepatitis, PCOS, and sleep apnea in adults [3–7]. Studies in young adults have shown that severity of metabolic syndrome is associated with a number of mediators within the inflammatory cascade including CRP, IL-18, IL-6 and decreased adiponectin. Importantly, this association is independent of insulin sensitivity, as determined by euglycemic hyperinsulinemic clamp, highlighting the direct role of inflammation in these disorders [26].
There is less data about the role of inflammation in the development of metabolic disease in children; however, studies in adolescents show a positive correlation between CRP, IL-6 and leptin with overweight status [27, 28]. Further, elements of the metabolic syndrome such as dyslipidemia, hypertension, and insulin resistance in children and adolescents are associated with IL-6, TNF-α, and IL-18 [29, 30]. Adiponectin has been shown to be negatively correlated with metabolic syndrome in obese or overweight adolescent girls, but not boys [31]. Other studies in prepubertal children have shown that obesity, elevated TG levels, and insulin resistance are positively correlated with CRP and leptin and negatively correlated with adiponectin [28].
Since it is well established that the pathologic lesions associated with atherosclerotic disease begins in childhood [32, 33], these initial data correlating inflammation with various components of metabolic disease and cardiovascular health in obese adolescents provide further evidence for the need to address inflammation as a target of obesity therapy.
3 Insulin resistance and the metabolic syndrome
3.1 Introduction and prevalence
Obesity, specifically visceral adiposity, is directly linked to insulin resistance in both adults and children [34, 35]. IR results from a number of pathophysiologic mechanisms including impaired insulin signaling, abnormal glucose transport and abnormal adipocyte cytokine production [34–36]. The resistance to insulin action at the cellular level requires increasing insulin secretion to maintain normal rates of glucose disposal. When pancreatic β-cells can no longer secrete the levels of insulin required to overcome inherent insulin resistance, abnormal glucose metabolism, and eventually T2DM results [34]. Not surprisingly, obese children are more insulin resistant than children with normal BMI [37, 38]. However, body size does not explain the entire risk for IR. Insulin resistance is negatively associated with physical activity when adjusted for BMI, age and fat mass [38], highlighting the importance of fitness.
The euglycemic-hyperinsulinemic clamp is the gold standard for measuring insulin resistance. However, this study is invasive and time-consuming [39]. The difficulty of applying this study to large groups of children and adults has lead to the development of surrogate markers of insulin resistance/sensitivity. These include the homeostasis model assessment (HOMA), the quantitative insulin sensitivity check index (QUICKI), the fasting glucose-to-insulin-ratio, the Matsuda method, the whole-body insulin resistance index, and fasting insulin levels alone [39, 40]. These measures are frequently used in the literature, although their correlation with results from euglycemic-hyperinsulinemic clamp has varied from borderline to highly significant in the few studies that address this in children [39, 41].
Metabolic syndrome (MS) refers to a clustering of clinical findings and metabolic disturbances including abdominal obesity, hypertension, dyslipidemia, and insulin resistance (IR). Obesity and insulin resistance predispose to the other metabolic abnormalities observed in MS. IR, independent of obesity (BMI), is associated with a greater risk for development of dyslipidemia and hypertension [42–44]. Presence of MS in childhood correlates to markers of early cardiovascular disease such as increased carotid intima-media thickness and elevated inflammatory markers observed in heart disease [45–47]. Perhaps most importantly, presence of MS in childhood predicts metabolic syndrome and the development of type 2 diabetes mellitus (T2DM) in adulthood as well as an increased rate of premature cardiovascular events [48], making it imperative that effective strategies are developed to prevent or reverse this clustering of metabolic abnormalities.
In adults, metabolic syndrome is defined by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III as the presence of 3 or more of: waist circumference >102 cm for men and >88 cm for women, triglycerides greater than 150 mg/dl, high density lipoprotein cholesterol <40 mg/dl for men and <50 mg/dl for women, hypertension (SBP > 120 or DBP >85) and elevated fasting glucose (>110 mg/dl) [49]. However, there is no agreed-upon definition for this syndrome in children (reviewed in [46]). It has also been proposed that nonalcoholic fatty liver disease (NAFLD) should be added to the diagnostic classification of the metabolic syndrome, given the high prevalence of NAFLD in MS [50]. The International Diabetes Federation and American Heart Association have both recently proposed criteria (Table 2), but no criteria have yet been universally accepted [46].
Table 2.
Metabolic syndrome criteria for pediatric populations
| Ages (years) | AHA Criteria 12–19 |
IDF Criteria
|
||
|---|---|---|---|---|
| 6 to 9 | 10–15 | 16+ (adult criteria) | ||
| Waist circumference | >75th percentile for age and gender | ≥90th percentile | ≥90th percentile Or adult cut off if lower | Adult ethnic norms (or BMI > 30) |
| Blood Pressure | >90th percentile for age, gender and height | Cannot diagnose metabolic syndrome, but screen if suggestive family history | Systolic ≥ 130 or Diastolic ≥ 85 | Systolic ≥ 130 or Diastolic ≥ 85 Or treatment of diagnosed hypertension |
| Triglycerides | ≥1.1 mmol/L (≥100 mg/dL) | ≥1.7 mmol/L (≥150 mg/dL) | ≥1.7 mmol/L (≥150 mg/dL) | |
| HDL-C | boys 15–19: <1.17 mmol/L (<45 mg/dL) | <1.03 mmol/L (<40 mg/dL) | Males: <1.03 mmol/L (<40 mg/dL) | |
| all others: <1.3 mmol/L (<50 mg/dl) | Females: <1.29 mmol/L (<50 mg/dL) Or treatment for dyslipidemia | |||
| Fasting Glucose | ≥6.1 mmol/L (≥110 mg/dL) | ≥5.6 mmol/L (≥100 mg/dL) If elevated FG, recommend OGTT | ≥5.6 mmol/L (≥100 mg/dL) Or diagnosis of T2DM | |
The lack of universally accepted criteria has hampered the interpretation of the literature as different studies have used many different definitions. This has also made establishing prevalence data challenging, and recent estimates have ranged greatly depending on the definition used and the population under study. A brief review of the literature finds general population rates of MS ranging from 0.2% in 10 year olds in Europe to 9.5% among the U.S. pediatric population as a whole from the NHANES [51–55]. Not surprisingly, the prevalence rates are significantly higher in samples evaluating only obese children, ranging from 12.4–44.2% [53, 54]. The rates of metabolic syndrome in children referred for obesity treatment are even higher, approaching 50% in some studies [56]. Children with T2DM have among the highest prevalence rates, with 76.9% of children with T2DM also meeting criteria for metabolic syndrome [49].
3.2 Lifestyle factors and metabolic syndrome
Multiple studies have explored the relationship between cardiovascular fitness, physical activity and the metabolic syndrome. This was reviewed in depth by Steele et al. [57], who concluded that physical activity and cardiorespiratory fitness are both independently and negatively associated with the metabolic syndrome. This association remained, even when adjusted for age and BMI, suggesting that the beneficial effects of fitness and exercise cannot be fully explained by their effect on adiposity [51–53, 58]. Increased amounts of screen time have also been associated with increased rates of the metabolic syndrome in adolescents, and interestingly, this effect was independent of physical activity [59].
As might be expected, diet also plays a critical role in risk for developing MS. Pan and Pratt analyzed a sample of 4,450 12- to 19-year-olds from the NHANES 1999–2002 data, scoring them for adherence to the USDA dietary recommendations (the “Healthy Eating Index” score). In this large population study, children and adolescents with healthier diets had a lower prevalence of metabolic syndrome [53]. In a small cross-sectional study of healthy children, Casazza et al. found that diet was more strongly correlated with presence of the metabolic syndrome or its individual components than physical activity [60]. They also found that the percent of calories ingested in the form of carbohydrates had a stronger association with increased rates of the components of the metabolic syndrome, whereas diets with higher percentages of calories from protein and fat were associated with decreases in the components of the metabolic syndrome [60].
3.3 Treatment of insulin resistance and metabolic syndrome in children
Each component of the MS has its own pharmacologic treatment options (outlined in their respective sections below). However, when considering the greater constellation of insulin resistance and metabolic syndrome, the optimal approach should encompass the adoption of lifestyle changes, similar to the general principles followed for treating obesity in general.
3.3.1 Dietary interventions to improve IR
Given the association of obesity and IR as inherent components of MS, weight loss efforts through dietary modification and/or improved physical activity are the first line treatment in adults [61, 62]. However, there are few clinical trials evaluating treatment of metabolic syndrome in children. One small study by Chen et. al. of a 2 week residential lifestyle modification in 16 children found significant improvement in BMI, waist circumference, percent body fat, blood pressure, serum lipids, and fasting insulin levels. Of the children who met criteria for metabolic syndrome at the beginning of the study, none met criteria at the end of the study [63]. This study, while somewhat limited in its design, is striking in the benefit shown despite relatively short treatment time. Monzavi et al. studied the effects of a twelve week family-centered intensive lifestyle intervention on a group of obese children who were referred for weight management. Unfortunately, 46% of their population dropped out of the program. However, of the children who were able to participate, they found a statistically significant decrease in BMI, leptin, systolic BP, total and LDL cholesterol, triglyceride and 2 h postprandial glucose levels [56]. Even though these studies are relatively small and few in number, they do indicate the likely effectiveness of dietary intervention.
3.3.2 Exercise interventions to improve IR
Exercise is beneficial to cardiovascular health [64]. The beneficial effects of exercise on metabolic disease are propagated through increased cardiovascular fitness, decreased body fat, and improved skeletal muscle function [65]. Studies in adults have identified an anti-inflammatory property of physical activity, with lower IL-6, IL-, and CRP levels in physically active adults [66, 67]. Several studies have shown an inverse relationship between exercise in children and the inflammatory markers IL-6, TNF- α and CRP levels [30]. Additionally, leptin levels consistently decrease in response to exercise interventions in children [13]. There is some debate about whether this is a direct effect or a result of lower levels of adiposity; however, multiple studies in children support the conclusion that fitness and fatness exert independent effects on both inflammatory markers and insulin sensitivity [65].
Several studies targeting obesity as a primary outcome measure have shed light on the effectiveness of specific types of lifestyle intervention programs on markers of insulin resistance. One school-based lifestyle intervention study randomized 55 obese children to a school fitness curriculum vs. standard physical education classes. The intervention group had significant improvements in markers of cardiovascular fitness, body fat, and insulin sensitivity [68]. Savoye, et.al. randomized 209 obese children 2:1 to either a standard weight-management clinic or a more intensive weight-management program, consisting of 50 min of high-intensity exercise two times per week coupled with nutrition and behavioral interventions for 40 min once a week [69]. Although a high drop out rate in the intensive group limited the results of the study, the intensive group displayed a significant decrease in BMI, percent body fat and total body fat as well as significant decrease in insulin resistance as measured by HOMA-IR [69]. A Korean study which randomized a small number of obese high school males to a jump rope class vs. regular education, found that six weeks of jump rope training resulted in decreased waist circumference, hip circumference, body weight, BMI, fat mass and percent fat mass and also decreased insulin levels and HOMA-IR [70]. Several non-randomized studies of lifestyle interventions in obese children have also shown that decreases in BMI or BMI-SDS are associated with improvements in insulin resistance [71]. These studies indicate that lifestyle interventions that improve BMI can improve markers of insulin resistance as well. Still, the success of weight reduction via lifestyle intervention in obese IR pediatric patients is limited compared to those children without IR [72].
Some studies have indicated that lifestyle modification, especially improvements in physical fitness, may improve insulin resistance even in the absence of a change in BMI. In a study by Rohrer et al., 22 children (10 of which had IR by HOMA at baseline) were enrolled in a one-year physical exercise program [73]. 5 of the IR children experienced a decrease in BMI SDS while 5 did not. However, at the end of the 1-year study period, all of the children demonstrated significantly improved physical fitness levels and none had abnormal HOMA values [73]. Although limited by small sample size and a basic measure of IR, it hints to the critical importance of fitness on IR. Another study of 21 obese adolescent girls was designed to specifically address the question of effects of BMI reduction on IR. The girls were purposely put on a weight-maintenance diet and participated in aerobic exercise training. They found that after 12 weeks of exercise training, insulin AUC decreased significantly in the 11/15 girls who completed the study, even though there was no decrease in BMI [74]. These studies indicate that increased physical fitness in children secondary to exercise intervention can improve insulin resistance even in children who do not have significant weight change.
3.3.3 Dietary interventions to reduce inflammation
Omega-3 fatty acids from dietary sources including fish oil and flaxseed oil have been identified as natural antagonists of inflammatory pathways. Various studies have explored the benefit of supplementing the diet with these fatty acids to reduce inflammation, improve dyslipidemia, and possibly improve metabolic profile. One prospective, randomized double-blind cross-over study in obese adults supplemented the diet with flaxseed flour, which contains omega-3 fatty acids. Flaxseed flour consumption was associated with a significant reduction in CRP. Another study showed significant reduction in LDL levels and HOMA-IR index, but no change in IL-6 or CRP levels [75, 76]. There is evidence that obese children and adolescents have lower levels of circulating antioxidants, such as the omega-3 fatty acids, and that this is associated with higher levels of the inflammatory markers, IL-6 and CRP. Obese children with metabolic syndrome also had lower omega-3 fatty acid levels, independent of body fat [77].
3.3.4 Metformin treatment to improve IR
When considering the treatment of insulin resistance and metabolic syndrome, lifestyle interventions must be balanced with consideration of pharmaceutical therapies focusing on the prevention of progression to T2DM. With the ensuing rise in the pediatric obesity epidemic there has been increasing interest in the use of insulin sensitizers in the treatment of obesity and associated IR in children.
Metformin is a biguanide that affects hepatic glucose output and peripheral insulin sensitivity. It has been the most commonly studied agent for treating IR and obesity in children, dating back to the 1970s [78]. Thus far, the results of metformin as a primary agent for treating IR in children have been mixed. Love-Osborne et al. studied 85 adolescents with insulin resistance (fasting insulin levels >25 mU/ml or HOMA >3.5 and 2 of 3 risk factors for T2DM). These children were randomized in a 2:1 ratio to receive metformin or placebo in addition to lifestyle intervention. Overall, there was no difference between the groups at the end of the study. However, girls who were adherent to therapy experienced a significant decrease in BMI in comparison to boys, highlighting the potential for gender specific or other intrinsic factors to influence treatment effectiveness [79]. Another trial which used a crossover design randomized 28 obese adolescent patients with insulin resistance to either 6 months of metformin followed by placebo or 6 months of placebo followed by 6 months of metformin with 2 weeks of washout. In this study, metformin improved weight, BMI, waist circumference, fasting insulin, fasting glucose, but did not significantly improve IR as measured by intravenous glucose tolerance test. Reductions in subcutaneous fat but not visceral fat were seen in the treatment group compared to placebo [80]. In yet another study, 28 hyperinsulinemic (fasting insulin > 30 mg/dL), normoglycemic obese children were randomized to metformin versus placebo. The metformin group had improved BMI, fasting insulin levels, and improved heart rate variability, a marker which has been correlated with poor cardiovascular outcomes in adults. However, when insulin resistance was adjusted for baseline levels, the improvement in insulin sensitivity was no longer significant. The authors also found that metformin reduced subcutaneous but not visceral fat mass [81].
Other studies have found a more positive benefit from metformin on insulin resistance in obese children. A group from Turkey randomized 120 adolescents with IR by HOMA-IR in a double-blind fashion to metformin or placebo coupled with lifestyle modification. The metformin group experienced a significant decrease in BMI, insulin levels and in insulin resistance (by HOMA-IR and QUICKI) [82]. Freemark and Bursey performed a doubleblind, randomized, placebo controlled trial in 32 obese adolescents with a first or second degree relative with T2DM and fasting insulin >15 mU/L [83]. The metformin group experienced significant decrease in BMI, insulin levels and insulin resistance (HOMA-IR) and an increase in insulin sensitivity (QUICKI). Another study in 24 obese children without impaired glucose tolerance at baseline randomly assigned them in a double-blind fashion to metformin versus placebo. The metformin group lost more weight, more body fat and had improved insulin sensitivity [84].
It is clear from the data above, with the exception of the crossover study by Srinivasan et al. that improvement in insulin resistance in children treated with metformin seems to occur in the setting of improvements in BMI. This begs the question of whether metformin addresses the insulin resistance, which improves weight loss, or whether metformin improves weight loss, which improves insulin resistance. Though it may also be difficult to tease out the potential effect of concomitant lifestyle changes, there is preliminary data to suggest that metformin may be a helpful adjunct for treating accompanying IR in pediatric obesity.
The relative efficacy of lifestyle-based approaches vs. metformin therapy for prevention of progression to T2DM is still an active area of research in children. In adults, lifestyle intervention was shown to be more effective than metformin for T2DM prevention in a high-risk population [85]. In children, the studies have been small and limited by high dropout rates; however, it is noteworthy that the youngest participants in the Diabetes Prevention Program (DPP) showed a similar response to both metformin and lifestyle intervention [86]. While the DPP was not powered to specifically look at this group, it raises the question of whether metformin could be at least as effective as lifestyle changes in this population for T2DM prevention. At this point, there is a need for further long-term studies to address the issue of metformin as a primary prevention for development of T2DM.
3.4 Summary
There is little data on the treatment of the metabolic syndrome in children. However, it seems clear that lifestyle modification including improvements in physical activity and dietary changes are the mainstay of treatment. Adjunctive therapies using omega-3 fatty acids may be beneficial in reducing inflammation and lowering long-term risk, but no long term evidence is available. Metformin is a relatively low-risk intervention that is associated with a trend towards improvement in IR and weight reduction in certain groups. There is ample opportunity for continued research to evaluate adjunctive treatments that confer long term improvement on progression to both type 2 diabetes and early heart disease, and to come to a consensus on a definition of the metabolic syndrome in children.
4 Co-morbid conditions associated with insulin resistance and the metabolic syndrome
4.1 Prediabetes and development of type 2 diabetes mellitus
4.1.1 Introduction
The development of type 2 diabetes likely encompasses a spectrum beginning with IR and progressing to prediabetes and finally frank type 2 diabetes. Worsening insulin resistance results in increased stress on β-cell secretory capacity to maintain normoglycemia. Disordered glucose metabolism, or prediabetes, will eventually occur in individuals who experience some degree of β-cell failure [34, 87]. Prediabetes includes both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). IFG is defined as a fasting glucose level between 100 and 125 while impaired glucose tolerance is defined as a blood glucose level 141–200 mg/dl 120 min after a standardized oral glucose load during an oral glucose tolerance test. IGT is considered a precursor to T2DM and indicates some degree of impairment in β-cell response to a glycemic load.
4.1.2 Prevalence and screening
The prevalence of IR and prediabetes in children has been rising alongside the obesity epidemic. Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2002, found that 52% of obese US adolescents were insulin resistant, based on HOMA-IR [41]. In an Israeli referral population, 80% of obese children had insulin resistance as defined by HOMA-IR > 2. Of this group, 13.7% had IGT [88]. In 1998–1994, NHANES data revealed 0.39% of 12–19 year olds had impaired fasting glucose, with a combined prevalence of 0.41% of both type 1 and type 2 diabetes mellitus [89]. More recently, a German study from 2004 found a prevalence of IFG and IGT in obese 8–20 year olds of 3.7% and 2.1%, respectively, with a prevalence of T2DM of 1.5% [90]. IGT has been found at rates as high as 25% in obese children [91, 92]. It must be remembered that OGTT testing has poor reproducibility in children [93], so borderline cases may retest in a normal range.
Although the absolute numbers of patients remains relatively low in many centers [94], a greater proportion of new pediatric DM cases are being identified as type 2. Prior to the 1990s, T2DM was rare in most Pediatric Endocrinology practices but by 2000, 8–45% of new cases of diabetes in children were type 2 [95]. The SEARCH for Diabetes in Youth study is tracking incidence and prevalence patterns within select locations in the United States, and has found that proportions of DM classified as type 2 vary greatly by ethnicity, from 6% in non-Hispanic whites to 76% in Native Americans [2]. The American Diabetes Association (ADA) guidelines for screening for type 2 diabetes mellitus in asymptomatic children are listed in Table 3. In addition to a fasting plasma glucose, strong consideration should be given to obtaining an HbA1c [96] as an additional screening tool for T2DM.
Table 3.
American Diabetes Association Recommendations for testing for type 2 diabetes in asymptomatic children [210]
| Primary Criteria — Overweight, defined as: |
|
| Plus any two of the following risk factors: |
|
| Age of initiation: Age 10 years or at onset of puberty, if puberty occurs at a younger age |
| Frequency: Every 3 years |
| Screening Test: Fasting plasma glucose preferred |
4.1.3 Treatment of prediabetes
There is a paucity of information in the literature to address the treatment of prediabetes in children. Szamosi et al. studied a 2 year lifestyle intervention in 53 boys and 61 girls divided into 3 groups based on their glucose tolerance: a normal glycemic group; a group with abnormal glucose levels at 180 min during oral glucose tolerance testing (OGTT) but normal glucose levels at 120 min; and those who met criteria for IGT [92]. BMI, systolic blood pressure, triglycerides and HOMA index all decreased significantly in the patients who participated in the intervention. Fasting plasma insulin levels decreased in those in the normal group, but not the others. Glucose parameters on OGTT also significantly decreased in all groups.
To this point, pharmaceutical intervention to prevent T2DM (metformin) has not been formally studied in the pediatric population. In adults, metformin does appear to be an effective adjunctive or primary therapy to prevent or delay the onset of type 2 diabetes in a prediabetic population [85]. Given the growing evidence of the potential benefit of metformin in the IR pediatric population, it should strongly be considered as an adjunct to lifestyle alteration.
4.1.4 Treatment of type 2 diabetes mellitus in children
Since pediatric T2DM is a fairly new entity, there is a paucity of data on the effectiveness and safety of pharmaceutical strategies that are commonly employed in adults. The only currently approved therapies forT2DM in childrenare limited to metformin and subcutaneous insulin. The most effective treatment for children with a formal diagnosis of T2DM is unknown, but a large-scale trial comparing lifestyle, metformin, and the thiazolidenedione, rosiglitazone, for T2DM in youth is well underway [86]. Other classes of agents used in adults, including sulfonylureas, thiazolidenediones, alpha glucosidase inhibitors, GLP-1 agonists, and DPP-4 inhibitors have not been well studied in children. Despite this, many of these oral medications are being used in pediatric patients with T2DM [97]. In a survey of practitioners in the UK, the most prevalent treatment used in children with T2DM was metformin, but insulin, sulfonylureas, and thiazolidinediones were also commonly used [98]. At the current time metformin is still the only oral agent FDA approved for the treatment of T2DM in the pediatric age group [99].
Initial treatment in children with T2DM is based on metabolic control at presentation. If the child is in poor metabolic control or diabetic ketoacidosis, the first line therapy is insulin [100]. Once metabolic stability has been achieved, lifestyle modifications, in conjunction with metformin, become the primary treatments [100–102].
Metformin
A single randomized, double blinded placebo controlled trial established the effectiveness of metformin for pediatric T2DM and led to its approval as a first line drug [103]. In this study, a total of 42 children aged 8–16 year old were enrolled to one of two arms. The trial was stopped early due to 70% of placebo patients requiring rescue medications. Metformin treated patients had significant reduction in fasting plasma glucose levels and hemoglobin A1C levels compared to placebo [103].
Sulfonylureas
Sulfonylureas bind to the sulfonylurea receptor on pancreatic beta cells resulting in cell depolarization and eventual secretion of insulin. One study has compared the effectiveness of the sulfonylurea glimepiride to metformin in a single-blind randomized trial among pediatric patients [99]. Two hundred, eighty-five children were randomized to glimepiride or metformin, with mean final dose for glimepiride of 3.8 mg/day and metformin of 1408 mg/day. There was a significant and equal reduction in A1C values at 24 weeks in both groups. However, there was a significant difference in BMI at the end of the study with the glimepiride group gaining weight (+0.26 kg/m2) while the metformin group lost weight (−0.33 kg/m2). There was a comparable rate of adverse events between groups, although this rate was high (nearly 60%) in both groups. Although somewhat hampered by loss to follow up and the single-blinding design, it did demonstrate the efficacy of this class of agent in ages 8–17 years.
Thiazolidinediones
Thiazolidinediones bind to a member of the nuclear hormone receptor family known as peroxisome proliferator-activated receptor γ (PPAR γ). The exact mechanism of action of these drugs is unknown, but they enhance insulin action in peripheral tissues and the liver and improve lipids, blood pressure, and endothelial function in adults (reviewed in [102]). There is no published data about the safety or efficacy of this class of agents in the pediatric population at this time. The TODAY study should help elucidate their potential role [86]. As always, enthusiasm for new therapies must be weighed against the potential for long-term harm in the pediatric population. This is especially important in light of the recent data showing a potential link for earlier cardiovascular events in adults who received rosiglitazone as part of an intensive therapy regimen [104].
Glitinides
Glitinides stimulate endogenous insulin secretion by activating the voltage-dependent calcium channel. They have a more rapid onset and shorter duration of action than sulfonylurea and are given with meals. They have not been as well studied in adults as the sulfonylureas and there is no data in children or adolescents (reviewed in [102]).
Incretin hormones
Glucagon-like peptide 1 agonists (exenatide and liraglutide) are the newest class of agents used in type 2 diabetes therapy. This class of drug mimics the effects of incretin hormones which are normally released in response to a meal and enhance insulin secretion, reduce hepatic glucose output via suppression of glucagon, and slow gastric emptying. Dipeptidyl peptidase 4 (DPP-4) is an enzyme that breaks down GLP-1 and other incretin hormones. The class of drugs known as DPP-4 inhibitors (sitagliptin and vitagliptin) prolongs the activity of endogenous incretins. Both GLP-1 agonists and DPP-4 inhibitors are effective in lowering HbA1c values in adults and GLP-1 agonists have a weight negative effect [105]. There have been no studies performed in the pediatric population on effectiveness or safety of GLP-1 agonists or DPP–4 inhibitors.
Future directions: non-steroidal anti-inflammatory drugs
Salicylates were first noted to improve hyperglycemia in the late 19th century, well before the introduction of insulin (reviewed in [106]). Recent studies in mice have built on this historical observation to show that salicylates reverse both TNF-α-induced insulin resistance in adipocytes as well as lipid-induced defects in insulin signaling in hepatocytes [107, 108]. Three more recent studies of salsalate therapy humans demonstrated reductions in fasting glucose, CRP, total cholesterol and triglycerides in diabetic adults [109] as well as decreased markers of inflammation, improved insulin-stimulated glucose uptake, and decreased hepatic glucose production in obese, nondiabetic adults [106, 110]. These preliminary studies indicate a possible role for NSAID therapy in IR and T2DM, and larger randomized placebo-controlled studies are underway.
4.2 Dyslipidemia
4.2.1 Introduction
A clear association between an atherogenic lipid profile (elevated LDL cholesterol and triglycerides with low HDL cholesterol) and obesity in children has been well established [111]. An atherogenic lipid profile is a known risk factor for development of fatty streaks in the aorta. Postmortem studies have demonstrated evidence of coronary artery disease in obese young men who had unfavorable lipid profiles and met criteria for metabolic syndrome [32, 112]. Lipid profiles (and other components of metabolic syndrome) track from childhood into adulthood [113–115]. Therefore, dyslipidemia, when present in youth, represents an opportunity to modify a risk factor that may have a dramatic effect in future cardiovascular disease.
4.2.2 Prevalence and screening
Obesity is listed as a risk factor to screen for lipid abnormalities in the most recent policy statement adopted by the American Academy of Pediatrics [116]. Up to 46% of a referral population of overweight 7–12 year old children had evidence for abnormalities in their lipid profile when compared to population based norms. Moreover the children with high TG and low HDL had significantly higher BMI-SDS and lower indices of fitness than the rest of the cohort [117]. Obese youth, when matched for age, gender, and pubertal stage, have a 2.5–7.1 fold increased risk for an atherogenic lipid profile and insulin resistance in comparison to their leaner counterparts [118]. In most obese children, the dyslipidemia observed is mixed and includes elevated TG, elevated LDL, and low HDL levels. Isolated hypertriglyceridemia has been reported in 10–20% of obese children [119].
The dyslipdemia seen in pediatric obesity has both genetic and environmental origins. However, a key risk factor for development of lipid abnormalities is concomitant insulin resistance. For the same BMI, adolescents with evidence for IR are more likely to have an abnormal lipid profile [120].
Treatment cut-offs have generally depended on LDL levels that are above a single acceptable value for children established by the National Cholesterol Education Panel or above age adjusted norms. An adaptation of current cut-offs for consideration of therapy is listed in Table 4. For obese children with LDL levels above this range, therapy is recommended. Due to the inherent increased risk for future cardiovascular disease, children diagnosed with T2DM have different thresholds to initiate more aggressive therapy once glycemic control is maximized.
Table 4.
Lipid treatment thresholds and dietary therapies for dyslipidemia in obese children
| Lipid Thresholds: |
| Dietary Strategies |
|
Patients with type 2 diabetes mellitus should first have glycemic control maximized.
Threshhold for patients with type 2 diabetes mellitus is LDL > 100 mg/dl
Lipid Threshholds adopted from most recent NCEP guidelines for children and adolescents and American Heart Association guidelines for Prevention of Atherosclerotic Disease in Childhood
4.2.3 Treatment
Therapeutic guidelines for treatment of lipid abnormalities in children are limited by the lack of long term evidence except in children with a genetic mutation resulting in known disorder of lipid metabolism such as familial hypercholesterolemia (FH). Most safety and efficacy data on use of HMG CoA reductase inhibitors or “statins” stems from trials in children with FH. In the FH population, statins have been demonstrated to have excellent short-term safety, and are effective at improving lipid profiles and non-invasive surrogates of cardiovascular disease such as carotid IMT and brachial flow dilatation [121].
Lifestyle
First line therapy for dyslipidemia among obese youth remains lifestyle intervention including aggressive reductions in dietary fat intake and increasing aerobic activity. Dietary recommendations are to limit total fat intake to 25% or less of daily calories, saturated fat intake to <7% of total calories, trans fats to <1% of total calories, and cholesterol to <200 mg daily. Implementation of such dietary changes can be expected to induce modest reductions in LDL levels [122]. Additional low risk dietary intervention strategies should include supplementation with omega-3 fatty acids (docosahexanoic acid [DHA] and eicosapentaenoic acid [EPA]) as they are an effective and safe therapy for treatment of hypertriglyceridemia [123]. Preliminary data in children shows a similar benefit in promoting a less atherogenic profile [124]. Addition of soluble fiber and/or plant stanols and sterols may also have a moderate impact on LDL levels [116]. An increase in exercise is generally recommended based on adult data. Pediatric data is less abundant and shows mixed results; however, previous studies have been limited by small sample sizes and lack of control for pubertal staging, type of exercise, and diet. In general, it is believed that exercise promotes modest increases in HDL cholesterol without significantly changing total cholesterol [125].
Pharmacologic therapy
For obese children > 10 years who comply with dietary therapy and have persistent elevation of LDL cholesterol > 130 or for children with T2DM and LDL >100 mg/dl, pharmacologic therapy should be considered.
Bile Acid Resins
The bile-acid resin, cholestyramine, has long been the first line pharmacologic agent for children with persistent hypercholesterolemia. Modest reductions in total cholesterol of 10–20% have been observed in the pediatric population [126]. Despite their favorable side effect profile due to a lack of systemic absorption, cholestyramine is limited by its poor tolerability and lack of adherence.
Fibrates
Fibrates are recommended for adults with hypertriglyceridemia for further cardiovascular risk reduction and prevention of pancreatitis. The general cut-off for adults is a triglyceride level of 450 mg/dl or greater. No data is available to demonstrate improved outcomes in treated pediatric patients, but guidelines for treatment of hypertriglyceridemia in the obese pediatric patient have recently been proposed. This includes a step wise approach using lifestyle changes and omega–3 fatty acids with the addition of a fibrate (gemfibrozil) if persistent elevation in TG or a statin if non-HDL levels are high [119].
Niacin
Niacin is rarely used in the pediatric population due to its side effect profile (flushing, hepatitis). However, it can be effective in lowering LDL while increasing HDL and should be considered in the older adolescent with evidence for metabolic syndrome. No randomized controlled trials are available in the pediatric population.
HMG CoA-Reductase Inhibitors
HMG CoA-reductase inhibitors or “statins” interfere with the normal synthesis of endogenous cholesterol, thereby upregulating LDL receptors and increasing clearance of LDL particles in the blood. Most experience with statin drugs stems from trials in children with familial hypercholesterolemia (FH) and this is the only pediatric population for which statins are FDA approved. In the FH population, various statin drugs have been demonstrated to have excellent short-term safety, and are effective at improving lipid profiles and non-invasive surrogates of cardiovascular disease [121]. Trials in the obese dyslipidemic population have not been performed to this point. However, given their effectiveness in adults and FH population, they should be considered in adolescents with more severe increases in LDL levels or certainly in children with T2DM. Monitoring is essential in anyone started on a statin as complications of therapy include elevated transaminase and creatine kinase levels. Guidelines for starting and monitoring statin drugs in the pediatric population have been previously published [111]. To this point, similar calls for initiation of statin therapy as first line treatment in the obese pediatric population with dyslipidemia have not been made.
4.3 Hypertension
4.3.1 Introduction
Hypertension is a well established risk factor for cardiovascular disease and management of hypertension is an essential component of preventative care in adults. Children who develop hypertension are more likely to have hypertension as an adult [127, 128], thereby leaving them at an increased risk for various forms of adult cardiovascular disease. Moreover, the presence of hypertension in childhood is one of the risk factors associated with early atherosclerotic change [33]. Obesity is a well established risk factor for hypertension in children [118, 129–131], and increases in measures of adiposity during childhood increase the risk for hypertension as a young adult [132, 133]. The origins of hypertension in obese youth remain to be clarified but are believed to be in part secondary to a combination of autonomic dysfunction, insulin resistance, and abnormal vascular structure and function (Reviewed in [134]). As an additional modifiable risk factor for future heart disease, it is important to identify and initiate a therapeutic program to treat hypertension either as an isolated finding or as a component of metabolic syndrome in obese children.
4.3.2 Prevalence and screening
In general, secondary forms of hypertension are more common among the pediatric population. However, the increased rates of pediatric obesity have been a proposed factor in the increasing rates of primary hypertension among children. Estimating true prevalence rates are difficult and fraught with potential for bias due to differences in technique (manual vs. electronic), single vs. multiple readings for diagnosis, and proper use of age, height, and gender based norms. In a large cohort of 10–19 year olds, the prevalence of hypertension on 3 separate occasions was 4.5% in the cohort but 10.7% among children with a BMI>95%[129]. In fact, current estimates may be well below actual prevalence in children [135].
Blood pressure measurements should be performed on three separate occasions using the correct size cuff as excessively large or small cuffs will give falsely low or high readings, respectively. BP measurements should be compared to age, gender, and height matched norms. Hypertension is defined a systolic or diastolic blood pressure (typically systolic elevation) that is greater than the 95th percentile for age, gender, and height in children on three separate occasions [136]. In most situations, hypertension in the obese child is primary in nature, though secondary causes of hypertension can be investigated if suggested by clinical, family history or physical exam. Obese children with hypertension should have an echocardiogram performed to evaluate for evidence of left ventricular hypertrophy (LVH) which would signify the beginnings of end-organ damage and place the child in a higher risk category.
4.3.3 Treatment
The long term consequences of untreated hypertension in childhood are unknown, but given the available data linking childhood hypertension to future cardiovascular disease, it is an important obesity related metabolic co-morbidity to address. Similar to other components of MS, therapy for hypertension begins with strategies to induce weight loss. Growing evidence points to the beneficial effect of weight loss on blood pressure. In a well designed study, Rocchini, et.al. demonstrated a significant decrease in both BMI and blood pressure in overweight adolescents who completed a diet plus exercise program as compared to a similar group of children who completed a diet only program or served as a control [137]. Similar results were demonstrated in a study of 207 obese adolescents who underwent 1 of year diet, exercise, and behavioral therapy. Of the children who completed the study (drop out rate of 14%) and decreased their BMI-SDS (72% of remaining participants), a significant decrease of mean SBP and DBP was achieved [138]. In a separate study, family directed therapy to induce dietary behavioral change in obese youth significantly reduced BMI by 2.6 units and mean blood pressure as compared to a control group [139].
Concomitant pharmacologic therapy should be considered at the time of diagnosis in higher risk obese children, which would include evidence for LVH on echocardiogram or in those with additional metabolic risk factors (dyslipdemia, type 2 diabetes). In addition, antihypertensive therapy should be considered in those obese children who have participated in a lifestyle intervention program and have persistent hypertension. A number of antihypertensive classes are approved for use in children; however, no evidence basis for selection of an initial class exists for the obese population. Current guidelines allow for use of ACE inhibitors (ACEI), β-blockers, angiotensin receptor blockers (ARB), calcium channel blockers, or diuretics [136]. ACEI or ARB should be considered as first line agents especially in obese youth with type 2 diabetes given their additional protective effect against nephropathy and retinopathy progression.
5 Polycystic ovarian syndrome (PCOS)
5.1 Introduction
PCOS is one of the most common endocrine disorders encountered in females, occurring in 3–10% of women [140]. PCOS is characterized clinically by anovulatory menstrual cycles and hirsutism or biochemical hyperandrogenism. Typical laboratory abnormalities in PCOS include elevations in free testosterone or dehydroepiandosterone sulfate (DHEAS) and decreases in sex hormone binding globulin [141]. Polycystic ovaries on ultrasound are also found in many women with PCOS, but their inclusion in the diagnostic criteria has been controversial [142]. In addition to the cosmetic problems that women can experience, serious sequelae of PCOS include problems with infertility [143]. There also is growing evidence that PCOS is strongly associated with features of the metabolic syndrome [144, 145], and markers for subclinical cardiovascular disease [146]. Although PCOS may occur in both normal weight and obese females, an overweight phenotype is more common and is associated with a more severe clinical picture, including a worse cardiovascular risk profile [147, 148]. The diagnosis of PCOS is exclusive to reproductively mature females, but childhood associations and risk factors have emerged. Pre-pubertal characteristics, including being small for gestational age and having a history of precocious pubarche, are proposed risk factors for later PCOS and metabolic syndrome [149–151].
5.2 Etiology of PCOS
The etiology of PCOS not completely understood, but it is likely multi-factorial with both genetic and environmental influences playing key roles [140]. The hyperandrogenism in PCOS appears to be of both adrenal and ovarian origin. 40–70% of women with PCOS have what is termed functional adrenal hyperandrogenism characterized by an exaggerated adrenal androgen response to ACTH [152]. Insulin resistance is believed to have a causative role in promoting both ovarian and adrenal hyperandrogenemia (reviewed in [153]) as well as a tendency toward glycemic intolerance.
Obese adolescents with PCOS are more likely to have severe insulin resistance, as demonstrated by hyperinsulinemic-euglycemic clamp, hyperglycemic clamp, and HOMA-IR [154, 155]. When compared to BMI matched girls without PCOS, obese girls with PCOS have measures of peripheral insulin sensitivity that are close to 50% lower [154]. IR is not completely dependent on measures of adiposity, however, as non-obese girls with PCOS have also been found to be markedly insulin resistant by HOMA [156].
Given the presence of underlying insulin resistance, it is not surprising that abnormalities in glucose tolerance have also emerged. Impaired fasting glucose and impaired glucose tolerance have been identified in adolescents with PCOS, with rates approaching 25% [157]. Arslanian et al. found that obese girls with PCOS and impaired glucose tolerance have a lower first phase insulin secretion and abnormal nocturnal blood pressure regulation suggesting high risk for progression to T2DM and a tendency towards metabolic syndrome and increased cardiovascular risk [158]. Not all studies have identified the same risk towards metabolic syndrome, however. Rossi et al. performed a cross-sectional study of overweight and obese girls 12–18 years old, 43 with PCOS and 31 BMI-matched controls and found no significant difference in the rate of metabolic syndrome between the two groups [159].
5.3 PCOS therapy
Several therapeutic strategies have been employed in adults with PCOS including lifestyle interventions, oral contraceptives, insulin sensitizers and antiandrogen therapies (reviewed in [160]). Each of these therapies is directed at one of the multiple disturbances in physiology, although several of these strategies are complementary. Ultimately, the goal of therapy for PCOS is to improve disturbances in physiology that cause troublesome signs and symptoms (hirsutism, oligomenorrhea, insulin resistance) and reduce future risks for metabolic syndrome and cardiovascular disease. Data in adolescents is less robust but does offer insight into effective therapies for this population as well.
5.3.1 Lifestyle interventions/weight loss
Lifestyle modification is considered the first line therapy for obese women and adolescents with PCOS, [161]. Lifestyle interventions addressing both weight loss and cardiorespiratory fitness have been found to be effective in reducing hyperandrogenemia, insulin resistance and cardiometabolic risk factors in adults [162]. However, there are few studies of lifestyle intervention alone in girls with PCOS. Hoeger et al. studied intensive lifestyle modification in a randomized fashion comparing metformin vs. oral contraceptive pills vs. placebo coupled with usual lifestyle advice. In this small study, although adolescents had difficulty complying with the intensive lifestyle modification, it was demonstrated that even small amounts of weight loss were associated with significant improvements in sex hormone binding globulin and markers of inflammation. Successful weight loss was associated with reduced total testosterone, reduced hirsutism and improved cholesterol markers [157].
5.3.2 Oral contraceptives
Oral contraceptives (OCPs) are effective in regulating menstrual cycles and decreasing ovarian hyperandrogenism, and therefore have long been one of the first line therapies for PCOS [163]. In more obese females, these effects may be more difficult to achieve (reviewed in [164]). Although OCPs have been successful in treating menstrual irregularities and hyperandrogenemia, it remains controversial whether they have any beneficial effect on the underlying metabolic derangements observed in PCOS. Hoeger et al. report a small randomized placebo-controlled trial comparing intensive lifestyle management, oral contraceptives and metformin in adolescents. The group randomized to oral contraceptives experienced a decrease in total testosterone, but an increase in total, LDL and HDL cholesterol and high-sensitivity CRP, a common marker of inflammation. In addition, OCPs had no apparent effect in insulin or glucose levels [157]. Allen et al. performed a randomized trial comparing use of oral contraceptives to metformin therapy in 35 adolescent women with hyperinsulinemia and found that both groups experienced significant improvement in BMI, menstrual regularity, insulin sensitivity and a reduction in total testosterone. There were no significant differences found between the two treatment groups and no placebo group was included [163]. Mastorakos et al. randomized 36 adolescent girls to OCPs containing either desogestrel or cyproterone acetate as the active progestin. After 12 months of treatment, both groups experienced significantly increased insulin resistance as measured by HOMA-IR, although there was no change in BMI or waist to hip ratio [165]. In a previous study by the same group, the two formulations of OCPs were comparable and both improved menstrual cyclicity and decreased hirsutism and androgen levels [166]. From these studies, it seems reasonable to state that OCPs are effective in inducing menstrual cyclicity and hyperandrogenemia, although their effect on insulin resistance may not be as positive.
5.3.3 Insulin sensitizers
Because insulin resistance is felt to be an essential part of the pathophysiology of PCOS, insulin sensitizers have been used as treatment modality to reduce potential progression towards T2DM and to induce ovulation and treat infertility. In adults, metformin has been shown to improve ovulation, hyperandrogenism and insulin sensitivity, but its effect on fertility and other cardiovascular risk factors is less clear[167, 168]. Metformin has also been used in adolescents with PCOS. Ibáñez et al. demonstrated metformin therapy improved hirsutism, hyperinsulinism and hyperandrogenism in10 non-obese girls with PCOS and insulin resistance. Of note, there was no comparison group in this study [169]. Arslanian et al. performed an open label trial of 850 mg of metformin twice a day in 15 adolescents for 3 months. BMI decreased significantly with significant improvements in glucose and insulin levels during oral glucose tolerance testing. Total and free testosterone decreased significantly and menstrual cycles improved [152]. In the study by Hoeger et al, randomization to metformin was associated with decreased waist circumference and significantly greater decrease in testosterone levels compared to placebo [157]. Glueck et al. studied 11 obese females with PCOS at a dose of 500 mg tid-850 mg three times daily and found that 91% of the girls resumed normal menses. There was reduction in total cholesterol, increased estradiol levels and a downward trend in testosterone levels even when adjusted for weight loss [170].
Thiazolidinediones, a class of agents that bind to the peroxisome proliferator activated receptor (PPAR) have been studied in adults with PCOS. Troglitazone, rosiglitazone and pioglitazone all improve insulin sensitivity, glycemic control, anovulation and hormonal abnormalities [171–173]. Troglitazone has now been taken off the market due to liver toxicity [171, 172, 174]. Pioglitazone has been trialed in 22 obese adolescent and young adult women with PCOS, aged 15–25. All were treated with open-label pioglitazone 30 mg once daily combined with advice on lifestyle changes. Although there was no significant change in BMI from baseline, there was significant improvement in mean fasting plasma glucose and decreased insulin resistance. Menstrual regularity improved, but androgen levels did not change [173].
5.3.4 Antiandrogens
Antiandrogens have been used to directly address the hyperandrogenism present in PCOS. Spirinolactone, an androgen receptor blocker, is effective in reducing the effects of elevated testosterone levels in adults, and can be employed as a second line or adjunct agent in severe hirsutism in adolescent females. There are no randomized trials evaluating its effectiveness as a primary therapy in the obese pediatric population, although flutamide in combination with metformin has been used in non-obese adolescents with PCOS [156]. Spironolactone is a teratogenic agent and it is therefore critical to discuss birth control or use an OCP when utilizing spironolactone.
5.3.5 Summary
There are a number of effective short-term treatment options for PCOS in obese adolescents. A current practical approach is to begin with lifestyle interventions to induce weight loss followed by or concomitant with oral contraceptive therapy. Strong consideration of primary metformin therapy may be made in girls with evidence for insulin resistance, impaired fasting glucose, or impaired glucose tolerance. For troublesome hirsute features that are recalcitrant to initial therapies outlined above, addition of spironolactone should be considered. Given the concerning long-term cardiovascular and metabolic risks that are associated with this syndrome, long-term effects of these therapies need to be studied more intensely to better guide future treatment of this syndrome in the pediatric population.
6 Non alcoholic fatty liver disease (NAFLD)
6.1 Background
Non alcoholic fatty liver disease (NAFLD) refers to the presence of hepatic steatosis in the absence of significant ethanol consumption, underlying liver disease, or inborn errors of metabolism. NAFLD is likely part of a continuum of disease beginning with asymptomatic changes of steatosis on liver biopsy, to active liver inflammation termed Non Alcoholic Steatohepatitis (NASH), which can eventually result in frank hepatic necrosis with cirrhosis [175, 176].
NAFLD most commonly occurs in obese individuals. Although NAFLD can be present in the absence of obesity, up to 90% of children with NAFLD are overweight or obese [177, 178]. Children with higher BMI are more likely to have more extensive inflammation and fibrosis on pathology [177, 179–181]. Children with NAFLD also typically have evidence for insulin resistance, even when controlling for BMI [177, 180]. In fact, insulin resistance is hypothesized to play an active role in the pathogenesis of accretion of hepatic fat [182] and is associated with more severe disease on liver pathology [181]. There is also a high prevalence of the other features of the metabolic syndrome in children with NAFLD including elevated triglycerides, an atherogenic lipid profile, and elevated blood pressure [50, 183]. Direct correlations between number of components of the metabolic syndrome and higher transaminase levels with more fibrosis on biopsy have been reported [177].
NAFLD causes few if any symptoms, even in the presence of markedly abnormal liver pathology [182]. Making the definitive diagnosis of NAFLD requires liver biopsy and evidence for pathologic changes. However, it has been argued that liver biopsy is not appropriate for general screening, given the invasiveness and theoretical risks involved with that procedure. An alternative proposed diagnostic criteria include elevated transaminase levels (ALT > AST) coupled with characteristic findings on ultrasound in the absence of other causes of liver disease [182]. Importantly, detection of elevated transaminase levels is not 100% sensitive, as the disease has been found in the absence of elevated transaminases [184].
6.2 Prevalence
Nonalcoholic fatty liver disease (NAFLD) is now recognized as the most common cause of liver disease in childhood [182, 185]. The prevalence of this disorder has been increasing alongside that of the obesity epidemic [182]. In the NHANES data from 1999–2004, an alarming 8.0% of all US children ages 12–19 had evidence for elevated liver enzymes in the absence of other causes for liver disease [186]. A predilection towards Mexican-American children and of the male gender was noted in this age range [186]. Rates from the NHANES data are consistent with autopsy studies where histologically proven NAFLD has been found in 9.6% of 2–19 year old children [187]. Rates are dramatically higher among obese populations. Some groups have reported incidence rates as high as 60%[188] though selection bias for the most severely affected children with other co-morbidities may inflate the incidence within many obesity treatment centers. Others have reported prevalence among obese youth of 14–24%. Autopsy data has demonstrated rates among obese youth of 38% [187]. These rates are influenced by ethnic background, severity of obesity, degree of visceral adiposity, concomitant insulin resistance, levels of triglycerides, gender, and markers of inflammation [183, 187].
The natural history of NAFLD has not been well characterized, especially in children, but the differentiation between NAFLD and NASH may have important prognostic implications [181]. Unlike adults, where NAFLD is known to progress to cirrhosis [177] the long-term outcome of NAFLD in pediatric populations has not been completely elucidated. There have been cases of cirrhotic liver disease reported in children requiring transplant [180, 189]. Given even a small risk of cirrhosis, there has been interest in trials targeting treatment of NAFLD in the pediatric population.
6.3 NAFLD treatment
There is no conclusively proven effective treatment for NAFLD [180, 182]. Lifestyle interventions appear to be the most effective. Two other therapies, antioxidants (primarily Vitamin E) and metformin, have been studied in children with mixed results.
6.3.1 Lifestyle interventions
Similar to the therapeutic approach in T2DM, weight loss through lifestyle modification is a key component to management of NAFLD. Achievement of significant weight loss by lifestyle intervention has consistently been associated with improvement in NAFLD disease scores [180, 181, 190, 191]. In 2006, Nobili et al. reported on 84 overweight children with biopsy-proven NAFLD, 57 of who completed 1 year of lifestyle intervention. A successful decrease in BMI was associated with significant improvement in liver enzymes, cholesterol, triglyceride levels, insulin sensitivity, and improved liver appearance on ultrasound [180]. Importantly, the greater the weight loss, the greater the reduction in ALT levels. A second study by the same group in 88 patients with biopsy-proven NAFLD showed similar improvement with weight loss through lifestyle intervention [179]. A randomized, controlled study in obese Chinese school children with elevated ALT and steatosis by liver ultrasound, compared a very strict lifestyle intervention in the form of a summer camp to both a group treated with Vitamin E and a control, non-intervention group. Compared to controls, both the camp group and vitamin E group experienced significant improvement in BMI, AST, ALT, and insulin resistance, but the intensive intervention group experienced significantly greater change in BMI and ALT [192]. Reinehr et al. have recently published a longitudinal study of a large cohort of obese children with NAFLD (defined by appearance on liver ultrasound) who were offered a lifestyle intervention program focused on nutrition, fitness, and behavioral modification. In the children who participated in the lifestyle intervention program, significant improvement in BMI-SDS, AST, ALT and in the percentage of patients who met criteria for NAFLD on ultrasound were found at 1 year compared to the group of children that did not participate. These benefits persisted through 2 years of follow-up. Even those with the smallest degree of weight loss were found to have significant decrease in ALT levels [193].
Dietary modifications, on their own, may not equate to weight loss in this population [190]. Rather, the combined effects of diet modification, increased fitness through activity change, and behavioral therapy all play some role in weight loss, improved insulin sensitivity, and improvement in NAFLD. Though these data are based on disease markers rather than clinical outcomes, it appears that lifestyle modification resulting in reductions in BMI is an effective treatment for NAFLD in children.
6.3.2 Anti-oxidants
One of the hypotheses behind the detrimental effects of fat accretion in the liver is the production of reactive oxygen species leading to increased inflammation, necrosis and fibrosis (reviewed in [191]). Therefore, it has been proposed that treating patients with anti-oxidants, specifically Vitamin E in the form of α-tocopherol, may reverse the liver disease and improve prognosis (reviewed in [182]). Thus far, the few trials in children have resulted in contradictory results. A randomized, placebo controlled trial of Vitamin E and C therapy in 53 children with biopsy-proven NAFLD showed no significant differences between Vitamin E, Vitamin C, and placebo. This trial, however, was confounded by the fact that the vitamin E group had higher glucose levels and worse insulin resistance at baseline [194]. In another randomized, double-blind trial of Vitamin E therapy plus diet in 28 patients with obesity, elevated transaminases, and hepatic steatosis on ultrasound, no differences between the groups with respect to change in aminotransferase levels were identified. When a post-hoc subgroup analysis stratified based on “compliance” (defined as successful weight loss for the diet group and two-fold increase in serum vitamin E levels for the group treated with vitamin E) was performed, a significant decrease in ALT values were found for those who were “compliant” with the diet, and for those compliant with vitamin E therapy despite the fact that no significant weight loss occurred in this subgroup [178]. At this point, high dose therapy with antioxidants cannot be routinely recommended, but a larger scale study that is adequately powered to identify a benefit is needed.
6.3.3 Insulin sensitizers
Metformin and pioglitazone have been trialed as therapies in the adult population. Perhaps due to the potential concerns for increased risk of cardiovascular events associated with thiazolidinediones in adult populations [195, 196], and a paucity of overall data in children with T2DM, only metformin has been considered appropriate for use in children. Thus far, there have been two trials using metformin in pediatric NAFLD. An open-label, single arm, phase two trial by Schwimmer et al. in ten obese children with biopsy-proven NAFLD found that metformin therapy at a dose of 500 mg bid was associated with a significant decrease in AST, ALT, liver fat, insulin sensitivity and quality of life compared to baseline [190]. However, the conclusions of this trial were limited by small sample size, lack of blinding, and most significantly, lack of a comparison group. Nobili et al. studied 28 children with NAFLD in an open label trial of metformin 1.5 g/day plus lifestyle intervention compared to a placebo arm from a separate, parallel study that also received lifestyle intervention. Both groups experienced significant decrease in weight and had significant improvement in ALT, AST and liver histology on repeat biopsy after 2 years of therapy [197]. There was no significant difference in the degree of improvement between the two groups. A randomized, controlled, blinded study of Vitamin E therapy and metformin therapy in children ages 8–17 (the TONIC trial) is currently ongoing and will further elucidate the role of these therapies as an adjunct to the management of NAFLD in children [198].
6.3.4 Other therapies
Ursodeoxycholic acid has been studied in one trial in children and two in adults, where it was found to be ineffective as monotherapy and in combination with diet [199–201]. Pentoxifylline, a methylxanthine that down regulates TNF-α production, has shown preliminary promising results as a novel therapy for NASH in adults [202], but has yet to be studied in children.
6.4 Conclusions
It seems clear at this point that the only evidence for effective treatment of NAFLD is via weight reduction through lifestyle intervention. The only caveat to this is that extreme and rapid weight loss via extreme caloric restriction in adults has been associated with worsening fibrosis in NAFLD [203]. It remains to be seen whether complementary strategies with metformin, antioxidants, or agents targeting inflammatory mediators will also be useful as adjunctive agents to reverse disease and prevent progression of disease or other metabolic complications.
7 Summary
The association between obesity related inflammation and the metabolic consequences of excess adiposity in children is an important focus of ongoing study. There is solid evidence that lifestyle changes, such as diet and exercise may be effective in mediating this inflammatory state. Unfortunately, in practice, our skill in achieving widespread lifestyle change in the population is lagging behind the rapid pace at which the pediatric population is developing metabolic complications of obesity. This highlights the importance of accurate, timely identification of such complications and consideration of pharmacologic treatment. Such steps are necessary to prevent further progression of disease and as a preventative measure against future cardiovascular disease. Further advances in our understanding of the pathologic mechanisms underlying these pathways will play a major role in our ability to impact the health and well-being of children in a society with increasing rates of obesity.
Contributor Information
Katie Larson Ode, Division of Pediatric Endocrinology, University of Minnesota, 420 Delaware Street SE, MMC 404, Minneapolis, MN 55455, USA.
Brigitte I. Frohnert, Division of Pediatric Endocrinology, University of Minnesota, 420 Delaware Street SE, MMC 404, Minneapolis, MN 55455, USA
Brandon M. Nathan, Division of Pediatric Endocrinology, University of Minnesota, 420 Delaware Street SE, MMC 404, Minneapolis, MN 55455, USA
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–69. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 4.González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metab Clin Exp. 2009;58:954–62. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savoia C, Schiffrin EL. Reduction of C-reactive protein and the use of anti-hypertensives. Vasc Health Risk Manag. 2007;3:975–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Rueda-Clausen CF, López-Jaramillo P, Luengas C, del Pilar Oubiña M, Cachofeiro V, Lahera V. Inflammation but not endothelial dysfunction is associated with the severity of coronary artery disease in dyslipidemic subjects. Mediators Inflamm 2009. 2009;2009:469169. doi: 10.1155/2009/469169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemet D, Wang P, Funahashi T, Matsuzawa Y, Tanaka S, Engelman L, et al. Adipocytokines, body composition, and fitness in children. Pediatr Res. 2003;53:148–52. doi: 10.1203/00006450-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Anty R, Bekri S, Luciani N, Saint-Paul M, Dahman M, Iannelli A, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101:1824–33. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 11.Bisoendial RJ, Birjmohun RS, Akdim F, Van ’t Veer C, Spek CA, Hartman D, et al. C-reactive protein elicits white blood cell activation in humans. Am J Med. 2009;122:582.e1, 9. doi: 10.1016/j.amjmed.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Venner AA, Lyon ME, Doyle-Baker PK. Leptin: a potential biomarker for childhood obesity? Clin Biochem. 2006;39:1047–56. doi: 10.1016/j.clinbiochem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 15.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 16.Bastard J, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 17.Plaisance EP, Grandjean PW, Judd RL, Jones KW, Taylor JK. The influence of sex, body composition, and nonesterified fatty acids on serum adipokine concentrations. Metab Clin Exp. 2009 doi: 10.1016/j.metabol.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–61. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 20.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, et al. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 2008;16:893–5. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]
- 21.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 22.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–75. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu SW, Qiao SB, Yuan JS, Liu DQ. Association of plasma visfatin levels with inflammation, atherosclerosis, and acute coronary syndromes in humans. Clin Endocrinol (Oxf) 2009;71:202–7. doi: 10.1111/j.1365-2265.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- 24.Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–15. doi: 10.1053/j.gastro.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–30. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalska I, Straczkowski M, Nikolajuk A, Adamska A, Karczewska-Kupczewska M, Otziomek E, et al. Insulin resistance, serum adiponectin, and proinflammatory markers in young subjects with the metabolic syndrome. Metab Clin Exp. 2008;57:1539–44. doi: 10.1016/j.metabol.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005;28:878–81. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 28.Valle M, Martos R, Gascón F, Cañete R, Zafra MA, Morales R. Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab. 2005;31:55–62. doi: 10.1016/s1262-3636(07)70167-2. [DOI] [PubMed] [Google Scholar]
- 29.Wärnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008;19:11–5. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- 30.Wärnberg J, Nova E, Romeo J, Moreno LA, Sjöström M, Marcos A. Lifestyle-related determinants of inflammation in adolescence. Br J Nutr. 2007;98(Suppl 1):S116–20. doi: 10.1017/S0007114507839614. [DOI] [PubMed] [Google Scholar]
- 31.Cizmecioglu F, Etiler N, Ergen A, Keser A, Hekim N, Hamzaoglu O, et al. Association of Adiponectin, Resistin and High Sensitive CRP Level with the Metabolic Syndrome in Childhood and Adolescence. Exp Clin Endocrinol Diabetes. 2009 doi: 10.1055/s-0028-1112151. [DOI] [PubMed] [Google Scholar]
- 32.McGill HC, McMahan CA. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Cardiol. 1998;82:30T–6. doi: 10.1016/s0002-9149(98)00720-6. [DOI] [PubMed] [Google Scholar]
- 33.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 34.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:21–9. doi: 10.1097/MED.0b013e3282f43d19. [DOI] [PubMed] [Google Scholar]
- 37.Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28:1175–81. doi: 10.2337/diacare.28.5.1175. [DOI] [PubMed] [Google Scholar]
- 38.Krekoukia M, Nassis GP, Psarra G, Skenderi K, Chrousos GP, Sidossis LS. Elevated total and central adiposity and low physical activity are associated with insulin resistance in children. Metab Clin Exp. 2007;56:206–13. doi: 10.1016/j.metabol.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz B, Jacobs DR, Moran A, Steinberger J, Hong C, Sinaiko AR. Measurement of Insulin Sensitivity in Children: Comparison Between the Euglycemic Hyperinsulinemic Clamp and Surrogate Measures. Diabetes Care. 2008;31:783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 40.Eyzaguirre F, Mericq V. Insulin resistance markers in children. Horm Res. 2009;71:65–74. doi: 10.1159/000183894. [DOI] [PubMed] [Google Scholar]
- 41.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–32. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 42.Sinaiko AR, Steinberger J, Moran A, Hong C, Prineas RJ, Jacobs DR. Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides, and high-density lipoprotein cholesterol at age 19. Hypertension. 2006;48:730–6. doi: 10.1161/01.HYP.0000237863.24000.50. [DOI] [PubMed] [Google Scholar]
- 43.Sinaiko AR, Jacobs DR, Steinberger J, Moran A, Luepker R, Rocchini AP, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–7. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 44.Williams DE, Cadwell BL, Cheng YJ, Cowie CC, Gregg EW, Geiss LS, et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics. 2005;116:1122–6. doi: 10.1542/peds.2004-2001. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira AC, Oliveira AM, Adan LF, Oliveira NF, Silva AM, Ladeia AM. C-reactive protein and metabolic syndrome in youth: a strong relationship? Obesity (SilverSpring) 2008;16:1094–8. doi: 10.1038/oby.2008.43. [DOI] [PubMed] [Google Scholar]
- 46.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young;Council on Cardiovascular Nursing;and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 47.Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–91. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 48.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Sanders BH, Lubsch LM, West DS. Prevalence and treatment of metabolic syndrome in adolescents with type 2 diabetes. Ann Pharmacother. 2006;40:1517–21. doi: 10.1345/aph.1H130. [DOI] [PubMed] [Google Scholar]
- 50.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–83. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekelund U, Anderssen S, Andersen LB, Riddoch CJ, Sardinha LB, Luan J, et al. Prevalence and correlates of the metabolic syndrome in a population-based sample of European youth. Am J Clin Nutr. 2009;89:90–6. doi: 10.3945/ajcn.2008.26649. [DOI] [PubMed] [Google Scholar]
- 52.Kelishadi R, Razaghi EM, Gouya MM, Ardalan G, Gheiratmand R, Delavari A, et al. Association of physical activity and the metabolic syndrome in children and adolescents: CASPIAN study. Horm Res. 2007;67:46–52. doi: 10.1159/000096121. [DOI] [PubMed] [Google Scholar]
- 53.Pan Y, Pratt CA. Metabolic syndrome and its association with diet and physical activity in US adolescents. J Am Diet Assoc. 2008;108:276–86. doi: 10.1016/j.jada.2007.10.049. discussion 286. [DOI] [PubMed] [Google Scholar]
- 54.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–70. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Jago R, Baranowski T, Buse J, Edelstein S, Galassetti P, Harrell J, et al. Prevalence of the metabolic syndrome among a racially/ethnically diverse group of U.S. eighth-grade adolescents and associations with fasting insulin and homeostasis model assessment of insulin resistance levels: Studies to Treat or Prevent Pediatric Type 2 Diabetes (STOPP-T2D) Prevention Study Group. Diabetes Care. 2008;31(10):2020–5. doi: 10.2337/dc08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monzavi R, Dreimane D, Geffner ME, Braun S, Conrad B, Klier M, et al. Improvement in risk factors for metabolic syndrome and insulin resistance in overweight youth who are treated with lifestyle intervention. Pediatrics. 2006;117:e1111–8. doi: 10.1542/peds.2005-1532. [DOI] [PubMed] [Google Scholar]
- 57.Steele RM, Brage S, Corder K, Wareham NJ, Ekelund U. Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol. 2008;105:342–51. doi: 10.1152/japplphysiol.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DuBose KD, Eisenmann JC, Donnelly JE. Aerobic fitness attenuates the metabolic syndrome score in normal-weight, at-risk-for-overweight, and overweight children. Pediatrics. 2007;120:e1262–8. doi: 10.1542/peds.2007-0443. [DOI] [PubMed] [Google Scholar]
- 59.Mark AE, Janssen I. Relationship between screen time and metabolic syndrome in adolescents. J Public Health (Oxf) 2008;30:153–60. doi: 10.1093/pubmed/fdn022. [DOI] [PubMed] [Google Scholar]
- 60.Casazza K, Dulin-Keita A, Gower BA, Fernandez JR. Differential influence of diet and physical activity on components of metabolic syndrome in a multiethnic sample of children. J Am Diet Assoc. 2009;109:236–14. doi: 10.1016/j.jada.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 62.Stone NJ, Saxon D. Approach to treatment of the patient with metabolic syndrome: lifestyle therapy. Am J Cardiol. 2005;96:15E–21. doi: 10.1016/j.amjcard.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metab Clin Exp. 2006;55:871–8. doi: 10.1016/j.metabol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–41. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 65.Sacheck J. Pediatric obesity: an inflammatory condition? JPEN J Parenter Enteral Nutr. 2008;32:633–7. doi: 10.1177/0148607108324876. [DOI] [PubMed] [Google Scholar]
- 66.Kadoglou NPE, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14:837–43. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 67.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100:93–9. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 68.Carrel AL, Clark RR, Peterson SE, Nemeth BA, Sullivan J, Allen DB. Improvement of fitness, body composition, and insulin sensitivity in overweight children in a school-based exercise program: a randomized, controlled study. Arch Pediatr Adolesc Med. 2005;159:963–8. doi: 10.1001/archpedi.159.10.963. [DOI] [PubMed] [Google Scholar]
- 69.Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 70.Kim ES, Im J, Kim KC, Park JH, Suh S, Kang ES, et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring) 2007;15:3023–30. doi: 10.1038/oby.2007.360. [DOI] [PubMed] [Google Scholar]
- 71.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:2287–93. doi: 10.1210/jc.2007-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinhas-Hamiel O, Lerner-Geva L, Copperman N, Jacobson MS. Insulin resistance and parental obesity as predictors to response to therapeutic life style change in obese children and adolescents 10–18 years old. J Adolesc Health. 2008;43:437–43. doi: 10.1016/j.jadohealth.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Rohrer TR, Rizzo VF, Cäsar JJ, Muelbredt O, Sprengart S, Gortner L, et al. Changes in hepatic risk factors, metabolic variables, body composition, and physical fitness in obese children after a one-year weight loss program. J Pediatr Endocrinol Metab. 2008;21:837–45. doi: 10.1515/JPEM.2008.21.9.837. [DOI] [PubMed] [Google Scholar]
- 74.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metab Clin Exp. 2005;54:1472–9. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Faintuch J, Horie LM, Barbeiro HV, Barbeiro DF, Soriano FG, Ishida RK, et al. Systemic inflammation in morbidly obese subjects: response to oral supplementation with alpha-linolenic acid. Obes Surg. 2007;17:341–7. doi: 10.1007/s11695-007-9062-x. [DOI] [PubMed] [Google Scholar]
- 76.Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, et al. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. 2008;27:65–74. doi: 10.1080/07315724.2008.10719676. [DOI] [PubMed] [Google Scholar]
- 77.Molnár D, Decsi T, Koletzko B. Reduced antioxidant status in obese children with multimetabolic syndrome. Int J Obes Relat Metab Disord. 2004;28:1197–202. doi: 10.1038/sj.ijo.0802719. [DOI] [PubMed] [Google Scholar]
- 78.Lütjens A, Smit JL. Effect of biguanide treatment in obese children. Helv Paediatr Acta. 1977;31:473–80. [PubMed] [Google Scholar]
- 79.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152:817–22. doi: 10.1016/j.jpeds.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–80. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 81.Burgert TS, Duran EJ, Goldberg-Gell R, Dziura J, Yeckel CW, Katz S, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9:567–76. doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 82.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339–48. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 83.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 84.Kay JP, Alemzadeh R, Langley G, D’Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metab Clin Exp. 2001;50:1457–61. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 85.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, et al. Treatment options for type 2 diabetes in adolescents and youth: astudyofthecomparativeefficacyofmetforminaloneorin combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gungor N, Hannon T, Libman I, Bacha F, Arslanian S. Type 2 diabetes mellitus in youth: the complete picture to date. Pediatr Clin North Am. 2005;52:1579–609. doi: 10.1016/j.pcl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Shalitin S, Abrahami M, Lilos P, Phillip M. Insulin resistance and impaired glucose tolerance in obese children and adolescents referred to a tertiary-care center in Israel. Int J Obes (Lond) 2005;29:571–8. doi: 10.1038/sj.ijo.0802919. [DOI] [PubMed] [Google Scholar]
- 89.Fagot-Campagna A, Saaddine JB, Flegal KM, Beckles GL. Diabetes, impaired fasting glucose, and elevated HbA1c in U.S. adolescents: the Third National Health and Nutrition Examination Survey. Diabetes Care. 2001;24:834–7. doi: 10.2337/diacare.24.5.834. [DOI] [PubMed] [Google Scholar]
- 90.Wabitsch M, Hauner H, Hertrampf M, Muche R, Hay B, Mayer H, et al. Type II diabetes mellitus and impaired glucose regulation in Caucasian children and adolescents with obesity living in Germany. Int J Obes Relat Metab Disord. 2004;28:307–13. doi: 10.1038/sj.ijo.0802555. [DOI] [PubMed] [Google Scholar]
- 91.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 92.Szamosi A, Czinner A, Szamosi T, Sallai A, Hatunic M, Berla Z, et al. Effect of diet and physical exercise treatment on insulin resistance syndrome of schoolchildren. J Am Coll Nutr. 2008;27:177–83. doi: 10.1080/07315724.2008.10719689. [DOI] [PubMed] [Google Scholar]
- 93.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93:4231–7. doi: 10.1210/jc.2008-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goran MI, Davis J, Kelly L, Shaibi G, Spruijt-Metz D, Soni SM, et al. Low prevalence of pediatric type 2 diabetes: where’s the epidemic? J Pediatr. 2008;152:753–5. doi: 10.1016/j.jpeds.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–54. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 96.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benavides S, Striet J, Germak J, Nahata MC. Efficacy and safety of hypoglycemic drugs in children with type 2 diabetes mellitus. Pharmacotherapy. 2005;25:803–9. doi: 10.1592/phco.2005.25.6.803. [DOI] [PubMed] [Google Scholar]
- 98.Shield JPH, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94:206–9. doi: 10.1136/adc.2008.143313. [DOI] [PubMed] [Google Scholar]
- 99.Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007;30:790–4. doi: 10.2337/dc06-1554. [DOI] [PubMed] [Google Scholar]
- 100.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–80. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- 101.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. ISPAD Clinical Practice Consensus Guideline 2006–2007. Type 2 diabetes mellitus in the child and adolescent. Pediatr Diabetes. 2008;9:512–26. doi: 10.1111/j.1399-5448.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 102.Jacobson-Dickman E, Levitsky L. Oral agents in managing diabetes mellitus in children and adolescents. Pediatr Clin North Am. 2005;52:1689–703. doi: 10.1016/j.pcl.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Jones KL, Arslanian S, Peterokova VA, Park J, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 104.Yeap BB. Controversies in type 2 diabetes—An update. Aust Fam Physician. 2009;38:22–5. [PubMed] [Google Scholar]
- 105.Chia CW, Egan JM. Incretin-based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2008;93:3703–16. doi: 10.1210/jc.2007-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 108.Park E, Wong V, Guan X, Oprescu AI, Giacca A. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol. 2007;195:323–31. doi: 10.1677/JOE-07-0005. [DOI] [PubMed] [Google Scholar]
- 109.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised doubleblind placebo-controlled study. Diabetologia. 2009;52:385–93. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCrindle BW, Urbina EM, Dennison BA, Jacobson MS, Steinberger J, Rocchini AP, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–67. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 112.Berenson GS, Srnivasan SR. Cardiovascular risk factors in youth with implications for aging: the Bogalusa Heart Study. Neurobiol Aging. 2005;26:303–7. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 113.Srinivasan SR, Frontini MG, Xu J, Berenson GS. Utility of childhood non-high-density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: the Bogalusa Heart Study. Pediatrics. 2006;118:201–6. doi: 10.1542/peds.2005-1856. [DOI] [PubMed] [Google Scholar]
- 114.Magnussen CG, Raitakari OT, Thomson R, Juonala M, Patel DA, Viikari JSA, et al. Utility of currently recommended pediatric dyslipidemia classifications in predicting dyslipidemia in adult-hood: evidence from the Childhood Determinants of Adult Health (CDAH) study, Cardiovascular Risk in Young Finns Study, and Bogalusa Heart Study. Circulation. 2008;117:32–42. doi: 10.1161/CIRCULATIONAHA.107.718981. [DOI] [PubMed] [Google Scholar]
- 115.Steinberger J, Moran A, Hong CP, Jacobs DR, Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr. 2001;138:469–73. doi: 10.1067/mpd.2001.112658. [DOI] [PubMed] [Google Scholar]
- 116.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 117.Korsten-Reck U, Kromeyer-Hauschild K, Korsten K, Baumstark MW, Dickhuth H, Berg A. Frequency of secondary dyslipidemia in obese children. Vasc Health Risk Manag. 2008;4:1089–94. doi: 10.2147/vhrm.s2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103:1175–82. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 119.Manlhiot C, Larsson P, Gurofsky RC, Smith RW, Fillingham C, Clarizia NA, et al. Spectrum and management of hypertriglyceridemia among children in clinical practice. Pediatrics. 2009;123:458–65. doi: 10.1542/peds.2008-0367. [DOI] [PubMed] [Google Scholar]
- 120.Steinberger J, Moorehead C, Katch V, Rocchini AP. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J Pediatr. 1995;126:690–5. doi: 10.1016/s0022-3476(95)70394-2. [DOI] [PubMed] [Google Scholar]
- 121.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Büller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004;292:331–7. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 122.Kwiterovich PO, Hartmuller G, Van Horn L, Christoffel KK, Gernhoffer N, Gidding S, et al. The Writing Group for the DISC Collaborative Research Group Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low-density lipoprotein cholesterol. The Dietary Intervention Study in Children (DISC): JAMA. 1995;273(18):1429–35. doi: 10.1001/jama.1995.03520420045036. [DOI] [PubMed] [Google Scholar]
- 123.Jonkers IJAM, Smelt AHM, Princen HMG, Kuipers F, Romijn JA, Boverhof R, et al. Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J Nutr. 2006;136:987–91. doi: 10.1093/jn/136.4.987. [DOI] [PubMed] [Google Scholar]
- 124.Engler MM, Engler MB, Malloy MJ, Paul SM, Kulkarni KR, Mietus-Snyder ML. Effect of docosahexaenoic acid on lipoprotein subclasses in hyperlipidemic children (the EARLY study) Am J Cardiol. 2005;95:869–71. doi: 10.1016/j.amjcard.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 125.Tolfrey K, Jones AM, Campbell IG. The effect of aerobic exercise training on the lipid-lipoprotein profile of children and adolescents. Sports Med. 2000;29:99–112. doi: 10.2165/00007256-200029020-00003. [DOI] [PubMed] [Google Scholar]
- 126.McCrindle BW, O’Neill MB, Cullen-Dean G, Helden E. Acceptability and compliance with two forms of cholestyramine in the treatment of hypercholesterolemia in children: a randomized, crossover trial. J Pediatr. 1997;130:266–73. doi: 10.1016/s0022-3476(97)70353-6. [DOI] [PubMed] [Google Scholar]
- 127.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–41. [PubMed] [Google Scholar]
- 128.Rademacher E, Jacobs D, Moran A, Steinberger J, Prineas R, Sinaiko A. Relation of blood pressure and body mass index during childhood to cardiovascular risk factor levels in young adults. J Hypertens. 2009;27:1766–74. doi: 10.1097/HJH.0b013e32832e8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113:475–82. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 130.Paradis G, Lambert M, O’Loughlin J, Lavallée C, Aubin J, Delvin E, et al. Blood pressure and adiposity in children and adolescents. Circulation. 2004;110:1832–8. doi: 10.1161/01.CIR.0000143100.31752.B7. [DOI] [PubMed] [Google Scholar]
- 131.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–4. 644.e1. doi: 10.1016/j.jpeds.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 132.Sinaiko AR, Donahue RP, Jacobs DR, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children’s Blood Pressure Study. Circulation. 1999;99:1471–6. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 133.Gidding SS, Bao W, Srinivasan SR, Berenson GS. Effects of secular trends in obesity on coronary risk factors in children: the Bogalusa Heart Study. J Pediatr. 1995;127:868–74. doi: 10.1016/s0022-3476(95)70020-x. [DOI] [PubMed] [Google Scholar]
- 134.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–7. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 135.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–9. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 136.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–76. 4th Report. [PubMed] [Google Scholar]
- 137.Rocchini AP, Katch V, Anderson J, Hinderliter J, Becque D, Martin M, et al. Blood pressure in obese adolescents: effect of weight loss. Pediatrics. 1988;82:16–23. [PubMed] [Google Scholar]
- 138.Reinehr T, de Sousa G, Toschke AM, Andler W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr. 2006;84:490–6. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- 139.Jiang JX, Xia XL, Greiner T, Lian GL, Rosenqvist U. A two year family based behaviour treatment for obese children. Arch Dis Child. 2005;90:1235–8. doi: 10.1136/adc.2005.071753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:162–8. doi: 10.1210/jc.2007-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 142.Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91:786–9. doi: 10.1210/jc.2005-2501. [DOI] [PubMed] [Google Scholar]
- 143.Boomsma CM, Eijkemans MJC, Hughes EG, Visser GHA, Fauser BCJM, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 144.Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab. 2006;91:1275–83. doi: 10.1210/jc.2005-1707. [DOI] [PubMed] [Google Scholar]
- 145.Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:205–25. doi: 10.1097/GRF.0b013e31802f3547. [DOI] [PubMed] [Google Scholar]
- 146.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–6. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 147.Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. 1998;51:415–22. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 148.Littlejohn EE, Weiss RE, Deplewski D, Edidin DV, Rosenfield R. Intractable early childhood obesity as the initial sign of insulin resistant hyperinsulinism and precursor of polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2007;20:41–51. doi: 10.1515/jpem.2007.20.1.41. [DOI] [PubMed] [Google Scholar]
- 149.de Zegher F, Ibáñez L. Prenatal growth restraint followed by catch-up of weight: a hyperinsulinemic pathway to polycystic ovary syndrome. Fertil Steril. 2006;86(Suppl 1):S4–5. doi: 10.1016/j.fertnstert.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 150.Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–96. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 151.Ibáñez L, Potau N, Carrascosa A. Insulin Resistance, Premature Adrenarche, and a Risk of the Polycystic Ovary Syndrome (PCOS) Trends Endocrinol Metab. 1998;9:72–7. doi: 10.1016/s1043-2760(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 152.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–9. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 153.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 154.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 155.Sawathiparnich P, Weerakulwattana L, Santiprabhob J, Likitmaskul S. Obese adolescent girls with polycystic ovary syndrome (PCOS) have more severe insulin resistance measured by HOMA-IR score than obese girls without PCOS. J Med Assoc Thai. 2005;88(Suppl 8):S33–7. [PubMed] [Google Scholar]
- 156.Ibáñez L, Ong K, Ferrer A, Amin R, Dunger D, de Zegher F. Low-dose flutamide-metformin therapy reverses insulin resistance and reduces fat mass in nonobese adolescents with ovarian hyperandrogenism. J Clin Endocrinol Metab. 2003;88:2600–6. doi: 10.1210/jc.2002-022002. [DOI] [PubMed] [Google Scholar]
- 157.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 159.Rossi B, Sukalich S, Droz J, Griffin A, Cook S, Blumkin A, et al. Prevalence of metabolic syndrome and related characteristics in obese adolescents with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4780–6. doi: 10.1210/jc.2008-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martin KA, Chang RJ, Ehrmann DA, Ibáñez L, Lobo RA, Rosenfield RL, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105–20. doi: 10.1210/jc.2007-2437. [DOI] [PubMed] [Google Scholar]
- 161.Legro RS. Detection of insulin resistance and its treatment in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2002;15(Suppl 5):1367–78. [PubMed] [Google Scholar]
- 162.Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3373–80. doi: 10.1210/jc.2008-0751. [DOI] [PubMed] [Google Scholar]
- 163.Allen HF, Mazzoni C, Heptulla RA, Murray MA, Miller N, Koenigs L, et al. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2005;18:761–8. doi: 10.1515/jpem.2005.18.8.761. [DOI] [PubMed] [Google Scholar]
- 164.Mastorakos G, Lambrinoudaki I, Creatsas G. Polycystic ovary syndrome in adolescents: current and future treatment options. Paediatr Drugs. 2006;8:311–8. doi: 10.2165/00148581-200608050-00004. [DOI] [PubMed] [Google Scholar]
- 165.Mastorakos G, Koliopoulos C, Deligeoroglou E, Diamanti-Kandarakis E, Creatsas G. Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril. 2006;85:420–7. doi: 10.1016/j.fertnstert.2005.07.1306. [DOI] [PubMed] [Google Scholar]
- 166.Mastorakos G, Koliopoulos C, Creatsas G. Androgen and lipid profiles in adolescents with polycystic ovary syndrome who were treated with two forms of combined oral contraceptives. Fertil Steril. 2002;77:919–27. doi: 10.1016/s0015-0282(02)02993-x. [DOI] [PubMed] [Google Scholar]
- 167.Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951–3. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 169.Ibáñez L, Valls C, Potau N, Marcos MV, de Zegher F. Sensitization to insulin in adolescent girls to normalize hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism after precocious pubarche. J Clin Endocrinol Metab. 2000;85:3526–30. doi: 10.1210/jcem.85.10.6908. [DOI] [PubMed] [Google Scholar]
- 170.Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve-Smith L. Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS) J Adolesc Health. 2001;29:160–9. doi: 10.1016/s1054-139x(01)00202-6. [DOI] [PubMed] [Google Scholar]
- 171.Aroda VR, Ciaraldi TP, Burke P, Mudaliar S, Clopton P, Phillips S, et al. Metabolic and hormonal changes induced by pioglitazone in polycystic ovary syndrome: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2009;94:469–76. doi: 10.1210/jc.2008-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brettenthaler N, De Geyter C, Huber PR, Keller U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3835–40. doi: 10.1210/jc.2003-031737. [DOI] [PubMed] [Google Scholar]
- 173.Narsing Rao L, Jacob JJ, Paul TV, Rajarathinam S, Thomas N, Seshadri MS. Effects of pioglitazone on menstrual frequency, hyperandrogenism and insulin resistance in adoloscents and young adults with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2009;22:91–5. doi: 10.1016/j.jpag.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 174.Stout DL, Fugate SE. Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy. 2005;25:244–52. doi: 10.1592/phco.25.2.244.56943. [DOI] [PubMed] [Google Scholar]
- 175.Angulo P. Nonalcoholic fatty liver disease. Rev Gastroenterol Mex. 2005;70(Suppl 3):52–6. [PubMed] [Google Scholar]
- 176.Marion AW, Baker AJ, Dhawan A. Fatty liver disease in children. Arch Dis Child. 2004;89:648–52. doi: 10.1136/adc.2003.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32:381–7. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 178.Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, et al. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48–55. doi: 10.1097/00005176-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 179.Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553–61. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 180.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–65. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 181.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971.e2. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Manco M, Bottazzo G, DeVito R, Marcellini M, Mingrone G, Nobili V. Nonalcoholic fatty liver disease in children. J Am Coll Nutr. 2008;27:667–76. doi: 10.1080/07315724.2008.10719744. [DOI] [PubMed] [Google Scholar]
- 183.Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–94. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 184.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–8. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 185.Strauss RS, Barlow SE, Dietz WH. Prevalence ofabnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–33. [PubMed] [Google Scholar]
- 186.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–20. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 188.Sagi R, Reif S, Neuman G, Webb M, Phillip M, Shalitin S. Nonalcoholic fatty liver disease in overweight children and adolescents. Acta Paediatr. 2007;96:1209–13. doi: 10.1111/j.1651-2227.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 189.Jankowska I, Socha P, Pawlowska J, Teisseyre M, Gliwicz D, Czubkowski P, et al. Recurrence of non-alcoholic steatohepatitis after liver transplantation in a 13-yr-old boy. Pediatr Transplant. 2007;11:796–8. doi: 10.1111/j.1399-3046.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 190.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–9. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 191.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]
- 192.Wang C, Liang L, Fu J, Zou C, Hong F, Xue J, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease:2-year follow-up study. Arch Dis Child. 2009;94:437–42. doi: 10.1136/adc.2008.143594. [DOI] [PubMed] [Google Scholar]
- 194.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–28. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 195.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 196.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–95. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 197.Nobili V, Manco M, Ciampalini P, Alisi A, Devito R, Bugianesi E, et al. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther. 2008;30:1168–76. doi: 10.1016/j.clinthera.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 198.Lavine JE, Schwimmer JB. Clinical Research Network launches TONIC trial for treatment of nonalcoholic fatty liver disease in children. J Pediatr Gastroenterol Nutr. 2006;42:129–30. doi: 10.1097/01.mpg.0000189332.77644.1f. [DOI] [PubMed] [Google Scholar]
- 199.Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–43. [PubMed] [Google Scholar]
- 200.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 201.Méndez-Sánchez N, González V, Chávez-Tapia N, Ramos MH, Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol. 2004;3:108–12. [PubMed] [Google Scholar]
- 202.Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634–8. doi: 10.1111/j.1440-1746.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- 203.Andersen T, Gluud C, Franzmann MB, Christoffersen P. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991;12:224–9. doi: 10.1016/0168-8278(91)90942-5. [DOI] [PubMed] [Google Scholar]
- 204.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–55. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 205.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–51. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Berg AH, Scherer PE. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 207.Guzik TJ, Mangalat D, Korbut R. Adipocytokines — novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57:505–28. [PubMed] [Google Scholar]
- 208.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 209.Alberti G, Zimmet P, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The IDF consensus definition of the metabolic syndrome in children and adolescents. International Diabetes Federation. 2007 doi: 10.1111/j.1399-5448.2007.00271.x. http://www.idf.org/met_syndromechildren Accessed 5 Aug 2009. [DOI] [PubMed]
- 210.American Diabetes Association. Standards of Medical Care in Diabetes–2009. Diabetes Care. 2009;32:S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]


