Abstract
Fluorescence-quenched nucleic acid probes with reactive moieties at both the 5′ and 3′ ends are synthesized and tested for reaction with two adjacent nucleophile-containing DNAs. These probes improve signal to background over singly reactive probes and can discriminate single nucleotide polymorphisms in the target DNA or RNA.
Templated reactions have been studied increasingly as a strategy for detection of nucleic acids in vitro1–5 and directly in cells.6–10 One of these approaches, quenched autoligation (QUAL) reactions, relies on an SN2 reaction between an oligonucleotide with a 3′-nucleophile and a quenched probe with a 5′-leaving group.11,12 The reaction releases the quencher and unquenches a nearby fluorophore, producing a signal. As with virtually all DNA detection methods, background signals can interfere at low analyte concentrations; in the case of QUAL probes, background signals can arise from less-than-complete initial quenching and from nonspecific hydrolysis.
Herein we describe a new reaction design for quenched autoligation probes and for DNA-templated reactions in general that can lower such sources of background. All previous approaches to templated nucleic acid detection have involved two probes binding adjacent to one another, bringing functional groups into proximity for reaction. Here we report a new geometry for templated reactions. In this new approach, a labeled oligonucleotide is functionalized with electro-philically-linked quenchers at both ends. Two activating nucleophile probes bind on either side of this quenched probe, performing simultaneous reactions to elicit a signal. We find that this double-reaction approach produces a lower initial background signal as well as a higher signal-to-background than earlier single-reaction strategies, and is capable of detecting rRNA in intact bacterial cells.
In this new templated reaction design, the doubly-functionalized quencher oligonucleotides are flanked by both a 5′-nucleophile oligonucleotide and a 3′-nucleophile oligonucleotide in order for the fluorophore to be fully unquenched (Fig. 1); thus they are referred to as “Sandwich” probes. For our experiments, the central quenched probe is a fluorescein-labeled 10-mer designed to be highly responsive to single nucleotide mismatches. The quencher-leaving groups at both ends of the quenched probe are “dabsylate” moieties. The dabsylate at the 5′-end of the probe was conjugated using a previously described butyl linker.12 At the 3′-end, a new linker was needed to present an electrophilically-linked quencher and allow for subsequent solid-phase DNA synthesis following the coupling of the linker to the solid support. To fulfill these criteria, a five-carbon linker was synthesized that contained a dabsylate quencher, DMT-protected alcohol, and a phosphoramidite group. After coupling this linker to the solid support through standard phosphoramidite chemistry, the DMT group could be removed and standard 3′ to 5′ solid-phase DNA synthesis could then proceed. The dabsylate group was stable to DNA synthesis conditions, as evidenced by the persistent bright orange color of the beads (and later characterization). The synthesis of this 3′-linker and the Sandwich oligonucleotide are described in the ESI,† as are the syntheses of the 3′ and 5′ nucleophile oligonucleotides. The structure of the Sandwich probe is shown in Fig. 1C; the 2-cyanoethyl group was purposely not cleaved from the 3′-phosphate group of the probe to reduce the probability of an intramolecular reaction between the phosphate and the quencher leaving group.13 The presence of the three conjugated groups in the quenched probe, as well as the intact cyanoethyl group, was confirmed by MALDI-mass spectrometry (see ESI†).
Fig. 1.
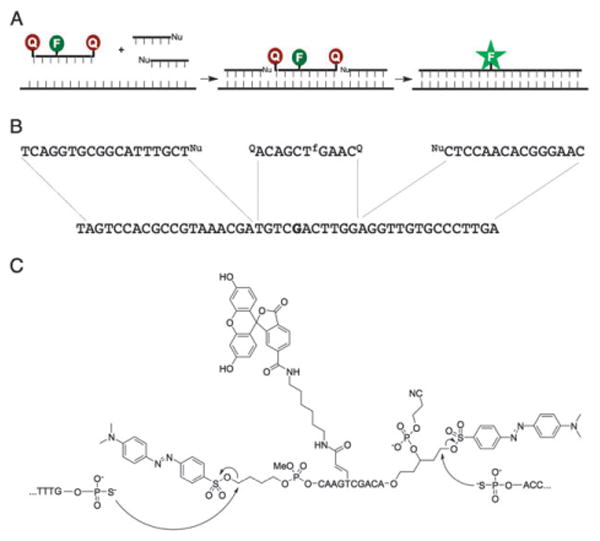
Schematics and structures of Sandwich probes. (A) Sandwich probes require two quencher displacement reactions (one at each end of the probe) to fully unquench the fluorophore and produce a signal. (B) Sequences of template, nucleophile and Sandwich probe oligonucleotides for in vitro experiments. The template corresponds to a sequence in bacterial rRNA and the base in bold is a position known to vary among different species. (C) Structure of Sandwich probe and expected reactions with adjacent 5′ and 3′ nucleophile-modified oligonucleotides.
Following the synthesis of the Sandwich probe, its initial background fluorescence was measured and compared to control probes containing either a single 5′ or 3′ dabsylate modification alone (Fig. S1A, ESI†). The results showed that the Sandwich probe exhibits better quenching in the unreacted state than either single dabsyl probes by between two- and six-fold.
Having a prototype Sandwich probe in hand, we proceeded to test whether it could undergo DNA-templated reactions in the presence of nucleophile-substituted probes. The displacement reactions were monitored by following the increase in fluorescence intensity of the reactions over time. The reactions were performed with both 5′ and 3′ nucleophile DNAs, and with controls containing either the 5′ or 3′ nucleophile DNAs separately. We also tested the effect of the template DNA explicitly by carrying out reactions with the three reacting probes but in the absence of template (Fig. 2A).
Fig. 2.
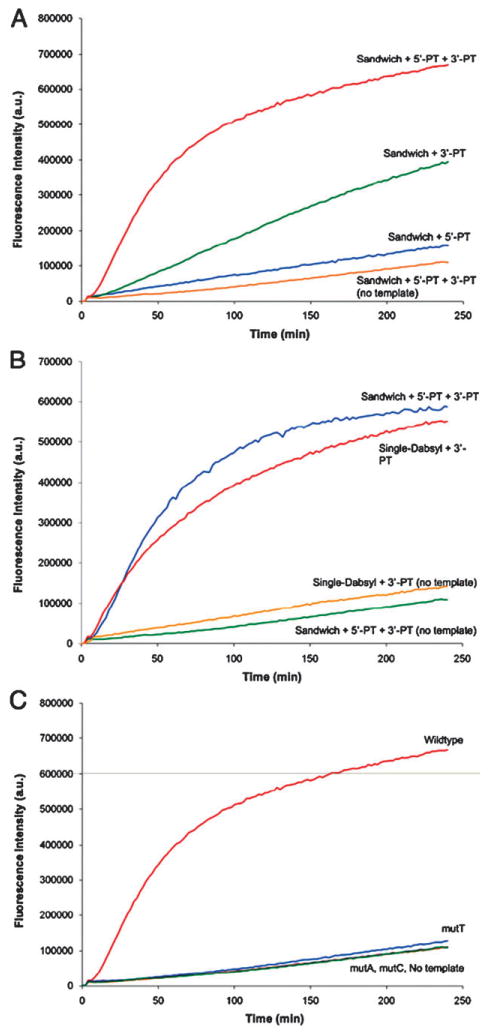
(A) Kinetics of Sandwich probe reactions with different nucleophile combinations; (B) comparison of kinetics for Sandwich and single dabsylate probe reactions with and without template; (C) kinetics of Sandwich probe reactions on templates with single nucleotide polymorphisms. Conditions: 100 nM quenched probe and template, 120 nM nucleophile probes, 70 mM PIPES, 10 mM MgCl2, 50 μM DTT for 4 h at 37 1C with 494 nm excitation and 522 nm emission. Quenched probes were added to reaction mixture after 4 min and nucleophile probes 4 min after that.
The experiments showed that the presence of both nucleophiles in the templated reaction produced a more rapid turn-on signal than with either individual nucleophile alone, which establishes that both nucleophiles play a cooperative role in developing the turn-on signal. The separate reactions with the 5′ or 3′ nucleophiles revealed that the 3′-nucleophile (reacting with 5′ dabsylate) is the faster reaction (by a factor of ∼2–3 after four hours; Fig. 2A), which may be due to differences in geometry and electrophilic linker structures. Significantly, in the absence of template, reaction of the three probes was much slower than in its presence, establishing the templated nature of the three-element reaction.
To test the overall reactivity of the Sandwich probe, we compared its reaction rate to that of a previously described, single-electrophile 5′-dabsylate probe. Interestingly, the reaction of the Sandwich probe with both nucleophiles had a similar rate as a control 5′-single dabsylate probe with the 3′-nucleophile (Fig. 2B), which is consistent with the fastest step of the Sandwich probe having the same reactive groups as this single-electrophile control. Notably, two reactions proceed in the Sandwich case in the same amount of time, despite the fact that the second set of reacting groups of the Sandwich probe (at its 3′ end) are inherently less reactive.
We quantified the fluorescence signal enhancements over time for the Sandwich reaction and 5′-dabsyl control. Importantly, the results showed that the new probe yields a substantially higher turn-on signal (48-fold at 4 h) than the singly reactive case (32-fold) (Fig. S1B, ESI†). We also measured signal-to-background (S/B) ratios as a function of time for the Sandwich configuration and singly reactive control (Fig. S2, ESI†). The new geometry yielded higher S/B (16 compared to 6) at the maximum, apparently due to a combination of lower initial fluorescence and reduced off-template signal due to the presence of two quenchers/leaving groups.
In order to verify that the Sandwich probe undergoes two ligation reactions, we used denaturing gel electrophoresis to evaluate the reaction products. The reactions were run for four hours at 37 °C, desalted, and loaded onto the gel. The Sandwich probes were added in reactions with neither nucleophile, both nucleophiles or only the 3′ nucleophile. As an additional control reaction, a quencher strand with only a single 5′ dabsyl was reacted with a 3′-nucleophile strand. The gel was imaged by fluorescence. Results (Fig. 3) showed that the lane containing only the Sandwich probe alone yielded one faintly fluorescent band corresponding to the unreacted or partially hydrolyzed probe. The control reactions containing only one of the two nucleophiles gave two bands on the gel, corresponding to the unligated and singly-ligated products. This pattern is also seen for the control probe containing only the 5′ dabsylate electrophile.
Fig. 3.
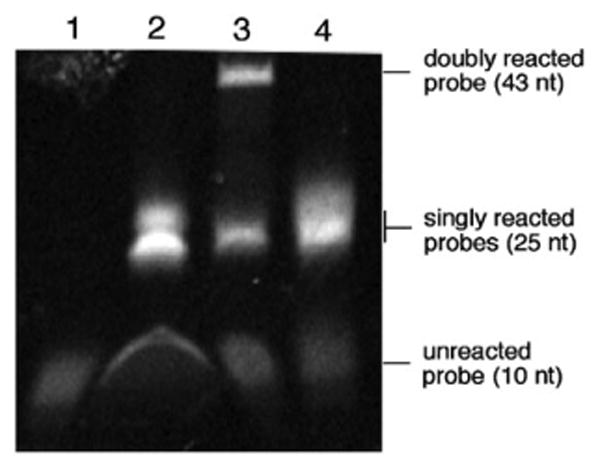
Fluorescence PAGE gel analysis of Sandwich probe reactions. Reactions contained 1 μM quenched probe, 1 μM template, and 1.2 μM nucleophile in 70 mM PIPES, 10 mM MgCl2, 50 μM DTT and were run for 4 h at 37 °C before being desalted and loaded onto the gel. Lane 1: Sandwich probe alone; Lane 2: Sandwich probe, template, 3′-nucleophile only; Lane 3: Sandwich probe, template, both 3′- and 5′-nucleophiles; Lane 4: Single dabsylate probe, template, 5′-nucleophile only.
Finally, the reaction containing the Sandwich probe with both nucleophiles showed three bands, corresponding to the unligated, singly-ligated, and doubly-ligated species.
To evaluate the selectivity of the Sandwich probe configuration, we tested probe reactivity on synthetic DNA targets corresponding to a site in the ribosomal RNA of E. coli, as well as mismatches at a single position that is known to vary in related bacteria. The central Sandwich probe was centered on the varied nucleotide, with nucleophile probes designed to flank this on both sides. The data show that the reaction proceeds with high selectivity (Fig. 2C). The reaction was run with both nucleophiles in the presence of the wild-type template, templates with single nucleotide mismatches and with no template. All the reactions performed with point mutations in the template showed similar kinetics to the reaction with no template at all. This confirms high selectivity, and suggests that all the signals in these experiments arose from very slow nontemplated reactions, rather than from reaction on the mismatched template.
To assess whether the results seen in buffer could translate to a cellular context, we tested the Sandwich probe reactivity on a ribosomal RNA target in intact E. coli cells. As a control for the background level of signal, the nucleophile probe or probes were omitted from the reactions. The probes were added to cells in the presence of 0.9 M sodium chloride and 90 mM sodium citrate (6× SSC) and 0.05% sodium dodecyl sulfate to aid in permeabilization, and were incubated 2 h without any preparation or washing steps prior to imaging under a fluorescence microscope. The results are shown in Fig. 4. The Sandwich probes yielded a visible green signal in the presence of both nucleophiles, but little or no signal in the presence of either single nucleophile. Virtually no background signal was seen in the absence of both nucleophile probes, revealing that two templated reactions are required to engender this signal.
Fig. 4.
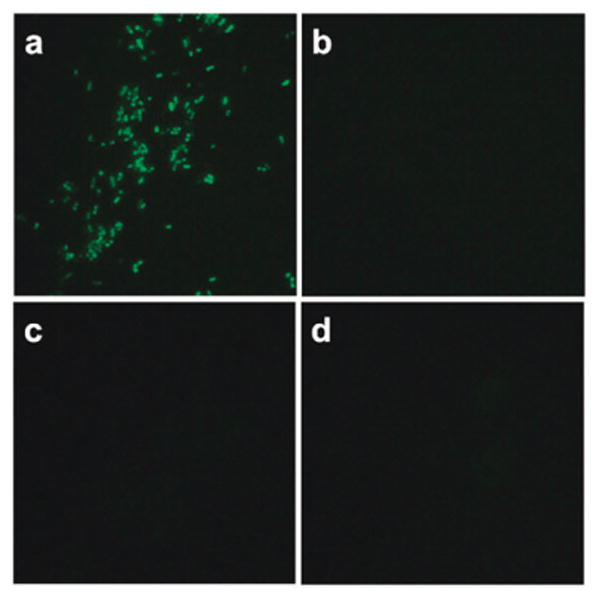
One-step fluorescence detection of rRNA in bacterial cells with Sandwich probes. The quenched probe (200 nM) and unlabeled helper DNA (3 μM (see ESI†)) were incubated in 6× SSC buffer with 0.05% SDS at 37 °C for 2 h with E. coli cells and 2 μM of (a) both 5′- and 3′-phosphorothioate nucleophile probes; (b) only 3′-phosphorothioate; (c) only 5′-phosphorothioate; and (d) no nucleophile. Images were taken with a black/white camera and false-colored green.
Taken together, the experiments demonstrate a new and viable architecture for nucleic acid templated reactions. The Sandwich probes show improved initial quenching and lower nontemplated signal due to the presence of two quencher groups, yield favorable signal to background kinetics, and can detect RNA sequences in intact bacterial cells. Surprisingly, the requirement for two reactions does not slow the overall turn-on of the probes. The findings suggest that increasing complexity in DNA templated reaction design14,15 may not necessarily lead to complex outcomes, and may in fact yield improved tools for research.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health (GM068122). We thank the NSF (CHE-0639053) for support of fluorescence instrumentation in the Department of Chemistry. We thank S. Tabakman for help with the gel experiments and B. Frezza for helpful discussions.
Footnotes
Electronic supplementary information (ESI) available: Experimental details, additional figures, characterization of synthetic intermediates. See DOI: 10.1039/c0cc01968b
Notes and references
- 1.Silverman AP, Kool ET. Chem Rev. 2006;106:3775–3789. doi: 10.1021/cr050057+. [DOI] [PubMed] [Google Scholar]
- 2.Pianowski ZL, Winssinger N. Chem Commun. 2007:3820–3822. doi: 10.1039/b709611a. [DOI] [PubMed] [Google Scholar]
- 3.Grossmann TN, Roglin L, Seitz O. Angew Chem, Int Ed. 2008;47:7119–7122. doi: 10.1002/anie.200801355. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Coull JM. J Am Chem Soc. 2008;130:3238–3239. doi: 10.1021/ja0753602. [DOI] [PubMed] [Google Scholar]
- 5.Jentzsch E, Mokhir A. Inorg Chem. 2009;48:9593–9595. doi: 10.1021/ic9006795. [DOI] [PubMed] [Google Scholar]
- 6.Silverman AP, Baron EJ, Kool ET. ChemBioChem. 2006;7:1890–1894. doi: 10.1002/cbic.200600278. [DOI] [PubMed] [Google Scholar]
- 7.Abe H, Kool ET. Proc Natl Acad Sci U S A. 2006;103:263–268. doi: 10.1073/pnas.0509938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzini RM, Kool ET. J Am Chem Soc. 2009;131:16021–16023. doi: 10.1021/ja904138v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa K, Abe H, Hibino K, Sako Y, Tsuneda S, Ito Y. Bioconjugate Chem. 2009;20:1026–1036. doi: 10.1021/bc900040t. [DOI] [PubMed] [Google Scholar]
- 10.Pianowski Z, Gorska K, Oswald L, Merten CA, Winssinger N. J Am Chem Soc. 2009;131:6492–6497. doi: 10.1021/ja809656k. [DOI] [PubMed] [Google Scholar]
- 11.Sando S, Kool ET. J Am Chem Soc. 2002;124:2096–2097. doi: 10.1021/ja017328s. [DOI] [PubMed] [Google Scholar]
- 12.Abe H, Kool ET. J Am Chem Soc. 2004;126:13980–13986. doi: 10.1021/ja046791c. [DOI] [PubMed] [Google Scholar]
- 13.Le Clezio I, Escudier JM, Vigroux A. Org Lett. 2003;5:161. doi: 10.1021/ol027222h. [DOI] [PubMed] [Google Scholar]
- 14.Snyder TM, Liu DR. Angew Chem, Int Ed. 2005;44:7379. doi: 10.1002/anie.200502879. [DOI] [PubMed] [Google Scholar]
- 15.Kleinbaum DJ, Miller GP, Kool ET. Bioconjugate Chem. 2010;21:1115–1120. doi: 10.1021/bc100165h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


