Abstract
Previous experiments have revealed a much greater efficiency of ammonium utilization by bean plants (Phaseolus vulgaris L.) when the acidity of the ambient medium was maintained at near-neutral conditions with carbonates or hydroxides. The present investigation, in which 15N-labeled ammonium was used, permitted an assessment of the origin of nitrogen in tissue nitrogen pools with and without acidity control (CaCO3 treated and untreated, respectively) in the root environment. Control of acidity resulted in greater ammonium uptake and greater incorporation into the amino fraction, amide, and ethanol-insoluble nitrogen by the root tissue. These differences were clearly evident by the fifth day after ammonium nitrogen had been applied.
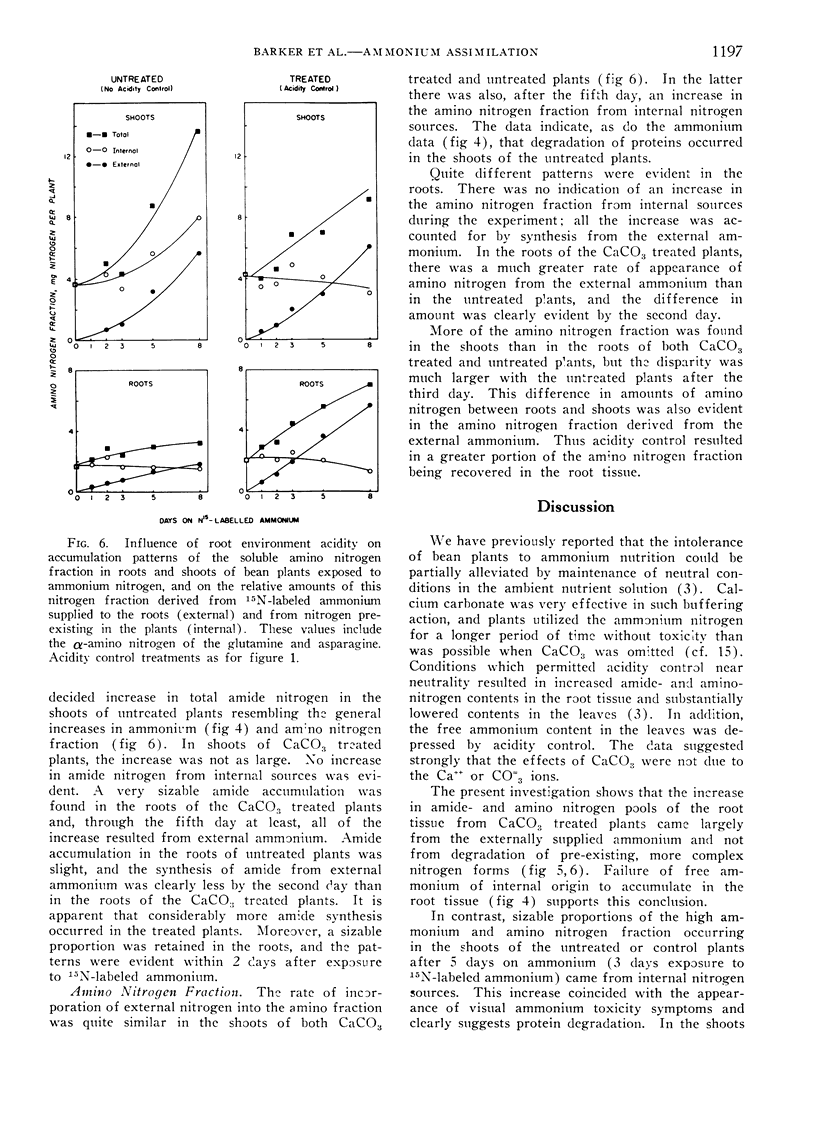
Shoots of the untreated plants rapidly accumulated free ammonium and amino nitrogen. A substantial portion of both fractions came from pre-existing nitrogen in the plants, indicating significant protein degradation. No evidence was found for such degradation in the roots of the untreated plants or in either roots or shoots of CaCO3 treated plants. The data indicate that control of ambient acidity in the root environment during ammonium absorption enhanced the conversion of entering ammonium to organic nitrogen compounds in the root tissue thereby restricting movement of free ammonium to shoots. Consequently, the detrimental effects of high ammonium concentrations in the leaves were largely prevented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Barnes R. L. Glutamine Synthesis & Translocation in Pine. Plant Physiol. 1962 May;37(3):323–326. doi: 10.1104/pp.37.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M., Calo N. Factors Affecting Light Induced Fixation of Carbon Dioxide by Isolated Spinach Chloroplasts. Plant Physiol. 1959 May;34(3):318–323. doi: 10.1104/pp.34.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanking B. M., Roberts S. Stimulation of protein synthesis in vitro by elevated levels of amino acids. Biochim Biophys Acta. 1965 Jul 8;104(2):427–438. doi: 10.1016/0304-4165(65)90348-x. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Racusen D., Foote M. Protein Turnover Rate in Bean Leaf Disks. Plant Physiol. 1962 Sep;37(5):640–642. doi: 10.1104/pp.37.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines H. M., Wedding R. T. Some Effects of Ammonia on Plant Metabolism and a Possible Mechanism for Ammonia Toxicity. Plant Physiol. 1960 Nov;35(6):820–825. doi: 10.1104/pp.35.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman G. S. Effect of Ammonium and Nitrate Nutrition on Protein Level and Exudate Composition. Plant Physiol. 1964 Nov;39(6):947–952. doi: 10.1104/pp.39.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]



