Abstract
Citrus macroptera (family Rutaceae), commonly known as Sat Kara, is a pharmacologically diverse medicinal plant. Various parts of this plant, specifically fruit, have an immense range of medicinal uses in folk medicine directed for a number of ailments. A plethora of active phytochemical constituents of this plant have been revealed so far, namely, limonene, beta-caryophyllene, beta-pinene, geranial edulinine, ribalinine, isoplatydesmine, and so forth. Several studies demonstrated the exploration of pharmacological potential of various parts such as fruits, leaves, and stems of C. macroptera as antioxidant, cytotoxic, antimicrobial, thrombolytic, hypoglycemic, anxiolytic, antidepressant, cardioprotective, and hepatoprotective. Furthermore, inhibition of in vitro α-amylase, inhibition of paracetamol induced hepatotoxicity, and potentiation of brain antioxidant enzyme are also ascertained. In present review, comprehensive study focused on knowledge regarding several phytopharmacological activities of Citrus macroptera has been described.
1. Introduction
Since time immemorial, mankind has searched for medicines to remove pain and cure various diseases. Evidence exists for the use of medicinal plants up to 60,000 years ago but more recently, a 5000-year-old Sumerian clay slab was discovered verifying the utilization of medicinal plants for the preparation of drugs [1]. Plants have different chemical compounds like secondary metabolites with many biochemical and bioactivity properties showing applications in various industries such as pharmaceuticals [2–6]. The interest in using natural sources or green medicine or medicinal plants is increasing worldwide due to their safety, efficacy, cultural acceptability, and lesser side effects as compared to synthetic drugs. At present, more than 80% of the global population depends on traditional plant-based medications for treating various human health problems [7–9]. More than 9000 native plants have been identified and recorded for their curative properties [10]. The genus Citrus contains many economically important fruits that are grown worldwide for their high nutritional and medicinal value [11]. Citrus is in the family Rutaceae, which is one of the largest families in order Sapindales. Flowers and leaves of Citrus are usually strong scented, the extracts of which contain many useful flavonoids and other compounds that are effective insecticides, fungicides, and medicinal agents [12–14]. Citrus genus includes some of the most important cultivated fruit trees worldwide [15]. C. macroptera is a semiwild species of Citrus native in Malesia and Melanesia [16]. The C. macroptera plant is grown in the places of most homesteads and hill tracts of the Sylhet division of Bangladesh [17]. The fruit of C. macroptera has significant cytotoxic, antimicrobial [18], antihypertensive, antipyretic, and appetite stimulant potentials [19, 20]. Additionally, significant hypoglycemic and neuropharmacological effects were confirmed in a rat model [21, 22]. It is reported that stem bark of C. macroptera possesses antioxidant activity [23] and essential oil obtained from the leave possesses antimicrobial activities [24]. Literature review suggested that plant extracts having antioxidant activities have health promoting effects and antiaging effects and are used for various metabolic and chronic diseases like cancer, liver diseases, inflammation, diabetes, arthritis, and stroke [25, 26].
However, no comprehensive review of this plant has been reported which demonstrates the efficacy of this plant in all dimensions. The present review is aimed at providing comprehensive and current information regarding the pharmacological potentials of C. macroptera.
1.1. Taxonomic Classification and Common Names
Kingdom: Plantae
Order: Sapindales
Family: Rutaceae
Genus: Citrus
Subgenus: Papeda
Species: C. macroptera
Common names include Melanesian papeda, wild orange [27], Cabuyao, and Satkara [28].
1.2. Introduction to Plant Profile
The fruit of C. macroptera is shown in Figure 1.
Figure 1.

Fruits of Citrus macroptera.
2. Morphological Studies
Citrus macroptera is a semiwild species of Citrus genus. It may be mentioned here that the English meaning of Satkara is “wild orange” [29]. Satkara (C. macroptera) fruits grow on trees, which are 5 m in height with thorns. The fruit is about 6-7 cm in diameter and the skin is fairly smooth [18]. It is a tree with abundant long spines on the stem, branches, and twigs. The dark green leaves of C. macroptera are 2.5–6.8 cm long and 2-3 cm thick. The round or oblong shaped green leaves of this plant are 2.5–3.8 cm in diameter [30]. The shape of fruit is spheroid with concave base and rounded apex and skin color of fruit is yellow with bumpy surface texture. In addition, seeds are of semideltoid shape with wrinkled surface and yellow in color [31].
3. Phytochemical Studies
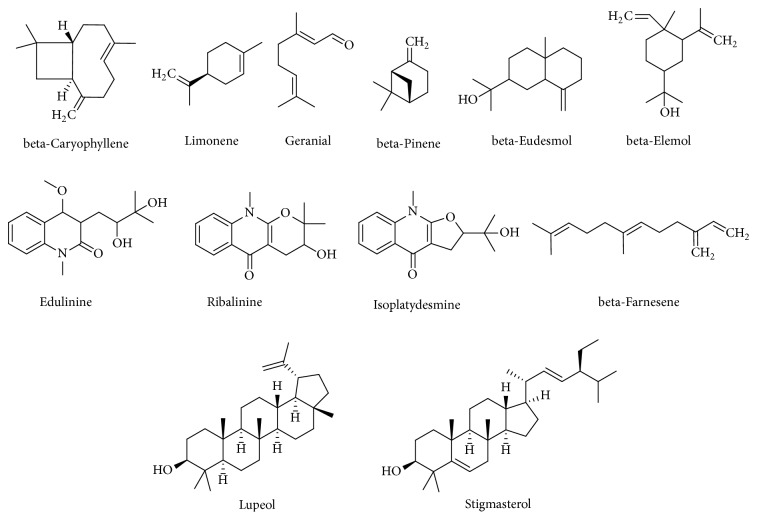
Rana and Blazquez (2012) reported that the essential oils obtained by hydrodistillation from the fresh peels of C. macroptera contained limonene, beta-caryophyllene, and geranial as main compounds [32]. Jantan et al. (1996) also revealed several phytochemicals from the peels of this plant like monoterpene hydrocarbon, namely, α-pinene, β-pinene, myrcene, α-phellandrene, limonene, and γ-terpinene as well as oxygenated monoterpene specially γ-elemene, linalool, terpinen-4-ol, α-terpineol, terpinolene, and geranyl acetate. Moreover, the peel of this plant has been found to contain some sesquiterpene hydrocarbons, namely, β-caryophyllene, (Z)-β-farnesene, aromadendrene, and α-guaiene, and oxygenated sesquiterpenes including elemol and β-eudesmol [33]. Waikedre et al. (2010) showed the beta-pinene as major component of essential oil of C. macroptera [24]. Gaillard et al. (1995) isolated edulinine, ribalinine, isoplatydesmine, and five aromatic compounds [34]. Yip and Dallman (1988) and Dallman et al. (1980) also revealed that the phytoconstituents of C. macroptera, such as polyphenols, flavonoids, and organosulfur compounds, were found to contribute to its neuropharmacological effects [35, 36]. Chowdhury et al. (2009) isolated two other important chemical compounds, namely, lupeol and stigmasterol [23]. Dreyer and Huey (1973) reported some coumarins like bergamottin, psoralen, marmin, severine, and geiparvarin [37]. However, the chemical structures of several important compounds isolated from different parts of C. macroptera are shown in Figure 2.
Figure 2.
Chemical structures of compounds isolated from Citrus macroptera.
4. Pharmacological Studies
The reported pharmacological activities of various parts of Citrus macroptera are detailed below.
4.1. Neuropharmacological Activities
The ethanolic extract of C. macroptera (EECM) fruit peels was examined for neuropharmacological activities using experimental animal models. Anxiolytic activity was assessed during Elevated Plus Maze (EPM) and Light and Dark Model (LDM) in mice. Acute oral toxicity studies of ethanolic extract of C. macroptera (EECM) fruit peels were carried out according to OECD-423 guidelines in mice and it was found to be nontoxic. Ethanolic extract of Citrus macroptera (EECM) fruit peels increases number of entries and time spent in light chamber in LDM in mice. Ethanolic extract of C. macroptera fruit peels (EECM) was found to possess anxiolytic activities. Moreover, antidepressant activity was assessed using Forced Swim Test (FST) and Tail Suspension Test (TST) in mice. Ethanolic extract of C. macroptera fruit peels (EECM) was found to possess antidepressant activities. Furthermore, this was also found to potentiate brain antioxidant enzyme level in experimental animal model [22].
4.2. Antioxidant Activities
The methanol and ethyl acetate extract of C. macroptera have been extensively studied to evaluate its antioxidant potential by various methods. It has been found that both extracts can scavenge the free radical and stop peroxidation process. The methanolic extract showed more antioxidant potential than ethyl acetate extract in DPPH, NO, and lipid peroxidation assay. However, in cupric acid reducing capacity and lipid peroxidation in human erythrocyte assay the ethyl acetate extract showed more potency than methanol extract [18]. It has also been found that hot methanol extract of stem bark of Citrus macroptera showed potential antioxidant activity (IC50: 178.96 μg/ml) whereas cold methanol and dichloromethane extracts of the stem bark showed moderate activity with IC50 value of 242.78 μg/ml and 255.78 μg/ml, respectively [23].
4.3. Paracetamol Induced Hepatorenal Toxicity Inhibition Activity
The ethanolic extract of this plant showed hepato- and nephroprotective activity induced by acetaminophen in rats. The treatment groups of rats were pretreated with extracts for 30 days at 250, 500, and 1000 mg/kg doses after acetaminophen administration. Over the similar period, Silymarin (100 mg/kg) was administered as a standard drug. In contrast to control, lipid peroxidation (TBARS) was increased two times which ultimately marked severe hepatic and renal injuries in association with oxidative stress. Interestingly, histopathological examinations demonstrated that treatment with the plant extract before acetaminophen administration improved all biological parameters studies, that is, transaminase activities, alkaline phosphatase, lactate dehydrogenase, γ-glutamyl transferase activities and total bilirubin, total cholesterol, triglyceride and creatinine, urea, uric acid, sodium, potassium and chloride ions, and TBARS levels. This extract showed potential activity at a dose 1000 mg/kg. Thus, C. macroptera was suggested to have potential activity probably through lipid peroxidation against acetaminophen-induced hepatonephrotoxicity [38].
4.4. Thrombolytic Activity
The methanolic extract of fruits of C. macroptera has been reported to possess considerable thrombolytic activity which exerted 48.47% lysis of the blood clot in thrombolytic activity test while 75.18% and 15.82% lysis were obtained in positive control (Streptokinase) and negative control, respectively [39].
4.5. In Vitro Cytotoxicity Bioassay
In Brine shrimp lethality bioassay, the methanolic extract of C. macroptera fruit showed a LC50 value of 30.90 μg/ml compared with the standard vincristine sulphate with LC50 value of 10.51 μg/ml [39]. The methanol and ethyl acetate extract of fruit of C. macroptera have been considered very toxic where the highest lethality was found for methanol extract which may be due to the presence of steroids, saponins, and terpenoids [18].
4.6. Antimicrobial Activities
The antimicrobial activity of methanolic and ethyl acetate extract of C. macroptera fruit extract was tested using the disc diffusion technique against six bacterial species where ethyl acetate extract showed broad spectrum antimicrobial activity against two gram positive Bacillus subtilis and Staphylococcus aureus and one gram negative Escherichia coli [18]. The volatile oil extracted from C. macroptera fruits was reported to possess reasonable antibacterial activity against four strains including Bacillus cereus, Bacillus subtilis, Escherichia coli, and Staphylococcus aureus but not against ampicillin resistant Escherichia coli and Salmonella typhi. The most potent activity was reported against both Bacillus species with a Minimum Inhibitory Concentration of 1.25 mg/ml [40].
4.7. Hypoglycemic Activity
The fruit extract of C. macroptera revealed moderate α-amylase inhibitory activity [IC50 value = (3.638 ± 0.190) mg/dl] as compared to acarbose. Moreover, at 500 mg/kg and 1000 mg/kg doses fruit extract significantly (P < 0.05 and P < 0.01 resp.) reduced fasting blood glucose level in normal rats as compared to glibenclamide (5 mg/kg). In oral glucose tolerance test, 500 mg/kg dose significantly reduced blood glucose level (P < 0.05) at 2 h but 1000 mg/kg dose significantly reduced blood glucose level at 2 h and 3 h (P < 0.05 and P < 0.01 resp.) whereas glibenclamide (5 mg/kg) significantly reduced blood glucose level at every hour after administration. These findings suggest that the plant may be a potential source for the development of new oral hypoglycemic agents [21]. In another study it was revealed that 250 mg/kg dose of methanolic extract of fruit of this plant significantly decreases the level of glycated hemoglobin (P < 0.05) leading to the potential hypoglycemic effect of C. macroptera [41].
4.8. Cardioprotective and Hepatoprotective Activities
An investigation of hematological and biochemical parameters in Sprague-Dawley female rats was performed which evaluated the potent cardioprotective activities of the methanolic extract of C. macroptera fruit. It is also claimed that the extract significantly reduced the levels of triglyceride, total cholesterol, low density lipoprotein, and very low density lipoprotein. The extract has also been reported with a significant decrease of alkaline phosphatase and a notable increase of high density lipoprotein cholesterol at a dose of 500 mg/kg and 1000 mg/kg, respectively, which also indicates the cardioprotective and moderate hepatoprotective activities of the fruit extract [41].
5. Discussions
The bioactive compounds of plants contain a wide variety of different substances like phytosterols, saponins, phenolic compounds, and terpenoids which have been found to be useful in the prevention and therapy of several diseases, including cancer, and also to have antimicrobial, antifungal, antiparasitic, antiviral antiallergic, antispasmodic, antihyperglycemic, anti-inflammatory, and immunomodulatory properties [42–45]. Encouragingly, current study reviewed several promising pharmacological activities which are intrigued by extensive variety of potential phytoconstituents of C. macroptera.
Recently C. macroptera was reported to have protective effects against hepatonephrotoxicity [38]. Treatment with paracetamol (APAP) is potentially attributed to hepatic injury and disturbances in the biosynthesis of some enzymes which leak out from the liver cytosol into the blood circulation, thus inducing necrosis and inflammatory responses due to the disruption of hepatocyte membrane permeability by APAP intoxication [46, 47]. This plant was found to maintain membrane integrity and restrict the leakage of hepatic enzymes [38]. Additionally, total bilirubin accumulation is indicative of the binding, conjugation, and excretory capacity of hepatic cells; raised level of it is an important indicator of the severity of necrosis [46, 48]. This plant was reported to restore the above diagnostic marker, thereby displaying its protective role in the liver [38].
Thrombolytic drugs block the pathway of thrombus formation with the help of plasmin that lyses clot by breaking down the fibrinogen and fibrin contained in a clot. Scientists revealed that flavanoids, among the plant metabolites, affect thrombosis and cardiovascular diseases by interfering with platelet activation which is a potential risk factor for cardiovascular disease [49]. In addition, it has been revealed earlier that terpenoids might have significant potentials of demonstrating thrombolytic activity [50, 51]. Admittedly, having abundant flavanoids and terpenoids might lead C. macroptera to exhibit thrombolytic activity.
Phytoconstituent showing toxicity is a major concern of scientist and our reviewed plant C. macroptera was found to show cytotoxicity which is most often related to chemoprevention. The cytotoxic activity of this plant may be mainly attributed to the compound lupeol which was reported previously as anticancer agent [52].
Some of the phytochemical compounds, for example, glycoside, saponins, tannins, flavanoids, terpenoids, and alkaloids, have been reported to have antimicrobial activity [53]. Several studies revealed that terpenoids are active against bacteria [54–63]. Noteworthy, the reviewed plant contains lupeol which is a pharmacologically active triterpenoid that displayed antimicrobial property earlier [52]. C. macroptera is profoundly enriched with terpenoids and henceforth may be leading to a remarkable antibacterial activity.
Several investigations revealed hypoglycemic potentials of C. macroptera. One study reported that lupeol shows inhibition of activity of α-amylase, which hydrolyses complex polysaccharides resulting in increased absorption through small intestine and ultimately enhanced postprandial glucose levels [64, 65]. Furthermore, stem bark of this plant contains two promising phytoconstituents; namely, lupeol and stigmasterol may have hypoglycemic potential. Another study suggested that these two constituents, lupeol and stigmasterol, may act as inhibitor of dipeptidyl peptidase-4 which plays a vital role in glucose metabolism being responsible for the degradation of incretins such as glucagon-like peptide [66, 67]. Moreover, limonene isolated from this plant may be a potential hypoglycemic agent [32].
Earlier, the reviewed plant exhibited potent antioxidant activity. It is well established that oxidative damage to biomolecules (lipids, proteins, and DNA), due to the overproduction of free radical plays an important role in the etiology of numerous diseases such as atherosclerosis, cancer, diabetes, rheumatoid arthritis, post-ischemic perfusion injury, myocardial infarction, cardiovascular diseases, chronic inflammation, stroke and septic shock, aging, and other degenerative diseases in humans [68, 69]. The oxidative stress can be effectively neutralized by enhancing cellular defense in the form of antioxidants which act as a persuasive therapeutic agent against different diseases [70]. Antioxidants exert their effects via several basic mechanisms, which include scavenging the species that initiate peroxidation, quenching singlet oxygen, chelating metals, breaking free radical chain reactions, and reducing the concentration of oxygen [71]. Again recent research suggests that the mechanisms of actions of antioxidants and their role in disease onset or progressions delve deep into cellular signaling processes and control of gene expression [72]. In vivo experiments demonstrated that antioxidants increase both the humoral and the cell-mediated immune response and immune surveillance against tumorigenesis [73–75]. Different studies regarding the potential therapeutic applications of antioxidants in free radical-related diseases led to the hypothesis of their use to slow down or reverse, for example, symptoms associated with neurodegenerative disorders, such as Alzheimer's disease (AD) and Parkinson's disease (PD); such effects could occur through a block of proinflammatory cytokines action and resulting oxidative damage [76–80]. The additional mechanism includes scavenging of a wide range of free radicals including the most active hydroxyl radicals, which initiate lipid peroxidation process [81].
6. Conclusion
Presently there is an increasing interest worldwide in herbal medicines accompanied with increased laboratory investigations into the pharmacological properties of the bioactive ingredients and their ability to treat various diseases. Enormous drugs have entered the international market through exploration of ethnopharmacology and traditional medicine. Admittedly, C. macroptera can be regarded as a versatile plant having a plethora of medicinal activities. This plant is inimitable source of a wide range of compounds having diverse medicinal properties. The current information regarding this medicinal plant may serve as the baseline data to enforce to do extensive studies for the discovery of new potent compounds and further investigations for their biological activities. Therefore, further research may be carried out on C. macroptera to explore their full therapeutic activity.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Summer J. The Natural History of Medicinal Plants. Vol. 16. London, UK: Timber Press; 2000. [Google Scholar]
- 2.Sarrafchi A., Bahmani M., Shirzad H., Rafieian-Kopaei M. Oxidative stress and Parkinson’s disease: new hopes in treatment with herbal antioxidants. Current Pharmaceutical Design. 2015;22(2):238–246. doi: 10.2174/1381612822666151112151653. [DOI] [PubMed] [Google Scholar]
- 3.Rabiei Z., Rafieian-Kopaei M. Neuroprotective effect of pretreatment with Lavandula officinalis ethanolic extract on blood-brain barrier permeability in a rat stroke model. Asian Pacific Journal of Tropical Medicine. 2014;7(1):S421–S426. doi: 10.1016/S1995-7645(14)60269-8. [DOI] [PubMed] [Google Scholar]
- 4.Dangl J. L., Jones J. D. G. Plant pathogens and integrated defence responses to infection. Nature. 2001;411(6839):826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 5.Yamane H., Konno K., Sabelis M., Takabayashi J., Sassa T., Oikawa H. In: Comprehensive Natural Products II. Mander L., Lui H. W., editors. Oxford, UK: Elsevier; 2010. p. 339. (385). [Google Scholar]
- 6.Eslami M., Bayat M., Mozaffari Nejad A. S., Sabokbar A., Anvar A. A. Effect of polymer/nanosilver composite packaging on long-term microbiological status of Iranian saffron (Crocus sativus L.) Saudi Journal of Biological Sciences. 2016;23(3):341–347. doi: 10.1016/j.sjbs.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumara Swamy M., Sudipta K. M., Lokesh P., et al. Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. International Journal of Phytomedicine. 2012;4(3):375–379. [Google Scholar]
- 8.Akhtar M. S., Degaga B., Azam T. Biological Sciences and Pharmaceutical research. Vol. 2. 2(1: 1-7; 2014. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: a review; p. p. 1. [Google Scholar]
- 9.Arumugam G., Swamy M. K., Sinniah U. R. Plectranthus amboinicus (Lour.) Spreng: botanical, phytochemical, pharmacological and nutritional significance. Molecules. 2016;21(4):p. 369. doi: 10.3390/molecules21040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swamy M. K., Sinniah U. R. Patchouli (Pogostemon cablin Benth.): botany, agrotechnology and biotechnological aspects. Industrial Crops and Products. 2016;87:161–176. doi: 10.1016/j.indcrop.2016.04.032. [DOI] [Google Scholar]
- 11.Su H.-J., Hogenhout S. A., Al-Sadi A. M., Kuo C.-H. Complete chloroplast genome sequence of omani lime (Citrus aurantiifolia) and comparative analysis within the rosids. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0113049.e113049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabberley D. J. Citrus (Rutaceae): A review of recent advances in etymology, systematics and medical applications. Blumea: Journal of Plant Taxonomy and Plant Geography. 2004;49(2-3):481–498. doi: 10.3767/000651904X484432. [DOI] [Google Scholar]
- 13.Tripoli E., Guardia M. L., Giammanco S., Majo D. D., Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chemistry. 2007;104(2):466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- 14.Ezeabara C. A., Okeke C. U., Aziagba B. O., Ilodibia C. V., Emeka A. N. Detremination of saponin content of various parts of six Citrus species. International Research Journal of Pure and Applied Chemistry. 2014;4:137–143. [Google Scholar]
- 15.Carbonell-Caballero J., Alonso R., Ibañez V., Terol J., Talon M., Dopazo J. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus citrus. Molecular Biology and Evolution. 2015;32(8):2015–2035. doi: 10.1093/molbev/msv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J. M., Che C. T., Lau C. B. S., Leung P. S., Cheng C. H. K. Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. International Journal of Biochemistry and Cell Biology. 2006;38(5-6):985–995. doi: 10.1016/j.biocel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Bose T. K., Mitra S. K. Fruits: Tropical and Subtropical. Calcutta, India: Naya Prokash; 1990. [Google Scholar]
- 18.Uddin N., Hasan M. R., Hossain M. M. Antioxidant, brine shrimp lethality and antimicrobial activities of methanol and ethyl-acetate extracts of Citrus macroptera Montr. fruit using in vitro assay models. British Journal of Pharmaceutical Research. 2014;4(14):1725–1738. doi: 10.9734/bjpr/2014/11235. [DOI] [Google Scholar]
- 19.Grover J. K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. Journal of Ethnopharmacology. 2002;81(1):81–100. doi: 10.1016/S0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 20.Rahmatullah M., Khatun M. A., Morshed N., et al. A randomized survey of medicinal plants used by folk medicinal healers of Sylhet Division, Bangladesh. Advances in Natural and Applied Sciences. 2010;4(1):52–62. [Google Scholar]
- 21.Uddin N., Hasan M. R., Hossain M. M., et al. In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit. Asian Pacific Journal of Tropical Biomedicine. 2014;4(6):473–479. doi: 10.12980/apjtb.4.2014c1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman H M. C., Eswaraiah., Dutta A. Neuropharmacological activities of ethanolic extract of Citrus macroptera (Varannamensis) fruit peels. Global Journal of Pharmacology. 2014;8(4):609–616. [Google Scholar]
- 23.Chowdhury S. A., Sohrab M. H., Datta B. K., Hasan C. M. Chemical and Antioxidant Studies of Citrus macroptera. Bangladesh Journal of Scientific and Industrial Research. 2009;43(4) doi: 10.3329/bjsir.v43i4.2235. [DOI] [Google Scholar]
- 24.Waikedre J., Dugay A., Barrachina I., Herrenknecht C., Cabalion P., Fournet A. Chemical composition and antimicrobial activity of the essential oils from new caledonian Citrus macroptera and Citrus hystrix. Chemistry and Biodiversity. 2010;7(4):871–877. doi: 10.1002/cbdv.200900196. [DOI] [PubMed] [Google Scholar]
- 25.Fusco D., Colloca G., Lo Monaco M. R., Cesari M. Effects of antioxidant supplementation on the aging process. Clinical Interventions in Aging. 2007;2(3):377–387. [PMC free article] [PubMed] [Google Scholar]
- 26.Willcox J. K., Ash S. L., Catignani G. L. Antioxidants and prevention of chronic disease. Critical Reviews in Food Science and Nutrition. 2004;44(4):275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 27.Manner H. I., Buker R. S., Ward D., Smith V. E., Elevitch C. R. Species Profiles for Pacific Island Agroforestry. New York: Permanent Agricultural Resources; 2006. [Google Scholar]
- 28.Hanelt P. Mansfeld's Encyclopedia of Agricultural and Horticultural Crops. first English edition. Springer; 2001. [DOI] [Google Scholar]
- 29.Elevitch C. R. Traditional trees of pacific islands: their culture, environment, and use. Hawaii. Permanent Agriculture Resources. 2007:243–276. [Google Scholar]
- 30.Ong H. C. Buah, Khasiat Makanan & Ubatan; Utusan Publications & Distributors: Kuala Lampur, Malaysia. Kuala Lampur: Malaysia; 2004. [Google Scholar]
- 31.Malik S. K., Chaudhury R., Dhariwal O. P., Kalia R. K. Collection and characterization of Citrus indica Tanaka and C. macroptera Montr.: wild endangered species of northeastern India. Genetic Resources and Crop Evolution. 2006;53(7):1485–1493. doi: 10.1007/s10722-005-7468-7. [DOI] [Google Scholar]
- 32.Rana V. S., Blazquez M. A. Compositions of the volatile oils of Citrus macroptera and C. maxima. Natural Product Communications. 2012;7(10):1371–1372. [PubMed] [Google Scholar]
- 33.Jantan I., Ahmad A. S., Ahmad A. R., Azah Mohd Ali N., Ayop N. Chemical composition of some citrus oils from Malaysia. Journal of Essential Oil Research. 1996;8(6):627–632. doi: 10.1080/10412905.1996.9701030. [DOI] [Google Scholar]
- 34.Gaillard E., Muyard F., Bevalot F., Regnier A., Vaquette J. Rutaceae from new caledonia: chemical composition of stem bark of Citrus macroptera Montr. Annales Pharmaceutiques Francaises. 1995;53(2):75–78. [PubMed] [Google Scholar]
- 35.Yip R., Dallman P. R. The roles of inflammation and iron deficiency as causes of anemia. The American Journal of Clinical Nutrition. 1988;48:1295–1300. doi: 10.1093/ajcn/48.5.1295. [DOI] [PubMed] [Google Scholar]
- 36.Dallman P., Siimens M., Stekekl A. Iron deficiency in infancy and childhood. The American Journal of Clinical Nutrition. 1980;33:86–118. doi: 10.1046/j.1467-3010.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 37.Dreyer D. L., Huey P. F. Coumarins of Citrus macroptera. Phytochemistry. 1973;12(12):3011–3013. doi: 10.1016/0031-9422(73)80536-9. [DOI] [Google Scholar]
- 38.Paul S., Islam M. A., Tanvir E. M., et al. Satkara (Citrus macroptera) fruit protects against acetaminophen-induced hepatorenal toxicity in rats. Evidence-based Complementary and Alternative Medicine. 2016;2016:11. doi: 10.1155/2016/9470954.9470954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karim N., Sakib A. H., Sarkar M. Y., Hossain M. S., Ansari M. A., Dhar R. Study on in-vitro cytotoxic and thrombolytic activity of methanolic extract of Citrus macroptera (Fruit) Journal of Scientific Research and Advances. 2015;2(2):90–94. [Google Scholar]
- 40.Miah M. N., Bachar S. C., Nahar L., et al. Composition of the volatiles of Citrus macroptera var. annamensis and evaluation of bioactivity. Journal of Essential Oil-Bearing Plants. 2010;13(2):211–218. doi: 10.1080/0972060X.2010.10643814. [DOI] [Google Scholar]
- 41.Uddin N., Hasan M. R., Hasan M. M., et al. Assessment of toxic effects of the methanol extract of Citrus macroptera Montr. fruit via biochemical and hematological evaluation in female Sprague-Dawley rats. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111101.e111101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabi T., Bishayee A. Terpenoids and breast cancer chemoprevention. Breast Cancer Research and Treatment. 2009;115(2):223–239. doi: 10.1007/s10549-008-0118-y. [DOI] [PubMed] [Google Scholar]
- 43.Wagner K.-H., Elmadfa I. Biological relevance of terpenoids: Overview focusing on mono-, di- and tetraterpenes. Annals of Nutrition and Metabolism. 2003;47(3-4):95–106. doi: 10.1159/000070030. [DOI] [PubMed] [Google Scholar]
- 44.Sultana N., Ata A. Oleanolic acid and related derivatives as medicinally important compounds. Journal of Enzyme Inhibition and Medicinal Chemistry. 2008;23(6):739–756. doi: 10.1080/14756360701633187. [DOI] [PubMed] [Google Scholar]
- 45.Shah B. A., Qazi G. N., Taneja S. C. Boswellic acids: a group of medicinally important compounds. Natural Product Reports. 2009;26(1):72–89. doi: 10.1039/B809437N. [DOI] [PubMed] [Google Scholar]
- 46.Afroz R., Tanvir E. M., Hossain M. F., et al. Protective effect of Sundarban honey against acetaminophen-induced acute hepatonephrotoxicity in rats. Evidence-Based Complementary and Alternative Medicine. 2014;2014:8. doi: 10.1155/2014/143782.143782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenoy S., Kumar H., Thasma N. V., Prabhu K., Pai P. Hepatoprotective activity of Plectranthus amboinicus against paracetamol induced hepatotoxicity in rats. International Journal of Pharmacology and Cliniacl Sciences. 2012;2:32–38. [Google Scholar]
- 48.Manokaran S., Jaswanth A., Sengottuvelu S. Hepatoprotective activity of Averva lanata Linn. Against paracetamol induced hepatotoxicicty in rats. Research Journal of Pharmacy and Technology. 2008;1(4):398–400. [Google Scholar]
- 49.Dwivedi S. Terminalia arjuna wight and arn: a useful drug for cardiovascular disorders. Journal of Ethnopharmacology. 2007;114(2):114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Chen C., Li S.-X., Wang S.-M., Liang S.-W. Investigation into the anti-thrombosis effect and contents of total saponins and flavonoids in the bioactive fraction of Naodesheng prescription. Journal of Ethnopharmacology. 2012;144(1):208–212. doi: 10.1016/j.jep.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Pietri S., Maurelli E., Drieu K., Culcasi M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract. Journal of Molecular and Cellular Cardiology. 1997;29(2):733–742. doi: 10.1006/jmcc.1996.0316. [DOI] [PubMed] [Google Scholar]
- 52.Wal P., Wal A., Sharma G., Rai A. K. Biological activities of lupeol. Systematic Reviews in Pharmacy. 2011;2(2):96–103. doi: 10.4103/0975-8453.86298. [DOI] [Google Scholar]
- 53.Wallace R. J. Antimicrobial properties of plant secondary metabolites. Proceedings of the Nutrition Society. 2004;63(4):621–629. doi: 10.1079/PNS2004393. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed A. A., Mahmoud A. A., Williams H. J., Ian Scott A., Reibenspies J. H., Mabry T. J. New sesquiterpene α-methylene lactones from the Egyptian plant Jasonia candicans. Journal of Natural Products. 1993;56(8):1276–1280. doi: 10.1021/np50098a011. [DOI] [PubMed] [Google Scholar]
- 55.Amaral J. A., Ekins A., Richards S. R., Knowles R. Effect of selected monoterpenes on methane oxidation, denitrification, and aerobic metabolism by bacteria in pure culture. Applied and Environmental Microbiology. 1998;64(2):520–525. doi: 10.1128/aem.64.2.520-525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barre J. T., Bowden B. F., Coll J. C., et al. A bioactive triterpene from Lantana camara. Phytochemistry. 1997;45(2):321–324. doi: 10.1016/s0031-9422(96)00805-9. [DOI] [PubMed] [Google Scholar]
- 57.Habtemariam S., Gray A. I., Waterman P. G. A new antibacterial sesquiterpene from Premna oligotricha. Journal of Natural Products. 1993;56(1):140–143. doi: 10.1021/np50091a022. [DOI] [PubMed] [Google Scholar]
- 58.Himejima M., Hobson K. R., Otsuka T., Wood D. L., Kubo I. Antimicrobial terpenes from oleoresin of ponderosa pine tree Pinus ponderosa: a defense mechanism against microbial invasion. Journal of Chemical Ecology. 1992;18(10):1809–1818. doi: 10.1007/bf02751105. [DOI] [PubMed] [Google Scholar]
- 59.Kubo I., Muroi H., Himejima M. Antibacterial activity of totarol and its potentiation. Journal of Natural Products. 1992;55(10):1436–1440. doi: 10.1021/np50088a008. [DOI] [PubMed] [Google Scholar]
- 60.Mendoza L., Wilkens M., Urzúa A. Antimicrobial study of the resinous exudates and of diterpenoids and flavonoids isolated from some Chilean Pseudognaphalium (Asteraceae) Journal of Ethnopharmacology. 1997;58(2):85–88. doi: 10.1016/s0378-8741(97)00084-6. [DOI] [PubMed] [Google Scholar]
- 61.Scortichini M., Rossi M. P. Preliminary in vitro evaluation of the antimicrobial activity of terpenes and terpenoids towards Erwinia amylovora (Burrill) Winslow et al. Journal of Applied Bacteriology. 1991;71(2):109–112. doi: 10.1111/j.1365-2672.1991.tb02963.x. [DOI] [Google Scholar]
- 62.Tassou C. C., Drosinos E. H., Nychas G. J. E. Effects of essential oil from mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in model food systems at 4° and 10°C. Journal of Applied Bacteriology. 1995;78(6):593–600. doi: 10.1111/j.1365-2672.1995.tb03104.x. [DOI] [PubMed] [Google Scholar]
- 63.Taylor R. S. L., Edel F., Manandhar N. P., Towers G. H. N. Antimicrobial activities of southern Nepalese medicinal plants. Journal of Ethnopharmacology. 1996;50(2):97–102. doi: 10.1016/0378-8741(95)01335-0. [DOI] [PubMed] [Google Scholar]
- 64.Ranilla L. G., Kwon Y.-I., Apostolidis E., Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresource Technology. 2010;101(12):4676–4689. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 65.El-Kaissi S., Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Current Diabetes Reviews. 2011;7(6):392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 66.Marques M. R., Stüker C., Kichik N., et al. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia. 2010;81(6):552–556. doi: 10.1016/j.fitote.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 67.Panda S., Jafri M., Kar A., Meheta B. K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 2009;80(2):123–126. doi: 10.1016/j.fitote.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Annals of the New York Academy of Sciences. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 69.Fang Y.-Z., Yang S., Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 70.Gilgun-Sherki Y., Melamed E., Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 71.Badannath A. V., Rao K. M., Chetty C. M. S., Ramkanth S., Rajan T. V. S., Gnanaprakash K. A review on in-vivo antioxidants methods: comparisons. Correlations and Considerations. 2010;2(2):1276–1285. [Google Scholar]
- 72.Wachtel-Galor S., Benzie I. F. F. Series Preface. In: Benzie I. F. F., Wachlet-Galor S., editors. Herbal Medicine Biomolecular and Clinical Aspects. 2nd. Boca Raton, Fla, USA: CRC Press; 2011. pp. 1–10. [PubMed] [Google Scholar]
- 73.Villamor E., Fawzi W. W. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clinical Microbiology Reviews. 2005;18(3):446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chew B. P., Park J. S. Carotenoid action on the immune response. Journal of Nutrition. 2004;134(1):257S–216S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 75.Stephensen C. B. Vitamin A, infection, and immune function. Annual Review of Nutrition. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 76.Mancuso C., Bates T. E., Butterfield D. A., et al. Natural antioxidants in Alzheimer's disease. Expert Opinion on Investigational Drugs. 2007;16(12):1921–1931. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]
- 77.Whitton P. S. Inflammation as a causative factor in the aetiology of Parkinson's disease. British Journal of Pharmacology. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. European Journal of Pharmacology. 2006;545(1):51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 79.Pham D. Q., Plakogiannis R. Vitamin E supplementation in Alzheimer's disease, Parkinson's disease, tardive dyskinesia, and cataract: part 2. Annals of Pharmacotherapy. 2005;39(12):2065–2072. doi: 10.1345/aph.1G271. [DOI] [PubMed] [Google Scholar]
- 80.Drisko J. A. The use of antioxidants in transmissible spongiform encephalopathies: a case report. Journal of the American College of Nutrition. 2002;21(1):22–25. doi: 10.1080/07315724.2002.10719189. [DOI] [PubMed] [Google Scholar]
- 81.Al-Attar A. M., Abu Zeid I. M. Effect of tea (camellia sinensis) and olive (olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed Research International. 2013;2013:6. doi: 10.1155/2013/461415.461415 [DOI] [PMC free article] [PubMed] [Google Scholar]