Abstract
Background
Previous studies regarding the cardioprotective effects of dipeptidyl peptidase 4 (DPP-4) inhibitors have not provided sufficient evidence of a relationship between DPP-4 inhibition and actual cardiovascular outcomes. This study aimed to evaluate the impact of DPP-4 inhibitors on the survival of diabetic patients after first acute myocardial infarction (AMI).
Methods
This was a nationwide, propensity score-matched, case–control study of 186,112 first AMI patients, 72,924 of whom had diabetes. A propensity score, one-to-one matching technique was used to match 2672 controls to 2672 patients in the DPP-4 inhibitor group for analysis. Controls were matched based on gender, age, and a history of hypertension, dyslipidemia, diabetes, peripheral vascular disease, heart failure, cerebrovascular accident, end-stage renal disease, chronic obstructive pulmonary disease, and percutaneous coronary intervention.
Results
DPP-4 inhibitors improve the overall 3-year survival rate (log rank P < 0.0001), whether male or female. Cox proportional hazard regression showed DPP-4 inhibitor is beneficial in diabetes patients after AMI (HR = 0.86; 95% CI 0.78–0.95), especially in those patients with hypertension (HR = 0.87; 95% CI 0.78–0.97; P = 0.0103) and cerebrovascular disease (HR = 0.83; 95% CI 0.72–0.97; P = 0.018), but without dyslipidemia (HR = 0.78; 95% CI 0.67–0.92; P = 0.0029), without peripheral vascular disease (HR = 0.86; 95% CI 0.78–0.96; P = 0.0047), without heart failure (HR = 0.84; 95% CI 0.73–0.96; P = 0.0106), without end stage renal disease (HR = 0.86; 95% CI 0.77–0.95; P = 0.0035), and without chronic obstructive pulmonary disease (HR = 0.87; 95% CI 0.78–0.97; P = 0.0096).
Conclusions
DPP-4 inhibitor therapy improved long-term survival in diabetic patients after first AMI, regardless of gender.
Keywords: DPP-4 inhibitor, Acute myocardial infarction, Diabetes
Introduction
Diabetes mellitus (DM) with hyperglycemia and insulin resistance is one of the main risk factors for cardiovascular disease. Dipeptidyl peptidase 4 inhibitors (DPP-4i) are one of oral hypoglycemic agents (OHA) commonly used in type 2 DM patients. The effects of DPP-4i are mediated through the incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide, by slowing gastric emptying, stimulating glucose-dependent insulin release from the pancreatic islets, and inhibiting inappropriate post-meal glucagon release [1].
In several animal studies, DPP-4 inhibitors were shown to achieve cardioprotective effects via several mechanisms. These effects included a reduction in reperfusion injury, an increase in threshold of ventricular fibrillation during the ischemic period, an induced antiapoptotic effect, a reduction in oxidative stress, a decrease in infarct size, stabilized cardiac electrophysiology, protected cardiac mitochondrial function, an inhibition of atherosclerosis, and vascular smooth muscle cell proliferation [2–7].
In human randomized, double-blind study studies, DPP-4i did not appear to increase the risk of major adverse cardiovascular events among patients with type 2 DM with established cardiovascular disease [8, 9]. However, definitive proof of an effect of DPP-4i in patients with acute coronary syndrome (ACS) is currently lacking. In a randomized trial, DPP-4i were shown to have a neutral effect on rates of major adverse ischemic cardiovascular events and to have increased the rate of hospitalization for heart failure in DM patients with ACS [10, 11]. Whereas, other prospective, open-labeled, randomized studies showed that DPP-4i improved coronary flow reserve and left ventricular election fraction, and achieved regression of coronary artery plaque or reduction in major cardiovascular events [12, 13].
This study aimed to evaluate the impact of DPP-4i on survival of diabetic patients after first acute myocardial infarction (AMI) through analysis of the data from the Taiwan National Health Insurance Research Database.
Methods
Data source
Since 1995, the National Health Insurance Program has provided healthcare to approximately 99% of the residents in Taiwan. The data for this study were collected from National Health Insurance Research Database (NHIRD) from January 2000 through December 2012.
The NHIRD includes detailed information from medical inpatient records including age, gender, diagnosis, interventional procedures, medical orders, and relevant survival data. The database provides researchers with de-identified data via encryption of the identification codes to preserve patient anonymity and has been extensively used in epidemiologic studies in Taiwan. This study was approved by the Human Research Committee of Kaohsiung Veterans General Hospital.
Definition of AMI population
A total of 186,326 patients admitted to hospitals in Taiwan between January 2000 and December 2012 with a primary diagnosis of AMI (ICD: 410–410.92) were retrieved from Taiwan’s NHIRD which consisted of data collected from more than 23,000,000 patients. From this group of 186,326 patients, those with a previous admission for AMI, those who were ≤18 or ≥120 years old, and those patients whose gender was undetermined were excluded. The remaining 186,112 patients were included in the analysis (Fig. 1).
Fig. 1.
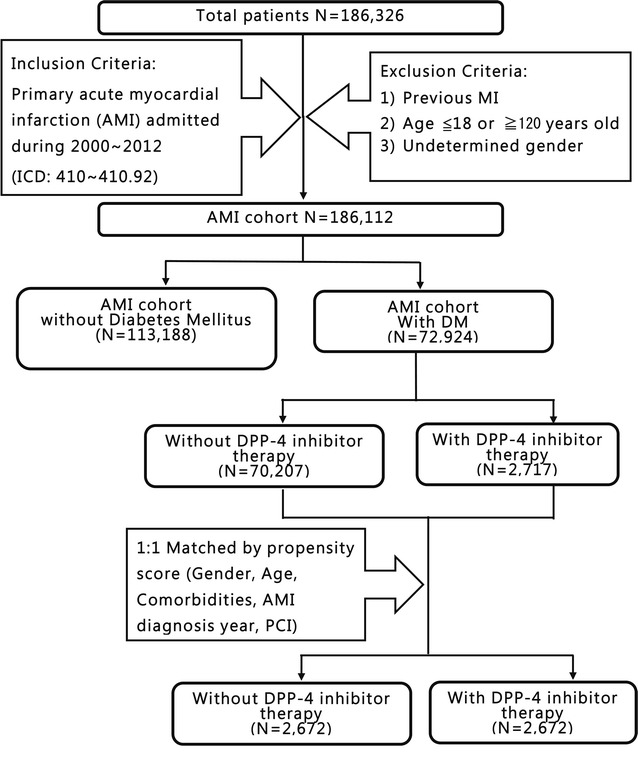
Flowchart outlining the various study cohorts. There were 186,326 patients in Taiwan between January 2000 and December 2012 with a primary diagnosis of acute myocardial infarction (AMI) (ICD codes: 410–410.92). From this group, patients were excluded who had a previous admission for AMI, who were ≤18 or ≥120 years old, and whose gender was undetermined. Among the remaining 186,112 cases with a primary diagnosis AMI, 72,924 cases had diabetes mellitus and underwent propensity score matching to controls to minimize baseline differences between the two groups. 2672 AMI patients with DPP-4i and 2672 matched controls were, therefore, included in our final analysis. AMI acute myocardial infarction, DM diabetes mellitus, DPP-4 dipeptidyl peptidase 4, PCI percutaneous coronary intervention
Study population
Among the 186,112 patients who were hospitalized for first AMI, 72,924 cases with DM were identified. The remaining 113,188 patients without DM were excluded. A propensity score matching technique was applied to minimize baseline differences between the control group and the DPP-4i group. One-to-one matching was performed using the following variables: gender, age, hypertension, dyslipidemia, diabetes, HF, peripheral vascular disease (PVD), cerebrovascular accident (CVA), end-stage renal disease (ESRD), chronic obstructive pulmonary disease (COPD), and percutaneous coronary intervention (PCI) (Table 1). The data from 2672 AMI patients receiving DPP-4i and 2672 matched controls were included in the final analysis (Fig. 1).
Table 1.
Patient characteristics first hospitalized for AMI with and without DPP-4 inhibitor
| Characteristics | Control group (N = 2672) | DPP-4 inhibitor group (N = 2672) | P value |
|---|---|---|---|
| Gender | |||
| Female | 1098 (41.09%) | 1098 (41.09%) | 1 |
| Male | 1574 (58.91%) | 1574 (58.91%) | |
| Age | |||
| Age <65 | 1064 (39.82%) | 1059 (39.63%) | 0.8468 |
| 65 ≤ age < 75 | 707 (26.46%) | 725 (27.13%) | |
| Age ≥75 | 901 (33.72%) | 888 (33.23%) | |
| Comorbidities | |||
| Hypertension | 2016 (75.45%) | 2005 (75.04%) | 0.7274 |
| Dyslipidemia | 1996 (74.7%) | 1996 (74.7%) | 1 |
| Peripheral vascular disease | 144 (5.39%) | 144 (5.39%) | 1 |
| Heart failure | 971 (36.34%) | 994 (37.2%) | 0.5141 |
| End stage renal disease | 187 (7%) | 187 (7%) | 1 |
| COPD | 231 (8.65%) | 234 (8.76%) | 0.8842 |
| Cerebrovascular accident | 789 (29.53%) | 796 (29.79%) | 0.8339 |
| Operations | |||
| Percutaneous coronary intervention | 1699 (63.59%) | 1699 (63.59%) | 1 |
| Coronary artery bypass graft | 260 (9.73%) | 297 (11.12%) | 0.0976 |
| IABP | 240 (8.98%) | 248 (9.28%) | 0.704 |
| Medication | |||
| Aspirin | 2330 (87.2%) | 2440 (91.32%) | <0.0001 |
| Clopidogrel | 2350 (87.95%) | 2454 (91.84%) | <0.0001 |
| Any anti-platelet drug | 2513 (94.05%) | 2596 (97.16%) | <0.0001 |
| ACEI or ARB | 1743 (65.23%) | 2007 (75.11%) | <0.0001 |
| Statin | 1616 (60.48%) | 1780 (66.62%) | <0.0001 |
| β-Blocker | 1587 (59.39%) | 1884 (70.51%) | <0.0001 |
| CCB | 980 (36.68%) | 1145 (42.85%) | <0.0001 |
| Heparin or low molecular weight heparin | 2174 (81.36%) | 2266 (84.81%) | 0.0008 |
| Spironolactone | 468 (17.51%) | 615 (23.02%) | <0.0001 |
| Nitrate | 2245 (84.02%) | 2366 (88.55%) | <0.0001 |
| Nicorandil | 284 (10.63%) | 401 (15.01%) | <0.0001 |
| α-Glucosidase | 197 (7.37%) | 602 (22.53%) | <0.0001 |
| Glinides | 311 (11.64%) | 644 (24.1%) | <0.0001 |
| Metformin | 781 (29.23%) | 1168 (43.71%) | <0.0001 |
| Sulfonylureas | 778 (29.12%) | 1420 (53.14%) | <0.0001 |
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, CCB calcium channel blocker, COPD chronic obstructive pulmonary disease
Outcome analysis
Survival was defined based on the difference between the date of hospitalization and the end date of National Health Insurance (NHI) coverage. Since the NHI premium is paid monthly, coverage can easily be canceled at the time of death. Measurement of mortality was valid via the record of the end date of NHI coverage, within a maximum error of 1 month [14, 15].
Statistical analysis
The SAS version 9.4 software (SAS Institute, Inc., Cary, NC) was used to analyze the data in this study. All variables were calculated using descriptive statistics. Categorical data were expressed as percentile values and continuous variables were expressed as a mean and standard deviation (SD). Paired t test for continuous variables and Chi squared test for categorical variables were applied to evaluate between-group differences. A P < 0.05 was considered statistically significant.
Cox proportional hazard regression analysis was used to calculate the hazard ratio (HR) and the associated 95% confidence intervals (95% CIs) for significant variables. Kaplan–Meier cumulative survival curves were used to compare survival between patients who received DPP-4i compared with those who did not receive DPP-4i in order to compare survival between the two groups as a whole, based on gender, and also based on age. Log-rank tests which used a P < 0.05 were considered statistically significant.
Results
The descriptive characteristics of the 2672 patients in the AMI patient group with diabetes who also received DPP-4i (the DPP-4i group) and the 2672 matched controls (the control group) are listed in Table 1. The two groups were comparable with regards to age, gender, comorbidities, and number and type of surgery. However, the patients in the DPP-4i group exhibited a higher use of anti-platelet drugs, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), β-blockers, heparin, low molecular weight heparin, spironolactone, nitrates, and nicorandil (Table 1). Furthermore, the patients in DPP-4i also received a greater proportion of the other classes of oral hypoglycemic agents compared with controls (Table 1).
Overall, the 3-year survival rate was significantly higher in the DPP-4i group when compared with the control group (log-rank P < 0.0001), regardless of gender (log-rank P = 0.0039 for males and log-rank P = 0.0002 for females) (Fig. 2).
Fig. 2.
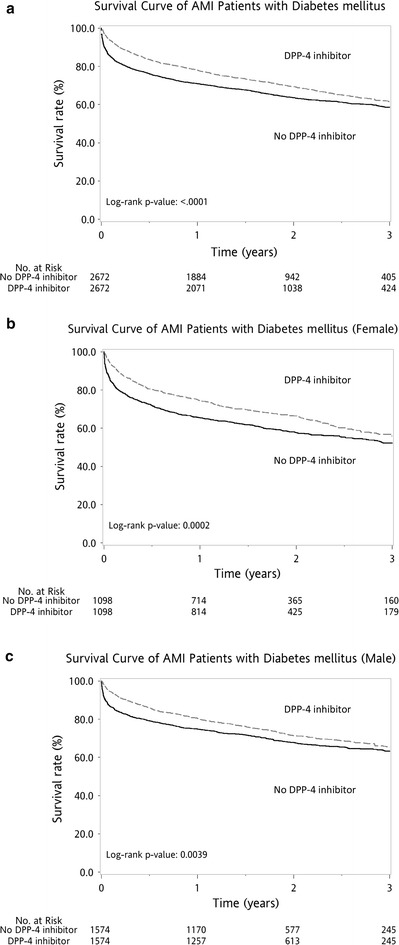
Kaplan–Meier survival curve after first acute myocardial infarction (AMI) for gender subgroup analysis. Overall, the 3-year survival rate was higher for the DPP-4i group than for the control group (log-rank P < 0.0001, a), regardless of gender [female (b) or male subgroup (c)]
The patients were divided into three subgroups based on age. The Kaplan–Meier cumulative survival curves revealed better survival in the DPP-4i group among all three age subgroups, i.e., age <65 years (log-rank P = 0.0322), 65 ≤ age < 75 years (log-rank P = 0.0069), and age ≥75 (log-rank P = 0.0002) (Fig. 3).
Fig. 3.
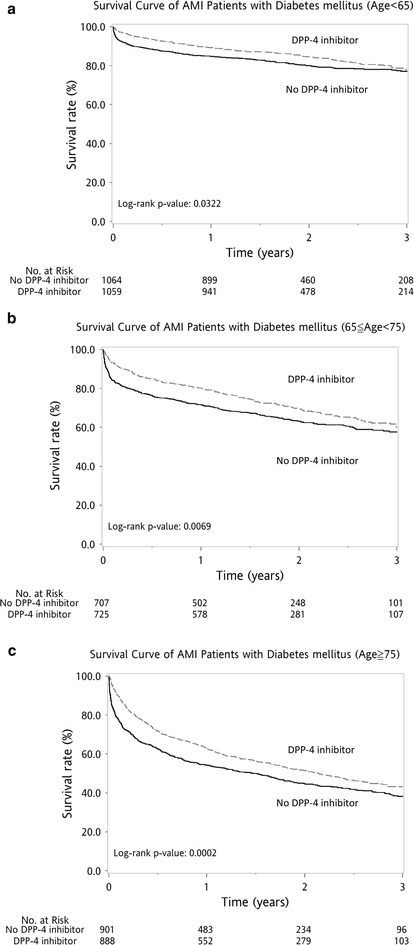
Kaplan–Meier survival curve after first acute myocardial infarction (AMI) for age subgroup analysis. Patients in the DPP-4i and control groups were subdivided into three subgroups by age. Kaplan–Meier cumulative survival curves revealed better survival in all three subgroups [age <65 years (log-rank P = 0.0322, a), 65 ≤ age < 75 years (log-rank P = 0.0069, b), and age ≥75 (log-rank P = 0.0002, c)]
Cox proportional hazard regression analysis was performed to further evaluate the impact of DPP-4i on the survival of DM patients after first AMI (Table 2). Overall, HRs for mortality were higher in patients who were relatively older, i.e., 65 ≤ age < 75 compared with age <65 (HR = 1.71; 95% CI 1.5–1.96) and age ≥75 compared with age <65 (HR = 2.51; 95% CI 2.2–2.85). In addition, mortality was higher in patients with hypertension (HR = 1.2; 95% CI 1.06–1.37), peripheral vascular disease (HR = 1.57; 95% CI 1.34–1.84), heart failure (HR = 1.35; 95% CI 1.23–1.49), ESRD (HR = 1.76; 95% CI 1.51–2.06), previous stroke (HR = 1.33; 95% CI 1.21–1.47), and COPD (HR = 1.33; 95% CI 1.16–1.52). PCI was shown to reduce the risk of mortality in DM patients after AMI (HR = 0.54; 95% CI 0.49–0.60).
Table 2.
Cox proportional hazard regression analysis on mortality of diabetic patients after first acute myocardial infarction
| Characteristics (all, N = 5344) | HR (95% CI) |
|---|---|
| Sex (male vs female) | 0.98 (0.89–1.07) |
| Age (65 ≤ age < 75 vs age <65) | 1.71 (1.5–1.96)*** |
| Age (age ≥75 vs age <65) | 2.51 (2.2–2.85)*** |
| Hypertension (yes vs no) | 1.2 (1.06–1.37)** |
| Peripheral vascular disease (yes vs no) | 1.57 (1.34–1.84)*** |
| Heart failure (yes vs no) | 1.35 (1.23–1.49)*** |
| End stage renal disease (yes vs no) | 1.76 (1.51–2.06)*** |
| Cerebrovascular disease (yes vs no) | 1.33 (1.21–1.47)*** |
| Chronic obstructive pulmonary disease (yes vs no) | 1.33 (1.16–1.52)*** |
| Percutaneous coronary intervention (yes vs no) | 0.54 (0.49–0.6)*** |
| Any antiplatelet (yes vs no) | 0.58 (0.49–0.7)*** |
| ACEI or ARB (yes vs no) | 0.72 (0.65–0.8)*** |
| β-Blocker (yes vs no) | 0.79 (0.71–0.87)*** |
| Heparin or low molecular weight heparin (yes vs no) | 1.02 (0.91–1.15) |
| α-Glucosidase (yes vs no) | 0.95 (0.83–1.08) |
| Glinides (yes vs no) | 1.05 (0.94–1.18) |
| Metformin (yes vs no) | 0.77 (0.68–0.86)*** |
| Sulfonylureas (yes vs no) | 0.91 (0.82–1.01) |
| Thiazolidinedione (yes vs no) | 0.79 (0.59–1.04) |
| DPP-4 inhibitor (yes vs no) | 0.86 (0.78–0.95)** |
** P < 0.01, *** P < 0.001
Regarding medications, DPP-4i therapy improved overall survival (HR = 0.86; 95% CI 0.78–0.95), and metformin also made contributions to overall survival (HR = 0.77; 95% CI 0.68–0.86). Other medications improved survival, including β-blockers (HR = 0.79; 95% CI 0.71–0.87), anti-platelet drugs (HR = 0.58; 95% CI 0.49–0.70), and ACEIs or ARBs (HR = 0.72; 95% CI 0.65–0.80) (Table 2).
DPP-4 inhibitors therapy is beneficial in both male and female patients (Fig. 4). In addition, a Forest plot of HRs for various patient characteristics with or without DPP-4i revealed that DPP-4i had better outcomes in patients with hypertension (HR = 0.87; 95% CI 0.78–0.97; P = 0.0103) and cerebrovascular disease (HR = 0.83; 95% CI 0.72–0.97; P = 0.018), but without dyslipidemia(HR = 0.78; 95% CI 0.67–0.92; P = 0.0029), without peripheral vascular disease (HR = 0.86; 95% CI 0.78–0.96; P = 0.0047), without heart failure (HR = 0.84; 95% CI 0.73–0.96; P = 0.0106), without end stage renal disease (HR = 0.86; 95% CI 0.77–0.95; P = 0.0035), and without chronic obstructive pulmonary disease (HR = 0.87; 95% CI 0.78–0.97; P = 0.0096) (Fig. 4).
Fig. 4.
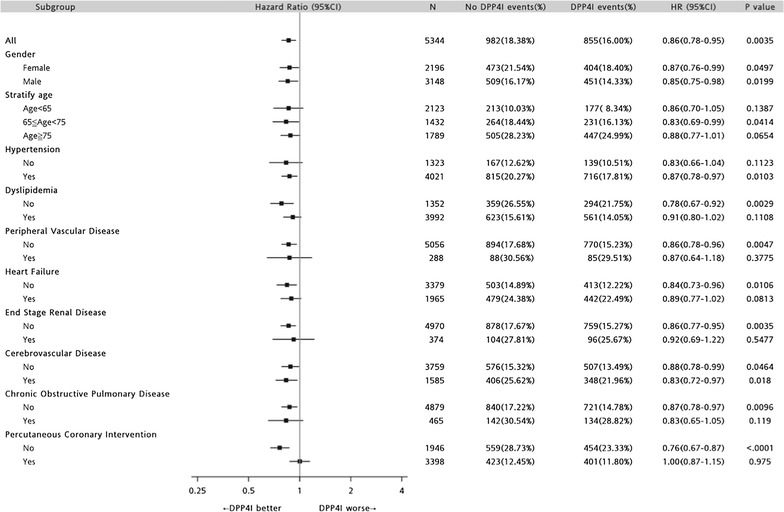
Forest plot of hazard ratios for various patient characteristics with or without DPP-4 inhibitor (DPP-4i). DPP-4 inhibitors therapy is beneficial in both male and female patients. DPP-4i therapy is beneficial in patients who are 65 ≤ age < 75 (HR = 0.83; 95% CI 0.69–0.99; P = 0.0414). In addition, a positive effect of DPP-4i can be seen in patients without dyslipidemia (HR = 0.78; 95% CI 0.67–0.92; P = 0.0029), without peripheral vascular disease (PVD) (HR = 0.86; 95% CI 0.78–0.96; P = 0.0047), without heart failure (HF) (HR = 0.84; 95% CI 0.73–0.96; P = 0.0106), without end stage renal disease (ESRD) (HR = 0.86; 95% CI 0.77–0.95; P = 0.0035), and those without chronic obstructive pulmonary disease (COPD) (HR = 0.87; 95% CI 0.78–0.97; P = 0.0096). DPP-4i are also beneficial in patients with hypertension (HR = 0.87; 95% CI 0.78–0.97; P = 0.0103) and cerebrovascular disease (HR = 0.83; 95% CI 0.72–0.97; P = 0.018)
Discussion
This study demonstrated that use of DPP-4i in AMI patients with diabetes resulted in improved 3-year survival rates. DPP-4i therapy was especially beneficial in hypertension and cerebrovascular disease, regardless of gender, and in patients without peripheral vascular disease, end stage renal disease and chronic obstructive pulmonary disease.
The impact of DPP-4 inhibitors on cardiovascular disease
Most of the prior literature has focused on the relationship between DPP-4i and cardiovascular safety. Several recent studies (including pre-clinical data, small mechanistic studies, and post hoc analyses of randomized clinical trials) support the benefit of DPP-4i in patients with cardiovascular disease [16–18]. In a nationwide longitudinal study, DPP4i-treated T2DM patients were shown to have a lower risk for cardiovascular disease as compared with non-DPP4i users [19]. Furthermore, in DM patients with pre-existing heart failure, the use of DPP-4i has resulted in a lower risk of mortality in patients with the combination of myocardial infarction and ischemic stroke [16]. Some studies have discussed the effect of DPP-4i on cardiovascular outcomes, but were limited regarding their ability to evaluate the impact of DPP-4i on the long-term outcomes after first AMI.
The impact of DPP-4 inhibitors on DM patients after AMI
In a murine experimental diabetes model, DPP-4 inhibition was shown to attenuate cardiac dysfunction and adverse remodeling in the post-MI setting [20]. Furthermore, chronic treatment with DPP-4i in an animal model reduced infarct size and improved LV function, via the GLP-1 receptor-protein kinase A pathway, in a glucose-dependent manner [21, 22]. Peak plasma troponin I did not differ between patients with myocardial infarction on DPP4i and those who did not receive such therapy [23]. Rather than a neutral or negative effect, our study provides evidence that DPP-4i therapy can increase the survival of patients following a first AMI regardless of gender. In this study, DPP-4i therapy after first AMI improved long-term survival rather than resulting in just a “do no harm” effect. Major prospective clinical trials are investigating the various uses and cardiovascular outcomes of DPP-4i in diabetic patients. These trials include the Sitagliptin Cardiovascular Outcome Study (TECOS), the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI), the cardiovascular outcomes study of alogliptin in subjects with type 2 diabetes and acute coronary syndrome (EXAMINE), and the cardiovascular outcome study of linagliptin versus glimepiride in patients with type 2 diabetes (CAROLINA) trial [24–26]. It would be an important milestone and significant influence on the management of diabetes if these trials confirm that DPP-4i can contribute to a reduction in the cardiovascular complications of DM.
The mechanisms underlying the DPP-4 inhibitor benefit
There are several possible explanations for the benefit of DPP-4i in patients with AMI. First, DPP4i may reduce reperfusion injury via protection of mitochondrial function [27]. DPP-4i has been shown to elevate the activity of reperfusion injury salvage kinase and reduce reperfusion injury caused by reperfusion-related cardiac tissue damage and instigated arrhythmias [2, 22, 27, 28]. Furthermore, mitochondria are both a major energy and oxidative stress production site. DPP-4i can rescue cardiac mitochondrial dysfunction and decrease reactive oxygen species production in those patients with obesity-related insulin resistance and DM with ischemia/reperfusion injury. In addition, DPP4i can reduce oxidative stress [27, 29]. Diabetes is one of the comorbidities that can induce a systemic inflammatory state, while DPP-4i can reduce the systemic proinflammatory state and decrease oxidative stress, which may explain why DPP-4i decrease coronary microvascular endothelial inflammation and make a contribution to post-AMI remodeling during advanced heart failure [20]. Improvements in survival after AMI in T2 DM might also arise from modification of autophagy in the non-infarcted region of the heart [30]. Diabetes carries a two-fold increased risk of heart failure following myocardial infarction, due to an excessive loss of cardiac microvasculature. Stromal cell-derived factor-1alpha (SDF-1alpha) is a chemokine that is elaborated by ischemic tissue but is rapidly degraded by DPP-4i, thus, DPP-4i attenuates cardiac dysfunction and adverse remodeling in the post-MI setting [20].
Finally, DPP4i might inhibit atherosclerosis and proliferation of vascular smooth muscle cell. The possible impact of DPP-4i on post-AMI survival may derive from their cardioprotective effects and their beneficial effect on cardiovascular risk factors such as atherosclerosis and hypertension [4–8, 31–36]. In Japanese patients with diabetes and multiple CV risk factors, DPP4i was shown to decrease blood pressure associated with an improvement in albuminuria in addition to glycemic control [17]. In a sub-analysis of the PROLOGUE study, DPP-4i was shown not to alter endothelial function at the 2-year follow-up [37], a result which was further confirmed by another study [38].
Benefit of DPP-4i-based combination therapy in AMI
Both DPP-4i and metformin have been shown to improve insulin resistance and attenuate myocardial injury caused by ischemia/reperfusion injury, and several studies have indicated that the combined therapy provided better outcomes than monotherapy with a reduction in arrhythmia scores and reduced all-cause mortality rates [39–43]. Previous studies revealed that both DPP-4i and metformin users had a significantly lower risk for composite cardiovascular diseases [18]. The combination therapy of DPP4i with conventional OHA led to an improvement in passive left ventricular compliance [20]. Interestingly, there were pleiotropic effects on cardiovascular protection using several OHAs, and their use was also associated with a lower risk of aortic aneurysm growth in Metformin-, sulfonylurea-, and TZD-treated patients but not in patients treated with DPP-4i or alpha-glucosidase inhibitors [19]. In our study, 1168 (43.71%) patients received both metformin and DPP-4i therapy, and the overall survival rates were better in those patients receiving DPP-4i. This benefit may be attributed to metformin (HR = 0.77; 95% CI 0.68–0.86) or DPP-4i (HR = 0.86; 95% CI 0.78–0.95). Furthermore, combined therapy with DPP-4i contributed to the improvement in survival rates independent of gender.
Study limitations
Our study had several limitations. We did not use an objective indicator, such as glycated hemoglobin (HbA1c), as a standard for assessing diabetic compensation and treatment, due to bias in the frequency and interval of HbA1c check-ups. In addition, the DPP-4i group had a higher proportion of patients prescribed β-blockers, ARBs, spironolactone, and OHAs, which implied intractable hypertension, poorly controlled DM, or an unstable status. Regardless of the severity of diabetes or underlying conditions in the DPP-4i group, DPP-4i still made an independent contribution to the long-term survival of patients, which was also confirmed by Cox proportional hazard regression analysis.
Strengths of this study
Previous studies analyzed relatively small samples, while our study enrolled 186,326 patients with first AMI, based on an entire population comprising 23,000,000 patients. Our large sample size reduced the variability in sampling statistics and also utilized a propensity score, one-to-one matching technique to minimize confounding factors between the DPP-4i and control groups. However, future prospective randomized studies are required to confirm our findings.
Conclusions
This nationwide study showed that DPP-4i therapy improved the long-term survival of diabetic patients after first AMI, regardless of gender. Furthermore, DPP-4i therapy was shown to be especially beneficial in patients without peripheral vascular disease, ESRD, or COPD.
Authors’ contributions
W-CH, C-PL, H-TC and G-YM conceived and designed the study. W-TH, C-CC, and J-SY conducted the research. P-LT analyzed the data. M-TW and S-CL wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Miss Hsiao-Chin Lin, Miss Tzu-Jung Chuang, Miss Hsiao Ching Kuo, and Chia-Jung Chin for their expert statistical assistance.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Bureau. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw).
Consent for publication
All authors give their consent for publication.
Ethics approval and consent to participate
The Institutional Review Board (IRB) of the Kaohsiung Veterans General Hospital approved this study (No. VGHKS14-CT7-07). We do not need to obtain written informed consent from the study patients because the NHI data set consists of de-identified secondary data for research purposes, and the IRB of Kaohsiung Veterans General Hospital issued a formal written waiver of the requirement for informed consent.
Funding
This study was supported by grants from the Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, i.e., Grant Nos. VGHKS 212347-23, 106-084, 106-142, 106-D01-3, 106-160, 106-156, 106-062, 105-139, 106-159 and the Ministry of Science and Technology, i.e., Grants Most 105-2314-B-075B-006 and Most 105-2314-B-075B-007.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ACS
acute coronary syndrome
- AMI
acute myocardial infarction
- ARB
angiotensin receptor blocker
- CAROLINA
cardiovascular outcome study of linagliptin versus glimepiride in patients with type 2 diabetes
- CCB
calcium channel blocker
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CVA
cerebrovascular accident
- DM
diabetes mellitus
- DPP-4
dipeptidyl peptidase 4
- DPP-4i
dipeptidyl peptidase 4 inhibitors
- ESRD
end-stage renal disease
- EXAMINE
cardiovascular outcomes study of alogliptin in subjects with type 2 diabetes and acute coronary syndrome
- GLP-1
glucagon-like peptide 1
- HbA1c
glycated hemoglobin A1c
- HF
heart failure
- HR
hazard ratio
- IRB
Institutional Review Board
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- OHA
oral hypoglycemic agent
- PCI
percutaneous coronary intervention
- PKA
protein kinase A
- PVD
peripheral vascular disease
- SAVOR-TIMI
saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction
- SD
standard deviation
- SDF-1alpha
stromal cell-derived factor-1alpha
- T2DM
type 2 DM
- TECOS
Sitagliptin Cardiovascular Outcome Study
Footnotes
Mei-Tzu Wang and Sheng-Che Lin contributed equally to this work
Contributor Information
Mei-Tzu Wang, Email: meitzuwang@gmail.com.
Sheng-Che Lin, Email: schelin@vghks.gov.tw.
Pei-Ling Tang, Email: pltang728@gmail.com.
Wang-Ting Hung, Email: wonderingines@gmail.com.
Chin-Chang Cheng, Email: chinchangcheng@gmail.com.
Jin-Shiou Yang, Email: PT136@fy.edu.tw.
Hong-Tai Chang, Email: htchang@vghks.gov.tw.
Chun-Peng Liu, Email: cpliu@vghks.gov.tw.
Guang-Yuan Mar, Email: gymar@vghks.gov.tw.
Wei-Chun Huang, Phone: 886-7-3468278, Email: wchuanglulu@gmail.com.
References
- 1.Fadini GP, Albiero M, Avogaro A. Direct effects of DPP-4 inhibition on the vasculature. Reconciling basic evidence with lack of clinical evidence. Vasc Pharmacol. 2015;73:1–3. doi: 10.1016/j.vph.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Chinda K, Palee S, Surinkaew S, Phornphutkul M, Chattipakorn S, Chattipakorn N. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia–reperfusion injury. Int J Cardiol. 2013;167(2):451–457. doi: 10.1016/j.ijcard.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Kubota A, Takano H, Wang H, Hasegawa H, Tadokoro H, Hirose M, Kobara Y, Yamada-Inagawa T, Komuro I, Kobayashi Y. DPP-4 inhibition has beneficial effects on the heart after myocardial infarction. J Mol Cell Cardiol. 2016;91:72–80. doi: 10.1016/j.yjmcc.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori R, et al. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology. 2013;154(3):1260–1270. doi: 10.1210/en.2012-1855. [DOI] [PubMed] [Google Scholar]
- 5.Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;58(2):157–166. doi: 10.1097/FJC.0b013e31821e5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, Wang L, He F, Xue Y. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol. 2014;13:32. doi: 10.1186/1475-2840-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirano T, Mori Y. Anti-atherogenic and anti-inflammatory properties of glucagon-like peptide-1, glucose-dependent insulinotropic polypepide, and dipeptidyl peptidase-4 inhibitors in experimental animals. J Diabetes Investig. 2016;7(Suppl 1):80–86. doi: 10.1111/jdi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 9.Wang SH, Chen DY, Lin YS, Mao CT, Tsai ML, Hsieh MJ, Chou CC, Wen MS, Wang CC, Hsieh IC, et al. Cardiovascular outcomes of sitagliptin in type 2 diabetic patients with acute myocardial infarction, a population-based cohort study in Taiwan. PLoS ONE. 2015;10(6):e0131122. doi: 10.1371/journal.pone.0131122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 11.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Fukui K, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Iwasawa T, Kimura K. Inhibition of DPP-4 by alogliptin improves coronary flow reserve and left ventricular systolic function evaluated by phase contrast cine magnetic resonance imaging in patients with type 2 diabetes and coronary artery disease. Int J Cardiol. 2016;223:770–775. doi: 10.1016/j.ijcard.2016.08.306. [DOI] [PubMed] [Google Scholar]
- 13.Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27(Suppl 3):57–64. doi: 10.1185/03007995.2011.602964. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. 2014;24(6):500–507. doi: 10.2188/jea.JE20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. 2015;201:96–101. doi: 10.1016/j.ijcard.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 16.Ou SM, Chen HT, Kuo SC, Chen TJ, Shih CJ, Chen YT. Dipeptidyl peptidase-4 inhibitors and cardiovascular risks in patients with pre-existing heart failure. Heart (British Cardiac Society) 2017;103(6):414–420. doi: 10.1136/heartjnl-2016-309687. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Iwanaga Y, Miyaji Y, Nohara R, Ishimura T, Miyazaki S. Cardiovascular efficacy of sitagliptin in patients with diabetes at high risk of cardiovascular disease: a 12-month follow-up. Cardiovasc Diabetol. 2016;15:54. doi: 10.1186/s12933-016-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou HT, Chang KC, Li CY, Wu JS. Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: a nation-wide longitudinal study. Cardiovasc Diabetol. 2016;15:41. doi: 10.1186/s12933-016-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu CY, Su YW, Chen YT, Tsai SH, Chang CC, Li SY, Huang PH, Chen JW, Lin SJ. Association between use of oral-antidiabetic drugs and the risk of aortic aneurysm: a nested case-control analysis. Cardiovasc Diabetol. 2016;15(1):125. doi: 10.1186/s12933-016-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connelly KA, Zhang Y, Advani A, Advani SL, Thai K, Yuen DA, Gilbert RE. DPP-4 inhibition attenuates cardiac dysfunction and adverse remodeling following myocardial infarction in rats with experimental diabetes. Cardiovasc Thera. 2013;31(5):259–267. doi: 10.1111/1755-5922.12005. [DOI] [PubMed] [Google Scholar]
- 21.Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, Mocanu MM, Yellon DM. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013;12:154. doi: 10.1186/1475-2840-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hocher B, Sharkovska Y, Mark M, Klein T, Pfab T. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol. 2013;167(1):87–93. doi: 10.1016/j.ijcard.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Nunes JP, Rodrigues JD, Melao F. Acute myocardial infarction associated to DPP-4 inhibitors. Heart Lung Vessels. 2014;6(3):180–186. [PMC free article] [PubMed] [Google Scholar]
- 24.Bethel MA, Engel SS, Green JB, Huang Z, Josse RG, Kaufman KD, Standl E, Suryawanshi S, Van de Werf F, McGuire DK, et al. Assessing the safety of sitagliptin in older participants in the trial evaluating cardiovascular outcomes with sitagliptin (TECOS) Diabetes Care. 2017;40(4):494–501. doi: 10.2337/dc16-1135. [DOI] [PubMed] [Google Scholar]
- 25.Scheen AJ. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors: from risk factors to clinical outcomes. Postgrad Med. 2013;125(3):7–20. doi: 10.3810/pgm.2013.05.2659. [DOI] [PubMed] [Google Scholar]
- 26.Paneni F. DPP-4 inhibitors, heart failure and type 2 diabetes: all eyes on safety. Cardiovasc Diagn Ther. 2015;5(6):471–478. doi: 10.3978/j.issn.2223-3652.2015.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang CJ, Yang J, Yang J, Fan ZX. DPP-4 inhibitors: a promising feasible therapeutic approach for myocardial ischemia–reperfusion injury. Int J Cardiol. 2015;201:253–254. doi: 10.1016/j.ijcard.2015.08.112. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, Xu G, Ng CF, Yao X, Gao Y, et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 2014;21(11):1571–1581. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murase H, Kuno A, Miki T, Tanno M, Yano T, Kouzu H, Ishikawa S, Tobisawa T, Ogasawara M, Nishizawa K, et al. Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc Diabetol. 2015;14:103. doi: 10.1186/s12933-015-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh TP, Vangaveti VN, Malabu UH. Dipeptidyl peptidase-4 inhibitors and their potential role in the management of atherosclerosis—a review. Diabetes Metab Syndr. 2015;9(4):223–229. doi: 10.1016/j.dsx.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Jialal I, Bajaj M. DPP-4 inhibitors and atherosclerosis: the promise. Atherosclerosis. 2013;227(2):224–225. doi: 10.1016/j.atherosclerosis.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Dai Y, Wang X, Ding Z, Dai D, Mehta JL. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol. 2014;51(3):471–478. doi: 10.1007/s00592-013-0541-3. [DOI] [PubMed] [Google Scholar]
- 34.Yang CJ, Fan ZX, Yang J, Yang J. DPP-4 inhibitors: a potential promising therapeutic target in prevention of atherosclerosis. Int J Cardiol. 2016;202:797–798. doi: 10.1016/j.ijcard.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 35.Dhindsa S, Jialal I. Potential anti-atherosclerotic effects of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus. Curr Diab Rep. 2014;14(2):463. doi: 10.1007/s11892-013-0463-z. [DOI] [PubMed] [Google Scholar]
- 36.Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, Osonoi T, Kaneto H, Kosugi K, Umayahara Y, et al. Rationale, design, and baseline characteristics of a clinical trial for prevention of atherosclerosis in patients with insulin-treated type 2 diabetes mellitus using DPP-4 inhibitor: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE) Diabetol Metab Syndr. 2014;6(1):35. doi: 10.1186/1758-5996-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruhashi T, Higashi Y, Kihara Y, Yamada H, Sata M, Ueda S, Odawara M, Terauchi Y, Dai K, Ohno J, et al. Long-term effect of sitagliptin on endothelial function in type 2 diabetes: a sub-analysis of the PROLOGUE study. Cardiovasc Diabetol. 2016;15(1):134. doi: 10.1186/s12933-016-0438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ida S, Murata K, Betou K, Kobayashi C, Ishihara Y, Imataka K, Uchida A, Monguchi K, Kaneko R, Fujiwara R, et al. Effect of trelagliptin on vascular endothelial functions and serum adiponectin level in patients with type 2 diabetes: a preliminary single-arm prospective pilot study. Cardiovasc Diabetol. 2016;15(1):153. doi: 10.1186/s12933-016-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheller NM, Mogensen UM, Andersson C, Vaag A, Torp-Pedersen C. All-cause mortality and cardiovascular effects associated with the DPP-IV inhibitor sitagliptin compared with metformin, a retrospective cohort study on the Danish population. Diabetes Obes Metab. 2014;16(3):231–236. doi: 10.1111/dom.12197. [DOI] [PubMed] [Google Scholar]
- 40.Apaijai N, Chinda K, Palee S, Chattipakorn S, Chattipakorn N. Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia–reperfusion injury in obese-insulin resistant rats. PLoS ONE. 2014;9(7):e102374. doi: 10.1371/journal.pone.0102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eriksson JW, Bodegard J, Nathanson D, Thuresson M, Nystrom T, Norhammar A. Sulphonylurea compared to DPP-4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all-cause mortality. Diabetes Res Clin Pract. 2016;117:39–47. doi: 10.1016/j.diabres.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Morgan CL, Mukherjee J, Jenkins-Jones S, Holden SE, Currie CJ. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality. Diabetes Obes Metab. 2014;16(10):977–983. doi: 10.1111/dom.12306. [DOI] [PubMed] [Google Scholar]
- 43.Yu OH, Yin H, Azoulay L. The combination of DPP-4 inhibitors versus sulfonylureas with metformin after failure of first-line treatment in the risk for major cardiovascular events and death. Can J Diabetes. 2015;39(5):383–389. doi: 10.1016/j.jcjd.2015.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Bureau. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw).


