Abstract
Background
Exposures to melanopsin-stimulating (melanopic) component-rich blue light enhance arousal level. We examined their effects in office workers.
Main body of abstract
Eight healthy university office workers were exposed to blue and orange lights for 30 min during lunch break on different days. We compared the effects of light color on autonomic arousal level assessed by heart rate variability (HRV) and behavioral alertness by psychomotor vigilance tests (PVT). Heart rate was higher and high-frequency (HF, 0.150.45 Hz) power of HRV was lower during exposure to the blue light than to orange light. No significant difference with light color was observed, however, in any HRV indices during PVT or in PVT performance after light exposure.
Short conclusion
Exposure to blue light during lunch break, compared with that to orange light, enhances autonomic arousal during exposure, but has no sustained effect on autonomic arousal or behavioral alertness after exposure.
Keywords: Alertness, Arousal, Blue light, Heart rate variability, Melanopsin, Non-image forming vision, Orange light, Organic light-emitting diode, Psychomotor vigilance
Background
Along with the development of lighting elements capable of various color-rendering, the introduction of color lighting in life environment is taking on reality. Such lightings may be used for adjusting our mind and physical states to suit our desired activities, such as work, study, rest, and sleep [1–4]. In previous studies [5, 6], we have demonstrated that exposure to melanopsin-stimulating (melanopic) component-rich blue light enhances autonomic arousal and behavioral alertness. These observations, however, were obtained in laboratory settings. In this study, we examined the effects of exposure to blue light or orange lights during the lunch break on autonomic arousal and behavioral alertness in office workers.
Main text
Subjects and protocols
We studied eight healthy university office workers (mean age ± SD, 27 ± 7 years, range 21–39 years, 2 females) with normal color vision who were not taking any medications for >2 weeks and displayed a normal sinus rhythm on electrocardiogram (ECG).
We compared the effects on heart rate variability (HRV) and psychomotor vigilance test (PVT) caused by exposures to blue and orange lights during the lunch break in workplace. We prepared a light chamber in the workplace with an organic light-emitting diode (OLED) ceiling light system with adjustable color and brightness (Fig. 1), whose details have been reported elsewhere [6]. Table 1 shows the optical characteristics of blue and orange lights.
Fig. 1.
Ceiling light chamber with organic light-emitting diode (OLED) when the lighting is a blue and b orange
Table 1.
Characteristics of organic light-emitting diode (OLED) lights used in this study
| Blue | Orange | |
|---|---|---|
| Illuminance, lx | 12.9 | 17.7 |
| Irradiance, μW/cm2 | 8.02 | 6.54 |
| Chromaticity (x, y) | 0.14, 0.16 | 0.56, 0.42 |
| Weighted mean wavelength, nm | 485 | 622 |
| PFD, μmol/m2/s | 0.323 | 0.333 |
| Melanopic PFD, μmol/m2/sa | 0.225 | 0.026 |
| Relative melanopic content, % | 69.6 | 7.9 |
Experiments with the two light colors were performed on separated days with an interval of 5–7 days for washout. The color orders were counterbalanced across subjects. On each experimental day, Holter ECG was recorded continuously during experiment. Subjects took a light lunch (the same menu on two experimental days) with a drink not containing caffeine or alcohol at the university cafeteria. During the last 30 min of lunch break, they were exposed to blue or orange light in the light chamber (air-conditioned at 24 ± 2 °C), sitting in a low chair with 60° backrest just below the ceiling light.
After the lunch break, a 5-min PVT was performed in their work office which was illuminated by fluorescent lamps with a correlated color temperature of 4010 K, an illuminance of 450 lx, a photon flux density of 6.7 μmol/m2/s, and a relative melanopic component of 20.4%. PVT was performed with validated software (PC-PVT) [7]. The detailed method for PVT has been reported elsewhere [6]. The anticipation was set at 100 ms, deadline at 65,000 ms, minor lapse at 500 ms, and total trial time at 300 s.
Data analysis
R-R interval time series obtained from Holter ECG were extracted for the durations of OLED light exposure (30 min) and PVT (5 min) for the analysis of HRV. HRV was analyzed by the methods reported elsewhere [8, 9]. The power of the low-frequency (LF, 0.04–0.15 Hz) and the high-frequency (HF, 0.15–0.40 Hz) components were computed by fast Fourier transformation. We used heart rate as an index of sympatho-vagal balance, HF power as cardiac vagal function, and LF-to-HF power ratio (LF/HF) as sympathetic predominance in cardiac autonomic functions. The power of LF and HF components was transformed into natural logarithmic value.
PVT performance was analyzed according to the earlier studies [10]. We measured the number of minor lapses (reaction time (RT) ≥500 ms) with transformation (), fastest and slowest 10th percentile RTs, and difference between fastest and slowest RTs.
We used the Statistical Analyses System version 9.4 (SAS institute Inc., Cary, NC, USA) for statistical analysis. To examine the effects of light color on HRV indices and PVT performance, we compared the values between blue and orange lights with paired t test. P < 0.05 was considered to be statistically significant.
Results
The HRV indices during exposures to blue and orange lights and during PVT after exposure in individual subjects are shown in Fig. 2. Heart rate was higher (P = 0.001) and HF power was lower (P = 0.03) during exposure to blue light than to orange light, whereas there was no significant difference with light color in any HRV indices during PVT after exposure. As shown in Fig. 3, no significant difference with light color was observed in any indices of PVT performance after exposure.
Fig. 2.
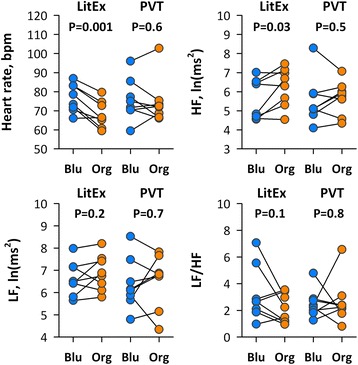
Differences between blue and orange lights in heart rate and heart rate variability (HRV) indices during light exposure (LitEx) and psychomotor vigilance test (PVT) after exposure in individual subjects (n = 8). Blu blue light, HF high-frequency component, LF low-frequency component, LF/HF LF-to-HF ratio in power, Org orange light
Fig. 3.
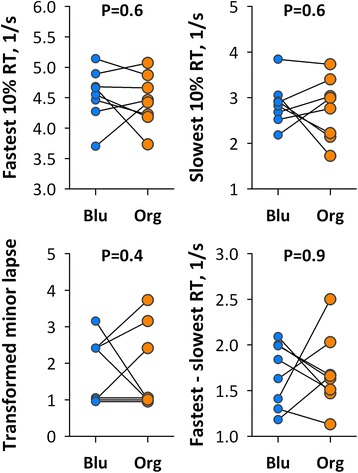
Differences between blue and orange lights in the performance of PVT after light exposure in individual subjects (n = 8). Blu blue light, Org orange light, RT reaction time
Conclusions
From these findings, we conclude that compared with orange light, blue light during lunch break enhances autonomic arousal during exposure, but causes no sustained differences in autonomic arousal or behavioral alertness after exposure.
In this study, we observed higher heart rate and lower HF power during exposure to blue light than orange light. These observations seem consistent with earlier studies suggesting autonomic arousal effects of melanopic component-rich blue light [1–6]. In contrast, we failed to detect significant difference with light color in autonomic indices during PVT or PVT performed after exposure. This seems inconsistent with those of an earlier study of Chellappa et al. [2] and of our previous study [6]. We performed PVT 30 min after light exposure in this study, while it was performed during light exposure in Chellappa’s study [2] and <5 min after exposure in our previous study [6]. The difference may be attributable to the duration of effects. In any case, however, exposure to blue light during lunch break may not cause substantial difference from orange light in behavioral alertness during afternoon’s work.
This study has several limitations. First, this study was performed in university office workers. Our observations may not be generalized for other types of workers. Second, the intensity of blue light we used was low (PFD, 0.323 μmol/m2/s), but the effects may differ depending on light intensity [11, 12]. Third, because we did not measure melatonin secretion, we were unable to determine whether the light exposures affect the entrainment to environmental light–dark cycles or not.
Acknowledgements
This study was performed as a part of the collaborative studies with Chemical materials Evaluation and Research Base (CEREBA), Japan.
Funding
This study was supported by the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Availability of data and materials
All data described in this study are freely available to any scientist wishing to use them in a way that ensures the participant’s confidentiality. The software described in this study is also available to any scientist wishing to use them. In any case, the data and software described in the manuscript are available for testing by reviewers in a way that preserves the reviewers’ anonymity.
Abbreviations
- ECG
Electrocardiogram
- HF
High frequency
- HRV
Heart rate variability
- LF
Low frequency
- LF/HF
LF-to-HF ratio
- OLED
Organic light-emitting diode
- PFD
Photon flux density
- PVT
Psychomotor vigilance test
- RT
Reaction time
Authors’ contributions
EY participated in the design and coordination of the study and drafted the manuscript. HO developed the software for the study and participated in the coordination of the study. YY participated in the data analysis and performed the statistical analysis. JH conceived the study, interpreted the results, and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed according to the protocol that was approved by the Institutional Review Board of Nagoya City University Graduate School of Medical Sciences and Nagoya City University Hospital (No. 60160164). All the subjects of this study gave their written informed consent to participate in this study.
Consent for publication
I assure you that all the authors have read the manuscript and approved its submission to the Journal of Physiological Anthropology.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emi Yuda, Phone: +81-52-853-8501, Email: emi21@med.nagoya-cu.ac.jp.
Hiroki Ogasawara, Phone: +81-52-853-8501, Email: ogas@med.nagoya-cu.ac.jp.
Yutaka Yoshida, Phone: +81-52-853-8501, Email: yyoshida@med.nagoya-cu.ac.jp.
Junichiro Hayano, Phone: +81-52-853-8501, Email: hayano@med.nagoya-cu.ac.jp.
References
- 1.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90(3):1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 2.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One. 2011;6(1):e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi CJ, Kim KS, Kim CM, Kim SH, Choi WS. Reactivity of heart rate variability after exposure to colored lights in healthy adults with symptoms of anxiety and depression. Int J Psychophysiol. 2011;79(2):83–8. doi: 10.1016/j.ijpsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Daneault V, Hebert M, Albouy G, Doyon J, Dumont M, Carrier J, Vandewalle G. Aging reduces the stimulating effect of blue light on cognitive brain functions. Sleep. 2014;37(1):85–96. doi: 10.5665/sleep.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuda E, Ogasawara H, Yoshida Y, Hayano J. Suppression of vagal cardiac modulation by blue light in healthy subjects. J Physiol Anthropol. 2016;35:24. doi: 10.1186/s40101-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuda E, Ogasawara H, Yoshida Y, Hayano J. Enhancement of autonomic and psychomotor arousal by exposures to blue wavelength light: importance of both absolute and relative contents of melanopic component. J Physiol Anthropol. 2017;36(1):13. doi: 10.1186/s40101-017-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khitrov MY, Laxminarayan S, Thorsley D, Ramakrishnan S, Rajaraman S, Wesensten NJ, Reifman J. PC-PVT: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46(1):140–7. doi: 10.3758/s13428-013-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camm AJ, Malik M, Bigger JT, Jr, Breithardt G, Cerutti S, Cohen RJ, Coumel P, Fallen EL, Kleiger RE, Lombardi F, Malliani A, Moss AJ, Rottman JN, Schmidt G, Schwartz PJ, Singer DH. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–65. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 9.Hayano J. Introduction to heart rate variability. In: Iwase S, Hayano J, Orimo S, editors. Clinical assessment of the autonomic nervous system. Japan: Springer; 2016. pp. 109–27. [Google Scholar]
- 10.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80(5):695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Kozaki T, Koga S, Toda N, Noguchi H, Yasukouchi A. Effects of short wavelength control in polychromatic light sources on nocturnal melatonin secretion. Neurosci Lett. 2008;439(3):256–9. doi: 10.1016/j.neulet.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Brainard GC, Hanifin JP, Warfield B, Stone MK, James ME, Ayers M, Kubey A, Byrne B, Rollag M. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res. 2015;58(3):352–61. doi: 10.1111/jpi.12221. [DOI] [PubMed] [Google Scholar]
- 13.Enezi J, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26(4):314–23. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- 14.Price LLA . Report on the First International Workshop on Circadian and Neurophysiological Photometry, 2013. International Commission on Illumination, 2015 CIE TN 003. 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data described in this study are freely available to any scientist wishing to use them in a way that ensures the participant’s confidentiality. The software described in this study is also available to any scientist wishing to use them. In any case, the data and software described in the manuscript are available for testing by reviewers in a way that preserves the reviewers’ anonymity.


