Abstract
The weakened tumour colonization of attenuated Salmonella has severely hampered its clinical development. In this study, we investigated whether an anti-inflammation and antiangiogenesis compound triptolide could improve the efficacy of VNP20009, a highly attenuated Salmonella strain, against mice melanoma. By comparing the effects of conventional VNP20009 monotherapy and a combination therapy that uses both triptolide and VNP20009, we found that triptolide significantly improved the tumour colonization of VNP20009 by reducing the number of infiltrated neutrophils in the melanoma, which led to a larger necrotic area in the melanoma. Moreover, the combination therapy suppressed tumour angiogenesis by reducing the expression of VEGF in a synergistic manner, retarding the growth of the melanoma. Our study revealed that triptolide could significantly enhance the antitumour effect of VNP20009 by modulating tumour angiogenesis and the host immune response, providing a new understanding of the strategy to improve Salmonella-mediated tumour therapy.
Keywords: Salmonella-mediated tumor therapy, melanoma, neutrophils, angiogenesis.
Introduction
Salmonella enterica serovar Typhimurium (S. Typhimurium), an oncolytic bacterium and tumour-specific vehicle for therapeutic genes 1, 2, has been genetically attenuated to obtain a safer antitumour effect with a higher specificity and a lower systematic toxicity by deleting genes involved in the synthesis of some key factors, such as adenine and lipopolysaccharide 3. Attenuated S. Typhimurium specifically colonizes solid tumours, which are more hypoxic than healthy tissue, inducing the necrosis and apoptosis of cancer cells through invasive bacterial infection or by expressing cytotoxic genes 3, 4. Even though attenuated S. Typhimurium achieved remarkable antitumour effects in animal models, little clinical success has been obtained in human cancer patients 5, 6, which is a result that is probably related to the high complexity of the human immune system and the overattenuation of S. Typhimurium, both of which have led to poor tumour colonization and weak biological effects.
The host immune response is one of the key factors that modulate Salmonella's antitumour effects 7-9. Salmonella triggers a cascade of cellular signalling events, including NFκB signalling, immediately upon its entry into the body, leading to the release of cytokines and chemokines, which recruit polymorphonuclear cells 10. These inflammatory cells play different roles in Salmonella-colonized tumours 8, 9, 11. Neutrophils directly cause the elimination of bacteria from the body 8, 11, while CD8+ T cells contribute to the tumour growth suppression effect of Salmonella 12, 13. Therefore, reducing the number of neutrophils and/or increasing the percentage of CD8+ T cells should improve the tumour suppression efficacy of S. Typhimurium.
The anaerobic tumour microenvironment and the chemokines secreted by tumour cells and/or adjacent tissues strongly attract Salmonella to colonize the necrotic and hypoxic regions in solid tumours 4, 14. This resident Salmonella can cause the lysis of tumour cells and the fragmentation of the nuclei, resulting in larger necrotic and hypoxic regions in tumours 15. Moreover, this resident Salmonella also suppresses intratumoural neoangiogenesis by inducing the apoptosis and necrosis of endothelial cells and by reducing the VEGF level and percentage of CD31 positive cells in tumours, resulting in a more hypoxic tumour microenvironment 4, 16. This positive feedback loop between the hypoxic tumour microenvironment and Salmonella colonization theoretically provides a way to improve the tumour suppression efficacy of Salmonella by suppressing intratumoural neoangiogenesis with angiogenesis inhibitors, which should create an ideal anaerobic microenvironment in the tumour that supplements the conventional monotherapy that uses Salmonella. The combination of an antiangiogenesis drug and Salmonella has achieved a significantly improved efficacy in a pancreatic cancer patient-derived orthotopic xenograft (PDOX) model 17.
Triptolide is a potent anti-inflammatory and antiangiogenesis diterpenoid triepoxide compound purified from Tripterygium wilfordii Hook. F. (Celastraceae), which has been used to treat autoimmune and inflammatory diseases in China for centuries 18. Triptolide has been shown to suppress the growth and metastasis of many kinds of solid tumours, including melanoma 19-21. As a broad-spectrum anticancer and antiangiogenesis drug 22, the combination therapy of triptolide and standard chemotherapy drugs, such as cisplatin, showed dramatic synergistic effects 23. However, whether the combination of triptolide and Salmonella has a similar synergistic effect has not been studied.
In this study, we used the highly attenuated S. Typhimurium VNP20009 variant, which has been proved to be highly effective in experimental models and highly safe in initial clinical trials 5, 6 but has failed to show any convincing therapeutic effects in later trials 5, 24. Unlike in mouse models, in which VNP20009 actively colonizes a tumour at low doses, only extremely high doses or the continuous administration of VNP20009 has led to tumour colonization in humans 5, 6. Therefore, we hypothesize that the therapeutic effect of VNP20009 can be optimized by combining it with triptolide, which modulates the host immune response and intratumoural neoangiogenesis.
In this study, we demonstrated that triptolide significantly improved the tumour colonization of VNP20009 by suppressing the anti-Salmonella host immune response, especially the infiltration of neutrophils. Triptolide also inhibited intratumoural angiogenesis to create a more hypoxic tumour microenvironment that favoured bacterial proliferation. Our findings enriched the understanding of key factors regulating the tumour colonization of Salmonella and improved the tumour-targeting Salmonella therapy for further clinical developments.
Materials and Methods
Cells, bacteria and colony formation assays
B16F10 melanoma cells and Jurkat cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and grown as previously described 16. Lipid A-modified (msbB-) auxotrophic (purI-) S. Typhimurium strain VNP20009 (ATCC) was grown at 37°C to the mid-logarithmic phase in LB broth (without sodium chloride). For colony formation assays, the number of colony forming units (CFUs) was determined as previously described 4, 16.
Animal studies
Six to seven week-old female C57BL/6 mice, which were purchased from the Laboratory Animal Center, Yangzhou University, Yangzhou, Jiangsu province, China, were housed in environmentally controlled conditions (22°C, a 12 h light/dark cycle with the light cycle from 6:00 to 18:00 and the dark cycle from 18:00 to 6:00) with ad libitum access to standard laboratory chow and water. The study protocol was approved by the local institution review boards and the animal study was carried out in accordance the established ethical guidelines for animal use and care at Nanjing University. C57BL/6 mice were inoculated subcutaneously on the mid-right flank with 5 ×105 B16F10 cells in 0.1 mL PBS and, at d 6 (or 8) post tumor inoculation, were treated intraperitoneally, intravenously or by direct local injection with S. Typhimurium strain VNP20009 at a dose of 105, 5×106 or 5 ×104 CFUs per mouse, respectively. Additionally, mice were treated intraperitoneally with triptolide at 20 μg/kg body per mouse from d 4 to d 14 post tumor inoculations. For the neutrophil-depletion experiment, mice were injected intraperitoneally with anti-Gr-1 monoclonal antibody (BD Biosciences, San Diego, CA) or non-immune mouse IgG (R & D Systems, Minneapolis MN) at 50 μg per mouse on d 6 and 8 post tumor inoculations. For the neutralization of VEGF in tumors, anti-VEGF neutralizing antibody (R & D Systems) or non-immune mouse IgG was directly injected into tumors at various locations at 10 μg per tumor on d 7, 9 and 10 post tumor inoculations. Tumor volume was determined as previously described 4 by using the formula: tumor volume = length × width2 × 0.52. Survival information was also recorded.
Flow-cytometric analysis
Tumor mass was digested with collagenase I (Invitrogen, Carlsbad, CA), collagenase IV (Sigma, St. Louis, MO), DNase I (Sigma) and hyaluronidase (Worthington Biochemical Corp, Lakewood, CA ) for 1 h at 37°C for the preparation of the single cell suspension. The cells were blocked and stained with anti-CD4-PE, anti-CD8-FITC, or anti-Gr-1-FITC antibodies (BD Biosciences), and were subjected to flow-cytometry as described 26.
Immunoblotting and RT-PCR assays
Cellular extracts of B16F10 melanoma cells or Jurkat cells were prepared in the cell extraction buffer as instructed by the manufacturer (Invitrogen). The Immunoblotting procedures were performed as depicted previously 4 and the following antibodies were used: mouse monoclonal antibodies against NF-κBp65 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit antibodies against phospho-p65, IκB and GAPDH (all from Cell Signaling, Beverly, CA). For RT-PCR assays, total cellular RNA was extracted using RNeasy Micro or Mini Kit following the manufacturer's protocol (QIAGEN). cDNA synthesis and RT-PCR were performed by using primers for VEGF and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described before 4.
Luciferase reporter assays
Jurkat cells were plated in 24-well plates at 104 cells/500 μL. After 24 h, they were transfected with polyethylenimine and 0.5 μg mouse NFκB p65 luciferase reporter plasmid and 0.1 μg Renilla luciferase reporter vector. After 24 h, the cells were treated with triptolide (10 ng/mL) for 6 h followed by treatment with fresh Salmonella VNP20009 for 15 min. The cells were then prepared for luciferase assays using the Dual-Luciferase system (Promega, Madison, WI). Data was expressed as relative luciferase activity representing the mean ± S.D. of duplicate experiments and were obtained by calculating the ratio of Firefly luciferase activity and Renilla luciferase activity.
Electrophoretic mobility shift assays (EMSA)
Nuclear extracts were prepared as described 4 and 10 μg nuclear extract was pre-incubated with 2 μg poly dI-dC on ice for 10 min before incubation with 0.05 pmol FAM-labeled oligonucleotide in binding buffer containing 10 mM Tris-HCl, (pH 7.5), 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, 4% glycerol, and 1% bovine serum albumin for 45 min. The DNA-protein complexes were resolved by running on a 4.5% polyacrylamide gel. The sequences of the double-stranded EMSA NFκB (p65) probes were: NFκB-forward (FAM), 5'-FAM-AGTTGAGGGGACTTTCCCAGGC-3', and NFκB-forward (cold), 5'-AGTTGAGGGGACTTTCCCAGGC-3'.
Bio-Plex Multiplex Suspension Array and ELISA
Tumor tissue was weighed and homogenized for 1 h on ice in 50 mM HEPES (pH 7.4), 100 mM NaCl, 50 mM NaF, 2 mM EDTA, 1% Triton-100 and 100 µg/mL phenylmethanesulfonylfluoride (PMSF). Cytokines and chemokines were measured using the Bio-Plex Suspension Array System (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's protocol. For ELISA, tissue homogenates were subjected to the ELISA procedure as described 4 by using the mouse VEGF ELISA kit (Boster, Wuhan, China).
Immunohistochemistry and fluorescent microscopy
Tumor tissues were fixed with 4% formaldehyde, embedded in paraffin and sectioned for hematoxylin and eosin (H&E) staining. For necrosis determination in tumors, the necrotic area was quantitated by software Image J (NIH, Bethesda, MD). Immuno-stainings were performed on frozen tumor sections with appropriate biotinylated antibodies according to standard histological procedures. Gr-1+ and CD31+ cells and VEGF in tumor tissues were stained, and CD31+ cells were quantitated as previously described 16. Macrophages in tumors were determined by anti-F4/80-FITC antibody, and GFP-expressing VNP and TUNEL assay were described previously 4.
Statistical analysis
Student's t test analysis was carried out on data using the SPSS software to assess statistical significance. Differences between experimental groups were considered to be significant when P < 0.05.
Results
Triptolide and VNP20009 synergistically suppressed melanoma growth in vivo by enlarging the necrotic area caused by VNP20009 colonization
The xenografts of murine B16F10 melanoma, which are fast growing and easy to monitor, are commonly used for the evaluation of cancer therapies 25. To test whether the combination therapy of triptolide and VNP20009 could act synergistically against the B16F10 melanoma, mice bearing melanoma were treated with triptolide alone, VNP200009 alone, or the combination of both. Both the intraperitoneal and intratumoural administration of triptolide alone or VNP20009 alone showed a moderate suppression of the growth of melanoma in mice, and the combination of triptolide and VNP20009 resulted in significantly better antitumour efficacy (Figure 1A, Figure S1A and S1B). Furthermore, the intraperitoneal administration of both triptolide and VNP20009 remarkably prolonged the survival length of the mice bearing subcutaneous melanoma, resulting in a 40-day survival rate of 85.7% for the mice that received the combination therapy, which was significantly better than the 14.3% 40-day survival rate experienced by mice that received the conventional monotherapy of VNP20009 alone (Figure 1B).
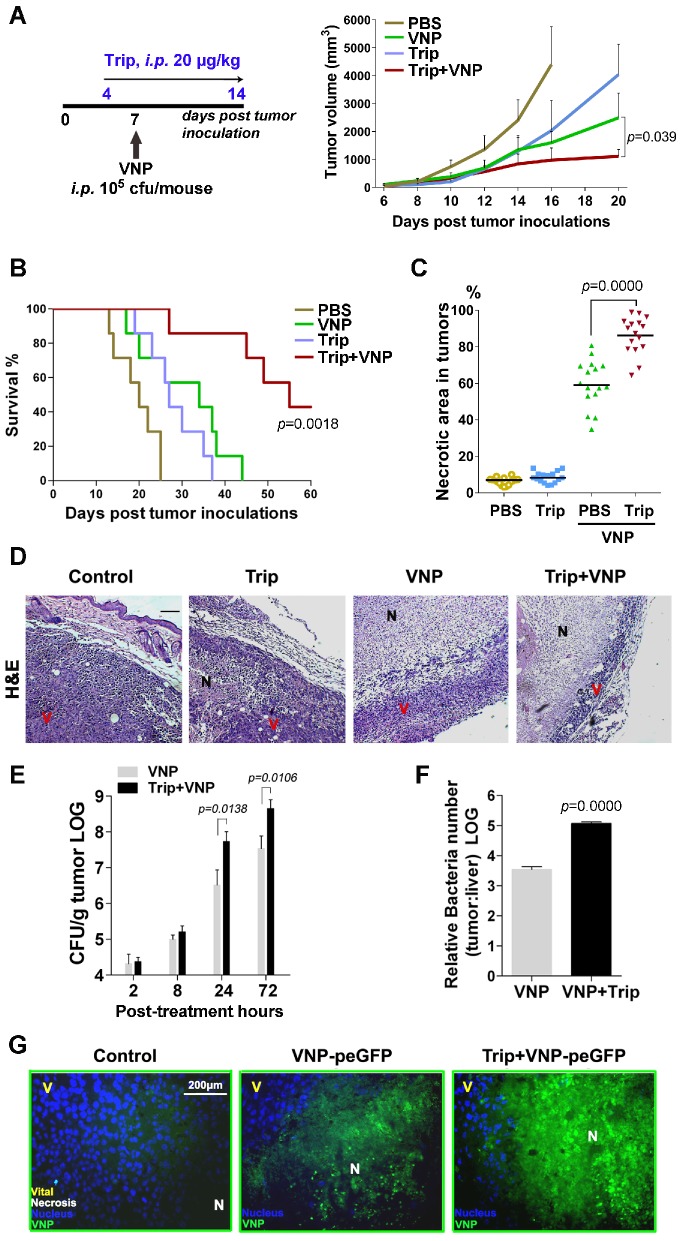
Figure 1.
Triptolide Enhances the Anti-tumour Effect of VNP20009 by Enlarging the Necrotic Area Caused by VNP Colonization A) The treatment schedule of triptolide and VNP20009 (VNP) for mice bearing B16F10 melanoma xenografts, and the volume of tumours in different treatment groups. Triptolide was given intraperitoneally once every day from d 4 to d 14 at 20 μg/kg in 200 μL PBS. Mean + S.D.. n=7. B) The Kaplan-Meier survival curves of mice bearing melanoma xenografts after intraperitoneal administration of triptolide and VNP, n=7, P value represents VNP versus the combination of triptolide and VNP. C) The quota of the percentage of necrotic areas in sections of tumours harvested on d 10 post treatment. In each section per tumour, four random areas were counted, and four tumours were harvested in each treatment group. The quota of the percentage of necrosis was determined using Image J software. Mean ± S.D., n=16. D) H&E staining (bar represents 300 μm) of tumours treated with triptolide, VNP, or the combination of triptolide and VNP. N: necrotic areas, V: viable tumour cells. E) The numbers of bacterium colonized in the tumors at 2, 8, 24 and 72 h post infection. Mean ± S.D., n=3, All the assays were repeated three times independently. F) The ratio of bacterial colonization in tumours and livers on d 3 post the intraperitoneal injection of VNP at 105 CFU/mouse. G) Fluorescent microscopy of melanoma tissues from mice receiving different treatments of triptolide and VNP expressing GFP. The nuclei were stained with DAPI, N: necrotic area, V: viable tumour cells.
The Hematoxylin and eosin (H&E) staining of tumour tissues showed that the combination therapy resulted in a sharper increase in the number of necrotic tumour cells than with the treatments consisting of either triptolide alone or VNP200009 alone (Figure 1C). Fewer viable tumour cells and a larger area of necrosis were observed in the centre of melanoma tissue from the mice receiving the combination therapy than in the other mice (Figure 1D), suggesting that the synergistic tumour inhibition effects of the combined regimen could be partially attributed to the enhanced necrosis of melanoma cells.
Colony formation assays of bacteria recovered from the tumour and liver tissues demonstrated that both the exact number of tumour-colonized bacteria and the relative tumour specificity of VNP20009 were improved by being combined with triptolide (Figure 1E and 1F). Fluorescent microscopy using VNP20009-expressing green fluorescent protein (VNP-peGFP) further demonstrated that a greater number of VNP-peGFP was localized to the necrotic portion of tumours in the VNP-peGFP-inoculated mice treated with triptolide (Figure 1G).
Triptolide amplified the antitumour effect of VNP20009 by inhibiting the tumour infiltration of inflammatory cells caused by VNP20009 infection
Salmonella-colonized tumours are always infiltrated by various inflammatory cells for the elimination of invading pathogens 26. The fluorescent immunostaining of F4/80 (a macrophages marker) and the Immunohistochemistry (IHC) staining of Gr-1 (a neutrophils marker) showed that tumours infected with VNP20009 attracted numerous macrophages and neutrophils, which were weakened by triptolide (Figure 2A). The blockage of neutrophils using either triptolide or a Gr-1-neutralizing antibody significantly enhanced the tumour inhibition effect of VNP20009 (Figure 2B and Figure S2). The IHC staining of tumour tissues from the VNP20009-inoculated mice exhibited numerous neutrophils between the necrotic and viable regions of tumours, which confined VNP20009 inside the necrotic area. By contrast, fewer neutrophils were present in the tumours obtained from mice that received the combination of VNP20009 and triptolide or the Gr-1-neutralizing antibody (Figure 2C). The reduction of Gr-1+ cells in tumours from mice receiving the combination therapies was also confirmed by flow cytometry analysis (Figure 2D). Colony formation assays showed that a significantly larger number of colonies were produced by the bacteria retrieved from the tumours of mice that received the combination of VNP20009 and triptolide or the Gr-1 neutralizing antibody (Figure 2E). These combination therapies also led to a dramatic expansion of the necrotic area in tumours treated with VNP20009 (Figure 2F).
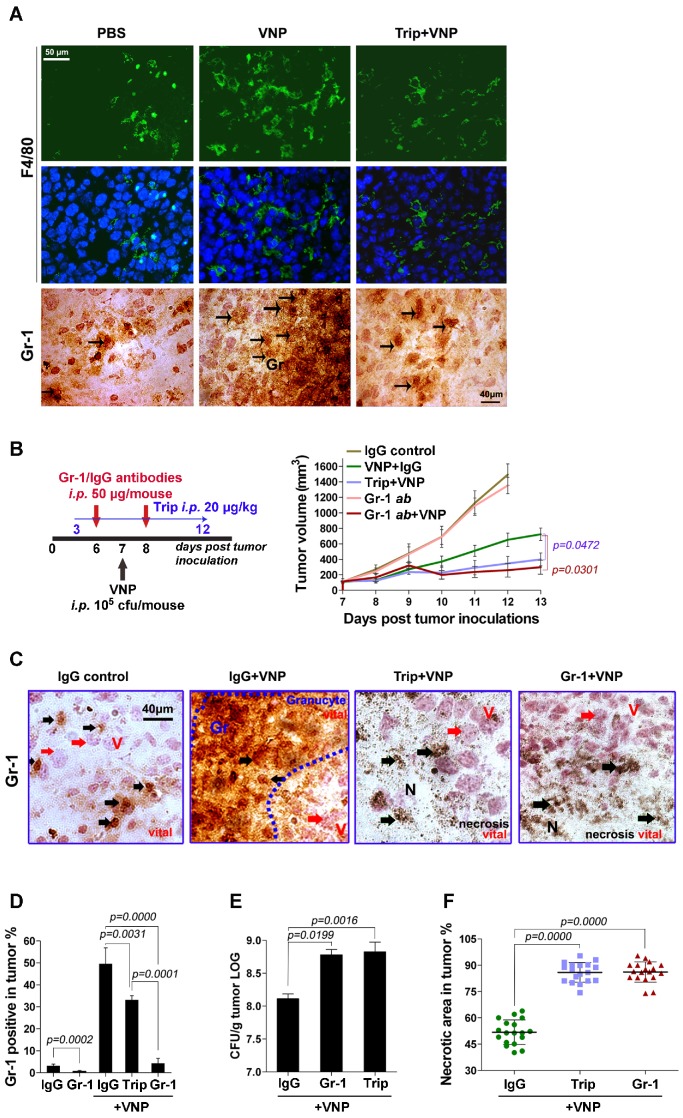
Figure 2.
Triptolide Amplifies the Anti-tumour Effect of VNP20009 by Inhibiting the Infiltration of Inflammatory Cells Caused by VNP20009 Infection. A) Fluorescent microscopy of F4/80+ macrophages and immunohistochemistry of Gr-1+ granulocytes in tumours. The F4/80 antibody was tagged with FITC and the nucleus was stained with DAPI. The Gr-1+ cells are indicated by arrows. B) The treatment schedule of neutrophil-depletion experiment in melanoma bearing mice, and the volume of tumours in different treatment groups. Gr-1 neutralizing antibody or its IgG control (50 μg/mouse) was injected twice intraperitoneally on d 6 and d 8, triptolide (20 μg/mouse) was injected once daily intraperitoneally from d 3 to d 12 and 105 CFU/mouse VNP20009 (VNP) was intraperitoneally injected on d 7 post tumour-inoculation. Mean ± S.D., n=5. C) IHC stainings of Gr-1 in tumours from the neutrophil- depletion experiment on d 7 post VNP infection. Black arrows indicate Gr-1+ cells. Red arrows indicate tumour cells. D) Intratumoral Gr-1+ cells were measured by the flow-cytometry on d 7 post VNP infection. Mean ± S.D., n=3. E) Relative tumour bacterial colonization on d 7 post VNP infection. Mean ± S.D., n=3. F) The quantification of necrotic area in tumours from the neutrophil-depleted mice. Three tumours were harvested in each group. Six parts at random locations were fixed and sectioned in each tumour, and the percentage of necrotic was calculated by Image J software. Mean ± S.D., n=16.
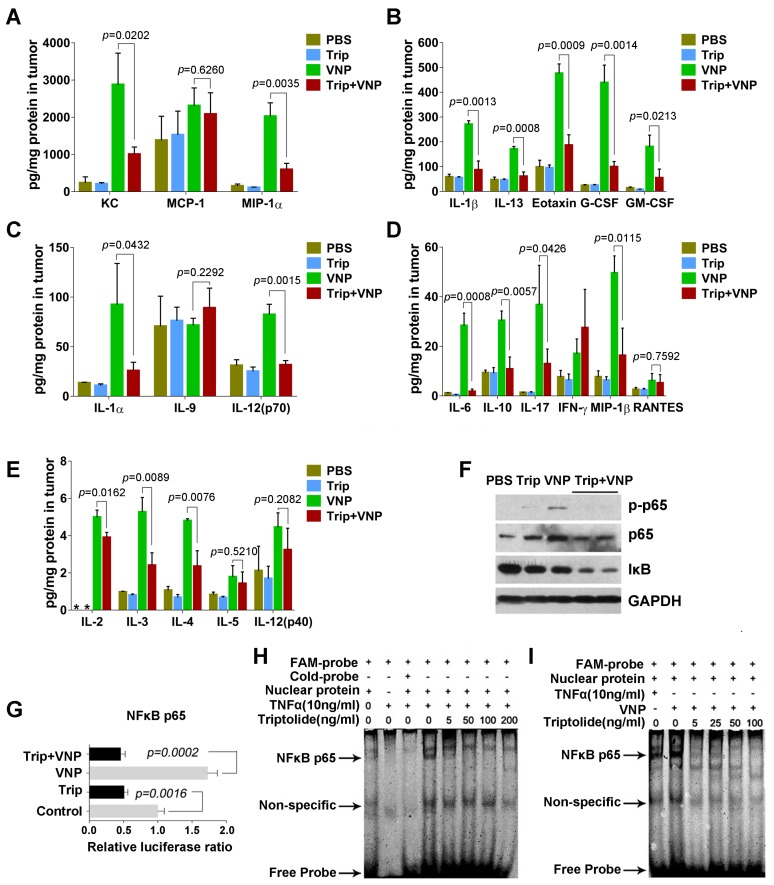
When infection by Salmonella occurs, the NF-kB pathway is immediately activated in lymphocytes, leading to the massive secretion of various proinflammatory factors 27. The cytokine and chemokine profiling of tumour tissues by the multiplex suspension array revealed that, compared with the mock treatment using PBS, VNP20009 significantly upregulated the production of proinflammatory factors, such as IL-6, IL-1α, IL-1β, IL-12p70, IL-17, and IL-13, and chemokines, such as G-CSF, GM-CSF, MIP-1α, MIP-1β, KC, and eotaxin, in the tumour microenvironment, which was significantly attenuated by triptolide (Figure 3A-3E). Jurkat cells, an immortalized line of human T lymphocyte cells, were treated with VNP20009 and triptolide to test their effects on the NF-kB pathway in vitro. Immunoblottings of cell lysates from VNP20009-treated Jurkat cells showed that VNP20009 caused rapid NFκB (p65) phosphorylation, which was strongly suppressed by triptolide (Figure 3F).
Figure 3.
Triptolide Inhibits the Production of Cytokines and Chemokines by Suppressing the NFκB Pathway of the Tumour-related Inflammatory Cells. A-E) The Bio-plex Multiplex Suspension Array of cytokines in tumours, Mean ± S.D., n=3, * below detection limits. F) Immunoblotting assays showed that triptolide inhibited the NFκBp65 phosphorylation induced by VNP infection in Jurkat cells. G) NFκBp65 luciferase reporter assays showed that triptolide inhibited the transcriptional activity of NFκBp65 which was activated by VNP infection in Jurkat cells. The mean value of the control group was defined as 1.0. Mean ± S.D., n=3. H) The EMSA assay showed that triptolide exhibited a dose-dependent inhibition on the TNF-α-induced DNA binding activity of NFκBp65 in Jurkat cells. I) The EMSA assay showed that triptolide exhibited a dose-dependent inhibition on the VNP-induced DNA binding activity of NFκBp65 in Jurkat cells.
Luciferase reporter assays further showed that VNP20009 rapidly induced the transcriptional activity of NFκB (p65), which could be significantly inhibited by triptolide (Figure 3G). Moreover, electrophoretic mobility shift assays demonstrated that VNP20009 enhanced the DNA binding activity of p65, while triptolide significantly attenuated the TNF-α- or VNP20009-induced DNA binding activity of p65 in Jurkat cells (Figure 3H and 3I). The results of these experiments suggest that the activation of lymphocytes by VNP20009 is weakened by triptolide, which is consistent with previous reports about triptolide's immunosuppressive functions 28, 29.
Our data shows that by suppressing the tumour infiltration of neutrophils and by downregulating the production of proinflammatory cytokines and chemokines, triptolide improves the survival and growth of VNP20009 inside melanoma tissues, leading to enhanced tumour necrosis.
Triptolide and VNP20009 exerted antineoangiogenesis effects by reducing CD31+ cells and inhibiting VEGF expression in melanoma
Angiogenesis is critical to tumour growth, metastasis, and neoplastic progression 30. A strong staining of CD31, an important neovascularization marker, indicates active angiogenesis 31, which could improve the influx of oxygen inside the tumour. However, a hypoxic tumour microenvironment is more conducive to the proliferation of Salmonella 14.
Triptolide has been characterized as a compound that suppresses angiogenesis 22, and there have been some successful instances of improving Salmonella therapy with antiangiogenesis drugs 17. In this study, we were interested in discovering whether triptolide could exert synergistic antiangiogenesis effects when used with VNP20009 to achieve a better inhibitory effect on tumour growth in melanoma-bearing mice. The IHC staining of CD31 showed that triptolide by itself reduced the number of CD31 positive cells in melanoma, which was further accentuated by VNP20009 (Figure 4A and 4B). Cancer cells secrete VEGF to induce angiogenesis inside the tumour to support the tumour's growth 35, so a high VEGF protein level is strongly correlated with poor prognosis 36. The IHC staining of VEGF in melanoma tissue, the RT-PCR analysis of the RNA level of VEGF in melanoma tissue, and the ELISA assay's measurement of the VEGF level in the lysates of melanoma tissue all showed that triptolide and VNP20009 synergistically inhibited VEGF expression in melanoma tissue (Figure 4A, 4C, and 4D), suggesting that triptolide and VNP20009 could strongly inhibit tumour angiogenesis in a synergistic manner.
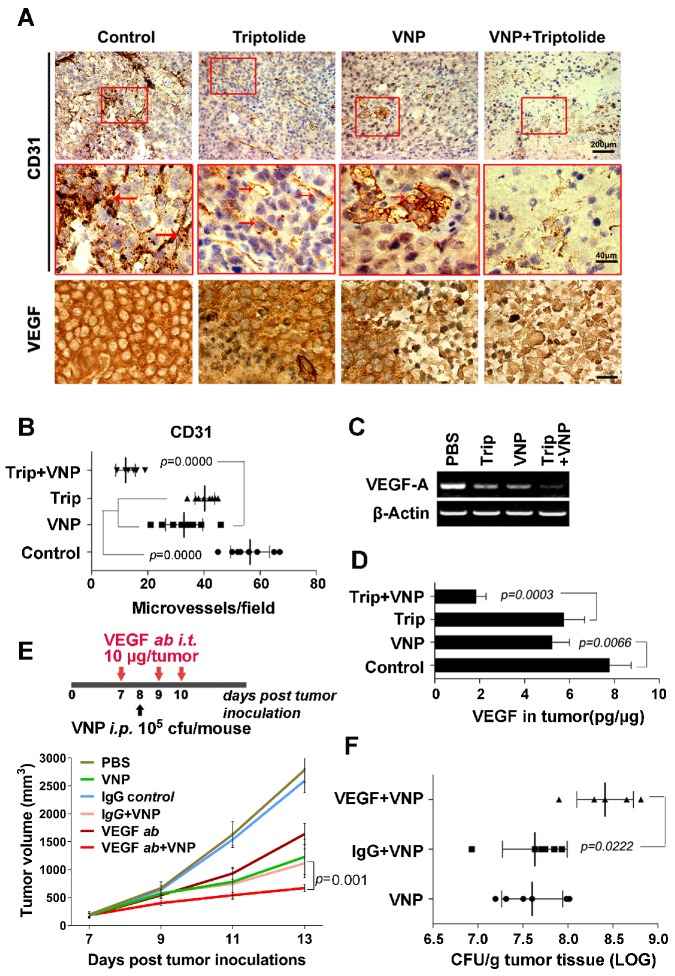
Figure 4.
Triptolide and VNP20009 Synergistically Inhibit the Tumour Angiogenisis by Suppressing the Production of VEGF. A) Immunohistochemistry of CD31+ cells and VEGF expression in melanoma tumours from mice receiving triptolide and/or VNP20009 (VNP) treatments on d 7 post infection. CD31+ cells are indicated by red arrows. Bars on VEGF sections represent 10 μm. B) The quantification of CD31+ microvessels in tumours. Two sections per tumour were determined and four tumours were harvested in each group. The number of the CD31+ cells was calculated. Mean ± S.D., n=8. C) VEGF expression in melanoma tumours was examined by RT-PCR. D) ELISA assays were performed to determine VEGF concentration in tumours. Mean ± S.D., n=4. E) The treatment schedule of the combination therapy of VNP and VEGF neutralizing antibody, and its effect on the growth of tumours. The VEGF neutralizing antibody (10 μg/ tumour) or the IgG control antibody (10 μg/ tumour) were injected directly into the tumours three times on d 7, 9 and 10. VNP was intraperitoneally injected on d 8 post tumour inoculation. Mean ± S.D., n=5. F) The bacterial colonization in the tumours was determined on d 5 post VNP infection in the VEGF neutralization assay. Mean ± S.D., n=5.
To test whether suppressing tumour angiogenesis could directly improve the antitumour effects of VNP20009, VEGF was specifically depleted by a neutralizing antibody in the VNP20009-inoculated mice, and the growth of tumour was closely monitored. The tumour growth was more significantly slowed by the combination therapy of the VEGF-neutralizing antibody and VNP20009 than with monotherapies consisting of the VEGF-neutralizing antibody alone or VNP20009 alone (Figure 4E and Figure S3). At the same time, a better colonization of VNP20009 was observed in the tumours of mice receiving the combination of VNP20009 and the VEGF-neutralizing antibody than in the tumours of mice receiving the combination of VNP20009 and the nonspecific antibody (Figure 4F), indicating that VEGF depletion directly improved VNP20009 therapy by enhancing the tumour colonization of the bacteria. Triptolide, which suppressed the expression of VEGF, could contribute to VNP20009 therapy partially through its antiangiogenesis activity.
In summary, our study showed that the combination therapy of triptolide and VNP20009 exhibited significantly improved efficacy compared with the conventional monotherapy of VNP20009 alone. Triptolide enhanced the tumour colonization of VNP20009 by suppressing the angiogenesis and the infiltration of inflammatory cells in the tumours, which were two important factors limiting the antitumour effects of highly attenuated VNP20009 (Figure S4).
Discussion
As a pathogen that causes typhoid fever, Salmonella has a dramatic influence on the immune system, quickly activating multiple host immune responses to fight the pathogen. A variety of bone marrow-derived phagocytes, including neutrophils, macrophages, and natural killer cells are recruited to the site of infection to phagocytose bacteria and to present the antigen to initiate an adaptive immune response 34. During Salmonella-mediated cancer therapy, such a process occurs, but it occurs in a comparatively milder manner due to the use of the attenuated strains. In this case, the host immune response, which tries to clear Salmonella from the body, is an important factor that limits the efficacy of the Salmonella-mediated cancer therapy—this response is a double-edged sword 11-13.
Tumour-targeting VNP20009 attracts various inflammatory cells, such as neutrophils, macrophages, and CD8+ T cells to a tumour 11-13. Neutrophils and macrophages suppress VNP20009 via their bactericidal activities 11, and CD8+ T cells can eliminate tumour cells by releasing cytotoxic granules 12, 13. However, CD8+ cytotoxic T cells are often dysfunctional in the tumour microenvironment due to the upregulation of Programmed cell death protein 1 (PD-1) and abnormally active regulatory T cells, which create an immunosuppressive microenvironment in the tumour 35, 36. Salmonella infection can break this immunosuppressive status by strongly activating the NFκB pathway in various lymphocytes 27. Salmonella can reportedly promote the tumour infiltration of CD8+ T cells 37. In this study, we also found that a low dose of triptolide (20 µg/kg) promoted the VNP20009-induced tumour infiltration of CD8+ T cells (Figure S5A and S5B), while a high dose of triptolide (≥100 µg/kg) exhibited a broad-spectrum suppressive effect on immunity, resulting in the loss of triptolide's synergistic antitumour effect (Figure S6A-S6E).
The antitumour effect of VNP20009 was weaker in immunodeficient mice than in mice with an intact immune system 8. However, another strain of S. Typhimurium, A1-R, which was specially isolated from A1-infected tumours in immunodeficient mice, showed strong antitumour effects in several kinds of PDOX immunodeficient mouse models, including pancreatic cancer, cervical cancer, soft-tissue sarcoma, and breast cancer 38-40. Apart from suppressing the growth of primary tumours, A1-R could also reduce brain and bone metastasis in experimental breast cancer metastatic mouse models 41, 42.
These observations indicate that host immunity is actively involved during the process of Salmonella-mediated cancer therapy even though the extent varies with strains. In the necrotic area, the lysis of tumour cells provides a large pool of tumour-specific antigens, which could trigger an adaptive immune response against tumour cells by the infiltrated phagocytes 26. As a strong stimulus for the host immune response and as a tumour-specific vehicle, Salmonella has been genetically engineered as a carrier of tumour-specific antigens for the cancer immune therapy, achieving complete tumour regression and the generation of a lifelong protective memory in an A20 lymphoma model 43.
Therefore, the careful manipulation of the host immunity, such as the suppression of Gr-1+ neutrophils and the activation of CD8+ T cells using either a low dose of triptolide or other compounds, can potentiate the colonization of VNP20009 inside tumours to maximize VNP20009's antitumour effect. At the same time, further studies on the Salmonella-mediated tumour therapy can provide important knowledge about the crosstalk between cancer and host immunity to promote the development of vaccinations against cancer.
Supplementary Material
Supplementary figures.
Acknowledgments
This study was supported in part by grants from the Doctoral Station Science Foundation by the Chinese Ministry of Education (20130091130003), the Chinese National Natural Sciences Foundation (81630092, 81573338, 81421091), the National Key Research Program by Ministry of Science and Technology (2014CB744501, 2016YFC0902700).
References
- 1.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–44. [PubMed] [Google Scholar]
- 2.Morrissey D, O'Sullivan GC, Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther. 2010;10:3–14. doi: 10.2174/156652310790945575. [DOI] [PubMed] [Google Scholar]
- 3.Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M. et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Wei D, Zhuang H, Qiao Y, Tang B, Zhang X. et al. Proteomic screening of anaerobically regulated promoters from Salmonella and its antitumor applications. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.009399. 009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–52. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C. et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737–44. doi: 10.1038/sj.cgt.7700634. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Wu CL, Chen SH, Shiau AL. Humoral immune responses inhibit the antitumor activities mediated by Salmonella enterica serovar choleraesuis. J Immunother. 2009;32:376–88. doi: 10.1097/CJI.0b013e31819d4ebc. [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, Hsieh JL, Wu CL, Hsu PY, Shiau AL. T cell augments the antitumor activity of tumor-targeting Salmonella. Appl Microbiol Biotechnol. 2011;90:1381–8. doi: 10.1007/s00253-011-3180-z. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Hsieh JL, Wu CL, Hsu HC, Shiau AL. B cells are required for tumor-targeting Salmonella in host. Appl Microbiol Biotechnol. 2011;92:1251–60. doi: 10.1007/s00253-011-3386-0. [DOI] [PubMed] [Google Scholar]
- 10.Saltzman DA, Katsanis E, Heise CP, Hasz DE, Vigdorovich V, Kelly SM. et al. Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent? J Pediatr Surg. 1997;32:301–6. doi: 10.1016/s0022-3468(97)90198-6. [DOI] [PubMed] [Google Scholar]
- 11.Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–60. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- 12.Domingos-Pereira S, Hojeij R, Reggi E, Derre L, Chevalier MF, Romero P. et al. Local Salmonella immunostimulation recruits vaccine-specific CD8 T cells and increases regression of bladder tumor. Oncoimmunology. 2015;4:e1016697. doi: 10.1080/2162402X.2015.1016697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roider E, Jellbauer S, Kohn B, Berchtold C, Partilla M, Busch DH. et al. Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer. Cancer Immunol Immunother. 2011;60:371–80. doi: 10.1007/s00262-010-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall DM, Srikanth CV, McCormick BA. Targeting tumors with salmonella Typhimurium- potential for therapy. Oncotarget. 2010;1:721–8. doi: 10.18632/oncotarget.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci U S A. 2007;104:10170–4. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia LJ, Wei DP, Sun QM, Jin GH, Li SF, Huang Y. et al. Tumor-targeting Salmonella typhimurium improves cyclophosphamide chemotherapy at maximum tolerated dose and low-dose metronomic regimens in a murine melanoma model. Int J Cancer. 2007;121:666–74. doi: 10.1002/ijc.22688. [DOI] [PubMed] [Google Scholar]
- 17.Hiroshima Y, Zhang Y, Murakami T, Maawy A, Miwa S, Yamamoto M. et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–57. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupchan SM, Court WA, Dailey RG Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194–5. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF. et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- 20.Wang C, Liu B, Xu X, Zhuang B, Li H, Yin J. et al. Toward targeted therapy in chemotherapy-resistant pancreatic cancer with a smart triptolide nanomedicine. Oncotarget. 2016;7:8360–72. doi: 10.18632/oncotarget.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang QW, Cheng KJ, Mei XL, Qiu JG, Zhang WJ, Xue YQ. et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He MF, Huang YH, Wu LW, Ge W, Shaw PC, But PP. Triptolide functions as a potent angiogenesis inhibitor. Int J Cancer. 2010;126:266–78. doi: 10.1002/ijc.24694. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Wang X, Xu X. Triptolide potentiates lung cancer cells to cisplatin-induced apoptosis by selectively inhibiting the NER activity. Biomark Res. 2015;3:17. doi: 10.1186/s40364-015-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum Gene Ther. 2001;12:1594–6. [PubMed] [Google Scholar]
- 25.Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol; 2001. Chapter 20: Unit 20.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V. et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–7. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 27.Johannessen M, Askarian F, Sangvik M, Sollid JE. Bacterial interference with canonical NFkappaB signalling. Microbiology. 2013;159:2001–13. doi: 10.1099/mic.0.069369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Krakauer T, Chen X, Howard OM, Young HA. Triptolide attenuates endotoxin- and staphylococcal exotoxin-induced T-cell proliferation and production of cytokines and chemokines. Immunopharmacol Immunotoxicol. 2005;27:53–66. doi: 10.1081/iph-51294. [DOI] [PubMed] [Google Scholar]
- 30.Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM. et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694–701. doi: 10.1038/sj.bjc.6600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Stockard CR, Harkins L, Lott P, Salih C, Yuan K. et al. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech Histochem. 2008;83:179–89. doi: 10.1080/10520290802451085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 33.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–53. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 34.Pham OH, McSorley SJ. Protective host immune responses to Salmonella infection. Future Microbiol. 2015;10:101–10. doi: 10.2217/fmb.14.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H. et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CH, Wu CL, Shiau AL. Salmonella choleraesuis as an anticancer agent in a syngeneic model of orthotopic hepatocellular carcinoma. Int J Cancer. 2008;122:930–5. doi: 10.1002/ijc.23047. [DOI] [PubMed] [Google Scholar]
- 38.Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F. et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell cycle. 2014;13:3958–63. doi: 10.4161/15384101.2014.964115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T. et al. Tumor-Targeting Salmonella typhimurium A1-R Arrests a Chemo-Resistant Patient Soft-Tissue Sarcoma in Nude Mice. PloS one. 2015;10:e0134324. doi: 10.1371/journal.pone.0134324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murakami T, Igarashi K, Kawaguchi K, Kiyuna T, Zhang Y, Zhao M, Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miwa S, Yano S, Zhang Y, Matsumoto Y, Uehara F, Yamamoto M. et al. Tumor-targeting Salmonella typhimurium A1-R prevents experimental human breast cancer bone metastasis in nude mice. Oncotarget. 2014;5:7119–25. doi: 10.18632/oncotarget.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Miwa S, Zhang N, Hoffman RM, Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget. 2015;6:2615–22. doi: 10.18632/oncotarget.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Hegazy WA, Guo L, Gao X, Courtney AN, Kurbanov S. et al. Effective cancer vaccine platform based on attenuated salmonella and a type III secretion system. Cancer Res. 2014;74:6260–70. doi: 10.1158/0008-5472.CAN-14-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.