Abstract
Catalytic nanomaterials with intrinsic enzyme-like activities, called nanozymes, have recently attracted significant research interest due to their unique advantages relative to natural enzymes and conventional artificial enzymes. Among the nanozymes developed, particular interests have been devoted to nanozymes with peroxidase mimicking activities because of their promising applications in biosensing, bioimaging, biomedicine, etc. Till now, lots of functional nanomaterials have been used to mimic peroxidase. However, few studies have focused on the Ni-based nanomaterials for peroxidase mimics. In this work, we obtained the porous LaNiO3 nanocubes with high peroxidase-like activity by inducing its 3+ oxidation state in LaNiO3 perovskite and optimizing the morphology of LaNiO3 perovskite. The peroxidase mimicking activity of the porous LaNiO3 nanocubes with Ni3+ was about 58~fold and 22~fold higher than that of NiO with Ni2+ and Ni nanoparticles with Ni0. More, the porous LaNiO3 nanocubes exhibited about 2-fold higher activity when compared with LaNiO3 nanoparticles. Based on the superior peroxidase-like activity of porous LaNiO3 nanocubes, facile colorimetric assays for H2O2, glucose, and sarcosine detection were developed. Our present work not only demonstrates a useful strategy for modulating nanozymes' activities but also provides promising bioassays for clinical diagnostics.
Keywords: nanozymes, peroxidase-like activity, LaNiO3 perovskite oxide, oxidation state, biomedical assays.
Introduction
Natural enzymes, with remarkable catalytic efficiency and extraordinary substrate specificity, have attracted researchers' enormous interest due to their important roles not only in living systems but also in biomedical diagnostics and therapeutics.1 However, the intrinsic shortcomings of natural enzymes such as high cost, low stability, and difficulty of recycling have impeded their practical applications. To address these drawbacks, great efforts have been devoted to searching for natural enzymes' alternatives called as “artificial enzymes”.2-5 Recently, nanomaterials with enzyme-like characteristics, called as “nanozymes”, have been developed to act as promising artificial enzymes along with the remarkable achievements in the field of nanotechnology.6-9 As a new type of artificial enzymes, nanozymes are advantageous over natural enzymes in several aspects such as low cost, long-term storage, and high stability. More, nanozymes are even superior to conventional artificial enzymes in their large surface area for bioconjugation, self-assembly capabilities, tunable catalytic activities, etc.10-12 Therefore, intensive efforts have been devoted to developing functional nanomaterials for enzyme mimics in recent years.13-27
Among the nanozymes developed, particular interests have been focused on nanozymes with peroxidase mimicking activities due to their great promise in biomedical diagnosis, bioimaging, antibacterial agents, antibiofouling medical devices, etc.28-38 Till now, lots of functional nanomaterials (e.g., carbon based, metal oxide based and metal based nanomaterials) have been explored to mimic peroxidase.15, 17, 39-41 Among them, transition metal oxide nanomaterials had been extensively studied due to their excellent catalytic activities, high stability, low cost and ease of preparation.6, 10, 11 For instance, Fe3O4 nanoparticles have been fabricated to mimic peroxidase for Ebola diagnosis and tumor immunostaining.30, 42 Despite of these progresses, very little attention has been paid to developing Ni-based nanomaterials as peroxidase mimics. A recent study reported that NiO nanoparticles modified with H2TCPP had an intrinsic peroxidase mimicking activity. However, the catalytic activity may be mainly from the H2TCPP ligand rather than the NiO nanoparticle itself.43 More, several seminal studies have demonstrated that the activities of nanozymes were strongly dependent on the size, composition, shape, surface ligand, and exposed facet of nanomaterials.12, 44-49 Previous studies have also showed that the oxidation state was very important for cerium oxides' and iron oxides' enzyme mimicking activities.50-53 However, very little information was available about the influence of nanostructured nickels' oxidation state on their enzyme mimicking activities. Such information, if available, would help to rationally design nanozymes with high enzyme mimicking activities (including peroxidase mimicking activities).
Here, we showed that high peroxidase mimicking activity of LaNiO3 perovskite could be achieved by inducing the 3+ oxidation state of nickel into the perovskite lattice. To find out the effect of nickel atom's oxidation state on the Ni-based nanomaterials' peroxidase mimicking activity, other Ni-based nanomaterials such as NiO and Ni nanoparticles with Ni2+ and Ni0 were also studied respectively. The peroxidase mimicking activity study revealed that the oxidation state of nickel in Ni-based nanomaterials was very important for the peroxidase mimicking activity and Ni3+ was superior to Ni0 and Ni2+ for the nanozymes' catalytic activities. Specifically, the peroxidase mimicking activity of the porous LaNiO3 was about 58~fold and 22~fold higher than that of NiO and Ni nanoparticles. More, the influence of LaNiO3 morphology on its peroxidase-like activity was also investigated. The result showed that the peroxidase-like activities of porous LaNiO3 nanocubes were about 2~fold higher than that of LaNiO3 nanoparticles. The latter were synthesized by a conventional sol-gel method. Finally, the porous LaNiO3 nanocubes with the highest peroxidase mimicking activity were employed to develop reliable diagnostic platforms for important biomolecular targets.
It has been established that bioactive small biomolecules, such as glucose and sarcosine, play critical roles in disease diagnosis.54-58 For example, glucose is not only a well-known indicator for diabetes but also closely associated with ischemia stroke and even cancers.59-65 Previous study also suggested that sarcosine may act as a metabolic biomarker for prostate cancer.66 The oxidation of glucose and sarcosine with the corresponding oxidase produces H2O2. The produced H2O2 can catalytically oxidize peroxidase substrates with LaNiO3 nanocubes-based peroxidase mimics to produce colored products for signaling. Therefore, we further developed facile bioassays for glucose and sarcosine detection by combining glucose oxidase (GOx) and sarcosine oxidase (SOx) with the porous LaNiO3 nanocubes-based peroxidase mimics.
Materials and Methods
Chemicals and Materials
Nickel nitrate hexahydrate, citric acid, sucrose, and glucose were obtained from Nanjing Chemical Reagent Co., Ltd. Ethylene glycol, lactose, sodium hydroxide, hydrogen peroxide, and ammonium hydroxide were purchased from Sinopharm Chemical Reagent Co., Ltd. TMB (3,3',5,5'-tetramethylbenzidine), OPD (o-phenylenediamine), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)), fructose, glycine, lanthanum nitrate hexahydrate, lanthanum oxide, Ni nanoparticles, and glucose oxidase (GOx from Aspergillus niger, >180 units/mg) were purchased from Aladdin Chemical Reagent Co., Ltd. Polyvinyl pyrrolidone (PVP, molecular weight=58000) and sarcosine oxidase (SOx) were obtained from Sigma-Aldrich. All chemical reagents were used as received without further purification. Deionized water produced by Millipore system was used in all experiments.
Instrumentation
Powder X-ray diffraction (XRD) data were collected at room temperature on a Rigaku Ultima diffractometer by using Cu Kα radiation. The diffractometer was operated at 40 kV and 40 mA with a scan rate of 2°/minute. Transmission electron microscopy (TEM) imaging was performed on a JEOL JEM-2100 transmission electron microscope at an acceleration voltage of 200 kV. Scanning electron microscopy (SEM) imaging was performed on a Hitachi S-4800 scanning electron microscope operating at 5 kV. UV-visible absorption spectra were collected on a UV-visible spectrophotometer (TU-9100, Beijing Purkinje General Instrument Co. Ltd., China). Nitrogen adsorption-desorption isotherms were performed on Micromeritics ASAP 2020 surface area and porosity analyzer, from which the nanozymes' surface areas were calculated with the Brunauer-Emmett-Teller (BET) method.
Synthesis of Porous LaNiO3 Nanocubes via a Hydrothermal Method
LaNiO3 nanocubes were synthesized as follows.67 Briefly, the lanthanum nitrate hexahydrate (1.0 mmol), nickel nitrate hexahydrate (1.0 mmol), and glycine (4 mmol) were dissolved in 25 mL of deionized water, followed by the adding 0.3 g of PVP. After stirring for 30 min, the pH of the solution was adjusted to about 7.0 via slowly adding ammonium hydroxide. The resulting solution was transferred into a 40 mL Teflon-lined autoclave and heated at 180 °C for 24 hours. The resulting product was centrifuged and washed with deionized water and ethanol for several times, which was then dried at 60 °C for overnight. The precursor powder was finally annealed at 650 °C for 2 hours with a ramp rate of 5 °C/minute to obtain the final porous LaNiO3 nanocubes.
Synthesis of LaNiO3-H2 Nanocubes
The LaNiO3-H2 was obtained by controlled reduction of as-prepared LaNiO3 porous nanocubes with H2 to partially form Ni2+ in the LaNiO3-H2 lattice. Specifically, the as-prepared LaNiO3 was heated to 350 °C at a ramp rate of 5 °C/minute and maintained at 350 °C for 2 hours under a forming gas of 5% H2 in Argon.
Synthesis of LaNiO3-H2-Air Nanocubes
To obtain the LaNiO3-H2-Air nanocubes, the as-prepared LaNiO3-H2 nanocubes were recalcined at 650 °C in the air for 2 hours with a ramp rate of 5 °C/minute.
Synthesis of LaNiO3 Nanoparticles via a Sol-Gel Method
The LaNiO3 nanoparticles were synthesized via a conventional sol-gel strategy.68 Briefly, lanthanum nitrate hexahydrate (1.5 mmol), nickel nitrate hexahydrate (1.5 mmol), and citric acid (12 mmol) were dissolved in 100 mL of deionized water, followed by adding 1.5 mL of ethylene glycol. Subsequently, the resulting transparent solutions were condensed at 90 °C into a gel under stirring, which were then decomposed at 180 °C for 5 hours to form solid precursors. The solid precursors were then decomposed at 400 °C for 2 hours to obtain foam-like precursors via removing the organic components. The precursors were further annealed at 700 °C for 5 hours with a ramp rate of 5 °C/minute to obtain the final LaNiO3 nanoparticles. The obtained LaNiO3 nanoparticles was denoted as LaNiO3-SG.
Synthesis of NiO
NiO nanoparticles were synthesized as follows.69 0.5 g of NaOH and 1.66 g of PVP were dissolved in 25 mL of deionized water and 1.45 g of nickel nitrate hexahydrate was dissolved in 10 mL of deionized water, respectively. The nickel nitrate aqueous solution was then added to the NaOH/PVP solution dropwise under stirring. The resulting solution was stirred for 3 hours to obtain the NiO precursor, followed by washing with H2O and ethanol for several times and drying at 60 °C overnight. Then, the precursor was annealed at 650 °C for 2 hours in air with a ramp rate of 5 °C/minute to form the final NiO nanoparticles.
Colorimetric Detection of H2O2, Glucose, and Sarcosine with the Porous LaNiO3 Nanocubes
H2O2 detection was carried out as follows: (1) 20 μL of porous LaNiO3 nanocubes (1 mg/mL), 80 μL of TMB (10 mM), and 800 μL of NaOAc buffer (0.2 M, pH 4.5) was added into a 1.5 mL tube. (2) 100 μL of H2O2 with various concentrations was added into the above reaction solution. (3) The mixed solution was incubated for 20 min at 37 °C and then the absorption spectra were measured when keeping the solution in an ice water bath.
Glucose detection was performed as follows: (1) 100 μL of GOx (1 mg/mL) and 100 μL of glucose with different concentrations in 0.2 M phosphate buffer (pH 7.0) were incubated for 30 min at 37 °C. (2) 20 μL of porous LaNiO3 nanocubes (1 mg/mL), 100 μL of TMB (10 mM), and 680 μL of NaOAc buffer (0.2 M, pH 4.5) were added into the above 200 μL of glucose and GOx reaction solution. (3) The mixed solution was incubated for 20 min at 37 °C and then the absorption spectra were measured when keeping the solution in an ice water bath.
Sarcosine detection was performed as follows: (1) 100 μL of SOx (2 mg/mL) and 100 μL of sarcosine with various concentrations in 0.2 M phosphate buffer (pH 7.0) were incubated for 90 min at 37 °C. (2) 60 μL of porous LaNiO3 nanocubes (1 mg/mL), 100 μL of TMB (10 mM), and 640 μL of NaOAc buffer (0.2 M, pH 4.5) was added into the above 200 μL sarcosine and SOx reaction solution. (3) The mixed solution was incubated for 20 min at 45 °C and then the absorption spectra were measured when keeping the solution in an ice water bath.
Cell Assays
Hela cells were incubated in High Glucose DMEM medium with 10% fetal bovine serum (FBS) at 37 °C with the atmosphere containing 5% CO2. Fresh cells were subcultured into 96 wells, and 10 μg/mL lipopolysaccharides (LPS) was used to incubate with cells overnight. Then, 40 μg/mL LaNiO3 and 0.5 mM TMB were added into the cells. The cells solution was incubated for 10 min and the absorbance values at 652 nm were measured.
Results and Discussion
Synthesis and Characterization of Porous LaNiO3 Nanocubes
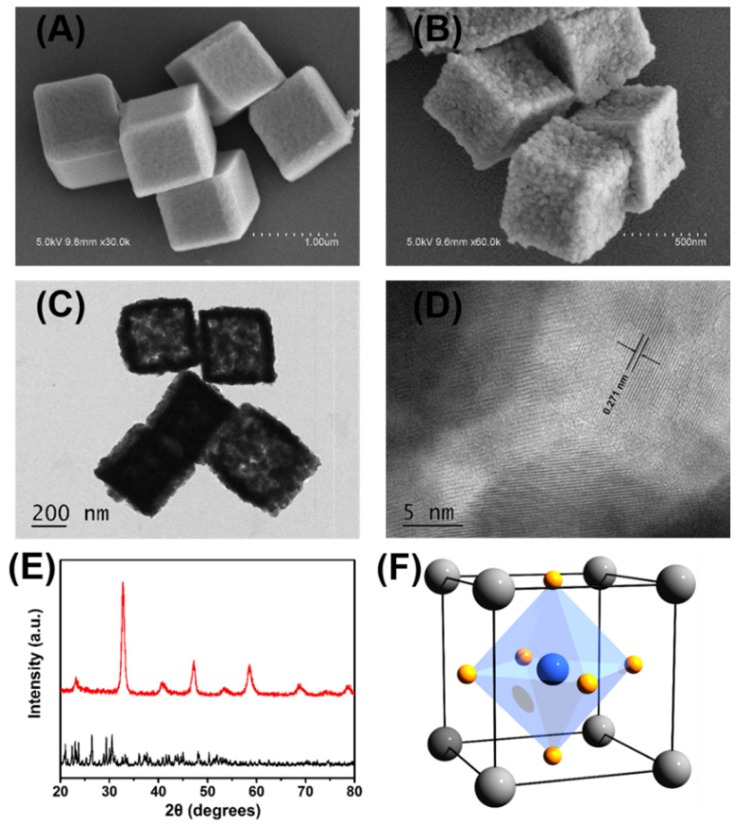
The porous LaNiO3 nanocubes were obtained by annealing the nanocube-like precursors at 650 °C for 2 hours with a ramp rate of 5 °C/minute. The SEM images of nanocube-like precursors shown in Figure 1A and Figure S1A revealed that the precursors had smooth surfaces with the size of about 800 nm. After annealing in air at 650 °C for 2 hours, the annealed products maintained the precursors' nanocube-like morphology. The annealed products had rough surfaces with the size about 600 nm as shown in Figure 1B and Figure S1B. As shown in the SEM and TEM images of Figure 1B and Figure 1C, lots of pores randomly distributed in the annealed products after annealing the precursors in air at 650 °C for 2 hours. The high-resolution TEM images of the porous LaNiO3 nanocubes showed in Figure S1D suggested that the annealed products were polycrystalline. As shown in Figure 1D, the distance of the adjacent fringes was 0.271 nm, which was corresponded to the lattice spacing of the (110) plane of LaNiO3 perovskite. X-ray diffraction patterns confirmed that the annealed products had a perovskite structure with high phase purity, as seen in Figure 1E. This result suggested that nanocube-like precursors were completely transformed into porous LaNiO3 perovskite oxide after annealing in air at 650 °C for 2 hours.
Figure 1.
Representative SEM images of (A) the obtained nanocube-like precursors and (B) the porous LaNiO3 nanocubes after annealing the precursors. (C) Representative TEM images of the porous LaNiO3 nanocubes. (D) High-resolution TEM images of the porous LaNiO3 nanocubes. (E) Powder X-ray diffraction patterns of the nanocube-like precursors (black line) and the porous LaNiO3 nanocubes (red line). (F) Schematic of LaNiO3 perovskite oxide structure (La in grey, Ni in blue, and O in yellow).
Evaluation of Peroxidase Mimicking Activity of Porous LaNiO3 Nanocubes
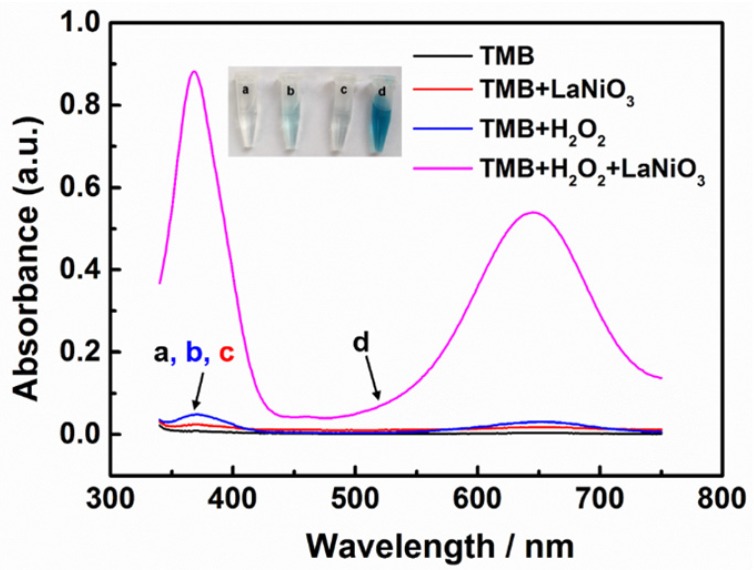
After establishing the successful fabrication of the porous LaNiO3 nanocubes, the peroxidase-like activity of the nanocubes was investigated. To evaluate the peroxidase mimicking activity of the porous LaNiO3 nanocubes, TMB was selected as the catalytic substrate because it was a typical chromogenic substrate for peroxidase. The oxidation of TMB generated the oxidized product (i.e., TMBox) with characteristic absorption peaks at 370 nm and 652 nm. As seen in Figure 2, the porous LaNiO3 nanocubes along with H2O2 and TMB incubated at room temperature for 10 min showed a deep blue color with strong absorbance at 370 nm and 652 nm. However, the other control experiments suggested that the solution contained TMB alone or H2O2 and TMB showed negligible color change. Note, the LaNiO3 and TMB system also showed a slight color change, which was probably due to the oxidase mimicking activity of LaNiO3 nanocubes. To further confirm the peroxidase-like activity of porous LaNiO3 nanocubes, the colorimetric experiments were also performed with the other two typical peroxidase substrates (i.e., OPD and ABTS). As confirmed in Figure S2, all three peroxidase substrates showed deep color changes with strong absorbance, which were attributed to the catalytic oxidation of the substrates with the porous LaNiO3 nanocubes in the presence of H2O2. These results confirmed that the porous LaNiO3 nanocubes possessed peroxidase-like activity.
Figure 2.
The absorption spectra of different reaction systems: (a) TMB only, (b) TMB + H2O2, (c) TMB + LaNiO3 nanocubes, and (d) TMB + H2O2 + LaNiO3 nanocubes.
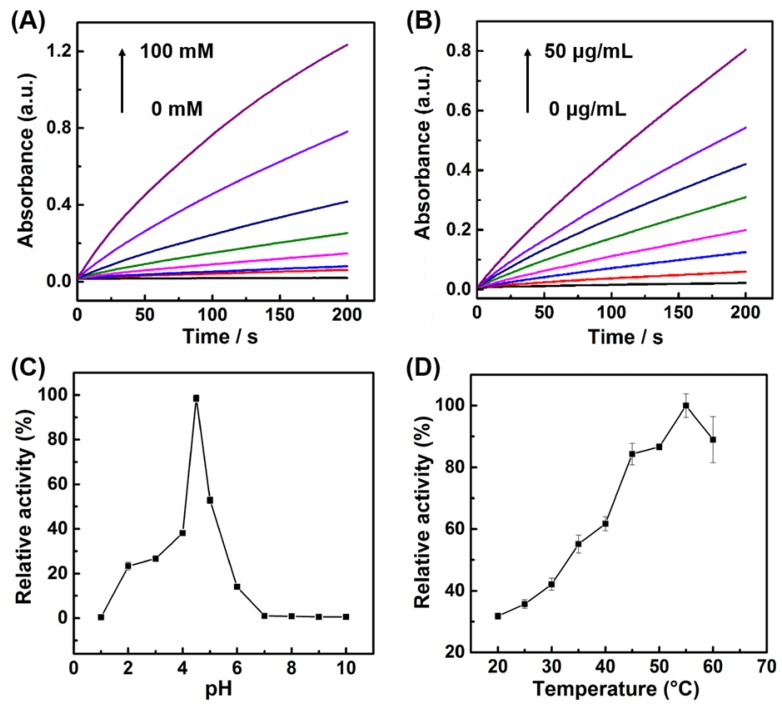
The activity of nature enzymes is dependent on substrate concentrations and reaction conditions. Similar with the nature enzymes, the influence of H2O2 concentrations, catalyst concentrations, temperature, and pH on the peroxidase-like activity of the porous LaNiO3 nanocubes was investigated. As seen in Figure 3A, the reaction rate increased with the increase of H2O2 concentrations, indicating that the peroxidase-like activity of porous LaNiO3 nanocubes was dependent on the H2O2 concentrations. The effect of catalyst concentrations was also evaluated by monitoring the time-dependent absorption spectra under various catalyst concentrations. The reaction rate dramatically increased with the increase of catalyst concentrations, demonstrating that the peroxidase-like activity of porous LaNiO3 nanocubes was dependent on the catalyst concentrations. More, the pH and temperature-dependent peroxidase-like activity were also investigated. As shown in Figure 3C, the peroxidase-like activity of porous LaNiO3 nanocubes was gradually increased in the pH range from 1 to 4.5, while the peroxidase-like activity was gradually decreased in the pH range from 4.5 to 10. The effect of temperature on the peroxidase-like activity of LaNiO3 nanocubes was evaluated in the range from 20 °C to 60 °C. The peroxidase-like activity was confirmed to be the optimal at 55 °C. In a word, the peroxidase-like activity of porous LaNiO3 nanocubes was dependent on the pH and temperature and the optimized pH and temperature were 4.5 and 55 °C, respectively.
Figure 3.
(A) Kinetic curves of A652 for monitoring the catalytic oxidation of 1 mM TMB with various concentrations of H2O2 in the presence of 10 μg/mL porous LaNiO3 nanocubes. (B) Kinetic curves of A652 for monitoring the catalytic oxidation of 1 mM TMB with various concentrations of porous LaNiO3 nanocubes in the presence of 10 mM H2O2. (C, D) pH and temperature-dependent peroxidase-like activities of the porous LaNiO3 nanocubes.
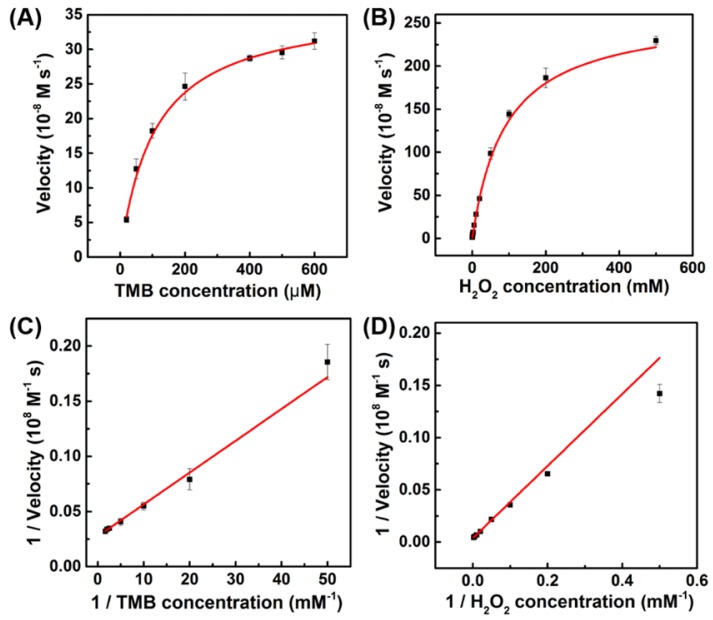
To investigate the catalytic mechanism and quantify the peroxidase-like activity of porous LaNiO3 nanocubes, the apparent steady-state kinetic parameters for the oxidation of TMB and H2O2 were determined. The kinetic data were obtained by varying the concentration of one substrate of H2O2 or TMB while keeping the other's concentration constant. In the suitable concentration range of H2O2 and TMB, typical Michaelis-Menten curves were obtained (Figure 4). The important enzyme kinetic parameters of maximum initial velocity (Vmax) and Michaelis-Menten constant (Km) were obtained using the Lineweaver-Burk plot. Km value showed the affinity of an enzymatic catalyst to its substrate, where the lower Km value represented the higher affinity. Vmax and Km values of porous LaNiO3 nanocubes, HRP, Pd-Ir cubes, graphene oxide (GO-COOH) and Fe3O4 were listed in Table S1. As seen in Table S1, the Km value of porous LaNiO3 nanocubes with H2O2 as the substrate was higher when compared to HRP, suggesting that the porous LaNiO3 nanocubes had a lower affinity with H2O2 than HRP. Low affinity between nanozymes and H2O2 is common for the nanozymes with peroxidase-like activities (such as the metal-, metal oxide-, and carbon-based nanozymes in Table S1). Interestingly, the Km value of porous LaNiO3 nanocubes with TMB as the substrate was lower when compared to HRP, which indicated the higher affinity between the porous LaNiO3 nanocubes and TMB than HRP. It is noteworthy that the porous LaNiO3 nanocubes showed higher Vmax values for both H2O2 and TMB when compared to other peroxidase-like nanozymes previously reported (Table S1). This demonstrated the excellent peroxidase mimicking activities of the currently developed LaNiO3 nanocubes.
Figure 4.
The steady-state kinetic assays of the porous LaNiO3 nanocubes. Plots of the velocity of the reaction versus different concentrations of TMB (A, 10 mM H2O2) or H2O2 (B, 0.8 mM TMB). Double reciprocal plots of the velocity versus varying concentration of (C) TMB and (D) H2O2.
Comparison of the Peroxidase-Like Activity of Porous LaNiO3 Nanocubes and Other Ni-based Nanomaterials
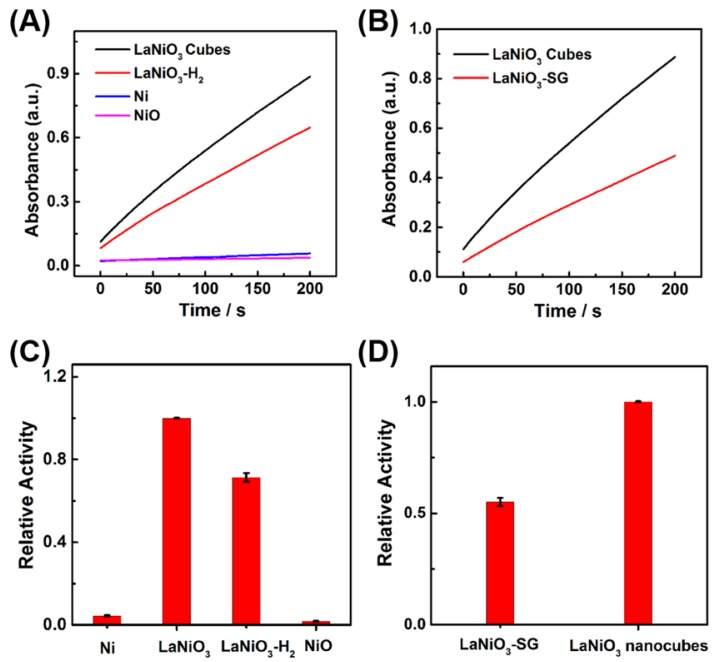
We investigated the influence of oxidation state of nickel atom in the Ni-based nanomaterials on their peroxidase-like activities. The peroxidase-like activities of Ni nanoparticles, LaNiO3-H2 nanocubes, and NiO with different oxidation state of nickel atom were studied and compared with that of the porous LaNiO3 nanocubes. LaNiO3-H2 nanocubes were obtained by annealing the porous LaNiO3 nanocubes at 350 °C for 2 hours under a forming gas with 5% H2 in argon, which partially reduced the Ni3+ to Ni2+. The successful synthesis of these Ni-based nanomaterials was confirmed by SEM and XRD (Figures S3-S6). The nickel atom in Ni nanoparticles was in the oxidation state of Ni0, while the nickel atom in LaNiO3 was Ni3+ and the nickel atom in NiO was Ni2+. As shown in Figure 5A and Figure 5C, the porous LaNiO3 nanocubes with Ni3+ exhibited the highest peroxidase-like activity while the Ni nanoparticles with Ni0 and NiO with Ni2+ showed negligible peroxidase-like activities. Interestingly, the LaNiO3-H2 nanocubes exhibited about 71% peroxidase-like activity of LaNiO3 nanocubes. The peroxidase-like activity of LaNiO3-H2 nanocubes decreased when compared to LaNiO3 nanocubes because the Ni3+ was partially reduced to Ni2+ in LaNiO3-H2 nanocubes. These results demonstrated that the oxidation state of nickel atom was very important for the peroxidase-like activity of Ni-based nanomaterials. More, the Ni3+ can be an optimal oxidation state for Ni-based nanomaterials with peroxidase mimicking activity. It is noteworthy that the peroxidase-like activity of LaNiO3-H2 nanocubes can be recovered when the LaNiO3-H2 nanocubes were reoxidized at 650 °C for 2 hours under the air to form LaNiO3-H2-Air. The peroxidase-like activity of LaNiO3-H2-Air was almost the same as that of the LaNiO3 nanocubes. The recovered catalytic activity of LaNiO3-H2-Air was attributed to the reoxidized Ni3+, further confirming the importance of Ni3+ to the Ni-based nanomaterials' peroxidase mimicking activities.
Figure 5.
(A, B) Kinetic curves of A652 for monitoring the catalytic oxidation of 1 mM TMB with 40 mM H2O2 in the presence of 10 μg/mL of (A) LaNiO3, LaNiO3-H2, Ni, and NiO; and (B) LaNiO3 and LaNiO3-SG. (C, D) Comparison of the peroxidase mimicking activities of (C) Ni, LaNiO3, LaNiO3-H2, and NiO; and (D) LaNiO3-SG and LaNiO3 nanocubes.
To study the effect of La atom on the catalytic activity of LaNiO3 nanocubes, the peroxidase mimicking activity of La2O3 nanoparticles with the same La3+ as LaNiO3 was investigated. As shown in Figure S7, La2O3 nanoparticles exhibited nearly negligible activity when compared with LaNiO3 nanocubes, suggesting that the Ni3+ instead of La3+ played the dominant role in the peroxidase mimicking activity of LaNiO3.
The peroxidase-like activity of LaNiO3-SG was also investigated and compared with that of the porous LaNiO3 nanocubes. The LaNiO3-SG was synthesized via a conventional sol-gel method to form LaNiO3 nanoparticles. Since the LaNiO3-SG and porous LaNiO3 nanocubes had the same 3+ oxidation state but different morphologies, it enabled us to study the morphology effect on the peroxidase-like activities of LaNiO3. Quantitative analysis showed that the porous LaNiO3 nanocubes exhibited about 2-fold higher activity when compared with LaNiO3-SG. The higher activity of porous LaNiO3 nanocubes than LaNiO3-SG may be attributed to the rough surfaces and rich porosity of nanocubes' morphology.
To exclude the effect of surface area on the catalytic activity, we investigated the surface area normalized peroxidase-like activities of Ni-based nanomaterials. As shown in Figure S8A, the surface area normalized results also showed that the oxidation state of nickel atom was very important for the peroxidase-like activity of Ni-based nanomaterials. The Ni3+ could be an optimal oxidation state for Ni-based nanomaterials with peroxidase-like activities. As shown in Figure S8B, the LaNiO3 nanocubes showed a higher surface area normalized activity than LaNiO3-SG, suggesting that the effect of morphology on the peroxidase-like activity of nanomaterials. In conclusion, the results obtained from the surface area normalized activity was well agreed with those obtained from mass normalized activity.
Therefore, the porous LaNiO3 nanocubes with the highest peroxidase mimicking activity were obtained by inducing its 3+ oxidation state of nickel atom in LaNiO3 perovskite and optimizing the morphology of nanomaterials.
Detection of H2O2, Glucose, and Sarcosine
On the basis of the highest intrinsic peroxidase-like activity of the porous LaNiO3 nanocubes, we developed colorimetric assays for H2O2, glucose, and sarcosine detection. As demonstrated above, the peroxidase-like activity of porous LaNiO3 nanocubes was dependent on the H2O2 concentrations, which can be used to detect H2O2 by monitoring the absorbance of TMB at 652 nm. As seen in Figure S10A and Figure S10B, the intensity of absorption peak at 652 nm increased with the increase of H2O2 concentration from 0 μM to 1000 μM. Figure S10C and Figure S10D exhibited good linearity relationships between the absorbance intensity at 652 nm and the H2O2 concentrations from 0 μM to 30 μM and 40 μM to 500 μM.
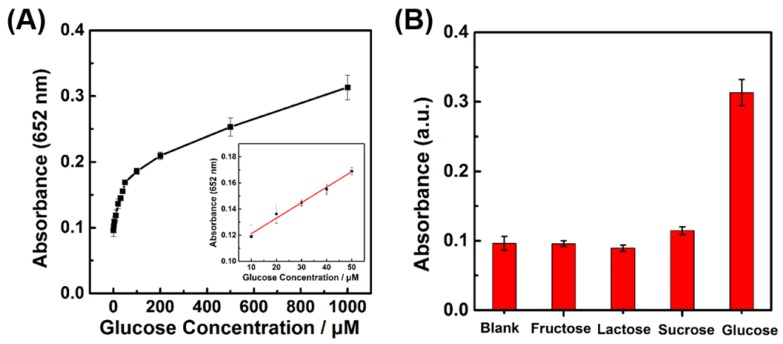
The glucose detection was then carried out by coupling cascade reactions of the glucose oxidation catalyzed by GOx and the TMB oxidation catalyzed by LaNiO3 nanocubes (Figure S11). First, GOx catalyzed the glucose oxidation with O2 to produce H2O2; then, the LaNiO3 nanocubes catalyzed the TMB oxidation with the in situ generated H2O2. Therefore, glucose could be determined by monitoring the absorbance of TMB at 652 nm. Figure 6A showed that the absorbance intensity exhibited a good response to glucose concentration from 2 μM to 1000 μM. A linear range from 10 μM to 50 μM and a detection limit of 8.16 μM were obtained for glucose detection. Control experiments were performed to evaluate the selectivity of the developed colorimetric assay for glucose by using other sugars such as fructose, lactose, and sucrose. As showed in Figure 6B, the other glucose analogues with a high concentration of 5 mM exhibited a negligible absorbance when compared with that of 1 mM glucose. This result demonstrated that the developed colorimetric assay had a good selectivity for glucose detection because of the high specificity of GOx for catalytic glucose oxidation.
Figure 6.
(A) Dependence of A652 for monitoring the catalytic oxidation of TMB on the concentration of glucose from 2 μM to 1 mM. The inset shows the linear calibration plot between the concentration of glucose and the absorbance at 652 nm. (B) Selectivity of glucose detection with the porous LaNiO3 nanocubes. Absorbance of TMB at 652 nm in the absence and presence of 5 mM fructose, 5mM lactose, 5 mM sucrose, and 1 mM glucose.
To demonstrate the practical applications of the developed colorimetric assay in real samples, four serum samples from patients were used for glucose detection. As shown in Table S3, the results obtained by our colorimetric assay agreed with the results obtained by a glucose meter, validating the developed colorimetric assay for glucose detection in complicated biomedical samples.
To show the general applications of the porous LaNiO3 nanocubes based colorimetric assays, the sarcosine detection was also performed. By using SOx and LaNiO3 nanocubes catalyzed cascade reaction, the sarcosine concentration could be determined by monitoring the absorbance of TMB at 652 nm (Figure S12). As shown in Figure S13, the absorbance intensity also exhibited a good response to sarcosine concentration from 0.5 μM to 500 μM. A linear range from 0.5 μM to 20 μM and a detection limit of 0.5 μM were obtained for sarcosine detection. The selectivity of the developed colorimetric assay for sarcosine detection was evaluated by using other amino acids such as glutamic acid, histidine, aspartic acid, and lysine. As shown in Figure S14, the other amino acids with a high concentration of 5 mM exhibited a negligible absorbance while sarcosine with a concentration of 1 mM showed a high absorbance, suggesting that the developed colorimetric assay had a good selectivity for sarcosine detection.
Conclusion
In conclusion, we obtained the porous LaNiO3 nanocubes as a peroxidase mimic by optimizing the oxidation state of nickel atom in Ni-based nanomaterials and the morphology of LaNiO3 perovskite oxide. For Ni-based nanomaterials, LaNiO3 perovskite oxide containing 3+ oxidation state showed excellent peroxidase-like activity while the Ni nanoparticles with Ni0 and NiO with Ni2+ exhibited negligible activities, confirming the importance of the oxidation state of nickel atom in Ni-based nanomaterials. More, the porous LaNiO3 nanocubes were more active than the LaNiO3 nanoparticles synthesized by a conventional sol-gel method. The porous LaNiO3 nanocubes followed the typical Michaeli-Menten kinetics and showed the dependence on the temperature, pH, catalyst concentration and H2O2 concentration. Based on the peroxidase-like activity of the porous LaNiO3 nanocubes, sensitive and selective colorimetric assays for H2O2, glucose, and sarcosine detection have been developed. The porous LaNiO3 nanocubes with high peroxidase-like activity exhibited promising applications in clinical diagnostics.
Supplementary Material
Supplementary figures and tables.
Acknowledgments
This work was supported by National Natural Science Foundation of China (21405081), Natural Science Foundation of Jiangsu Province (BK20130561), 973 Program (2015CB659400), PAPD program, Shuangchuang Program of Jiangsu Province, Open Funds of the State Key Laboratory of Analytical Chemistry for Life Science (SKLACLS1704), Open Funds of the State Key Laboratory of Electroanalytical Chemistry (SKLEAC201501), and Thousand Talents Program for Young Researchers.
References
- 1.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 5th edition. New York: W. H. Freeman and Company; 2008. pp. 183–229. [Google Scholar]
- 2.Breslow R. Artificial Enzymes. Science. 1982;218:532–7. doi: 10.1126/science.7123255. [DOI] [PubMed] [Google Scholar]
- 3.Murakami Y, Kikuchi J, Hisaeda Y, Hayashida O. Artificial enzymes. Chem Rev. 1996;96:721–58. doi: 10.1021/cr9403704. [DOI] [PubMed] [Google Scholar]
- 4.Breslow R. Artificial enzymes. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim; 2005. [Google Scholar]
- 5.Cheng H, Wang X, Wei H. Artificial Enzymes: The Next Wave. In: Wang Z, editor. Encyclopedia of Physical Organic Chemistry, First Edition. John Wiley & Sons, Inc; 2017. pp. 3885–3948. [Google Scholar]
- 6.Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–93. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 7.Ragg R, Tahir MN, Tremel W. Solids Go Bio: Inorganic Nanoparticles as Enzyme Mimics. Eur J Inorg Chem. 2016:1906–15. [Google Scholar]
- 8.Gao L, Yan X. Nanozymes: an emerging field bridging nanotechnology and biology. Sci China Life Sci. 2016;59:400–2. doi: 10.1007/s11427-016-5044-3. [DOI] [PubMed] [Google Scholar]
- 9.Melemenidis S, Jefferson A, Ruparelia N, Akhtar AM, Xie J, Allen D. et al. Molecular Magnetic Resonance Imaging of Angiogenesis In Vivo using Polyvalent Cyclic RGD-Iron Oxide Microparticle Conjugates. Theranostics. 2015;5:515–29. doi: 10.7150/thno.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Hu Y, Wei H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3:41–60. [Google Scholar]
- 11.Wang X, Guo W, Hu Y, Wu J, Wei H. Nanozymes: Next Wave of Artificial Enzymes. Springer; 2016. [Google Scholar]
- 12.Liu B, Liu J. Surface modification of nanozymes. Nano Research. 2017;10:1125–48. [Google Scholar]
- 13.Manea F, Houillon FB, Pasquato L, Scrimin P. Nanozymes: Gold-nanoparticle-based transphosphorylation catalysts. Angew Chem Int Ed. 2004;43:6165–9. doi: 10.1002/anie.200460649. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–83. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv Mater. 2010;22:2206–10. doi: 10.1002/adma.200903783. [DOI] [PubMed] [Google Scholar]
- 16.Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R. et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol. 2012;7:530–5. doi: 10.1038/nnano.2012.91. [DOI] [PubMed] [Google Scholar]
- 17.Xia X, Zhang J, Lu N, Kim MJ, Ghale K, Xu Y. et al. Pd-Ir Core-Shell Nanocubes: A Type of Highly Efficient and Versatile Peroxidase Mimic. ACS Nano. 2015;9:9994–10004. doi: 10.1021/acsnano.5b03525. [DOI] [PubMed] [Google Scholar]
- 18.Cai R, Yang D, Peng S, Chen X, Huang Y, Liu Y. et al. Single Nanoparticle to 3D Supercage: Framing for an Artificial Enzyme System. J Am Chem Soc. 2015;137:13957–63. doi: 10.1021/jacs.5b09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Hu S, Yin J, He W, Lu W, Ma M. et al. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J Am Chem Soc. 2016;138:5860–5. doi: 10.1021/jacs.5b12070. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Zhu C, Su S, Li D, He Y, Huang Q. et al. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano. 2010;4:7451–8. doi: 10.1021/nn102592h. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Liu Q, Jing C, Li Y, Li D, Luo W. et al. Catalytic Gold Nanoparticles for Nanoplasmonic Detection of DNA Hybridization. Angew Chem Int Ed. 2011;50:11994–8. doi: 10.1002/anie.201105121. [DOI] [PubMed] [Google Scholar]
- 22.Vernekar AA, Sinha D, Srivastava S, Paramasivam PU, D'Silva P, Mugesh G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat Commun. 2014;5:5301. doi: 10.1038/ncomms6301. [DOI] [PubMed] [Google Scholar]
- 23.Shen X, Liu W, Gao X, Lu Z, Wu X, Gao X. Mechanisms of Oxidase and Superoxide Dismutation-like Activities of Gold, Silver, Platinum, and Palladium, and Their Alloys: A General Way to the Activation of Molecular Oxygen. J Am Chem Soc. 2015;137:15882–91. doi: 10.1021/jacs.5b10346. [DOI] [PubMed] [Google Scholar]
- 24.Tonga GY, Jeong Y, Duncan B, Mizuhara T, Mout R, Das R. et al. Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat Chem. 2015;7:597–603. doi: 10.1038/nchem.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Huang Y, Zhu H, Zhu Q, Xia Y. Three-in-One: Sensing, Self-Assembly, and Cascade Catalysis of Cyclodextrin Modified Gold Nanoparticles. J Am Chem Soc. 2016;138:16645–54. doi: 10.1021/jacs.6b07590. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Li Z, Chen Z, Ren J, Qu X. Mesoporous silica-encapsulated gold nanoparticles as artificial enzymes for self-activated cascade catalysis. Biomaterials. 2013;34:2600–10. doi: 10.1016/j.biomaterials.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Ren J, Qu X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv Mater. 2014;26:4200–17. doi: 10.1002/adma.201400238. [DOI] [PubMed] [Google Scholar]
- 28.Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008;80:2250–4. doi: 10.1021/ac702203f. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Han X, Liu J. Iron oxide nanozyme catalyzed synthesis of fluorescent polydopamine for light-up Zn2+ detection. Nanoscale. 2016;8:13620–6. doi: 10.1039/c6nr02584f. [DOI] [PubMed] [Google Scholar]
- 30.Fan K, Cao C, Pan Y, Lu D, Yang D, Feng J. et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat Nanotechnol. 2012;7:459–64. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 31.Tao Y, Ju E, Ren J, Qu X. Bifunctionalized Mesoporous Silica-Supported Gold Nanoparticles: Intrinsic Oxidase and Peroxidase Catalytic Activities for Antibacterial Applications. Adv Mater. 2015;27:1097–104. doi: 10.1002/adma.201405105. [DOI] [PubMed] [Google Scholar]
- 32.Xue T, Peng B, Xue M, Zhong X, Chiu C-Y, Yang S. et al. Integration of molecular and enzymatic catalysts on graphene for biomimetic generation of antithrombotic species. Nat Commun. 2014;5:3200. doi: 10.1038/ncomms4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao L, Giglio KM, Nelson JL, Sondermann H, Travis AJ. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale. 2014;6:2588–93. doi: 10.1039/c3nr05422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim CK, Kim T, Choi I-Y, Soh M, Kim D, Kim Y-J. et al. Ceria Nanoparticles that can Protect against Ischemic Stroke. Angew Chem Int Ed. 2012;51:11039–43. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- 35.Kwon HJ, Cha M-Y, Kim D, Kim DK, Soh M, Shin K. et al. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer's Disease. ACS Nano. 2016;10:2860–70. doi: 10.1021/acsnano.5b08045. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang Z, Li X, Wang L, Yin M, Wang L. et al. Dietary Iron Oxide Nanoparticles Delay Aging and Ameliorate Neurodegeneration in Drosophila. Adv Mater. 2016;28:1387–93. doi: 10.1002/adma.201503893. [DOI] [PubMed] [Google Scholar]
- 37.Chen ZW, Yin JJ, Zhou YT, Zhang Y, Song L, Song MJ. et al. Dual Enzyme-like Activities of Iron Oxide Nanoparticles and Their Implication for Diminishing Cytotoxicity. ACS Nano. 2012;6:4001–12. doi: 10.1021/nn300291r. [DOI] [PubMed] [Google Scholar]
- 38.Xiong F, Wang H, Feng Y, Li Y, Hua X, Pang X, Cardioprotective activity of iron oxide nanoparticles. Sci Rep; 2015. p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong J, Song L, Yin J-J, He W, Wu Y, Gu N. et al. Co3O4 Nanoparticles with Multi-Enzyme Activities and Their Application in Immunohistochemical Assay. ACS Appl Mat Interfaces. 2014;6:1959–70. doi: 10.1021/am405009f. [DOI] [PubMed] [Google Scholar]
- 40.Wu G, He S, Peng H, Deng H, Liu A, Lin X. et al. Citrate-Capped Platinum Nanoparticle as a Smart Probe for Ultrasensitive Mercury Sensing. Anal Chem. 2014;86:10955–60. doi: 10.1021/ac503544w. [DOI] [PubMed] [Google Scholar]
- 41.Shi W, Wang Q, Long Y, Cheng Z, Chen S, Zheng H. et al. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun. 2011;47:6695–7. doi: 10.1039/c1cc11943e. [DOI] [PubMed] [Google Scholar]
- 42.Duan D, Fan K, Zhang D, Tan S, Liang M, Liu Y. et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron. 2015;74:134–41. doi: 10.1016/j.bios.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Liu Q, Yang Y, Li H, Zhu R, Shao Q, Yang S. et al. NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2 and glucose detection. Biosens Bioelectron. 2015;64:147–53. doi: 10.1016/j.bios.2014.08.062. [DOI] [PubMed] [Google Scholar]
- 44.He W, Wu X, Liu J, Hu X, Zhang K, Hou S. et al. Design of AgM Bimetallic Alloy Nanostructures (M = Au, Pd, Pt) with Tunable Morphology and Peroxidase-Like Activity. Chem Mater. 2010;22:2988–94. [Google Scholar]
- 45.Liu S, Lu F, Xing R, Zhu J. Structural Effects of Fe3O4 Nanocrystals on Peroxidase-Like Activity. Chem Euro J. 2011;17:620–5. doi: 10.1002/chem.201001789. [DOI] [PubMed] [Google Scholar]
- 46.Ge C, Fang G, Shen X, Chong Y, Wamer WG, Gao X. et al. Facet Energy versus Enzyme-like Activities: The Unexpected Protection of Palladium Nanocrystals against Oxidative Damage. ACS Nano. 2016;10:10436–45. doi: 10.1021/acsnano.6b06297. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Chen W, Liu A, Hong L, Deng H, Lin X. Comparison of the Peroxidase-Like Activity of Unmodified, Amino-Modified, and Citrate-Capped Gold Nanoparticles. ChemPhysChem. 2012;13:1199–204. doi: 10.1002/cphc.201100906. [DOI] [PubMed] [Google Scholar]
- 48.Fan K, Wang H, Xi J, Liu Q, Meng X, Duan D. et al. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun. 2016;53:424–7. doi: 10.1039/c6cc08542c. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H, Lin S, Muhammad F, Lin Y-W, Wei H. Rationally Modulate the Oxidase-like Activity of Nanoceria for Self Regulated Bioassays. ACS Sensors. 2016;1:1336–43. [Google Scholar]
- 50.Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29:2705–9. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS. et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46:2736–8. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, Huang Z, Liu J. Boosting the oxidase mimicking activity of nanoceria by fluoride capping: rivaling protein enzymes and ultrasensitive F- detection. Nanoscale. 2016;8:13562–7. doi: 10.1039/c6nr02730j. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X-Q, Gong S-W, Zhang Y, Yang T, Wang C-Y, Gu N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J Mater Chem. 2010;20:5110–6. [Google Scholar]
- 54.Xiong R, Soenen SJ, Braeckmans K, Skirtach AG. Towards Theranostic Multicompartment Microcapsules: in-situ Diagnostics and Laser-induced Treatment. Theranostics. 2013;3:141–51. doi: 10.7150/thno.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng H, Wang X, Wei H. Ratiometric Electrochemical Sensor for Effective and Reliable Detection of Ascorbic Acid in Living Brains. Anal Chem. 2015;87:8889–95. doi: 10.1021/acs.analchem.5b02014. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Guo S, Chung C-S, Zhu W, Sun S. A Sensitive H2O2 Assay Based on Dumbbell-like PtPd-Fe3O4 Nanoparticles. Adv Mater. 2013;25:132–6. doi: 10.1002/adma.201203218. [DOI] [PubMed] [Google Scholar]
- 57.Qian C, Chen Y, Zhu S, Yu J, Zhang L, Feng P. et al. ATP-Responsive and Near-Infrared-Emissive Nanocarriers for Anticancer Drug Delivery and Real-Time Imaging. Theranostics. 2016;6:1053–64. doi: 10.7150/thno.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P, Chen Y, Zeng Y, Shen C, Li R, Guo Z. et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci USA. 2015;112:E6129–E38. doi: 10.1073/pnas.1505799112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng H, Zhang L, He J, Guo W, Zhou Z, Zhang X. et al. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal Chem. 2016;88:5489–97. doi: 10.1021/acs.analchem.6b00975. [DOI] [PubMed] [Google Scholar]
- 60.Liu B, Sun Z, Huang P-JJ, Liu J. Hydrogen Peroxide Displacing DNA from Nanoceria: Mechanism and Detection of Glucose in Serum. J Am Chem Soc. 2015;137:1290–5. doi: 10.1021/ja511444e. [DOI] [PubMed] [Google Scholar]
- 61.Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S. et al. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano. 2013;7:6758–66. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 62.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112:8260–5. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He H, Xu X, Wu H, Jin Y. Enzymatic Plasmonic Engineering of Ag/Au Bimetallic Nanoshells and Their Use for Sensitive Optical Glucose Sensing. Adv Mater. 2012;24:1736–40. doi: 10.1002/adma.201104678. [DOI] [PubMed] [Google Scholar]
- 64.Chen L, Li H, He H, Wu H, Jin Y. Smart Plasmonic Glucose Nanosensors as Generic Theranostic Agents for Targeting-Free Cancer Cell Screening and Killing. Anal Chem. 2015;87:6868–74. doi: 10.1021/acs.analchem.5b01260. [DOI] [PubMed] [Google Scholar]
- 65.Fan W, Lu N, Huang P, Liu Y, Yang Z, Wang S. et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew Chem Int Ed. 2017;56:1229–33. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]
- 66.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J. et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 67.Zhang J, Zhao Y, Zhao X, Liu Z, Chen W. Porous Perovskite LaNiO3 Nanocubes as Cathode Catalysts for Li-O2 Batteries with Low Charge Potential. Sci Rep. 2014;4:6005. doi: 10.1038/srep06005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H, More R, Grundmann H, Cui C, Erni R, Patzke GR. Promoting Photochemical Water Oxidation with Metallic Band Structures. J Am Chem Soc. 2016;138:1527–35. doi: 10.1021/jacs.5b10215. [DOI] [PubMed] [Google Scholar]
- 69.Mahaleh YBM, Sadrnezhaad SK, Hosseini D. NiO Nanoparticles Synthesis by Chemical Precipitation and Effect of Applied Surfactant on Distribution of Particle Size. J Nanomater. 2008;2008:470595. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.