Abstract
Background
Donor organ shortage represents a major problem in lung transplantation. Donation after cardiac death could help to expand the pool of organs, but the additional period of warm ischemia after cardiac arrest aggravates primary graft dysfunction. The pulmonary endothelium of the graft constitutes an important source and target of reactive oxygen species generated during ischemia and reperfusion. Targeted protection of graft pulmonary endothelial cells by the antioxidant enzyme catalase, conjugated with a platelet/endothelial cell adhesion molecule-1 (PECAM-1) antibody to nanosized particles (anti-PECAM/catalase conjugates), might improve outcome in lung transplantation using donors after cardiac death and prolonged hypothermic preservation.
Methods
Left lung transplantation was performed in 18 pigs. Before cardiac arrest, donors received anti-PECAM/ catalase, unconjugated component mixture or vehicle solution. After 90-min warm and 18-hr hypothermic ischemia, lungs were transplanted, and function was assessed during 6 hr after reperfusion. Samples of bronchoalveolar lavage fluid and lung tissue were taken thereafter. Six sham-operated animals served as controls.
Results
During 6-hr reperfusion, anti-PECAM/catalase significantly ameliorated graft function, evidenced by major improvements of gas exchange and reduced intrapulmonary shunt fraction. Furthermore, lipid peroxidation, alveolar leakage, and edema formation were reduced in protected grafts. Similarly moderate lung pathology was seen after transplantation.
Conclusions
Augmentation of the antioxidant capacity of graft pulmonary endothelial cells with anti-PECAM/catalase nanoparticles represents a straightforward approach to enable a safe transplantation of prolonged preserved donation after cardiac death lungs. Anti-PECAM/catalase protection alleviated oxidative stress and allowed immediate reconstitution of normal gas exchange and pulmonary microcirculation, a prerequisite for improved graft and patient outcome.
Keywords: Lung transplantation, Vascular targeting, Ischemia/reperfusion injury, Antioxidants, Donors after cardiac death, Nanoparticle
Critical organ shortage has revived the interest in lung grafts procured from donors after cardiac death (1–3). Indeed, donation after cardiac death (DCD) today represents the fastest growing category in organ donation (4, 5). In The Netherlands, a statistical analysis revealed that an effective involvement, especially of Maastricht category 3 (controlled) DCD organs, could provide as much as 20% of the patients on the waiting list with a lung graft (6). In contrast to brain dead, heart-beating donors, lungs from DCD. are recovered after cessation of circulation (7), resulting in significant additional injury as a result of the period of detrimental warm ischemia before organ retrieval.
Inclusion of extended criteria donor lungs, that is, lungs procured within an ischemic period longer than 8 hr and DCD lungs, would alleviate allocation and enlarge the donor pool. However, without effective mitigation of the ischemia/reperfusion injury (IRI), which still represents a key limitation to successful outcomes in the early postoperative period, the incidence of primary graft failure would likely increase (8–11). In fact, primary graft failure grade 3 (International Society for Heart and Lung Transplantation) is reported with an incidence as high as 13% (12) to 29% (13) in DCD lung transplant recipients, indicating the importance of this complication.
High levels of reactive oxygen species (ROS, including H2O2) play a pivotal role in lung transplant–associated IRI (8). Cessation of pulmonary perfusion results in immediate ROS production in the pulmonary endothelium (14), because lungs are recovered inflated with oxygen (nonhypoxic ischemia (15). During hypothermic preservation, ROS generation continues (16) and further increases on reperfusion (17). Hence, with the pulmonary endothelium constituting both source and target of ROS, vascular oxidative stress is central in acute lung IRI. After transplantation, endothelial dysfunction can cause microvascular failure and increased vascular permeability (18, 19), resulting in hypoxemia, increased shunt perfusion, and edema (20). In particular, poor restoration of the pulmonary microcirculation causes diminished tissue oxygen supply and necessitates prolonged ventilator treatment. Thus, endothelial antioxidant protection might enhance ischemic tolerance and facilitate appropriate gas exchange in DCD lung transplantation.
Antioxidant enzymes, such as catalase that decomposes H2O2 into water, are candidate drugs for augmented graft resistance toward IRI; however, their protective effects are insufficient because of inadequate endothelial delivery. This problem might be overcome by vascular immunotargeting (21–23): nanosized immunoconjugates of catalase and antibodies that are directed against the endothelial determinant platelet/endothelial cell adhesion molecule-1 (PECAM-1; i.e., anti-PECAM/catalase conjugates) accumulate in the pulmonary vasculature after intravenous administration, bind, and eventually enter the pulmonary endothelial cell, thus providing defense against oxidative stress (24). The potential of anti-PECAM/catalase conjugates reducing IRI in DCD lungs, using a robust large animal transplant model, has not been investigated so far. Previously, we have analyzed the feasibility of DCD lung transplantation in a porcine model that mimics closely the clinical scenario of single lung transplantation (25–27). The goal of the current study was to test anti-PECAM/catalase nanoparticles in this standardized DCD model after long-term preservation, a translational approach to evaluating protective utilities of vascular immunotargeting in humans.
RESULTS
Animal Model
Baseline values (before sternotomy or thoracotomy/ transplantation) showed no significant differences in gas exchange, hemodynamics, and body weight between donor and recipient animals or between the study groups. Time of hypothermic preservation did not differ significantly between treated cohorts (conjugate: 18.3±0.1 hr; mixture: 18.2±0.3 hr; vehicle: 18.3±0.1 hr). During the 6-hr reperfusion period, no significant changes in leukocyte count and hemoglobin concentration were noted among the groups.
Donor Treatment
Donor animals were monitored to evaluate possible pharmacologic effects of the experimental therapeutic interventions. Significant group differences concerning hemodynamics or gas exchange were absent. Leukocyte counts remained unaffected in all groups.
Pulmonary Graft Function
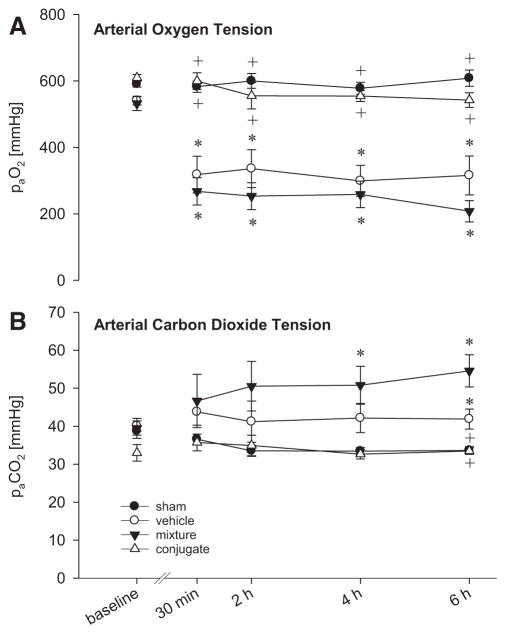
Transplantation resulted in major gas exchange abnormalities (Fig. 1A, B). Animals transplanted with vehicle-treated DCD lungs showed a significant reduction of paO2 by 45% to 65% and a significant paCO2 retention compared with the sham group. These changes were not improved by treatment with the mixture of unconjugated anti-PECAM and catalase. In marked contrast, donor treatment with anti-PECAM/catalase conjugates significantly improved recipients’ DCD graft lung function. Already, 30 min after reperfusion, paO2 reached a level of 599 mm Hg and remained there during the whole reperfusion period, comparable with oxygenation in the sham group. Similarly, paCO2 in the anti-PECAM/catalase conjugate group remained unchanged compared with the sham group.
FIGURE 1.
Gas exchange in single-lung ventilation (FiO2= 1.0) and perfusion of the native lung before transplantation (baseline, native lung) and of pulmonary DCD grafts after 30 min, 2, 4, and 6 hr reperfusion. Arterial oxygen tension (A; paO2) and carbon dioxide tension (B; paCO2) in grafts pretreated with anti-PECAM/catalase conjugates were unchanged compared with sham-operated animals. Vehicle- and mixture-treated animals revealed severely impaired gas exchange with significantly reduced paO2 and paCO2 retention. Data given as mean±standard error of the mean. *P<0.05 vs. sham. +P<0.05 vs. vehicle.
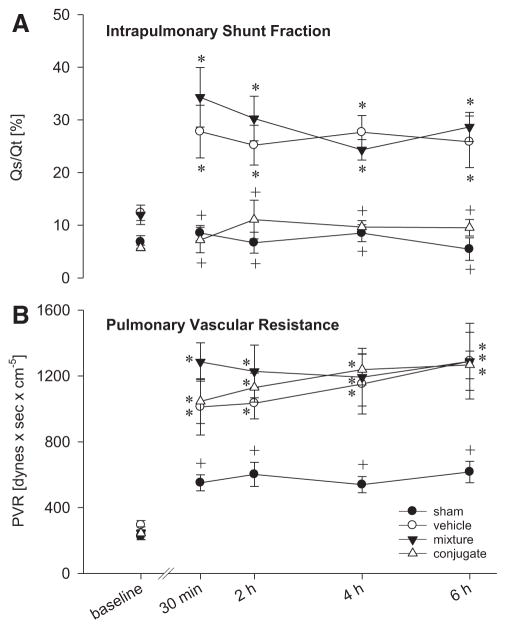
Furthermore, intrapulmonary shunt fraction, Qs/Qt, showed significant differences (Fig. 2A). Compared with the sham group (range: 5–9%), grafts from mixture- and vehicle-treated animals had significantly increased shunt fractions (24–34%), whereas Qs/Qt in anti-PECAM/catalase conjugate–treated grafts remained unchanged (7–11%). Pulmonary vascular resistance (PVR) did not differ significantly between the transplant groups but was increased compared with the sham group (Fig. 2B). Concerning systemic hemodynamics, no significant group differences were detected.
FIGURE 2.
Pulmonary shunt fraction (A; Qs/Qt) and pulmonary vascular resistance (B; PVR) before (baseline, native lung) and after transplantation of DCD grafts and 6 hr reperfusion. Anti-PECAM/catalase conjugate–pretreated graft lungs revealed a significantly reduced shunt fraction, indicating more homogeneous perfusion. PVR was markedly increased in all transplant groups. Data given as mean±standard error of the mean. *P<0.05 vs. sham. + P<0.05 vs. vehicle.
Morphologic Changes in Lung Tissue
Histologic examination of cold-preserved, nontransplanted donor lungs resulted in similar pathology scores in the three treatment groups (vehicle: 0.6±0.1; mixture: 0.5±0.1; conjugate: 0.5±0.1) and the control lung (0.9±0.2), indicating modest damage at this time point (before transplantation). In lung DCD grafts, moderate damage without significant group differences was detected after 6 hr reperfusion. Alveolar leukocyte accumulation and interstitial edema were visually enhanced in all DCD grafts versus lungs of sham-operated animals (Table 1).
TABLE 1.
Histologic examination of lung graft tissue specimens (semiquantitative score)
| Sham | Vehicle | Mixture | Conjugate | |
|---|---|---|---|---|
| Endothelial alteration | 1.7±0.2 | 2.3±0.2 | 2.6±0.3 | 2.7±0.2 |
| Vascular leukocytes | 2.7±1.7 | 2.0±0.3 | 2.3±0.3 | 2.3±0.2 |
| Alveolar leukocytes | 1.0±0.1 | 1.8±0.4a | 2.3±0.3a | 2.8±0.2a |
| Alveolar macrophages | 1.0±0.1 | 1.0±0.1 | 1.5±0.2 | 1.2±0.2 |
| Alveolar cell desquamation | 0 | 0 | 0.8±0.2 | 0.8±0.2 |
| Interstitial edema | 1.0±0.1 | 1.5±0.2a | 1.7±0.2a | 2.0±0.1a |
| Intra-alveolar edema | 0 | 0.2±0.2 | 0.3±0.2 | 0 |
| Fibrin exudate | 0.3±0.2 | 0.7±0.5 | 1.7±0.5 | 1.0±0.1 |
| Necrosis | 0 | 0 | 0 | 0 |
| Total score | 0.9±0.2 | 1.1±0.1 | 1.5±0.2 | 1.4±0.1 |
Histopathologic examination of hematoxylin-eosin–stained lung graft tissue samples after 6-hr reperfusion. According to a semiquantitative score, morphologic alterations were assessed as 0, normal/absent; 1, mild; 2, moderate; and 3, severe. Alveolar leukocytes and interstitial edema formation were markedly enhanced in grafts compared with sham-operated animals. Data shown as mean±standard error of the mean.
P<0.05 vs. sham.
Wet-to-Dry Weight Ratio
Wet-to-dry weight (W/D) ratio revealed the lowest edema formation in anti-PECAM/catalase conjugate–treated grafts, which seemed unchanged in comparison with the sham group, whereas in the vehicle and mixture group, a significant increase was noted (Fig. 3A).
FIGURE 3.
Wet-to-dry weight ratio in tissue specimens of DCD grafts and sham-operated lungs (A) and total protein content in bronchoalveolar lavage fluid (BALF) from DCD grafts and sham-operated lungs (B) after 6 hr reperfusion. Both W/D ratio and total protein content were lowest in conjugate-treated DCD grafts. Data given as mean ± standard error of the mean. *P<0.05 vs. sham.
Bronchoalveolar Lavage Fluid Analysis
Bronchoalveolar lavage fluid (BALF) protein content was lowest in the anti-PECAM/catalase conjugate group and remained unchanged in comparison with the sham group (Fig. 3B). Thiobarbituric acid reactive substances (TBARS) concentration in BALF was significantly increased in the vehicle group, whereas it remained unchanged in the anti-PECAM/catalase conjugate and mixture group compared with the sham group (Fig. 4A). Compared with control lungs, TBARS in cold-preserved donor lungs (before transplantation) were significantly increased in the vehicle and mixture group, but not in the anti-PECAM/catalase conjugate group (Fig. 4B). BALF cell differentials from graft lungs yielded increased neutrophil counts without significant differences between the study groups (Fig. 4C). In BALF smears from control and cold-preserved donor lungs, mainly macrophages were detected; significant group differences were absent (Fig. 4D).
FIGURE 4.
BALF thiobarbituric acid reactive substances (TBARS) and cytological analyses in DCD grafts and sham-operated lungs after 6 hr reperfusion (A, C) and of cold-preserved DCD lungs (nontransplanted) and (untreated) control lungs (B, D). Lipid peroxidation in BALF of the conjugate group remained unchanged both after cold preservation and after transplantation and 6 hr reperfusion. BALF of DCD grafts was characterized by a significant neutrophil influx, whereas in cold-preserved lungs and lungs from sham-operated animals predominantly macrophages were found. Data given as mean±standard error of the mean. *P<0.05 vs. sham/control.
DISCUSSION
Contrary to other solid organ grafts, IRI in lung transplantation is not caused by anoxia/hypoxia and reoxygenation. Lungs maintain aerobic metabolism during ischemia using oxygen from the alveolar space (28). Especially in the situation of DCD, nonhypoxic ischemia might be advantageous to keep lung cells viable (29). Indeed, tissue ATP levels in oxygen-inflated DCD lungs remain unchanged even during long-term storage (26). However, cessation of the circulation immediately initiates ROS production from sources, including graft endothelial NADPH oxidase and mitochondria (14, 30), leading to vascular oxidative stress already during ischemia (16). In line with that, we found increased TBARS concentrations in BALF of the vehicle and mixture groups before transplantation, indicating ongoing lipid peroxidation during cold ischemia. On reperfusion, oxygen and blood are reintroduced, further boosting ROS production. Hence, the pulmonary endothelium represents a principal target of the IRI in lung transplantation.
Particularly, in DCD lungs, where the warm ischemic period rapidly leads to detrimental damage, protective measures should ideally be initiated during organ procurement. According to the Maastricht criteria, four different categories of DCD are defined (7). We simulated the situation of a type III, controlled donor in the intensive care unit, where the patient is withdrawn from vital support because of terminal illness. Hence, cardiac arrest can be anticipated, allowing the pretreatment. Because lung graft function is directly related to the integrity of the alveolocapillary network (19), we tested whether reinforcement of the antioxidative capacity of the pulmonary endothelium, attained by means of targeted delivery of anti-PECAM/catalase to the donor, is beneficial in DCD lungs. In fact, reduced BALF-TBARS concentrations before transplantation suggested effective protection from oxidative stress already during warm ischemia and hypothermic preservation.
Moreover, anti-PECAM/catalase nanoparticles provided significant improvements of gas exchange in recipients. Oxygen tension in the anti-PECAM/catalase conjugate–treated group reached levels of nontransplanted animals in the initial analysis after 30-min reperfusion, demonstrating that alleviation of endothelial oxidative stress during warm and cold ischemic intervals helps to preserve graft function immediately after reperfusion. In the ensuing period, arterial oxygen tension persisted at that excellent level, whereas arterial carbon dioxide tension remained unchanged toward sham, indicating a sustained effect. In control DCD groups, oxygenation was significantly worse during the entire reperfusion period and carbon dioxide retention was noted. These data are of particular importance because gas exchange is directly related to the integrity of the alveolocapillary network and represents a central parameter of lung graft function. Furthermore, immediate adequate oxygenation is a key requirement after lung transplantation, improving graft and patient outcome: In the clinical arena, gas exchange after 6-hr reperfusion best correlates with 30-day mortality and is closely related to the duration of intubation or stay in the intensive care unit (31, 32). In addition, a correlation between graft ischemia time and gas exchange within the first 6 hr after reperfusion has been described (33). Indeed, IRI-associated initial hypoxia after transplantation is often difficult to handle and requires prolonged ventilator treatment, while increasing the risk for the development of an acute respiratory distress syndrome (20). Furthermore, severe IRI may go along with an increased incidence of the bronchiolitis obliterans syndrome, a substantial problem for long-term outcome (34). Of course, in view of questions such as development of the bronchiolitis obliterans syndrome, further investigations, using a chronic experimental setting, are needed.
In line with the improvements of graft pulmonary gas exchange, we observed alleviated oxidative stress and edema formation in anti-PECAM/catalase–treated lungs. Diminished W/D ratio and low BALF protein content indicated reduced microvascular and alveolar leakage, suggesting better preserved endothelial integrity after conjugate treatment and transplantation. In addition, decreased lipid peroxidation, as indicated by reduced TBARS concentration in the lavage fluid of those grafts after hypothermic preservation and after reperfusion, squares well with reduced damage to the alveolo-capillary network (14).
These results collectively point out that beneficial effects of anti-PECAM/catalase conjugates were resulting from the protection of graft microvascular endothelial cells against oxidative stress during both warm and cold ischemia and graft reperfusion. Endothelial protection finally facilitated an instantaneous and homogeneous reconstitution of the graft microcirculation, a pivotal requirement in transplantation for improved early and long-term graft function. Optimization of the microcirculation was indicated by a continuously low intrapulmonary shunt perfusion in the anti-PECAM/ catalase group. In contrast, in the vehicle and mixture groups, up to one third of the cardiac output shunted through the lung grafts without participating in oxygenation, pointing to persistent microvascular perfusion deficits.
Interestingly, catalase targeting did not affect graft leukocyte recruitment. Although endothelial function remained preserved with regard to pulmonary gas exchange, edema protection and ROS-related lipid peroxidation, other events within the postischemic cascade, such as leukocyte recruitment, obviously seemed to proceed. Nevertheless, in view of the functional improvements, cell recruitment seemed relatively innocuous at that time point, suggesting that leukocytes were not fully activated yet. Particularly, with regard to acute oxidative stress, such a dissociation between leukocyte infiltration and lung injury has been described (35). In line with that, only moderate morphologic changes were found in the graft lungs of all groups at that early time point of the reperfusion period. Actually, lung IRI typically presents as biphasic inflammatory process, where damage in the early phase is mainly ROS mediated, targeting endothelial functional integrity, whereas leukocyte-dependent alterations become evident later (8, 18). Consistently with that, rapid gas exchange normalization in the conjugate group indicated that protective effects were primarily associated with a functional preservation of the endothelium before the manifestation of pronounced morphologic group differences. Notably, improved microcirculation in the anti-PECAM/ catalase group itself might have alleviated leukocyte influx. Since more alveoli were perfused because of better restitution of the microcirculation, the chance of leukocyte extravasation to the alveolar space increases.
Despite improved microcirculation after anti-PECAM/ catalase treatment, we observed disturbed vasoreactivity in all transplant groups, evidenced by an increased PVR. Especially, the combination of 90-min warm ischemia and 18 hr hypothermic preservation herein represents an enormous challenge for the vasculature of the grafts, resulting in reduced vessel elasticity. This might be related particularly to altered vascular smooth muscle cell functions as recently demonstrated in an isolated perfused kidney DCD model (36). In addition, deficiency of nitric oxide (NO), which is reported for DCD lung transplantation (37), leading to pulmonary arteriolar constriction, could have contributed to this effect. Catalase detoxifies H2O2 but does not alleviate NO deficiency that is primarily a result of NO quenching by the superoxide anion (38). In this context, it will be interesting to test the recently designed anti-PECAM/SOD conjugate (39) per se and along with anti-PECAM/catalase. Graft function would also potentially profit from an additional intervention supplementing NO or its precursor L-arginine during early reperfusion (27, 40). NO may inhibit neutrophil activation and platelet aggregation, thus attenuating graft cell recruitment. Furthermore, NO represents a potent vasodilator acting through the increase of cyclic guanosine monophosphate in smooth muscle cells, thereby potentially improving pulmonary vasomotor functions (41).
In the current study, it was demonstrated for the first time in a large animal model that targeted antioxidant protection of pulmonary endothelial cells in DCD lungs has the potential to improve graft function significantly. Nanosized anti-PECAM/catalase is effective even after warm ischemia, long-term hypothermic preservation, and transplantation. Our translational approach may thus contribute to donor pool enlargement via inclusion of marginal organs and by possibly increasing the amount of time that lungs can successfully be transplanted. Vascular immunotargeting with antioxidants should be considered along with other novel approaches, such as targeting of antithrombotic drugs (42), supplementation of NO (27, 40), or ex vivo donor lung reconditioning (43, 44).
MATERIALS AND METHODS
Preparation of Nanosized Anti-PECAM/Catalase Conjugates
Anti-PECAM/catalase was synthesized as described previously (30). In brief, catalase (20 kU/mg; Sigma, St. Louis, MO) and murine monoclonal antibody 62, recognizing human and porcine PECAM-1 (anti-PECAM; Centocor, Malvern, PA), were biotinylated (NHS-LC-Biotin; Pierce, Rockford, IL) and conjugated at a molar ratio of 1:1 using neutravidin (Pierce). Molar ratio of neutravidin:b-protein was 1:1.25 to produce conjugates with 100 to 300 nm mean diameter, as assessed by dynamic light scattering (45). Immunoconjugates were stored at −20°C in phosphate-buffered saline, pH 7.4 containing 50% glycerol solution. To verify the biologic effectiveness of each immunoconjugate preparation, in vitro tests with human umbilical vein endothelial cells were performed before injection into the donor animals (see Figure, Supplemental Digital Content 1, http://links.lww.com/TP/A466).
Animal Model
All experiments were approved by the local government (Regierung von Oberbayern, AZ 209.1/211-2531-15/02) and performed at the Institute for Surgical Research, Walter-Brendel-Centre for Experimental Medicine, at Munich University in accordance with the Principles of Laboratory Animal Care, National Institutes of Health publication Vol. 25, No. 28, revised 1996. In total, 42 native bred pigs (19.7±0.3 kg; group size, n=6) were used. All procedures followed a standardized protocol as described previously (25–27, 40). In brief, six animals were randomly assigned to each group, trache-ostomized, and intubated. Intermittent positive pressure ventilation (Servo 900, Siemens, Elema, Sweden) was applied at a respiratory volume/min of 240 mL/kg, a respiratory rate of 12/min, 5 cm H2O positive end-expiratory pressure, and an inspired oxygen fraction (FiO2) of 1.0. Recipients were intubated with a 35-F double lumen left bronchocatheter (Mallinckrodt, London, United Kingdom). Single lung ventilation was performed by disconnecting the contralateral lung from ventilation and retaining the respiratory volume/min by increasing the respiratory rate to 24/min. In addition to arterial and central venous lines, a Swan-Ganz catheter (CritiCath thermodilution catheter 7F; Ohmeda, Erlangen, Germany), connected to a REF-1 CO-Computer (Baxter, Santa Ana, CA) was placed into recipients’ pulmonary arteries.
Experimental Groups
Donor animals were randomly assigned to three groups. In the conjugate group, animals received anti-PECAM-1 antibodies crosslinked with catalase to nanosized particles. Those antibody/catalase complexes recognize the endothelial antigen PECAM-1. Multivalent binding is followed by rapid internalization, facilitating intracellular endothelial protection. To exclude unspecific effects of the single components, in the mixture group, anti-PECAM-1 antibodies and catalase were applied in an unbound form, and in the vehicle group, the carrier solution glycerol alone was infused.
Donor Procedure
After sternotomy, conjugates, mixture, or vehicle were infused into the pulmonary artery over a period of 5 min (2-mL stock in 50 mL NaCl). Animals in the conjugate and mixture group received 0.2 mg/kg catalase. After 30 min, 10.000 IU heparin was injected intravenously, and cardiac arrest was induced by injecting potassium chloride into the coronary arteries. Subsequently, lungs remained in situ for a period of 90 min warm ischemia, with the tracheal tube clamped at half maximal inspiration. Thereafter, ventilation started again, and flush perfusion was performed with low potassium dextran (60 mL/kg, 50 cm H2O, Perfadex; Vitrolife, Goeteborg, Sweden). After recovery, lungs were stored inflated with oxygen in 4°C cold Ringer’s solution for topical cooling. After 18 hr preservation, left lungs were transplanted.
Recipient Procedure
In brief, after left-sided pneumonectomy, a tourniquet was placed around the right pulmonary artery. The pneumonectomized lung (termed “control lung”) was used for BALF analysis. Next, 10,000 IU heparin was given intravenously, and left lung transplantation performed as described previously (40). Animals of the sham group underwent the complete recipient procedure, except pneumonectomy and transplantation.
Animal Experimental Protocol
Parameters of systemic hemodynamics (mean arterial pressure, heart rate, cardiac output, and central venous pressure) and pulmonary hemodynamics (mean pulmonary arterial pressure and pulmonary capillary wedge pressure) were recorded. Lung function was assessed by analysis of gas exchange (pa/vO2, pa/vCO2, Sa/vO2, pHa/v). Blood samples were taken for hemoglobin and white blood cell count measurements. PVR and intrapulmonary shunt fraction (Qs/Qt) were calculated as described previously (25).
Baseline measurements were recorded before sternotomy or thoracotomy and transplantation. Graft function was analyzed after 5 min of isolated ventilation and perfusion (temporary tourniquet occlusion of the right pulmonary artery) at 30 min, 2, 4, and 6 hr after reperfusion. Tissue specimens and BALF were taken from nontransplanted donor lungs (right wing) after hypothermic preservation, from control lungs (recipients’ native left lung) and from graft and sham lungs after 6 hr reperfusion. Animals were killed in deep anesthesia.
Histologic Examination
Tissue samples were fixed in 4% paraformaldehyde, paraffin embedded, cut into 4-μm sections, and stained with hematoxylin-eosin. Blinded with a code, sections were evaluated in 20 high-power fields using a semiquantitative score described previously (40). In brief, endothelial alteration, leukocyte accumulation, alveolar cell desquamation, edema formation, fibrin exudation, and necrosis were graded (0–3) by a blinded pathologist (I.B.).
W/D Ratio
For calculation of the W/D ratio, specimens were weighed before and after 72 hr drying at 100°C.
BALF Analysis
BALF analyses were performed as described previously (40). In brief, BALF (mean recovery rate, 59±2%; cell viability, >90%) was filtered and centrifuged. After total cell count, monolayer cell preparations were stained according to Pappenheim. Analysis of the cell smears was based on 200 cells and differentiated between alveolar macrophages, neutrophils, and lymphocytes. In the supernatant, the amount of nonsedimentable protein was measured by a modified Lowry method (46). Furthermore, lipid peroxidation was analyzed using the TBARS assay (40), quantified with a standard curve of malondialdehyde (Sigma Chemie, Deisenhofen, Germany).
Statistics
Results are expressed as means±standard error of the mean. One-way analysis of variance on ranks followed by Student-Newman-Keuls test was applied for the assessment of group differences. For time interactions repeated-measures analysis of variance on ranks followed by Dunn’s test was used. Differences were considered significant at a P<0.05 level.
Acknowledgments
This work was supported by a grant of University of Munich (FöFoLe, Reg-Nr.:265) and University of Mainz (MAIFOR 8728132) and NHLBI RO1 HL073940.
The authors thank A. Allmeling, G. Hoebel, N. Koplin, S. Muenzing, and A. Schropp for their excellent technical assistance. The authors are grateful to Prof. G. Enders, MD, Prof. A. Baethmann, MD, Prof. R. Buhl, MD, Prof. C. Hammer, MD, DVM, Prof. M. Schmidt, PhD, and L.H. Schmidt, MD, for scientific and logistical support and fruitful discussion. The authors also thank M. Nakada, MD, for the generous gift of the antibody.
Footnotes
The authors declare no conflicts of interest.
Muzykantov and Wiewrodt shared senior co-authorship.
G.P. participated in research design, writing of the manuscript, research performance, and data analysis; F.L. participated in writing of the manuscript, research performance, and data analysis; I. Huff, U.E., I.B., I. Hermanns, and J.C.K., participated in research performance and data analysis; V.V.S. participated in research performance and contributed new reagents; K.F. participated in research performance and contributed analytical tools; M.E. participated in research design, performance, and data analysis; H.W. and K.W.J. participated in writing of the manuscript; S.M.A. participated in data analysis and writing of the manuscript; and V.R.M. and R.W. participated in research design, writing of the manuscript, research performance, data analysis, and contributed new reagents.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
References
- 1.De Vleeschauwer S, van Raemdonck D, Vanaudenaerde B, et al. Early outcome after lung transplantation from non-heart-beating donors is comparable to heart-beating donors. J Heart Lung Transplant. 2009;28:380. doi: 10.1016/j.healun.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Erasmus ME, Verschuuren EA, Nijkamp DM, et al. Lung transplantation from nonheparinized category III non-heart-beating donors. A single-centre report. Transplantation. 2010;89:452. doi: 10.1097/TP.0b013e3181c46a74. [DOI] [PubMed] [Google Scholar]
- 3.Mason DP, Thuita L, Alster JM, et al. Should lung transplantation be performed using donation after cardiac death? The United States experience. J Thorac Cardiovasc Surg. 2008;136:1061. doi: 10.1016/j.jtcvs.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrook R. Organ donation after cardiac death. N Engl J Med. 2007;357:209. doi: 10.1056/NEJMp078066. [DOI] [PubMed] [Google Scholar]
- 5.Summers DM, Counter C, Johnson RJ, et al. Is the increase in DCD organ donors in the United Kingdom contributing to a decline in DBD donors? Transplantation. 2010;90:1506. doi: 10.1097/TP.0b013e3182007b33. [DOI] [PubMed] [Google Scholar]
- 6.Nijkamp DM, van der Bij W, Verschuuren EA, et al. Non-heart-beating lung donation: How big is the pool? J Heart Lung Transplant. 2008;27:1040. doi: 10.1016/j.healun.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Kootstra G. The asystolic, or non-heartbeating, donor. Transplantation. 1997;63:917. doi: 10.1097/00007890-199704150-00001. [DOI] [PubMed] [Google Scholar]
- 8.de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 9.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: Opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172:944. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: An important predictor of survival after lung transplantation. J Heart Lung Transplant. 1996;15:160. [PubMed] [Google Scholar]
- 11.Van Raemdonck D. Thoracic organs: Current preservation technology and future prospects; part 1: Lung. Curr Opin Organ Transplant. 2010;15:150. doi: 10.1097/MOT.0b013e3283373b7e. [DOI] [PubMed] [Google Scholar]
- 12.Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant. 2008;8:1282. doi: 10.1111/j.1600-6143.2008.02231.x. [DOI] [PubMed] [Google Scholar]
- 13.de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007;26:529. doi: 10.1016/j.healun.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 14.al Mehdi AB, Zhao G, Dodia C, et al. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ Res. 1998;83:730. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 15.al Mehdi AB, Shuman H, Fisher AB. Intracellular generation of reactive oxygen species during nonhypoxic lung ischemia. Am J Physiol. 1997;272:L294. doi: 10.1152/ajplung.1997.272.2.L294. [DOI] [PubMed] [Google Scholar]
- 16.Fisher AB, Dodia C, Tan ZT, et al. Oxygen-dependent lipid peroxidation during lung ischemia. J Clin Invest. 1991;88:674. doi: 10.1172/JCI115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher AB, al Mehdi AB, Muzykantov V. Activation of endothelial NADPH oxidase as the source of a reactive oxygen species in lung ischemia. Chest. 1999;116:25S. doi: 10.1378/chest.116.suppl_1.25s. [DOI] [PubMed] [Google Scholar]
- 18.Eppinger MJ, Deeb GM, Bolling SF, et al. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol. 1997;150:1773. [PMC free article] [PubMed] [Google Scholar]
- 19.Novick RJ, Gehman KE, Ali IS, et al. Lung preservation: The importance of endothelial and alveolar type II cell integrity. Ann Thorac Surg. 1996;62:302. doi: 10.1016/0003-4975(96)00333-5. [DOI] [PubMed] [Google Scholar]
- 20.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 21.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): A strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA. 1999;96:2379. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherpereel A, Rome JJ, Wiewrodt R, et al. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther. 2002;300:777. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 23.Nowak K, Weih S, Metzger R, et al. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293:L162. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 24.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, et al. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2003;285:L283. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 25.Loehe F, Mueller C, Annecke T, et al. Pulmonary graft function after long-term preservation of non-heart-beating donor lungs. Ann Thorac Surg. 2000;69:1556. doi: 10.1016/s0003-4975(00)01234-0. [DOI] [PubMed] [Google Scholar]
- 26.Loehe F, Mueller C, Annecke T, et al. Tissue damage of non-heartbeating donor lungs after long-term preservation: Evaluation of histologic alteration, bronchoalveolar lavage, and energy metabolism. Shock. 2002;17:502. doi: 10.1097/00024382-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Loehe F, Preissler G, Annecke T, et al. Continuous infusion of nitroglycerin improves pulmonary graft function of non-heart-beating donor lungs. Transplantation. 2004;77:1803. doi: 10.1097/01.tp.0000131155.81609.37. [DOI] [PubMed] [Google Scholar]
- 28.Date H, Matsumura A, Manchester JK, et al. Changes in alveolar oxygen and carbondioxide concentration and oxygen-consumption during lung preservation—The maintenance of aerobic metabolism during lung preservation. J Thorac Cardiovasc Surg. 1993;105:492. [PubMed] [Google Scholar]
- 29.D’Armini AM, Roberts CS, Griffith PK, et al. When does the lung die? I. Histochemical evidence of pulmonary viability after “death”. J Heart Lung Transplant. 1994;13:741. [PubMed] [Google Scholar]
- 30.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, et al. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21:392. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 31.Oto T, Levvey BJ, Pilcher DV, et al. Evaluation of the oxygenation ratio in the definition of early graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2005;130:180. doi: 10.1016/j.jtcvs.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Chatila WM, Furukawa S, Gaughan JP, et al. Respiratory failure after lung transplantation. Chest. 2003;123:165. doi: 10.1378/chest.123.1.165. [DOI] [PubMed] [Google Scholar]
- 33.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: A multicenter analysis. Am J Respir Crit Care Med. 2005;171:786. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 34.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 35.Perkowski S, Scherpereel A, Murciano JC, et al. Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1050. doi: 10.1152/ajplung.00067.2006. [DOI] [PubMed] [Google Scholar]
- 36.Maathuis MH, de Groot M, Ploeg RJ, et al. Deterioration of endothelial and smooth muscle cell function in DCD kidneys after static cold storage in IGL-1 or UW. J Surg Res. 2009;152:231. doi: 10.1016/j.jss.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Egan TM, Hoffmann SC, Sevala M, et al. Nitroglycerin reperfusion reduces ischemia-reperfusion injury in non-heart-beating donor lungs. J Heart Lung Transplant. 2006;25:110. doi: 10.1016/j.healun.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Pinsky DJ, Naka Y, Chowdhury NC, et al. The nitric oxide/cyclic GMP pathway in organ transplantation: Critical role in successful lung preservation. Proc Natl Acad Sci USA. 1994;91:12086. doi: 10.1073/pnas.91.25.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuvaev VV, Tliba S, Nakada M, et al. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323:450. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 40.Preissler G, Loehe F, Ebersberger U, et al. Recipient treatment with L-arginine attenuates donor lung injury associated with hemorrhagic shock. Transplantation. 2009;87:1602. doi: 10.1097/TP.0b013e3181a52ce1. [DOI] [PubMed] [Google Scholar]
- 41.Cooke JP, Tsao PS. Cytoprotective effects of nitric oxide. Circulation. 1993;88:2451. doi: 10.1161/01.cir.88.5.2451. [DOI] [PubMed] [Google Scholar]
- 42.Ding BS, Hong N, Murciano JC, et al. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111:1999. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung JC, Cypel M, Waddell TK, et al. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques—Non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19:261. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009;87:255. doi: 10.1016/j.athoracsur.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 45.Wiewrodt R, Thomas AP, Cipelletti L, et al. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99:912. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 46.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]