Abstract
Objective
Previous studies determined, using between arms position matching assessments, that at least one-half of individuals with stroke have an impaired position sense. We investigated whether individuals with chronic stroke who have impairments mirroring arm positions also have impairments identifying the location of each arm in space.
Methods
Participants with chronic hemiparetic stroke and age-matched participants without neurological impairments (controls) performed a between forearms position matching task based on a clinical assessment and a single forearm position matching task, using passive and active movements, based on a robotic assessment.
Results
12 out of our 14 participants with stroke who had clinically determined between forearms position matching impairments had greater errors than the controls in both their paretic and non-paretic arm when matching positions during passive movements; yet stroke participants performed comparable to the controls during active movements.
Conclusions
Many individuals with chronic stroke may have impairments matching positions in both their paretic and non-paretic arm if their arm is moved for them, yet not within either arm if these individuals control their own movements.
Significance
The neural mechanisms governing arm location perception in the stroke population may differ depending on whether arm movements are made passively versus actively.
Keywords: Position sense, Stroke, Evaluation methodology, Robotics
1. Introduction
By 2030, approximately 10 million American adults will have been affected by a stroke, an estimated 84% of these individuals will survive, and many of these individuals will move on to longterm disability facing challenges in coordinating and controlling movements (Mozaffarian et al., 2015). According to clinical assessments, more than half of these stroke survivors may have a compromised position sense (Connell et al., 2008; Winward et al., 2002) that can result in devastating effects on their ability to control their movements (Cole, 1995; Ghez et al., 1990). Even so, our understanding about the reason for observed impairments during the clinical assessment is limited since the measurements: (1) lack sensitivity to identify the degree of an impairment (e.g., ratings are unimpaired, mildly impaired, severely impaired), (2) are subjective (e.g., a rater determines task performance based on visual inspection), (3) may not be reliable (e.g., ratings may differ depending on the rater and testing session), and (4) may be confounded by additional impairments (Carey et al., 1996; Sullivan and Hedman, 2008).
To address the limitations of currently available clinical sensory assessments, a number of research groups are employing robotic systems that standardize and automate the assessment of position sensing capabilities in individuals with stroke (Dukelow et al., 2010; Simo et al., 2014). Robotic systems offer numerous advantages including that sensors affixed to the robotic device can monitor the user’s interaction and data can be processed off-line.
Here, we characterized the ability of individuals with chronic hemiparetic stroke to match forearm positions using two approaches: a between forearms position matching clinical assessment and a single forearm position matching automated robotic assessment. Our aim was to determine whether individuals with chronic hemiparetic stroke, who have impairments matching positions between forearms, also have impairments matching positions within a single forearm. Based on our findings, we suggest that a large number of individuals with stroke who have a compromised ability to mirror arm positions on a clinical between arms position matching assessment may not have impairments identifying each arm’s location, separately, if these individuals actively control their arm movements. We also note that if the arms of individuals with stroke are moved for them, these individuals may have impairments identifying the location of both their paretic arm and their non-paretic arm. We conclude that the neural mechanism(s) causing impairments on clinical between forearms position matching assessments in individuals with stroke is not known.
2. Methods
Neural mechanisms contributing to a position matching task may differ when positions are matched between arms versus within a single arm since the body’s sensors and body’s schemas must relay comparable information for each arm during the between arms task, yet not during the single arm task (Adamo et al., 2007; Goble, 2010; Hirayama et al., 1999; Proske et al., 2014). First, we characterized participant performance during a between forearms position matching task using a clinical assessment in both participants with chronic hemiparetic stroke (i.e., participants with stroke) and age-matched participants without neurological impairments (i.e., controls) (see Fig. 1). Next, we quantified task performance in both of these populations during a single forearm position matching task using a robotic assessment to determine whether impairments arise when participants match positions within each forearm. Participants’ position matching performances within both the left forearm and the right forearm were measured, since prior work demonstrated that one should not assume that the ipsilesional forearm is unaffected (Carey, 1995; Carey et al., 1996; Hughes et al., 2015; Niessen et al., 2008) and since arm dominance may impact position sense (Goble and Brown, 2007; Goble, 2010; Nishizawa and Saslow, 1987). Additionally, participant task performance was tested during a passive experiment and an active experiment since the literature provides various views as to whether individuals’ perceptual capabilities differ when a movement is active versus passive (Fuentes and Bastian, 2010; Gritsenko et al., 2007; Paillard and Brouchon, 1968; Proske et al., 1993; Proske et al., 2000; Proske and Gandevia, 2012).
Fig. 1.
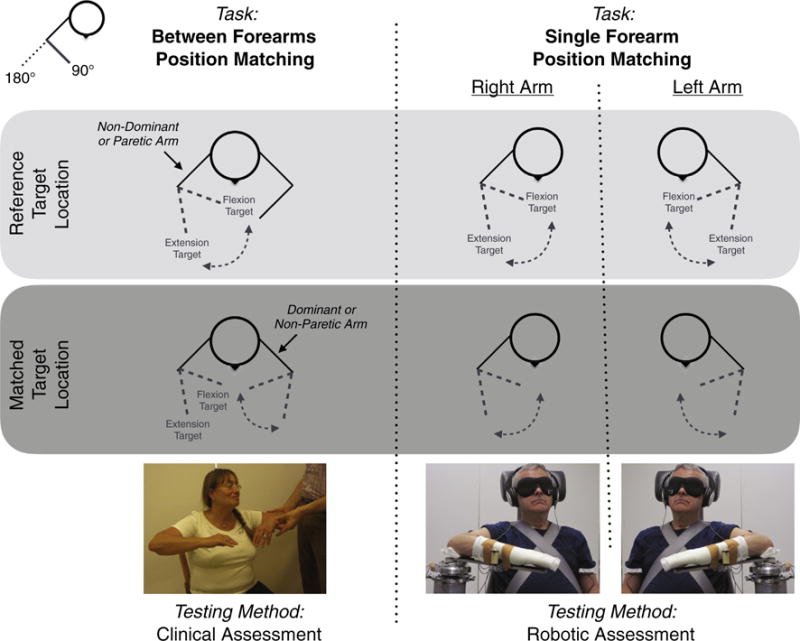
Experimental Methods. The ability of a participant to match forearm positions is tested (Left) during a between forearms position matching task and (Right) during a single forearm position matching task. For each task, (Light Gray Box) the participant remembers a reference target location and (Dark Gray Box) then tries to match the reference target location without receiving feedback about task performance. The between forearms position matching task is performed using a clinical assessment (revised Nottingham Sensory Assessment); a licensed physical therapist places the participant’s non-dominant (in controls) or paretic (in participants with stroke) forearm at the reference target location, and the participant then matches this reference target location by moving their dominant (in controls) or non-paretic (in participants with stroke) forearm to the mirrored location. The single forearm position matching task is performed using a robotic assessment, and the participant’s task performance is quantified for both the left arm and the right arm.
2.1. Between forearms position matching task: clinical assessment
Each participant was examined by a licensed physical therapist for clinically determined forearm position sensing impairments using a between arms limb matching task from the revised Nottingham Sensory Assessment (rNSA) (Lincoln et al., 1998). The physical therapist rotated the participant’s non-dominant (in controls) or paretic (in participants with stroke) forearm to an angular position without moving any of the other joints; the participant rotated their dominant (in controls) or non-paretic (in participants with stroke) forearm until he or she felt that both arms were positioned at the same mirrored configuration. The participant’s eyes were closed throughout the testing. This task was repeated for at least three different angular positions, and the participant’s task performance was assessed based on visual inspection. A score of 3 was given if the participant could successfully match all of the forearm positions, 2 if the participant could detect movement directions but the position error was greater than 10” for at least one angular position, 1 if the participant could detect the movements but not their directions nor positions, and 0 if the participant could not detect the movements. The same physical therapist also identified the level of motor impairments in all of the participants with stroke using the Upper-Extremity Fugl-Meyer Motor Assessment (FMA) scale (Fugl-Meyer et al., 1975).
2.2. Single forearm position matching task: robotic assessment
Each participant’s forearm position matching capabilities were quantified within the left arm and the right arm using a robotic device and an ipsilateral remembered position matching task (Adamo et al., 2007) during a passive experiment and then during an active experiment.
2.2.1. Robotic setup
The custom one-degree-of-freedom robotic device shown in Fig. 2 both rotated the participant’s forearm about the elbow joint and monitored the participant’s movements. The robotic device included a Harmonic Drive FHA-17C-100 motor with an attached US250 encoder (Peabody, MA, USA), which measures angular positions, and an OMEGA Engineering Inc. LCM201-300 load sensor (Stamford, CT, USA), which measures interaction forces. The motor rotated the participant’s forearm to set locations for the passive experiment, whereas the motor was controlled to create a low inertia and low damping virtual haptic virtual environment for the active experiment. Further details about the robotic device and controller for the active experiment are provided in Euving et al. (2016).
Fig. 2.
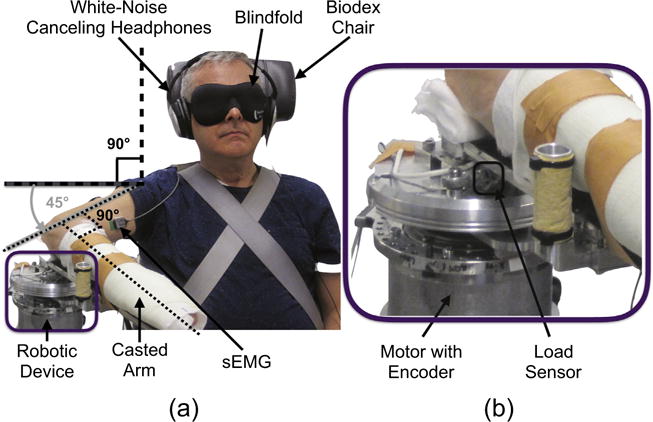
Experimental Setup. (a) The participant sat in the Biodex Chair, and their casted arm was attached to the robotic device with a shoulder abduction angle of 90°, horizontal shoulder flexion angle of 45°, and elbow flexion angle of 90°. The participant wore white-noise canceling headphones and a blindfold so that their task performance would not be influenced by auditory and visual cues. Surface electromyography (sEMG) sensors were placed on the biceps brachii, lateral head of triceps brachii, and brachioradialis so that muscle activity of the elbow flexor and extensor muscles could be monitored. (b) The robotic device includes a motor with encoder and load sensor, which allowed for the creation of virtual haptic environments and the monitoring of angular positions and interaction forces.
Elbow flexor and extensor muscle activity was monitored throughout the testing to ensure that participants were not contracting their muscles. Specifically, the biceps brachii, lateral head of triceps brachii, and brachioradialis were monitored using the Delsys Bagnoli™-8 and DE-2.1 surface electromyography sensors (sEMGs) (Natick, MA, USA); signals were amplified by a factor of 1,000 using the Delsys system. The sEMGs’ measurements were displayed in real-time to a Sharp Electronics Corporation 60” Class 1080P LED Smart TV with Quatron Model LC-60LE857U (Abeno-ku, Osaka, Japan).
The robotic device control loop ran at 4 kHz, and data were stored at 1 kHz.
2.2.2 Single forearm position matching task
Participant task performance was quantified when the forearm was (passive experiment) rotated by the robotic device or (active experiment) rotated by the participant. A summary of the trial timeline for each experiment is summarized in Fig. 3. The participant’s goal during the position matching task was to remember the forearm position at a reference target location based on haptic cues, and then to return to this forearm position after a series of random movements. For the passive and active experiment, the participant remained at the reference target location for 10 s and 4 s, respectively, since this amount of time was determined from empirical testing as long enough for the participant to fix the forearm position in memory (Goble et al., 2012); for the active experiment, 4 s was selected to avoid possible positional drift of the forearm (Ghez et al., 1990). To match the reference target location, the participant verbally indicated when the forearm was positioned at the matched target location by stating out loud ‘Target’, marking completion of the trial. Random movements to random angles were included to avoid the possibility that a participant could identify the reference target location by using timing cues (i.e., by counting).
Fig. 3.
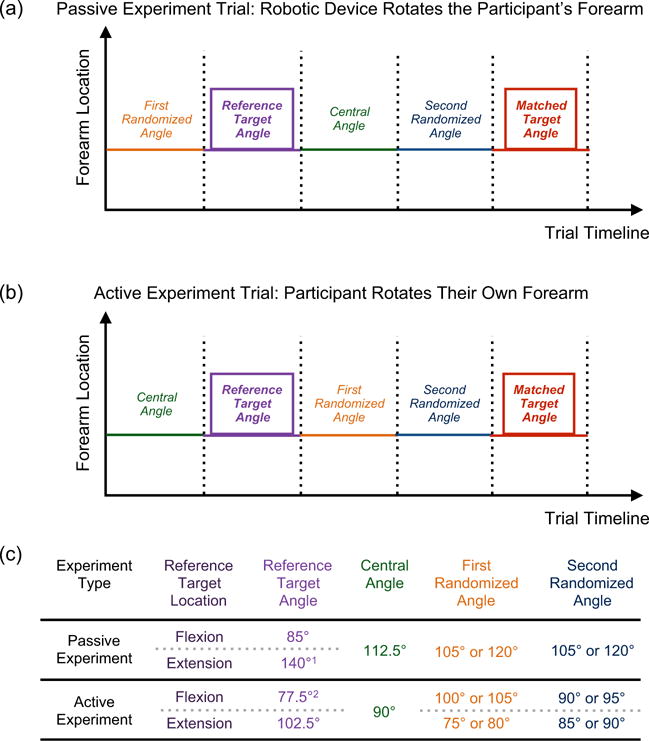
Single Forearm Position Matching Task – Trial Timeline. Trials were run for two experiment types: a passive experiment and an active experiment. (a) For the passive experiment, the robotic device rotated the participant’s forearm to the desired locations while the participant remained relaxed. (b) For the active experiment, the participant rotated their own forearm to the desired locations while affixed to the robotic device by following commands played on the white-noise canceling headphones (i.e., ‘In’, ‘Out’, ‘Hold’, ‘Remember this target location’, ‘Find the target location’). As noted in Fig. 1, the participant’s goal was to remember a reference target location, and then to return to that position (matched target location). In addition to these two locations, the participant’s forearm also rotated to a number of randomized locations to ensure that the participant could not rely on timing cues (i.e., counting) to determine the matched target location. (c) The table identifies the angles to which the participant’s forearm was rotated during both experiments for each flexion and extension reference target location. 1The extension reference target angle for the passive experiment was adjusted to 125° and 130° for Stroke 8 and Stroke 10, respectively, since these two participants had a limited passive range-of-motion in extension. 2The flexion reference target angle for the active experiment was adjusted to 85° for Stroke 7 and Stroke 11 since these two participants had a limited passive range-of-motion in flexion.
The participant wore a blindfold and white-noise canceling headphones to prevent visual and auditory cues from influencing judgment. Additionally, throughout the experiment the weight of the participant’s testing arm was fully supported by the robotic device, maximizing the available active range-of-motion in the paretic arm of the participants with stroke by decreasing the effects of synergistic muscle activation caused by shoulder abduction loading (Sukal et al., 2007).
2.2.3. Passive experiment trial
To begin a trial, the robotic device rotated the participant’s forearm to the first randomized angle (105° or 120°). Then, the robotic device rotated the participant’s forearm to the reference target angle (85° or 140°) and held the arm still at the reference target angle for 10 s. Next, the robotic device rotated the participant’s forearm to the central angle (112.5°), followed by the second randomized angle (105° or 120°). Last, the participant instructed the experimenter how to have the robotic device move their forearm in order to match the reference target angle by stating out loud ‘In’, ‘Out’, and ‘Stop’ to indicate that their arm should be flexed, extended, or held still, respectively. The participant identified that the reference target angle had been matched by stating out loud ‘Target’. The participant’s forearm rotated at 6°/s before reaching the central angle and at 2°/s after reaching the central angle to avoid the possibility that the participant could identify the reference target location based on timing cues (i.e., by counting) and to encourage that position sense was comparable across all participants and conditions (Proske et al., 2000).
2.2.4. Active experiment trial
To begin a trial, the robotic device rotated the participant’s forearm to the central angle (90°). Next, the participant followed auditory commands played on the white-noise canceling headphones (i.e., ‘In’, ‘Out’, ‘Hold’, ‘Remember this target location’, ‘Find the target location’) in order to perform the desired movements and tasks. First, the participant was instructed how to move to the reference target angle (77.5° or 102.5°), and then was instructed to hold their arm still and to remember the target location. Next, the participant was instructed how to move their forearm to the first randomized angle (100° or 105° for the flexion reference target location, 75° or 80° for the extension reference target location), followed by the second randomized angle (90° or 95° for the flexion reference target location, 85° or 90° for the extension reference target location). Last, the participant was instructed to match their forearm to the reference target angle that was held in memory. The participant indicated when the reference target angle had been matched by stating out loud ‘Target’. The participant’s forearm rotational speed was restricted to <10°/s to encourage that position sense (Proske et al., 2000) and haptic rendering (Gurari and Baud-Bovy, 2014) was comparable across all participants and conditions; each participant repeated all trials when the maximum speed was exceeded. Additionally, trials were repeated if the participant could not hold their forearm within 2.5° of the reference target location.
2.2.5. Experimental procedures
The passive and active experiment each spanned two 2–3 h sessions, during which the participant’s forearm position matching capabilities were quantified (one randomized arm tested per session).
At the beginning of the first session for each experiment type, the participant’s dominant (in controls) or non-paretic (in participants with stroke) arm was loosely attached to the robotic device, and the participant was instructed how to perform the forearm position matching task. Training was finished once the participant successfully completed two trials.
Next, across all sessions the participant’s testing arm was outfitted with a cast and the participant was strapped to a System 3 Pro™ Biodex chair (Shirley, NY, USA). The participant’s casted forearm was rigidly fixed to and fully supported by the robotic device (Fig. 1), and his or her elbow joint was situated in the robotic device’s cup such that rotations of the participant’s forearm were about the medial epicondyle. The location of the Biodex chair was adjusted so that the participant had 90° of shoulder abduction, 45° of horizontal shoulder flexion, and 90° of elbow flexion, as indicated in Fig. 2. Shoulder abduction motion occurred in the frontal plane around an anterior-posterior axis centered through the head of the humerus and was measured as the angle between the humerus and a true vertical line (shown by the solid black lines). Horizontal shoulder flexion motion occurred in the transverse (horizontal) plane around a superior-inferior axis centered through the gleno–humeral joint and was measured as the angle between the humerus and an imaginary line that connected the acromions of both arms (shown by the dashed gray lines). Elbow flexion, in our setup, was occurring in the horizontal plane around a superior-inferior axis centered through the elbow joint and was measured as the angle between the medial humerus and the midline of the forearm (shown by the dotted black lines). This configuration was selected so that the participant’s forearm rotated in the horizontal plane.
For the active experiment only, we had each participant perform a speed training task during which the participant learned how fast he or she would be permitted to rotate their arm throughout a position matching trial. For the speed training, the participant was required to rotate their forearm three times between 110° and 70° at a speed <10°/s in order to train the allowable range of movement speeds.
Subsequently, we assessed the range-of-motion about the elbow joint for each participant to ensure that the participant could reach beyond both the flexion and extension reference target locations. Both a flexion and an extension reference target location was chosen to investigate whether position sense may have been altered in the participants with stroke at an extended location, since their forearm may have an increased resistance to movement at this location when compared to the controls. During the passive experiment, we verified that each participant could reach the reference target locations by rotating the participant’s forearm to 10° beyond the extension and flexion reference target locations; the extension target was adjusted for Stroke 8 and Stroke 10 since these two participants with stroke had a limited passive range-of-motion, such that the difference between the flexion and extension targets was reduced from 55° to 40° and 45°, respectively. During the active experiment, we verified that each participant could reach beyond the reference target locations and hold their arm still by asking the participant to rotate their forearm to 2.5° beyond the extension and flexion reference target locations and to hold their arm still for 4 s. The flexion target was adjusted for Stroke 7 and Stroke 11 since we limited the range-of-motion of the robotic device to more conservative safety angles in order to avoid the possibility of these participants feeling pain during the flexion movements; therefore, the difference between the flexion and extension targets was reduced from 30° to 27.5°.
After the flexion and extension reference target locations were identified for each participant, the robotic device stretched each participant’s testing arm between extended and flexed locations at 120°/s with a 10 s hold at each location. This series of extension and flexion movements was repeated at least 20 times in order to significantly reduce the stretch reflex activity of the elbow flexor and extensor muscles in the participants with stroke (Schmit et al., 2000). An added benefit to this stretching is that the participant’s arm was conditioned for the position matching task. The history of a muscle’s activity can affect the sensitivity of the muscle spindles; therefore, this initial conditioning encouraged that each tested arm for every participant was in a similar mechanical state when the position matching trials began (Brown, 1969; Proske et al., 1993).
Next, we characterized each participant’s position matching capabilities using the single forearm position matching task. Participants performed eight trials for each of the two reference target locations (16 trials total – presentation of reference target locations were randomized), with a mandatory minimum break of one minute after eight successive trials. Participants were always positioned to approach the extension target with an extension movement and the flexion target with a flexion movement.
Upon completion of all testing trials, the participant provided a rating between 1 (guessing) and 10 (100% sure) to indicate how confident he or she felt that the matched target locations were at the same positions as the reference target locations.
Last, following the active experiment we investigated whether working memory impairments in the participants with stroke may have resulted in differences in task performance when compared to the controls. Our task to assess working memory capabilities was inspired by Kessels et al. (2000), Sebastian et al. (2008) and Cockrell and Folstein (2002). Each participant performed four working memory trials. For each trial, the participant’s forearm was rotated by the robotic device in four directions (e.g., flexion, extension, extension, flexion), then the participant was given by the experimenter a word with which he or she would have to rhyme another word (e.g., ‘pan’), the participant gave a rhyming word (e.g., ‘can’), and then the participant rotated the forearm in the four directions that he or she had originally felt (e.g., flexion, extension, extension, flexion). The participant successfully completed a trial if he or she identified a rhyming word and reproduced the correct directions of forearm movements.
2.3. Data analysis
Task performance for each participant and for every condition was measured using the metrics of constant error, absolute error, and variable error (Henry, 1974; Schutz and Roy, 1973). Constant error, CE, identified how accurately the participant matched the reference target location; specifically, the constant error provided information about whether across all trials the participant tended to overshoot (CE > 0), undershoot (CE < 0), or not show a directional bias (CE = 0) when identifying the reference target location. Absolute error, AE, also identified how accurately the participant matched the reference target location without placing importance on whether the participant undershot or overshot the reference target location; specifically, the absolute error identified whether the participant was matching at an angle near the reference target location, with perfect performance being AE = 0, or whether the participant was matching at an angle far from the reference target location. Variable error, VE, identified how precisely the participant matched the reference target location; specifically, the variable error identified whether across all trials the participant returned to the same location or to a wide range of locations (a larger variable error indicates a poorer ability to return to the same location, or a working memory impairment (Goble et al., 2012).
To obtain these metrics, the reference target location, , and the matched target location, , were identified for each trial i. For the active experiment, the participant may have initially made small movement adjustments, so and were defined as the mean angular position based on the last two seconds (of four seconds) when holding the forearm still at each respective location.
Next, the target error, or the difference between the matched target location, , and the reference target location, , was calculated for every trial. Flexion target errors were multiplied by −1, so that a positive and negative target error always corresponded to the reference target location being overshot and undershot, respectively. In other words, undershooting an extension target or overshooting a flexion target indicates that a participant matched at a more flexed location than the reference target location, whereas overshooting an extension target or undershooting a flexion target indicates that a participant matched at a more extended location than the reference target location.
Then, the constant error, absolute error, and variable error were calculated at every reference target location for each arm of every participant and of both experiment types. Constant error is defined as the mean target error across all extension or flexion trials for each participant and testing arm, or Absolute error is defined as the mean absolute target error across all extension or flexion trials for each participant and testing arm, or . Variable error is defined as the standard deviation across the same extension or flexion trials for each participant and testing arm, or .
Finally, we used analysis of variance (ANOVA) tests to identify the effect of each tested factor on participant task performance (i.e., constant error, absolute error, and variable error). Fixed factors were group (controls, participants with stroke), used arm (control – dominant, non-dominant; participants with stroke – non-paretic, paretic), and reference target location (extension, flexion), with participants being a random factor. Within each experiment type and group, we ran a repeated measures two-way ANOVA to determine the impact of the used arm and reference target location on participant task performance. We then combined the paretic arm and the non-paretic arm for the participants with stroke and the dominant and the non-dominant arm for the controls, since a significant effect of used arm and a significant effect of the interaction between used arm and reference target location was not found within each group; by combining both arms within each group of participants, we could then compare the task performance of the participants with stroke to the task performance of the controls. We ran a between subjects two-way ANOVA on the participants’ data for each experiment type to determine whether task performance differed across the two tested groups and reference target locations during the passive experiment and during the active experiment, respectively. A significant effect was based on α = 0.05.
We also used the Kruskal–Wallis H test to identify whether participant confidence ratings and performance on the working memory task differed across the fixed factors of group and used arm.
2.4. Participants
The Northwestern University Institutional Review Board granted approval to run both experiments, and each participant provided written informed consent. Non-Northwestern University employees were monetarily compensated for their time. Inclusion criteria for participants with chronic hemiparetic stroke included: (1) stroke was at least six months prior to the experiment testing date, (2) ability to understand and perform the task, (3) paresis confined to one side, (4) absence of serious upper extremity injury that may interfere with task performance, (5) no use of agents that may impact task performance, (6) absence of severe or proprioceptive-related medical concerns, and (7) capacity to provide informed consent.
The tested participants with stroke were heterogeneous in terms of hand dominance and affected hemisphere, and they all had moderate to severe motor impairments with FMA scores spanning between 11 and 35. Information about each tested participant with stroke is summarized in Table 1.
Table 1.
Participants with Stroke. This table summarizes information about our participants with stroke. Given is general information (i.e., gender, age, dominant arm, years since stroke), clinical information (i.e., revised Nottingham Sensory Assessment forearm position sense score, upper-extremity Fugl-Meyer Motor Assessment (FMA) score, lesion location), the minimum and maximum location at which each participant matched the reference target location during the active experiment, and the minimum and maximum angle to which each participant could actively rotate their forearm (data were obtained from a separate ongoing study). All participants demonstrated during a speed training task that they could reach 70° and 110°. A dash indicates that data were not collected. The participants with stroke who had an impaired forearm position sense yet unimpaired direction of movement sense according to the clinical assessment are highlighted by a bold font and are analyzed for the remainder of the paper. Lesion locations were identified from medical records and T1/T2 MRI scans – TH: thalamus, IC: internal capsule, BG: basal ganglia, F: frontal lobe, FP: frontal/parietal lobes, PO: parietal/occipital lobes; I: insula, T: temporal lobe, O: occipital lobe, SF: sylvian fissure, Po: pons.
| Participants with Stroke | Between Forearms Assessment rNSA Forearm Position Sense | Gender | Age | Dominant arm/Paretic arm (L – Left, R – Right) | Years since stroke | Upper-extremity FMA | Lesion location (L – Left, R – Right) | Min/max matching target Location of paretic arm during active experiment | Min/max active range-of- motion of paretic arm |
|---|---|---|---|---|---|---|---|---|---|
| Stroke 1 | Unimpaired Position Sense and | F | 67 | R/R | 12 | 11 | L: TH, IC, BG | 57.37B0/126.01° | |
| Stroke 2 | Direction of Movement Sense (3) | M | 61 | L/L | 6 | 17 | R: IC, Po | 61.54°/124.45° | |
| Stroke 3 | Impaired Position Sense and Unimpaired Direction of Movement Sense (2) | M | 60 | R/L | 4 | 20 | R: BG, F | 75.74°/107.34° | 63.42 °/150.71° |
| Stroke 4 | M | 49 | L/R | 27 | 26 | Not available1 | 71.57°/111.47° | 50.97 °/165.99° | |
| Stroke 5 | F | 46 | R/L | 9 | 28 | R: IC, SF, FP, T | 81.16°/116.00° | 66.01 °/186.52° | |
| Stroke 6 | M | 46 | R/L | 11 | 35 | R: TH, IC | 75.59°/103.12° | 46.72° /151.06° | |
| Stroke 7 | M | 69 | L/R | 21 | 12 | L: TH, IC, BG, I | 82.01°/101.60° | 69.33° /128.33° | |
| Stroke 8 | M | 61 | R/L | 7 | 15 | R: IC, BG | 72.33°/100.93° | −/− | |
| Stroke 9 | M | 50 | R/R | 17 | 30 | L: TH, IC, BG, PO | −/− | −/− | |
| Stroke 10 | M | 59 | R/L | 9 | 25 | R: IC, BG | −/− | 62.10° /107.11° | |
| Stroke 11 | F | 62 | R/R | 29 | 13 | L: TH, IC, BG | 83.12°/103.11° | 67.38° /129.81° | |
| Stroke 12 | F | 60 | R/L | 12 | 20 | R: TH, IC, T/F/O | −/− | 66.17°/171.42° | |
| Stroke 13 | Impaired Position Sense and Direction of Movement Sense (1) | M | 57 | L/R | 7 | 28 | L: TH, IC | −/− | −/− |
| Stroke 14 | Absent Position Sense and Direction of Movement Sense (0) | M | 62 | R/R | 8 | 25 | L: TH, IC, BG | −/− | −/− |
This participant is not MRI compatible, and his medical records were destroyed when the hospital where he was treated closed down.
All 14 participants with stroke performed the passive experiment, and eight participants with stroke (Stroke 3–9,11) who had an impaired position sense yet unimpaired direction of movement sense based on the clinical between forearms position matching task (rNSA score of 2) performed the active experiment. We do not report results for Stroke 13 and Stroke 14 when the reference target locations were matched using their paretic arm during the passive experiment, since these participants could not identify the direction of movement of their paretic arm and, in turn, could not perform the task.
For the passive experiment, nine age-matched controls participated and were comprised of five females and four males with ages ranging between 45 and 66 years (mean ± standard deviation: 56.2 ± 6.8 years). For the active experiment, eight controls participated in the experiment; however, one control was removed from the study due to time constraints. Therefore, seven controls completed the experiment and were comprised of three females and four males with ages ranging between 55 and 66 years (mean ± standard deviation: 59.6 ± 3.7 years). All controls were dominantly right-handed and had an unimpaired rNSA forearm position sense (i.e., score of 3).
3. Results
Results reveal that participants with stroke who have impairments mirroring forearm positions on a clinical assessment may not have impairments matching forearm positions within an arm if they actively control the movements of their arm. Additionally, findings indicate that impairments may arise in both the paretic arm and the non-paretic arm of individuals with stroke when matching positions within an arm if these individuals do not control their arm movements.
3.1. Between forearms position matching task: clinical assessment
The main finding is that 12 out of our 14 participants with stroke showed impairments on the between forearms position matching clinical assessment. The rNSA was performed on all participants; two out of the 14 participants with stroke had a lack of or an impaired position sense and direction of movement sense (score of 0 or 1), ten had an impaired position sense yet unimpaired direction of movement sense (score of 2), and two had an unimpaired position sense and direction of movement sense (score of 3). Example forearm position matching errors are shown in Fig. 4 for (a) our participants with an unimpaired forearm position sense and direction of movement sense and (b) our participants with an impaired forearm position sense yet unimpaired direction of movement sense. In particular, we point out that forearm position matching errors for a clinically determined impaired position sense yet unimpaired direction of movement sense were, based on visual inspection, at least 10°.
Fig. 4.
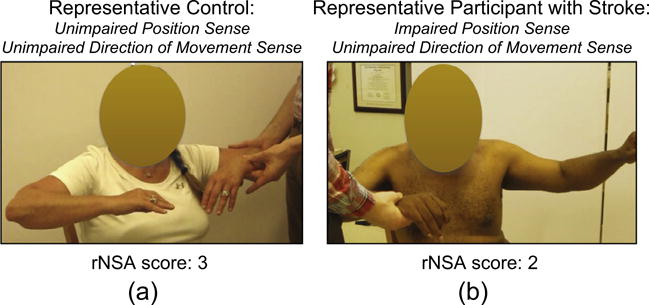
Between Forearms Position Matching Task – Revised Nottingham Sensory Assessment. Here, we illustrate forearm position matching errors during the clinical assessment for our participants that we discuss in the remainder of the manuscript. (a) All controls had an unimpaired forearm position sense and direction of movements sense (i.e., rNSA score of 3), as shown by the representative forearm position matching configuration in the left image. (b) Ten out of the 14 participants with stroke (Stroke 3 through 12 in Table 1) had an impaired forearm position sense yet unimpaired direction of movement sense (i.e., rNSA score of 2), as shown by the representative forearm position matching configuration in the right image.
3.2. Single forearm position matching task: robotic assessment
In this section, we report results for our participants with stroke who had, according to the clinical assessment, an impaired position sense yet unimpaired direction of movement sense (Stroke 3–12 in Table 1). Fig. 5 summarizes participant task performance as a function of group and used arm for the (Left) passive experiment and (Right) active experiment. We focus on significant findings for group and used arm, since we are primarily interested in comparing task performance of the participants with stroke to the controls and of the paretic (in participants with stroke) or non-dominant (in controls) arm to the non-paretic (in participants with stroke) or dominant (in controls) arm. We include information about participant task performance as a function of reference target location in Fig. 6, and main significant effects of reference target location on participant task performance are indicated by a line with a star above.
Fig. 5.
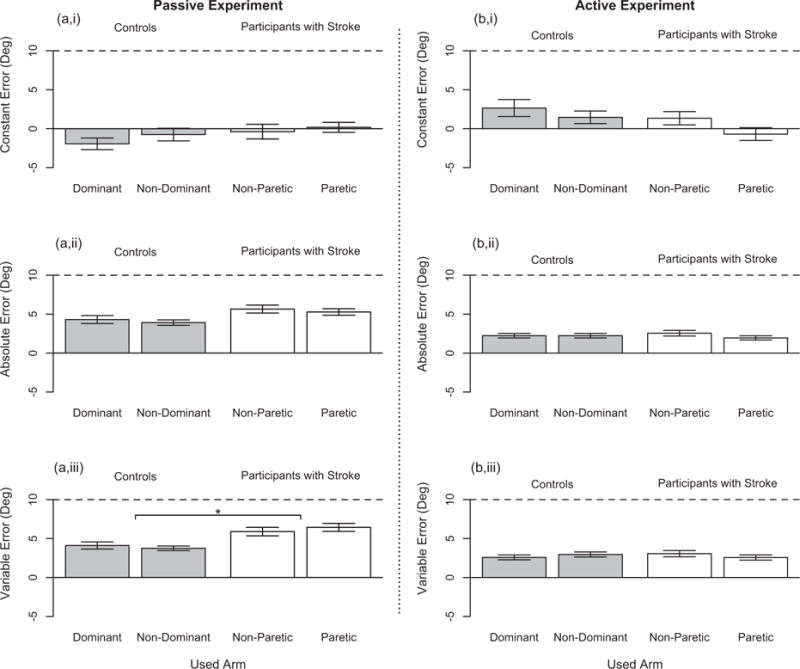
Single Forearm Position Matching Task – Participant Task Performance. Participant task performance during the single arm robotic assessment is given for the (a,i/a,ii/a,iii) passive experiment and (b,i/b,ii/b,iii) active experiment across our two tested groups – controls with an unimpaired forearm position sense and direction of movement sense according to the between arms clinical assessment (in Gray) and participants with stroke having an impaired forearm position sense yet unimpaired direction of movement sense according to the between arms clinical assessment (in White). Participant task performance is shown for both testing arms: dominant arm and nondominant arm in the controls and non-paretic arm and paretic arm in the participants with stroke. The ability of the participants to accurately match the reference target location is identified by the (a,i/b,i) constant error and (a,ii/b,ii) absolute error, where a value of 0° indicates perfect accuracy or that the participant did not overshoot or undershoot the reference target location when matching across all trials. (a,iii/b,iii) The ability of the participants to precisely match the reference target location is identified by the variable error, where a value of 0° indicates perfect precision or that the participant always returned to the same exact location when matching the reference target location. Each bar height represents the mean value, each error bar represents the standard error, and each horizontal black dashed line identifies a position matching error of 10°. The line with a star above indicates a significant main effect of group on participants’ variable error.
Fig. 6.
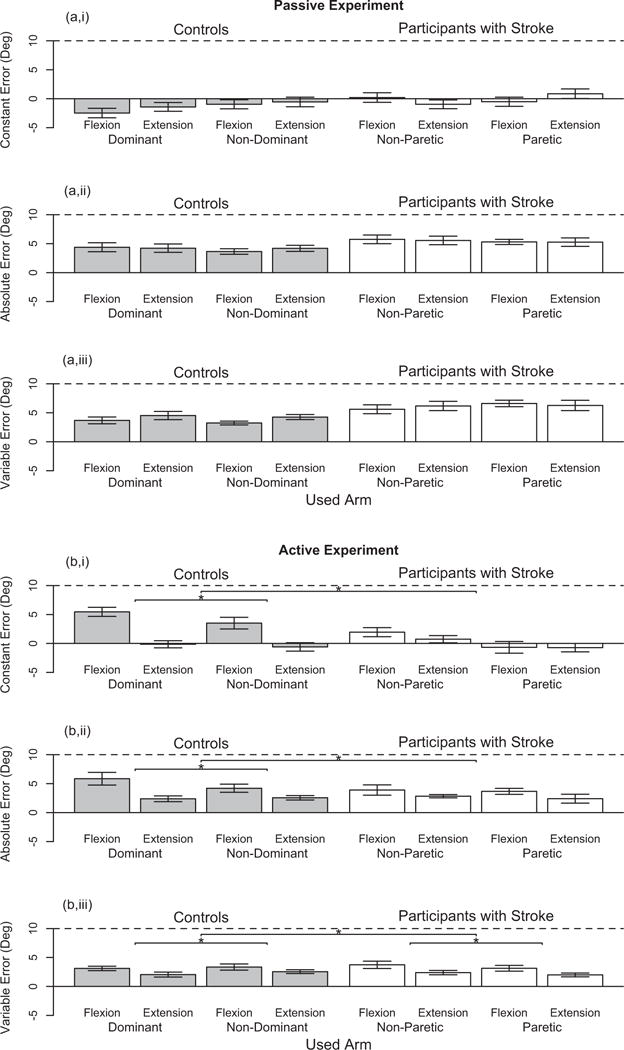
Single Forearm Position Matching Task – Participant Task Performance as a Function of Reference Target Location. Participant task performance during the single arm robotic assessment is given for the (a,i/a,ii/a,iii) passive experiment and (b,i/b,ii/b,iii) active experiment across two reference target locations – flexion and extension – and for our two tested groups – controls (in Gray) and participants with stroke (in White) – in both arms. The ability of the participants to accurately match the reference target location is identified by the (a,i/b,i) constant error and (a,ii/b,ii) absolute error, and the ability of the participants to precisely match the reference target location is identified by the (a,iii/b,iii) variable error. Each bar height represents the mean value, each error bar represents the standard error, and each horizontal black dashed line identifies a position matching error of 10°. Each line with a star above indicates a significant main effect of reference target location on participant task performance either within a group or between the tested groups.
3.2.1. Passive experiment
Prior to running the analyses, we removed data for one trial that was identified as an outlier, since the participant had an error of <1° with respect to the first and second randomized locations of 120° yet an error of 35° with respect to the reference target location of 85°; this participant accurately identified all of the remaining trials. Additionally, we note that the maximum allowable range-of-motion for the robotic device was reached for two trials; Stroke 4 reached the minimum angle of 65° using the non-paretic arm during one trial, and Stroke 5 reached the maximum angle of 160° using the paretic arm during one trial.
Both the participants with stroke and the controls did not significantly differ across arms in their task performance. An analysis of the controls (n = 9) and of the participants with stroke (n = 10), separately, did not reveal that the used arm, reference target location, or their interaction significantly affected task performance of either group.
We combined the data for both testing arms prior to comparing the task performance of the participants with stroke to the controls. The only significant effect found was that the variability in the matched target locations was greater in the participants with stroke than in the controls, respectively (F(1,34) = 9.92, = 0.23, p = 0.003). No significant effect of reference target location or the interaction between group and reference target location was found.
We also demonstrate that participants with stroke exceeded a magnitude of error of 10° in both the paretic arm and the nonparetic arm more often than the controls. Participants with stroke exceeded 10° of error for 13.2% and 13.8% of all trials when using their paretic arm (representing eight of ten participants) and their non-paretic arm (representing six of ten participants), respectively, whereas controls exceeded 10° of error for 3.5% and 6.3% of all trials when using their non-dominant arm (representing three of nine participants) and their dominant arm (representing five of nine participants), respectively.
To summarize, the participants with stroke were more variable in matching positions within both their paretic arm and their nonparetic arm when compared to the controls. Additionally, task performance for the participants with stroke, as well as for the controls, was comparable across arms.
3.2.2. Active experiment
First, we note that Stroke 4 and Stroke 9 completed a subset of the 16 testing trials (eight and five trials, respectively) due to time constraints. We removed data for Stroke 9 from the following analyses, since this participant did not have enough trials from which to obtain a good estimate for the variable error.
Then, we verified that the performance of the participants with stroke on the position matching task was not affected by a limited active range-of-motion of their paretic arm. In Table 1, we report the minimum and maximum location across all trials at which each participant with stroke matched the reference target location with their paretic arm. We also report the active range-of-motion in the paretic arm for our participants with stroke who have participated in a separate ongoing study. Given that the active range-of-motion in the paretic arm for each participant with stroke exceeded the range of locations to which these participants moved their paretic arm during the active experiment, we conclude that the results in the position matching task were not confounded by a motor impairment. Across all trials, the participants never reached the maximum allowable range-of-motion set for the robotic device.
Both the participants with stroke and the controls did not significantly differ in their task performance across arms. An analysis of the controls (n = 7) and the participants with stroke (n = 7), separately, revealed that the constant error, absolute error, and variable error were not significantly affected by the arm used within each group of participants. Reference target location significantly affected the variable error in the controls (F(1,6) = 8.18, = 0.58, p = 0.029) and participants with stroke (F(1,6) = 16.74, =0.74, p = 0.006), as well as the constant error (F(1,6) = 21.66, = 0.78, p = 0.003) and absolute error (F(1,6) = 10.01, = 0.63, p = 0.020) in the controls.
We combined the data for both testing arms prior to comparing the task performance of the participants with stroke to the controls at each reference target location. The main factor of group had no significant effect on participant task performance, as determined by the constant error, absolute error, and variable error. The main factor of reference target location significantly affected the constant error (F(1,24) = 8.413, = 0.26, p = 0.008), absolute error (F (1,24) = 10.40, =0.16, p = 0.004), and variable error (F(1,24) = 9.64, = 0.29, p = 0.005). Additionally, the interaction of group and reference target location significantly impacted the constant error (F(1,24) = 4.99, = 0.17, p = 0.035).
We also point out that the percentage of trials for which the participants with stroke exceeded a magnitude of 10° of error was less than the percentage of trials for which the controls exceeded a magnitude of 10° of error. Participants with stroke exceeded 10° of error for 0.9% and 2.9% of all trials when using their paretic arm (representing one of seven participants) and their non-paretic arm (representing two of seven participants), respectively, and controls exceeded 10° of error for 2.7% and 8.0% of all trials when using their non-dominant arm (representing two of seven participants) and their dominant arm (representing four of seven participants), respectively.
To summarize, the participants with stroke identified the location of both their paretic forearm and their non-paretic forearm in space just as well as the controls when actively controlling their arm movements. Moreover, task performance was comparable across arms for the participants with stroke and for the controls.
3.2.3. Confidence ratings and working memory task performance
The main finding is that the participants with stroke did not significantly differ from the controls in their confidence ratings (see Fig. 7) and working memory task performance. The analysis of the participant’s confidence ratings was run on the n = 9 controls and n = 10 participants with stroke who partook in the passive experiment, and the n = 7 controls and n = 7 participants with stroke (Stroke 7 was omitted since a rating was missing due to experimenter error) who partook in the active experiment. No significant effect of group or used arm was found on the participants’ self-reported confidence ratings. The analysis of the number of successful working memory trials was run on the n = 7 controls and n = 8 participants with stroke who partook in the active experiment. No significant effect of group or used arm was found on the participants’ working memory task performance. The mean number of successful working memory trials out of 4.0 was 3.2 across all participants and both testing arms.
Fig. 7.
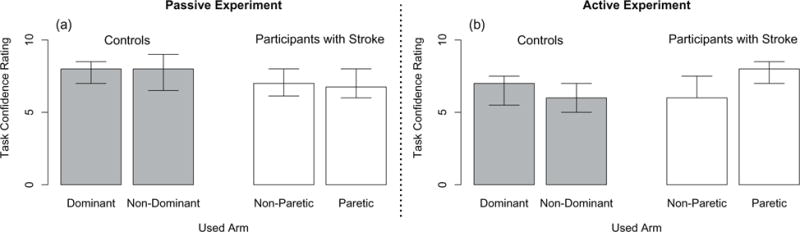
Single Forearm Position Matching Task – Participant Confidence Ratings. Participants identified on a scale of 1 (guessing) to 10 (100% sure) how confident they were that their matched target locations were positioned at the reference target locations. Given are the median values (bar height) and lower and upper quartile values (error bars) for the controls (in Gray) and participants with stroke (in White) in their dominant and non-dominant arm and paretic and non-paretic arm, respectively, during the (a) passive experiment and (b) active experiment. No significant differences in self-reported ratings were found between the participants with stroke and the controls and between each of the participants’ used arms.
4. Discussion
Findings from this study indicate that individuals with stroke may avoid impairments in locating their arm in space if they actively control their arm movements rather than have their arm moved for them. Additionally, the results suggest that impairments may arise in not only the paretic arm, but also in the non-paretic arm of individuals with stroke when these individuals’ arms are moved for them. 12 out of our 14 participants with stroke had forearm position matching impairments during a between forearms position matching clinical assessment. Moreover, these 12 participants with stroke matched forearm positions with larger magnitudes of error than the controls in both their paretic arm and their non-paretic arm when their arms were moved for them. However, a comparison of task performance of the seven tested participants with stroke and the seven tested controls who performed the active experiment found that the participants with stroke matched forearm positions just as well as the controls.
Position sense is created from numerous afferent signals including muscle spindles, joint mechanoreceptors, and cutaneous mechanoreceptors (Proske and Gandevia, 2012). Additionally, position sense is derived from an individual’s perception of their body schema, or the perception of the size and shape of their body segments (Longo and Haggard, 2010; Proske and Gandevia, 2012). Although still controversial, an individual’s perceptual capabilities may improve during active movements due to both alpha-gamma motor neuron coactivation, which heightens the sensitivity of muscle spindles (Prochazka et al, 1985; Prochazka and Gorassini, 1998), and an efference copy, or a copy of the motor commands which may provide additional positional information (Gritsenko et al., 2007; Paillard and Brouchon, 1968); however, findings may differ depending on whether the position matching task is performed using a single arm versus both arms (Allen, 2010). Differences in task performance during an active and passive position matching task may also arise due to muscle thixotropy, or a history-dependent change in a muscle’s passive mechanical properties (Proske et al., 1993; Proske and Gandevia, 2012; Tsay et al., 2015; Tsay and Giummarra, 2016).
Our results corroborate those of Fuentes and Bastian (2010) in that the precision with which our participants matched the reference target location was better during the active movements than during the passive movements (see Fig. 5). The difference in the magnitude of our participants’ variable errors between the passive and active experiments may have arisen for a number of reasons including that: (1) participants did not have complete control to start and stop their arm exactly when they wanted during the passive experiment, in contrast to the active experiment, since the participants were instructing an experimenter when to start and stop their arm; (2) differences arose due to the flexion and extension reference target locations not being exactly the same during the passive and active experiments – participant position matching abilities change depending on the target location (Vely et al., 1989); (3) the muscle spindles’ sensitivities were heightened during the active movement due to alpha-gamma motor neuron coactivation (Prochazka et al., 1985; Prochazka and Gorassini, 1998); and (4) efferent copies contributed additional positional information during the active movement that was not available during the passive movement (Fuentes and Bastian, 2010; Gritsenko et al., 2007; Paillard and Brouchon, 1968).
The discovery that position matching performance was unimpaired during an active single forearm position matching task in our participants with stroke who had between forearms position matching impairments indicates that these individuals’ abilities to accurately and precisely identify positions within an arm is not compromised when they control their arm movements despite damage occurring to the central nervous system of each of these participants. The discovery that an inability to mirror arm locations emerges when these participants with stroke perform a clinical between forearms position matching task indicates that impairments become noticeable during tasks requiring a reliance on passively imposed movements and/or interlimb coordination. Given the aforementioned findings, we have evidence to suggest that the neural mechanism contributing to between arms position matching impairments may have occurred for one of the following reasons. Impairments may have been observed during the clinical between arms assessment because the participants with stroke had an uncertainty about the location of their reference arm, or paretic arm, since their paretic arm was moved by the clinician to the reference target location (i.e., the participants with stroke had an uncertainty about their paretic arm’s location in space since they were not controlling the movements of their paretic arm). Also, cross-hemispherical communication between the sensorimotor cortices may have been compromised, affecting the ability of the sensory information from an individual’s two arms to effectively communicate with one another. Additionally, differing positional information may have been relayed from each arm of the participants with stroke due to a shorter muscle fascicle length in the paretic forearm when compared to the non-paretic forearm; as indicated by Hall and McCloskey (1983), “…proprioceptive performance at the elbow joint is unified by analysis in terms of the lengths of fascicles of the muscles which operate those joints. This strongly suggests a common mechanism based upon this variable.” Ongoing work in our lab suggests that muscle fascicle lengths in elbow flexor and extensor muscles of the paretic forearm of individuals with chronic stroke are shorter than in their non-paretic arm (possibly due to years of disuse) (Nelson et al., 2015), while a prior study demonstrated that muscle fascicle lengths in the paretic medial gastrocnemius muscle of individuals with chronic stroke are indeed shorter than in the non-paretic medial gastrocnemius muscle (Gao et al., 2009). Additional possible reasons for the between arms position matching impairments in our participants with stroke include that the efference copy was altered on one side of the brain causing changes in the estimation of the positional information about the contralateral arm (Paillard and Brouchon, 1968; Fuentes and Bastian, 2010), as well as the body schema for forearm positions in either/both arm(s) was altered (Longo and Haggard, 2010; McCloskey, 1973; Lackner, 1988).
Despite having knowledge about lesion locations for 13 of our 14 participants with stroke, this lesion location information does not elucidate which of our participants with stroke have what impairments. Currently, the location of a motor impairment can be determined based on the hemisphere in which a lesion is located. However, our results indicate that impairments in identifying an arm’s location in space, unlike the motor task, are not related to the hemisphere that was affected in individuals with stroke. Rather, both arms are seemingly either affected or are not affected. Additionally, we indicate that the thalamus, internal capsule, and/or basal ganglia were affected in every participant with stroke for which lesion location was obtained, whereas each participant with stroke had varying levels of position matching impairments. Therefore, we hypothesize that lesion location is not a good predictive indicator for sensory and/or interlimb processing impairments. Future work can investigate whether single forearm position matching impairments and between forearms position matching impairments can be identified in individuals with stroke based on the integrity of neural tracts. By using a magnetic resonance (MR) tractography method, such as diffusion tensor imaging, we can evaluate how various pathways (e.g., sensory, interhemispheric collosal) are affected in each participant with stroke and how these pathways may be linked to within and between arm position matching impairments.
Based on our results and the work of others (Connell et al., 2008; Dukelow et al., 2010; Hirayama et al., 1999), we propose that a large number of individuals with stroke may be misdiagnosed as having a sensory impairment according to a between arms position matching task. In a study run by Connell et al., individuals were assessed immediately after a stroke for a forearm position matching impairment using the same between forearms clinical assessment that we had used in our work. The authors found that of 70 patients with a first stroke, 37% had an unimpaired position sense and direction of movement sense (score of 3), 34% had an impaired position sense and unimpaired direction of movement sense (score of 2), 20% had an impaired position sense and direction of movement sense (score of 1), and 10% had completely lost both their position sense and direction of movement sense (score of 0) (Connell et al., 2008). Furthermore, Dukelow et al. found that 49% of their 45 participants with stroke had position sensing impairments based on a between arms position matching robotic assessment (Dukelow et al., 2010).
To conclude, we suggest that clinical proprioceptive assessment designs and rehabilitative treatments may benefit from focusing on interventions where individuals with stroke are encouraged to actively control their paretic arm. As demonstrated in this study, participants with stroke had a larger variability than the controls when matching their arm locations if their arms were moved for them, whereas these same participants performed comparably to the controls if they were permitted to move their arms. The ability of individuals with stroke to identify the location of their arm in space during passive movements may be related to an increased motoneuronal excitability at the spinal cord (McPherson et al., 2008), resulting in a hyperactive stretch reflex or spasticity. In our robotic assessment, we greatly reduced the presence of the hyperactive stretch reflex by repetitively stretching the paretic arm prior to running the single forearm position matching trials (Schmit et al., 2000). Future work may benefit from developing both clinical assessments and rehabilitation treatments that encourage an individual with stroke to perform active as opposed to passive range-of-motion exercises with their arms, so as to avoid such scenarios when an uncertainty about the location of their arm in space may arise.
5. Conclusions
Our results demonstrate that individuals with stroke who have impairments matching positions between forearms do not necessarily have impairments matching positions within a single forearm. Importantly, these findings underscore the notion that position sense within an individual’s forearm may involve different neural circuits than when matching forearm positions between arms, and that position sense can depend on whether or not the individual controls their limb movements. Therefore, each task needs to be assessed separately, and more targeted and effective proprioceptive assessments need to be developed for clinical use. Implications of this work include that we do not know whether proprioceptive rehabilitation is sensible for individuals with chronic stroke solely based on the findings from a between arms clinical assessment. Finally, we propose that individuals with stroke may benefit from active range-of-motion as opposed to passive range-of-motion exercises of their paretic arm as part of their neurorehabilitation therapy.
HIGHLIGHTS.
Participants with chronic stroke had impairments locating each forearm during passive movements.
Chronic stroke participants were unimpaired in locating each forearm during active movements.
We do not know what neural impairment is assessed by common clinical forearm position matching tasks.
Acknowledgments
We thank Carolina Carmona, Meriel Owen, Erik Euving, and Fabian David for their assistance with carrying out this work, and we are grateful to the participants who partook in and inspired this study. We also thank the anonymous reviewers for their feedback. We acknowledge Northwestern University’s Department of Physical Therapy and Human Movement Sciences for its financial support.
Footnotes
Conflict of interest statement: None of the authors have potential conflicts of interest to be disclosed.
References
- Adamo DE, Martin BJ, Brown SH. Age-related differences in upper limb proprioceptive acuity. Percep Mot Skills. 2007;104:1297–309. doi: 10.2466/pms.104.4.1297-1309. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Leung M, Proske U. The effect of fatigue from exercise on human limb position sense. J Phys. 2010;588:1369–77. doi: 10.1113/jphysiol.2010.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Goodwin GM, Matthews PBC. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Phys. 1969;205:677–94. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM. Somatosensory loss after stroke. Phys Rehab Med. 1995;7:51–91. [Google Scholar]
- Carey LM, Oke LE, Matyas TA. Impaired limb position sense after stroke: A quantitative test for clinical use. Arch Phys Med Rehab. 1996;77:1271–8. doi: 10.1016/s0003-9993(96)90192-6. [DOI] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-mental state examination. Princ Prac Ger Psych. 2002:140–41. [Google Scholar]
- Cole J. Pride and a daily marathon. The MIT Press; 1995. [Google Scholar]
- Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: Frequency of different deficits and their recovery. Clin Rehabil. 2008;22:758–67. doi: 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- Dukelow SP, Herter TM, Moore KD, Demers MJ, Glasgow JI, Bagg SD, Norman KE, Scott SH. Quantitative assessment of limb position sense following stroke. Neurorehab Neur Rep. 2010;24:178–87. doi: 10.1177/1545968309345267. [DOI] [PubMed] [Google Scholar]
- Euving EJ, Gurari N, Drogos JM, Traxel S, Stienen AHA, Dewald JPA. Int Conf Hum Hap Sens Touch Enab Comp App. Springer International Publishing; 2016. Individuals with chronic hemiparetic stroke correctly match forearm position within a single arm: Preliminary findings; pp. 122–33. [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophys. 2010;103:164–71. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gao F, Grant TH, Roth EJ, Zhang L-Q. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. 2009;90:819–26. doi: 10.1016/j.apmr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF, Christakos CN, Cooper SE. Roles of proprioceptive input in the programming of arm trajectories. Cold Spring Harb Symp Quant Biology. 1990:837–47. doi: 10.1101/sqb.1990.055.01.079. [DOI] [PubMed] [Google Scholar]
- Goble DJ. Proprioceptive acuity assessment via joint position matching: From basic science to general practice. Phys Ther. 2010;90:1176–84. doi: 10.2522/ptj.20090399. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res. 2007;180:693–704. doi: 10.1007/s00221-007-0890-7. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Mousigian MA, Brown SH. Compromised encoding of proprioceptively determined joint angles in older adults: The role of working memory and attentional load. Exp Brain Res. 2012;216:35–40. doi: 10.1007/s00221-011-2904-8. [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Krouchev NI, Kalaska JF. Afferent input, efference copy, signal noise, and biases in perception of joint angle during active versus passive elbow movements. J Neurophys. 2007;98:1140–54. doi: 10.1152/jn.00162.2007. [DOI] [PubMed] [Google Scholar]
- Gurari N, Baud-Bovy G. Customization, control, and characterization of a commercial haptic device for high-fidelity rendering of weak forces. J Neurosci Meth. 2014;235:169–80. doi: 10.1016/j.jneumeth.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Hall LA, McCloskey DI. Detections of movements imposed on finger, elbow, and shoulder joints. J Phys. 1983;335:519–33. doi: 10.1113/jphysiol.1983.sp014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry FM. Variable and constant performance errors within a group of individuals. J Mot Behav. 1974;6:149–54. doi: 10.1080/00222895.1974.10734991. [DOI] [PubMed] [Google Scholar]
- Hirayama K, Fukutake T, Kawamura M. ‘Thumb localizing test’ for detecting a lesion in the posterior column-medial lemniscal system. J Neur Sci. 1999;167:45–9. doi: 10.1016/s0022-510x(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Hughes CML, Tommasino P, Budhota A, Campolo D. Upper extremity proprioception in healthy aging and stroke populations, and the effects of therapist-and robot-based rehabilitation therapies on proprioceptive function. Front Hum Neurosci. 2015;9:1–11. doi: 10.3389/fnhum.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RPC, van Zandvoort MJE, Postma A, Kappelle LJ, deHaan EHF. The Corsi block-tapping task: Standardization and normative data. Appl Neuropsych. 2000;7:252–8. doi: 10.1207/S15324826AN0704_8. [DOI] [PubMed] [Google Scholar]
- Lackner JR. Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain. 1988;111:281–97. doi: 10.1093/brain/111.2.281. [DOI] [PubMed] [Google Scholar]
- Lincoln NB, Jackson JM, Adams SA. Reliability and revision of the Nottingham Sensory Assessment for stroke patients. Physiotherapy. 1998;84:358–65. [Google Scholar]
- Longo MR, Haggard P. An implicit body representation underlying human position sense. Proc Nat Acad Sci. 2010;107:11727–32. doi: 10.1073/pnas.1003483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI. Position sense after surgical disconnexion of the cerebral hemispheres in man. Brain. 1973;96:269–76. doi: 10.1093/brain/96.2.269. [DOI] [PubMed] [Google Scholar]
- McPherson JG, Ellis MD, Heckman CJ, Dewald JPA. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophys. 2008;100:3236–43. doi: 10.1152/jn.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Dewald JPA, Murray WM. Comb Sec Meet Am Phys Ther Assoc. APTA; 2015. Musculoskeletal adaption after hemiparetic stroke: In vivo measurements of shortened biceps brachii fascicle lengths and increased passive elbow stiffness; p. 95. [Google Scholar]
- Niessen MH, Veeger DH, Koppe PA, Konijnenbelt MH, van Dieën J, Janssen TW. Proprioception of the shoulder after stroke. Arch Phys Med Rehabil. 2008;89:333–8. doi: 10.1016/j.apmr.2007.08.157. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Saslow CA. Lateralization of kinesthetically guided spatial perception. Cortex. 1987;23:485–94. doi: 10.1016/s0010-9452(87)80009-6. [DOI] [PubMed] [Google Scholar]
- Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. Neuropsych Spat Orient Behav. 1968;11:37–55. [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Phys. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, Appenteng K. ‘Fusimotor set’: New evidence for α-independent control of γ-motoneurones during movement in the awake cat. Brain Res. 1985;339:136–40. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Phys Rev. 2012;92:1651–97. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: A review. Prog Neurobiol. 1993;41:705–21. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Proske U, Tsay A, Allen T. Muscle thixotropy as a tool in the study of proprioception. Exp Brain Res. 2014;232:3397–412. doi: 10.1007/s00221-014-4088-5. [DOI] [PubMed] [Google Scholar]
- Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol. 2000;60:85–96. doi: 10.1016/s0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Dewald JPA, Rymer WZ. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil. 2000;81:269–78. doi: 10.1016/s0003-9993(00)90070-4. [DOI] [PubMed] [Google Scholar]
- Schutz RW, Roy EA. Absolute error: The devil in disguise. J Mot Behav. 1973;5:141–53. doi: 10.1080/00222895.1973.10734959. [DOI] [PubMed] [Google Scholar]
- Sebastian M, Mayas J, Manso AJ, Ballesteros S. Int Conf Hum Hap Sens Touch Enab Comp App. Berlin Heidelberg: Springer; 2008. Working memory for visual and haptic targets: A study using the interference paradigm; pp. 395–9. [Google Scholar]
- Simo L, Botzer L, Ghez C, Scheidt RA. A robotic test of proprioception within the hemiparetic arm post-stroke. J NeuroEng Rehabil. 2014;11:1–12. doi: 10.1186/1743-0003-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukal TM, Ellis MD, Dewald JPA. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: Neuroscientific implications. Exp Brain Res. 2007;183:215–23. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JE, Hedman LD. Sensory dysfunction following stroke: Incidence, significance, examination, and intervention. Top Stroke Rehabil. 2008;15:200–17. doi: 10.1310/tsr1503-200. [DOI] [PubMed] [Google Scholar]
- Tsay A, Allen TJ, Proske U. Position sense at the human forearm after conditioning elbow muscles with isometric contractions. Exp Brain Res. 2015;233:2635–43. doi: 10.1007/s00221-015-4334-5. [DOI] [PubMed] [Google Scholar]
- Tsay AJ, Giummarra MJ. Position sense in chronic pain: Separating peripheral and central mechanisms in proprioception in unilateral limb pain. J Pain. 2016;17:815–23. doi: 10.1016/j.jpain.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Velay J-L, Roll R, Paillard J. Elbow position sense in man: Contrasting results in matching and pointing. Hum Mov Sci. 1989;8:177–93. [Google Scholar]
- Winward CE, Halligan PW, Wade DT. The Rivermead Assessment of Somatosensory Performance (RASP): Standardization and reliability data. Clin Rehabil. 2002;16:523–33. doi: 10.1191/0269215502cr522oa. [DOI] [PubMed] [Google Scholar]


