Abstract
Tight coupling of reproduction to environmental factors and physiological status is key to long-term species survival. In particular, highly conserved pathways modulate germline stem cell lineages according to nutrient availability. This Chapter focuses on recent in vivo studies in genetic model organisms that shed light on how diet-dependent signals control the proliferation, maintenance, and survival of adult germline stem cells and their progeny. These signaling pathways can operate intrinsically in the germline, modulate the niche, or act through intermediate organs to influence stem cells and their differentiating progeny. In addition to illustrating the extent of dietary regulation of reproduction, findings from these studies have implications for fertility during aging or disease states.
Introduction
Organisms face frequent challenges to their homeostasis, and sensing and responding appropriately to these challenges is essential for their survival and successful reproduction. Diet and various stressors in the external environment help determine the levels of many circulating factors, including nutrients, metabolites and hormones, which in turn can influence the germ line, a special lineage that gives rise to gametes and allows species propagation (Ables et al. 2012; Hubbard 2011). Constant evolutionary pressure on reproduction has therefore led to very tight coupling of nutrient availability, metabolic status and other aspects of whole-body physiology to the biology of germ cells.
In many systems, germline stem cells (GSCs) support gametogenesis throughout most of adult life. Germ cell development from the stem cell stage to fully differentiated gametes is energetically costly and entails a large number of cellular processes that impose varying metabolic demands. It is not surprising, therefore, that multiple steps of gametogenesis are regulated by diet and other physiological factors (Ables et al. 2012; Hubbard 2011; Gracida and Eckmann 2013b; Busada and Geyer 2016).
Over the past 15 years, many studies have tackled the complex question of how whole-body physiology controls adult GSC lineages by taking advantage of in vivo model systems amenable to genetic manipulation. In this Chapter, we summarize and discuss the progress in this field, with a special focus on diet-dependent mechanisms that modulate adult GSC lineages in Drosophila melanogaster, Caenorhabditis elegans, and mammals.
1. Responses of germline stem cell lineages to diet
By coupling reproduction to nutrient availability, organisms avoid the costly metabolic investment of reproduction under suboptimal conditions and improve evolutionary fitness. Diet-dependent regulation of germ cells is therefore found in a wide range of systems regardless of life history strategy or germline organization. For example, abalone produce fewer gametes under nutrient stress (Rogers-Bennett 2010), starved zebrafish slow down egg production (Wang et al. 2006), and women with anorexia or excessively low body fat do not ovulate (Group 2006; Rojas et al. 2015). In cases where germline stem cells (GSCs) support gametogenesis, the germline can be influenced by diet-dependent signaling in GSCs or their progeny, in the niche (a specialized microenvironment that maintains stem cells), or in intermediate organs that provide relay signals.
1.1 The Drosophila melanogaster ovary
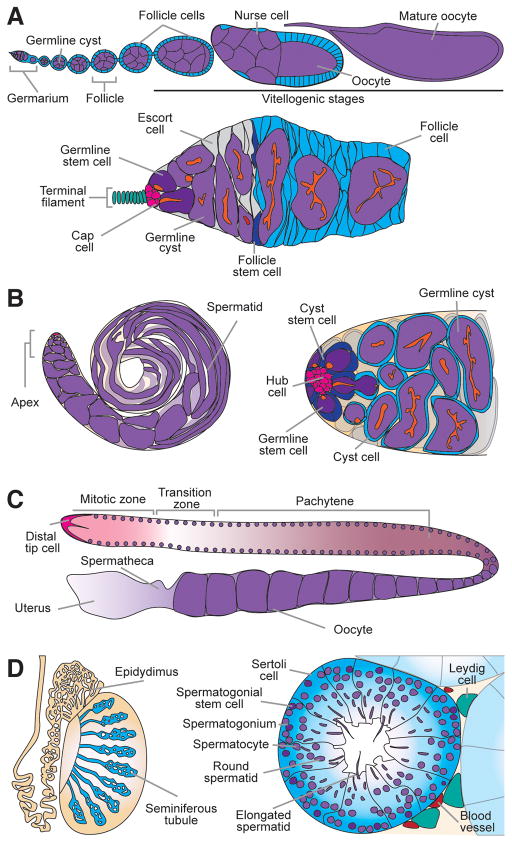
The Drosophila ovary has a well-described cell biology (Spradling 1993). Each ovary contains 15 to 20 ovarioles, composed of progressively more developed egg chambers (or follicles) formed in an anterior germarium, which houses GSCs and follicle stem cells (FSCs) (Figure 1A). Two to three GSCs are closely associated with a group of somatic cap cells, which are the major cell type in the GSC niche. Cap cells produce bone morphogenetic protein (BMP) signals that maintain the GSC fate by repressing a differentiation factor, while the physical association between cap cells and GSCs requires E-cadherin. Anterior to cap cells, a row of terminal filament cells also contributes to the niche. GSCs typically divide asymmetrically to self-renew and generate daughter cystoblasts. Cystoblasts divide four additional times with incomplete cytokinesis to form a 16-cell cyst: one of these cyst cells acquires an oocyte fate; the others support oocyte development as nurse cells. GSCs and their early progeny are easily identifiable based on the morphology of a specialized structure, the fusome. In GSCs, the fusome contacts the cap cell interface and remains round most of the time; as the cystoblast divides to form 16-cell cysts, the fusome becomes progressively more branched (Xie 2008). Early germ cells are closely associated with escort cells (also known as inner germarial sheath cells), which are required for the proper formation of 16-cell cysts (Kirilly et al. 2011). Two FSCs (abutting the posterior-most escort cells) give rise to follicle cells that envelop each 16-cell cyst to give rise to a follicle that buds off the germarium and proceeds through fourteen developmental stages (Xie 2008).
Figure 1.
GSC lineages. (A) Diagram of a Drosophila ovariole (top), which contains growing follicles. Each follicle is composed of a germline cyst surrounded by follicle cells and is produced from stem cell populations in the germarium (bottom). Germline stem cells (GSCs; dark purple) are juxtaposed to a somatic niche consisting primarily of cap cells (pink) and terminal filament cells (teal). GSCs divide asymmetrically, and their progeny generate 16-cell germline cysts (light purple) containing one oocyte and 15 nurse cells. The fusome (orange) becomes progressively more branched as cysts divide. Germline cysts initiately associate with escort cells (gray), and are subsequently enveloped by follicle cells (light blue) generated by follicle stem cells (dark blue) to form folicles. (B) The Drosophila testis (left) is a blind-end tube. GSCs (dark purple) reside at its apical end in close association with hub cells (pink) and cyst stem cells (CySCs, dark blue) (right). GSCs and CySCs divide asymmetrically, and their progeny (germline cysts and cyst cells, respectively) remain associated with each other during spermatogenesis. (C) Diagram showing one of the two gonad arms of adult C. elegans hermaphrodites. A niche comprising the distal tip cell (DTC; pink) maintains progenitor cells in the mitotic, proliferative zone. As progenitor cells move away from the niche, they enter meiosis. Sperm produced during larval stages are stored in the spermatheca; oocytes (purple) generate later are fertilized by stored sperm (or sperm introduced by mating) before progressing to the uterus. (D) In the mouse testis (left), spermatogenesis takes place in seminiferous tubules. Cross-section of a seminiferous tubule (right) showing different stages of the lineage supported by basally located spermatogonial stem cells (SSCs, dark purple). SSCs divide to produce mitotically active differentiating progeny (spermatogonia), which undergo meiosis (spermatocytes) and spermiogenesis (spermatids), and are released into the lumen of the tubule. Sperm undergo further maturation in the epidydimus, where they are eventually stored. Leydig cells (teal), blood vessels (red), and Sertoli cells (blue) plays important roles in support the SSC lineage.
Drosophila oogenesis is energetically demanding and highly regulated by diet (Ables et al. 2012). On a yeast-rich diet, each female lays an average of over 80 eggs per day, but upon shifting to a yeast-free (poor) diet, egg laying rates drop to just one or two eggs daily (Drummond-Barbosa and Spradling 2001). This largely reversible response to diet occurs within 18 to 24 hours, and reflects the concerted regulation of multiple processes in oogenesis. The proliferation rates of GSCs and FSCs, and the proliferation and growth of their progeny decrease, and follicles develop two- to three-fold more slowly on a poor diet (Drummond-Barbosa and Spradling 2001). An additional effect of starvation in developing follicles is the accumulation of large aggregates of processing bodies and cortically enriched microtubules; this is a reversible response that requires microtubule motor proteins (Burn et al. 2015). GSC and cap cell loss over time is also accelerated by a poor diet (Drummond-Barbosa and Spradling 2001; Hsu and Drummond-Barbosa 2009). In addition, early germline cysts die at an increased frequency within the germarium, follicles entering vitellogenesis degenerate, and ovulation is largely blocked, causing an accumulation of mature stage 14 egg chambers within the ovary (Drummond-Barbosa and Spradling 2001).
1.2 The Drosophila testis
Drosophila male GSCs reside at the testis apex in a niche, the hub, composed of 10–15 somatic cells (Figure 1B). Six to nine GSCs are closely associated with approximately twice as many somatic cyst stem cells (CySCs) at the hub. The cytokine Unpaired (Upd) produced by the hub is required for adhesion of GSCs to the hub, and for maintaining the CySC fate. Additional signals contribute to GSC and CySC maintenance, including BMPs and Hedgehog, respectively (Greenspan et al. 2015). GSCs divide asymmetrically to generate gonialblasts that form two-, four-, eight- and 16-cell cysts, collectively referred to as spermatogonia. CySCs give rise to postmitotic cyst cells; a pair of cyst cells envelops the gonialblast and remains associated with the resulting germline cyst as it develops. Meiotic divisions produce a cyst with 64 spermatids, followed by their individualization and mature sperm generation (Fuller 1993).
Despite the obvious size and energy storage differences between oocytes and sperm, early stages of Drosophila spermatogenesis are also regulated by diet. Upon protein starvation, the number of GSCs and their division rates decrease, and these effects are reversible (McLeod et al. 2010). A reduction in protein and sugar levels also slows down GSC proliferation, at least in part as a result of increased rates of centrosome misorientation (Roth et al. 2012). A reduction in overall food intake without specific removal of nutrients has been reported to result in a slight delay in GSC loss with age (Mair et al. 2010), suggesting that specific dietary manipulations can impact GSC maintenance in unique ways. More recent studies examining the kinetics of the protein-starvation response show that although GSC proliferation and numbers decrease initially, after about a week, GSC numbers stabilize and proliferation rates return to normal levels. During this response, early spermatogonial cells die at the two- to four-cell stage, and their death requires caspase activity in surrounding cyst cells. When death of cysts cells is blocked by dronc caspase knockdown or DIAP1 overexpression, GSCs are no longer maintained during prolonged starvation, suggesting that elimination of cyst cells/spermatogonial units has a protective effect against protein starvation (Yang and Yamashita 2015).
1.3. The Caenorhabditis elegans gonad
The hermaphrodite C. elegans has a gonad with two arms each containing ~1000 germ cells and capped by a single somatic distal tip cell (DTC) at each end (Figure 1C). Notch ligands produced by the DTC maintain a population of ~225 proliferating germ cells, including a stem cell pool of ~35–70 GSCs, in the distal mitotic zone (Kimble and Seidel 2008). As germ cells move proximately, they enter meiosis as a result of reduced Notch signaling and complex posttranscriptional regulation. Following intense oocyte growth, ovulation occurs, followed by fertilization (by sperm produced during larval development or introduced through mating) and egg laying (Hubbard and Greenstein 2005; L’Hernault 2009) (Figure 1C).
Complete removal of food in adult hermaphrodites can lead to different phenotypes (Angelo and Van Gilst 2009). Maternal death may occur due to internal development of progeny (who will eat their way out of the mother) following reduced rates of egg laying. Animals that escape matricide undergo instead an adult reproductive diapause, where over the initial 10 days of starvation, the germ line is reduced to ~35 germ cells (presumably GSCs) that resist continued starvation for over 30 days and are able to reconstitute a fully functional germ line within 72 hours of refeeding (Angelo and Van Gilst 2009). A more recent study showed that germ cells in the proliferative zone stop dividing and arrest in the G2 phase of the cell cycle, and meiotic entry is inhibited within a few hours of starvation of early adults, and these effects are reversible upon refeeding. Interestingly, GSCs retain their stemness independently of Notch signaling during this starvation-induced arrest (Seidel and Kimble 2015).
1.4. The mouse testis
Mouse spermatogenesis takes place in the epithelial lining of seminiferous tubules in the testis (Figure 1D)(de Rooij and Russell 2000). Large somatic Sertoli cells span the entire epithelium thereby contacting all stages of germ cell development, and they secrete glial cell line-derived neurotrophic factor (GDNF), required for GSC self-renewal (Franca et al. 2016). GSCs, called spermatogonial stem cells (SSCs), represent a subset of undifferentiated Asingle spermatogonia located basally in the epithelium. SSCs give rise to daughters that undergo four rounds of incomplete divisions remaining in clusters of two cells (Apaired), and four, eight, 16, and up to 32 cells (collectively called Aaligned spermatogonia), subsequently differentiating into B spermatogonia (de Rooij and Russell 2000). B spermatogonia differentiate into spermatocytes, which undergo meiosis to form haploid spermatids that differentiate into sperm released into the lumen. A highly vascularized interstitium surrounding seminiferous tubules contains peritubular myoid cells, macrophages, and testosterone-producing Leydig cells, all of which support spermatogenesis (Oatley and Brinster 2012; DeFalco et al. 2015).
Although specific effects on SSCs have not been carefully analyzed, several studies point to possible connections with diet. For example, diet-induced obesity and vitamin D or zinc deficiency can lead to a decrease in male fertility in mice (Fan et al. 2015; Sun et al. 2015; Croxford et al. 2011). Adult mice deprived of dietary vitamin A show testicular degeneration as a result of increased apoptosis and sloughing of immature germ cells into the lumen in more severe cases (Boucheron-Houston et al. 2013). Several of these observations have parallels in humans: obese men are more likely to be infertile (Campbell et al. 2015), and there is a positive correlation between vitamin D and zinc and sperm quality in adult men (Blomberg Jensen 2014; Colagar et al. 2009).
2. Nutritional control of GSC lineages
GSC lineages sense and respond to nutritional inputs through multiple mechanisms integrated into a seamless physiological output. GSC lineages can directly receive dietary information through cellular energy sensors, or nutrient transport and sensing. These same inputs can act in the niche, indirectly influencing GSCs and their progeny. Finally, remote endocrine cells can produce hormones in a diet-dependent manner with broad physiological influence over the organism, including direct effects on the niche, GSCs, or their differentiating progeny, or indirect effects through one or more intermediate organs.
2.1 Nutrient sensors control GSC lineages
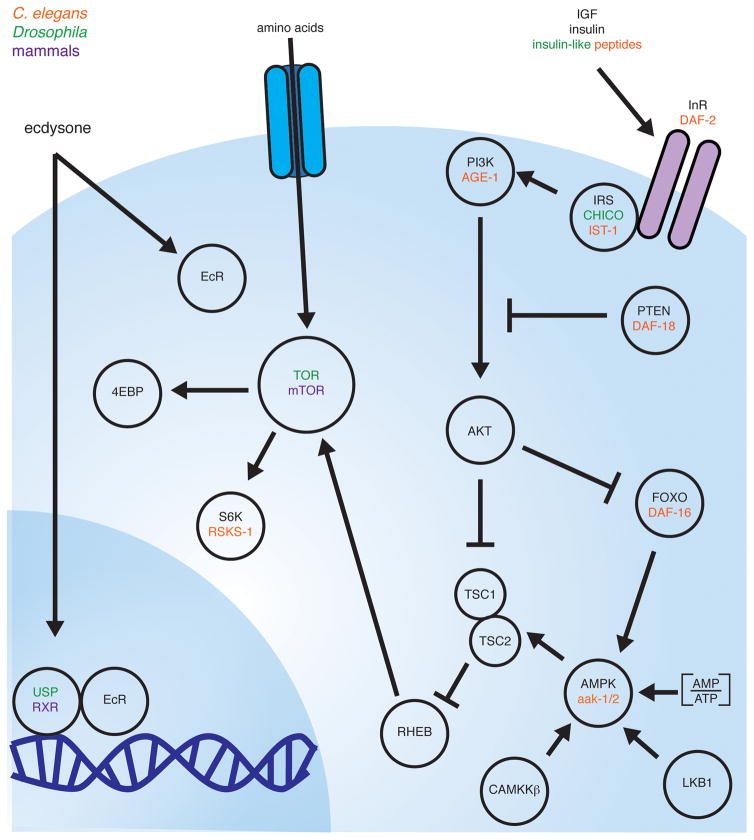
Highly conserved nutrient-sensing pathways operate in a wide range of cells, including those in GSC lineages; yet, several examples illustrate how these pathways can control distinct processes depending on the cellular context. The energy sensor adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a heterotrimeric protein, composed of a catalytic α and regulatory β and γ subunits. When ATP levels are low (for example, due to low nutrients or prolonged exercise), AMP and ADP binding to the γ subunit lead to activation of AMPK, which also requires phosphorylation of the α subunit by liver kinase B1 (LKB1) or, in a few cases, by calmodulin-dependent protein kinase kinase β (CAMKKβ) (Hardie et al. 2016) (Figure 2). AMPK activation inhibits anabolism and cell growth and promotes catabolic processes to restore cellular energetic balance (Hardie 2015; Hardie and Ashford 2014). AMPK inhibits growth in part through the inhibition of the kinase Target of Rapamycin (TOR, or mTOR in mammals). TOR exists as part of two distinct complexes, TORC1 and TORC2, which differ in their regulation and downstream roles (Bar-Peled and Sabatini 2014; Huang and Fingar 2014; Devreotes and Horwitz 2015). TORC1 is the best-understood complex and integrates diverse upstream inputs, including extracellular signals (e.g. insulin signaling) and intracellular cues (e.g. amino acid levels, AMPK activity), to control a variety of downstream cellular processes, including cell growth (Hindupur et al. 2015) (Figure 2).
Figure 2.
Conserved nutrient sensing pathways. Cells respond to intrinsic energy and nutrient levels and external stimulation by hormones to activate an interdependent, conserved cellular response to diet. Species-specific protein names are indicated in orange (C. elegans), green (Drosophila), and purple (mammalian); common names are shown in black.
TOR signaling is required in Drosophila female GSCs. TOR activity promotes GSC proliferation via G2 independently of insulin signaling (see below). Intriguingly, maintenance of female Drosophila GSCs requires very precise regulation of TOR signaling levels. Tor mutation decreases GSC numbers (LaFever et al. 2010), and loss of Tsc1 function causes a significantly more severe GSC loss phenotype (LaFever et al. 2010; Sun et al. 2010), indicating the either low or high TOR activity levels are detrimental to stem cell maintenance. Tsc1 GSCs have low levels of BMP signaling, suggesting an impaired ability to respond to niche signals (Sun et al. 2010). Regulation of the C. elegans germline progenitor pool by AMPK and TOR is mechanistically distinct in developing and adult worms. During earlier nutrient-dependent developmental checkpoints, the homologs of mammalian AMPK, aak-1 and aak-2, suppress germline proliferation, and their simultaneous mutation leads to germline hyperplasia (Fukuyama et al. 2012; Narbonne and Roy 2006a). The C. elegans homolog of the TOR substrate (S6K), rsks-1, is required for germ cell proliferation during the fourth larval instar (Korta et al. 2012). In stark contrast, rsks-1 is dispensable for progenitor proliferation in adults (Korta et al. 2012), and AMPK mutants are still competent to undergo GSC quiescence in response to starvation (Seidel and Kimble 2015). The mechanisms controlling the response of adult C. elegans proliferative germ cells to diet remain largely unknown, and appear to be distinct from those controlling adult female Drosophila GSCs.
TOR is also required for the proliferation, growth and survival of differentiating progeny of Drosophila female GSCs (LaFever et al. 2010). Tor mutant dividing germline cysts in mosaic germaria show increased death, and follicles containing Tor mutant cysts grow at a markedly decreased rate (LaFever et al. 2010). Conversely, follicles carrying homozygous mutant cysts for Tsc1, an upstream inhibitor of TOR, grow significantly faster (LaFever et al. 2010). Under amino acid starvation, TORC1 inhibition by Nitrogen permease regulator like 2 and 3 (Nprl2 and Nprl3) protects pre-vitellogenic follicles from apoptosis, and knockdown of nprl2 and nprl3 in the germline prevents recovery of oogenesis following amino acid starvation (Wei and Lilly 2014). Mutations in follicle cells, in addition to affecting follicle cell growth itself, can also non-autonomously influence the growth of the underlying wild type germline, with Tor and Tsc1 mutant follicle cells slowing down or accelerating follicle growth, respectively (LaFever et al. 2010). AMPK activity also controls follicle cells, and ampk mutant follicle cells are larger than wild type cells (Haack et al. 2013). Follicles carrying Tor mutant cysts eventually arrest at or prior to vitellogenesis, depending on allele strength, and degenerate (Pritchett and McCall 2012; LaFever et al. 2010). In another dipteran species, the yellow fever mosquito Aedes aegypti, where ovarian follicles remain in a previtellogenesis arrest until blood ingestion (Attardo et al. 2005), entry into vitellogenesis is also TOR-dependent, suggesting evolutionary conservation. Specifically, ovarian TORC1 activity is stimulated by a blood meal (Hansen et al. 2005; Roy and Raikhel 2012), and global knockdown of the TOR downstream effector ribosomal protein S6 kinase (S6K) inhibits yolk deposition (Hansen et al. 2005). Finally, TORC1 activity also regulates meiotic entry in the Drosophila ovary, as Tor mutant germline clones enter meiosis prematurely before 16-cell cysts are formed and the amino acid sensing GATOR1 complex promotes meiotic entry in the early germline by inhibiting TOR activity. Since global amino acid deprivation is not a condition of meiotic entry in the Drosophila ovary, however, GATOR1 may be acting in a nutrient-independent role (Wei et al. 2014)
Optimal levels of mTOR activity are also required in the mouse testis. TOR inhibition by rapamycin in neonatal mice reduces testis size, likely due to decreased germ cell proliferation and a block to meiosis (Busada et al. 2015). On the other hand, activation of mTORC1 as a result of global mutation of promyeolocytic leukemia zinc finger (Plzf) leads to compromised GDNF signaling and progressive germ cell loss, and these defects are rescued by rapamycin feeding (Hobbs et al. 2010). Nutrient-sensing pathways in Sertoli cells also influence the germline. For example, Sertoli cell-specific overactivation of TOR via Tsc1 or Tsc2 deletion or through decreased AMPK activity by conditional Lkb1 knockout leads to progressive germ cell loss and loss of Sertoli cell quiescence (Tanwar et al. 2012). These studies suggest that tight regulation of TOR is a recurring theme in the regulation of GSC lineages. Intriguingly, men with Peutz-Jeghers syndrome, frequently associated with LKB1 mutations (Hemminkl 1998), are at risk for germ cell loss and Sertoli cell tumors, suggesting a conserved role for LKB1 in the human testis (Venara et al. 2001; Gourgari et al. 2012).
2.2. Diet-dependent hormones affect GSC lineages
Long-range, diet-dependent signals are integral components of the control of GSC lineages. As discussed below, hormones can regulate GSC lineages through direct actions on the germline itself or indirectly, via the niche or other somatic support tissues. Crosstalk among different hormonal systems and their integration within specific cellular contexts can further refine downstream responses.
Activation of insulin/insulin-like signaling is a highly conserved hormonal response to nutrient availability, and it can also affect downstream production of other hormones, such as the Drosophila steroid hormone 20-hydroxyecdysone (20E). In mammals, insulin secreted by pancreatic β cells in response to stimulation by glucose or amino acids signals through the insulin receptor (InR) (Figure 2). Insulin-like growth factors 1 and 2 (IGF1 and IGF2) are primarily synthesized in the liver and act through their receptors IGFR-1 and IGFR-2 to control cell growth (Siddle 2011). Activation of InR/IGFR receptors leads to multiple downstream events, including increased phosphoinositide-3 kinase (PI3K) activity and downstream phosphorylation and inhibition of the transcriptional factor FOXO. The Drosophila genome encodes eight insulin-like peptides (ILPs), whereas C. elegans has as many as 40, but in both cases, single homologs of InR and other downstream effectors transduce these signals (Kannan and Fridell 2013; Murphy and Hu 2013). 20E, the major steroid hormone in Drosophila, is produced from dietary cholesterol or ergosterol. In females, late stage egg chambers produce 20E in a diet and insulin-dependent manner, whereas the source of the much lower titers of 20E in male hemolymph remains unidentified (Schwedes and Carney 2012). 20E acts through a nuclear hormone receptor composed of the ecdysone receptor [EcR, the homolog of farnesoid X receptor and liver X receptor (King-Jones and Thummel 2005)] and Ultraspiracle (Usp, the homolog of mammalian retinoid X receptor, RXR) to induce a wide range of downstream direct and indirect targets, including the early response genes E74, E75, and broad (Uryu et al. 2015).
ILPs directly stimulate GSC proliferation in Drosophila. In females, neural ILPs act directly on the germ line to control the G2 phase of the GSC cell cycle via PI3K but independently of dFOXO (LaFever and Drummond-Barbosa 2005; Hsu et al. 2008). While down-regulation of the insulin pathway preferentially extends G2, in females on a poor diet, both the G1 and G2 phases of the GSC division cycle are lengthened, suggesting the existence of a yet unknown diet-dependent signal that regulates G1 (Hsu et al. 2008). In male GSCs, germline-specific knockdown or inhibition of insulin signaling increases centrosome misorientation by disrupting localization of Apc2, a cortical protein required for centrosome anchoring (Roth et al. 2012). Conversely, constitutive activation of InR in the male germline rescues diet-induced centrosome misorientation. Further, an intact centrosome orientation checkpoint is required for the slow down of GSC division in males on a poor diet (Roth et al. 2012), suggesting a central role for this mechanism in the testis.
These studies in Drosophila are in contrast to findings in C. elegans. Although larval germ cells or adult germ cell tumors require insulin signaling for proliferation, normal adult germ cells proliferate in an insulin-independent manner (Michaelson et al.; Hubbard 2011; Dillin et al. 2002). Nevertheless, in adult C. elegans, insulin signaling couples nutrient availability to oocyte development and progression through meiosis I through the activation of MAPK/ERK signaling (and, notably, independent of FOXO/DAF-16) (Lopez et al. 2013), indicating that germ cells in different developmental stages use distinct branches of insulin signaling.
Drosophila GSC maintenance also requires insulin signaling. In females, the mechanisms involved are clearly distinct from those controlling proliferation. Insulin signaling is not required in GSCs themselves for their maintenance. Instead, ILPs act directly on cap cells to promote Notch signaling, which is required for cap cell maintenance (Song et al. 2007), through FOXO inhibition; under low insulin signaling, FOXO induces high levels of the glycosyltransferase Fringe, leading to inhibition of the Notch receptor (Hsu and Drummond-Barbosa 2009, 2011; Yang et al. 2013). In addition, ILPs directly stimulate the physical association between cap cells and GSCs through E-cadherin, independently of Notch signaling (Hsu and Drummond-Barbosa 2009). By contrast, in males, insulin signaling is intrinsically required in GSCs for their maintenance, as indicated by the higher frequency of loss of homozygous mutant InR GSCs in mosaic testes. Full rescue of GSCs loss induced by starvation, however, requires the constitutive activation of InR in both the germline and hub cells, suggesting that insulin signaling in the niche may also contribute to GSC maintenance (McLeod et al. 2010). These studies imply that conserved signaling pathways regulating similar processes (e.g. GSC proliferation or maintenance) can evolve distinct mechanisms to achieve that goal even between the different sexes of a given species.
20E directly controls GSC proliferation and maintenance in the Drosophila ovary, and it also has additional roles in ovarian and testicular somatic cells. Temperature-sensitive EcR mutants show increased loss and reduced proliferation rates of GSCs, and both of these phenotypes reflect an intrinsic requirement for ecdysone signaling in GSCs, based on genetic mosaic analyses (Ables and Drummond-Barbosa 2010). Ecdysone signaling controls GSCs independently of insulin signaling by modulating the responsiveness of GSCs to BMP ligands from the niche. This role requires the downstream target E74 [a member of the ets proto-oncogene family (Burtis et al. 1990; Karim et al. 1990)] specifically, as GSCs mutant for usp or E74, but not E75 or broad, show low levels of a BMP signaling reporter and are rapidly lost from the niche (Ables and Drummond-Barbosa 2010). E75 [a homolog of mammalian peroxisome proliferator-activated receptor γ PPARγ)(King-Jones and Thummel 2005)] is also required in escort cells for GSC maintenance, and expression of a dominant negative form of EcR in escort cells disrupts female germ cell differentiation (Konig et al. 2011; Morris and Spradling 2012), underscoring the complexity of 20E regulation of the early female germ line. In later follicle development, E78, an E75-related gene that is also an early ecdysone target (Stone and Thummel 1993), is cell autonomously required for germline survival and functionally interacts with ecdysone signaling (Ables et al. 2015). Many additional targets of ecdysone contribute to its various roles in the female GSC lineage, although more in depth analysis is needed (Ables submitted). In the testis, EcR knockdown in the somatic lineage leads to CySC and GSC loss, and death of differentiating germ cells, although the specific mechanisms involved remain unclear (Li et al. 2014).
Progression through vitellogenesis is a major nutritional checkpoint in Drosophila and other insects. Drosophila temperature-sensitive EcR mutants and E75 mutant germline clones fail to progress through vitellogenesis (Carney and Bender 2000; Buszczak et al. 1999), indicating a germline requirement for ecdysome signaling. Drosophila InR global mutants are defective in ecdysteroid production and vitellogenesis, and these defects appear to be at least partially rescued by treatment with a juvenile hormone analog (Tu et al. 2002). Follicles containing germline clones that are mutant for InR or defective for PI3K signaling, however, have reduced rates of growth and blocked vitellogenesis, clearly indicating that insulin signaling through PI3K is required in the germline itself for these processes (Hsu et al. 2008; LaFever and Drummond-Barbosa 2005). Unlike for GSC proliferation, however, insulin signaling does not involve FOXO and, instead, feeds into TOR signaling for the control of follicle growth (Hsu et al. 2008). Interestingly, insulin/TOR signaling in follicle cells is required for the processing body and microtubule rearrangements that occur in the underlying germ line in response to diet (Burn et al. 2015). In A. aegypti, vitellogenesis also requires insulin signaling (Brown et al. 2008; Gulia-Nuss et al. 2011), and the ovary expresses EcR and shows induction of ecdysone response genes, including E75, upon blood feeding (Cho et al. 1995; Swevers 2009; Pierceall et al. 1999), suggesting conserved mechanisms of vitellogenesis control. After vitellogenesis in the Drosophila ovary, a reduction in insulin signaling induces a metabolic shift toward glycogen storage and mitochondrial quiescence, preparing the oocyte for fertilization (Sieber et al. 2016).
Multiple hormones also regulate mammalian GSC lineages, although the connection to diet is not always well understood. Retinoic acid (RA) is derived from dietary vitamin A and signals through retinoic acid receptors (RARs) and their partners, RXRs (Blomhoff and Blomhoff 2006). The multiple isoforms of RAR and RXR are differentially expressed, lending an additional level of specificity to RA signaling. Analogously to ecdysone signaling in Drosophila, RA signaling is required in both the germline and somatic support cells of the mouse testis (Hogarth and Griswold 2013). Knockout of all three RAR or RXR isoforms specifically in the germ line suppressed spermatogonia proliferation, consistent with the role of vitamin A in maintaining spermatogenesis (Gely-Pernot et al. 2012; Ghyselinck et al. 2006). Furthermore, Sertoli cell-specific knockout of retinaldehyde dehydrogenases, which are required for RA synthesis, blocks sperm meiotic entry (Tong et al. 2013; Raverdeau et al. 2012). Testicular RA titers are also reduced in mice genetically depleted of macrophages, resulting in spermatogonial differentiation defects (DeFalco et al. 2015). Conversely, ectopic RA administration induces differentiation and meiotic entry in specific, poised subsets of spermatogonia (Endo et al. 2015). While spermatogonia can directly respond to RA in vivo (Zhou et al. 2008), it is unlikely that RAR/RXR are the sole mediators of its activity. For example, spermatogonia lacking all three isoforms of either RAR or RXR can still enter meiosis, albeit at a low frequency (Gely-Pernot et al. 2015), hinting that RA could act independently of the nuclear hormone receptor dimer or act indirectly through other testicular cell types (Gely-Pernot et al. 2015). Indeed, Sertoli cell-specific RARα knockout in RARγ mutant mice have a complete block to meiosis, suggesting that the RA-dependent meiosis signal requires paracrine signaling from Sertoli cells (Gely-Pernot et al. 2015). While spermatogonial RAR and RXR isoforms are dispensable for germ cell survival (Gely-Pernot et al. 2015), Sertoli cell RARα promotes germ cell survival in aging animals (Vernet et al. 2006). Sertoli cell-specific knockout of RARα leads to progressive testis deterioration, with death of spermatocytes and spermatids (Vernet et al. 2006). Intriguingly, this phenotype is not recapitulated in Sertoli cell knockout of all isoforms of RXR, invoking again an RXR-independent RA signaling mechanism. Bioinformatic analysis further suggests that several genes responsible for establishing tight junctions to create the blood testis barrier contain retinoic acid response elements, suggesting that RAR activity in Sertoli cells may regulate the integrity of the blood-testis barrier (Chung et al. 2010).
The hypothalamic-pituitary-gonadal axis is a major regulator of mammalian spermatogenesis, and there is evidence to suggest that diet and obesity can impact its function. Gonadotropin releasing hormone (GnRH) secreted from the brain induces tsecretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary, which act on Sertoli and Leydig cells, respectively, to control SSC lineage activity (O’Shaughnessy 2014). LH induces testosterone production by Leydig cells, and testosterone signals via the androgen receptor (AR), a nuclear hormone receptor predominantly expressed in Sertoli cells (Sar et al. 1990; reviewed in Walker and Cheng 2005), although recent evidence suggests the presence of a functional AR in human sperm (Aquila et al. 2007). Aromatase activity in the testis, and to a lesser extent in peripheral tissues, catalyzes the synthesis of estradiol, the most potent biological estrogen, from androgens (Marcus 1976). Estrogens are integral regulators of spermatogenesis, in part because they feed back on the brain to regulate LH and FSH secretion (Simpson et al. 1999; Simpson et al. 2000). While our current understanding of nutrient inputs to the hypothalamic-pituitary-gonadal axis is complicated by the tissue dysfunction associated with its disruption, studies in rats suggest that GnRH release decreases in fasted animals (Gruenewald and Matsumoto 1993). Administration of insulin to female volunteers produces a serum pulse of LH, consistent with insulin acting on the GnRH neurons (Moret et al. 2009). Conflicting reports in mouse models, however, debate whether insulin acts directly on GnRH neurons (Evans et al. 2014; DiVall et al. 2015), and the response to insulin could be sexually dimorphic (Kovacs et al. 2002; Castellano et al. 2006). Furthermore, Leydig cell testosterone production is perturbed in mice with Sertoli cell-specific InR or Igf1r knockout, suggesting that diet-dependent signaling controls the hypothalamic-pituitary-gonadal axis at the testis level (Pitetti et al. 2013b). Igf1 knockout mice also have reduced spermatogenesis, although it is unclear whether there is a developmental contribution to this phenotype (Baker et al. 1996). Metabolic state influences the hypothalamic-pituitary-gonadal axis in adult men and women. Women with diet-induced amenorrhea have reduced plasma levels of estrogen, FSH, and LH (Couzinet et al. 1999), and high aromatase expression in post-menopausal obese women leads to more circulating estrogen (Baglietto et al. 2009). Additionally, men with type 2 diabetes or obesity are more likely to be testosterone deficient, further suggesting a possible connection between the hypothalamic-pituitary-gonadal axis and diet (Kelly and Jones 2013; Wang et al. 2011).
The hypothalamic-gonadal-pituitary axis regulates both the germline and somatic support tissues in the mammalian testis. Testosterone and FSH act semi-redundantly through their receptors (AR and FSHR, respectively) on Sertoli cells to promote germ cell survival (Walker and Cheng 2005). Estrogen signaling is also critical for germline survival during spermatogenesis, as shown in a knockin ER mutant mouse that does not respond to estrogen (Sinkevicius et al. 2008; Sinkevicius et al. 2009). In accordance, global knockout of aromatase in mice results in increased germ cell apoptosis in the testis (Robertson et al. 1999). In both estrogen-signaling incompetent and aromatase mutant mice, circulating LH and FSH levels were slightly higher and unchanged, respectively, suggesting that germ cell apoptosis is not simply a downstream effect of estrogens controlling LH and FSH secretion (Sinkevicius et al. 2009; Robertson et al. 1999; Simpson et al.; Simpson et al. 2000). FSH is required for testis growth in both mice and humans (Kumar et al. 1997; Phillip et al. 1998), and FSHR knockout mice have impaired spermatid elongation (Krishnamurthy et al. 2000). AR is absolutely required in Sertoli cells for completion of meiosis (Abel et al. 2008; De Gendt et al. 2004). Furthermore, mice with a conditional knockout of AR in peritubular myoid cells have significantly reduced sperm counts, suggesting multiple sites of action for testosterone (Zhang et al. 2006).
2.3. Additional interorgan communication influences GSC lineages
Beyond the more “traditional” hormone examples discussed above, the extent of GSC lineage regulation by signals originating in other organs, with distinct organs providing various types of information, is just beginning to be appreciated. The molecular mechanisms of many of these signaling axes remain unknown, but proteohormones, signaling lipids or metabolites, and mobilized nutrients from one organ to another are likely participants.
The nervous system regulates physiological circuits that marry inputs from the external environment, including diet, to whole-body physiology either through changes in organismal behavior or more directly. As discussed above, Drosophila ILPs are diet-dependent neuropeptides that directly regulate GSC lineages and nearby somatic support cells (Hsu and Drummond-Barbosa 2009; LaFever and Drummond-Barbosa 2005). Ecdysone produced by ovarian follicles acts on the brain to promote female-specific feeding behavior, increasing nutrient uptake and supporting oogenesis (Sieber and Spradling 2015). Octopaminergic neurons innervate the ovaries and reproductive tract and are essential for Drosophila ovulation (Lee et al. 2003; Monastirioti 2003; Deady and Sun 2015). Interestingly, a subset of octopaminergic neurons becomes hyperactive under starvation (and promotes foraging behavior) (Yang et al. 2015), suggesting a potential molecular link between ovulation and nutrient availability. During C. elegans development, transforming growth factor β (TGFβ/DAF-7) expression in chemosensory neurons is activated by food availability and low dauer pheromone, which is involved in sensing population density (Ren et al. 1996; Schackwitz 1996; Dalfo et al. 2012). daf-7 mutant worms enter meiosis prematurely, thereby reducing progenitor number, and downstream signaling pathway components are required in the DTC, indicating a niche-mediated role for TGFβ in maintaining a large germline progenitor pool from which gametes can be generated (Dalfo et al. 2012). As mentioned earlier, GnRH released from the mammalian hypothalamus stimulates pituitary FSH and LH release, which in turn act on testis somatic cells to control the germline (O’Shaughnessy 2014). In adult men, even short periods of fasting can suppress GnRH, leading ultimately to a fall in LH-induced testosterone production (Trumble et al. 2010). Thus, nutritional cues may be transmitted to the germline via the brain in multiple organisms, although specific strategies can vary.
GSC lineages are also controlled by endocrine signals from other organs, including the adipose tissue. Accordingly, obese men are more likely to be subfertile (Martin 2014; Kawwass et al. 2015), and diet-induced obesity in rats is linked to decreased sperm motility (Fernandez et al. 2011; Palmer et al. 2012). Mammalian proteohormones secreted from adipocytes, or adipokines, modulate homeostasis by regulating multiple processes (Cao 2014). For example, adiponectin regulates the sensitivity of peripheral tissues to insulin (Yamauchi and Kadowaki 2013), while leptin signals satiety to the brain and controls metabolism in peripheral tissues (Moran and Phillip 2003). Several lines of evidence also suggest that adipokines might influence reproduction, although reports are conflicting (Kawwass et al. 2015). Adiponectin receptors 1 and 2 are expressed in the human and mouse hypothalamus (Dupont et al. 2014), and their transcripts are detected in Leydig cells and the testicular epithelium of rats (Caminos et al. 2008). Adiponectin knockout mice, however, are fertile, while mice lacking adiponectin receptor 2 do not produce sperm (Bjursell et al. 2007), suggesting possible adiponectin-independent roles for this receptor. The leptin receptor is also expressed in the mammalian testis (Landry et al. 2013), and leptin-deficient mice have increased germ cell death and fertility defects (Mounzih et al. 1997; Bhat et al. 2006). Conversely, spermatogenesis defects in men show an association with increased expression of leptin and its receptor in the testis (Ishikawa et al. 2007). Deletion of leptin receptor specifically in the hypothalamus recapitulates many of the phenotypes of leptin-deficient mice, however, suggesting that leptin acts via the brain to control fertility (Ahima et al. 2006). Although these studies are important steps towards our understanding of how mammalian adipokines influence the germline (Kawwass et al. 2015), much remains to be learned about how these and other adipocyte factors control reproduction.
Adipocyte factors also contribute to the control of GSC lineages in invertebrate model systems. In Drosophila, the fat body, an organ composed of adipocytes and hepatocyte-like oenocytes (Gutierrez et al. 2007), has endocrine roles that control development, metabolism and behavior (Arrese and Soulages 2010). Although C. elegans lack a distinct fat storage organ, dedicated lipid storage cells are found in the intestine and epidermis (Mullaney and Ashrafi 2009). Adipokine signaling modules are conserved in Drosophila and C. elegans, and they influence the GSC lineages of these organisms (Rajan and Perrimon 2012; Laws et al. 2015; Kwak et al. 2013; Svensson et al. 2011). The sole adiponectin receptor in Drosophila, AdipoR, is intrinsically required by ovarian GSCs for their maintenance (Laws et al. 2015). In C. elegans, deletion of adiponectin receptor homologs paqr-1,2, and 3 causes extensive defects, including reduced brood size (Svensson et al. 2011). Adiponectin-like ligands have not yet been identified in C. elegans or Drosophila; nevertheless, ex vivo cultures of fly larval brains respond to stimulation by recombinant mammalian adiponectin (Kwak et al. 2013), suggesting that the Drosophila receptor recognizes an endogenous ligand with conserved three-dimensional structure. Additionally, Unpaired 2 (Upd2) is secreted from Drosophila adipocytes and stimulates brain ILP secretion (Rajan and Perrimon 2012), presumably indirectly affecting oogenesis. Human leptin transgenic expression or feeding rescues the upd2 mutant phenotype, suggesting that Upd2 is the functional equivalent of leptin despite lack of primary sequence homology (Rajan and Perrimon 2012).
Studies in Drosophila also show that adipocytes play an important role in reproduction by transmitting nutritional information to the ovary. In Drosophila females, a slight decrease in amino acid levels within adult adipocytes through the knockdown of single amino acid transporters significantly increases the rate of GSC loss from the niche and partially blocks ovulation through distinct mechanisms (Armstrong et al. 2014). Low amino acid levels trigger the evolutionarily conserved amino acid response pathway through unloaded tRNA-mediated activation of the GCN2 kinase within adipocytes to cause GSC loss, whereas amino acids modulate TOR to regulate oocyte ovulation (Armstrong et al. 2014). Adipocyte factors acting downstream of the amino acid response pathway or TOR to control the GSC lineage, however, remain unidentified. In A. aegypti, global knockdown of amino acid transporters reduces egg laying (Carpenter et al. 2012), and TOR activity in the fat body is induced after blood feeding (Hansen et al. 2005) and required for ovarian follicle vitellogenesis (Hansen et al. 2004; Hansen et al. 2005; Roy and Raikhel 2012, 2011; Carpenter et al. 2012). Fat body transcription of vitellogenin, which is physiologically triggered by 20E following a blood meal, can be induced by ex vivo treatment of cultured fat bodies with amino acids, but this effect is suppressed by TOR inhibition (Hansen et al. 2004; Hansen et al. 2005). In vivo, knockdown of components of the TOR pathway reduces egg laying and can impair yolk uptake and egg viability (Hansen et al. 2004; Hansen et al. 2005), although these effects likely reflect potential roles of TOR in multiple locations, as is the case in Drosophila.
Many additional organs are important regulators of physiology; therefore, it would be logical to explore their potential roles in contributing to the control of GSC lineages. For example, nutrients are absorbed at the intestine, and in Drosophila females, feeding conditions change the physiology of the midgut (Cognigni et al. 2011). Mating also leads to extensive remodeling of the midgut and increased lipid metabolism, and these changes are required for normal levels of fecundity (Reiff et al. 2015). Although some of these effects likely reflect changes in efficiency of digestion and nutrient absorption, it is conceivable that more active signaling occurs between the intestine and GSC lineages. It is also important to consider the effect of the gut microbiome in reproduction. The type of bacteria ingested by C. elegans influences its brood size (Yu et al. 2015). Also, in the absence of the nuclear hormone receptor nhr-114 (the homolog of HNF4), worms are sterile when fed a specific strain of bacteria (Gracida and Eckmann 2013a). nhr-114 activity is detected in both the germline and gut, but it does not appear to be required n the germline. Tryptophan supplementation rescues this phenotype, suggesting that nhr-114 may help buffer dietary changes in the gut (Gracida and Eckmann 2013a). Interestingly, germ-free mice have impaired blood-testis barrier and lumen formation in seminiferous tubules, and reduced levels of serum LH and FSH and testicular testosterone. Exposure of these mice to a strain of bacteria that secrete high levels of the short-chain fatty acid butyrate restored integrity of the blood-testis barrier (Al-Asmakh et al. 2014). Recent studies have also shown that muscles secrete peptide hormones, or myokines, in Drosophila and mice (Demontis et al. 2013; Demontis and Perrimon 2010; Demontis et al. 2014), and genetic manipulations in muscles affect the physiology of the fly (Demontis and Perrimon 2010). In mice, osteocalcin secreted from bones modulates spermatogenesis by promoting testosterone production in Leydig cells (Oury et al. 2011). Further, in insects, sex peptides transferred during mating trigger a host of physiological changes, including many upstream of GSC lineage activity (Soller et al. 1997; Kubli 2003). In C. elegans, major sperm protein (MSP) released by sperm promotes oocyte growth, meiotic maturation and ovulation in proximal oocytes via several mechanisms (Miller et al. 2001; Harris et al. 2006; Govindan et al. 2009; Kim et al. 2013). Future studies should consider many possible modes of action for various signals coming from multiple organs in regulating reproductive lineages.
3. Discussion
As reviewed here, highly conserved diet dependent pathways control GSC lineages, revealing interesting similarities and differences in their specific roles in different contexts (Figure 3). As the diet-dependent molecular, cellular and physiological mechanisms controlling GSC lineages are further investigated in multiple models, common themes and more specific strategies shaped by evolution will become clearer. As this field advances, the continued use of tissue- and cell-type-specific manipulations in an in vivo setting will be crucial to understand the full range of contributions of any given hormone or other factor to the regulation of the germline.
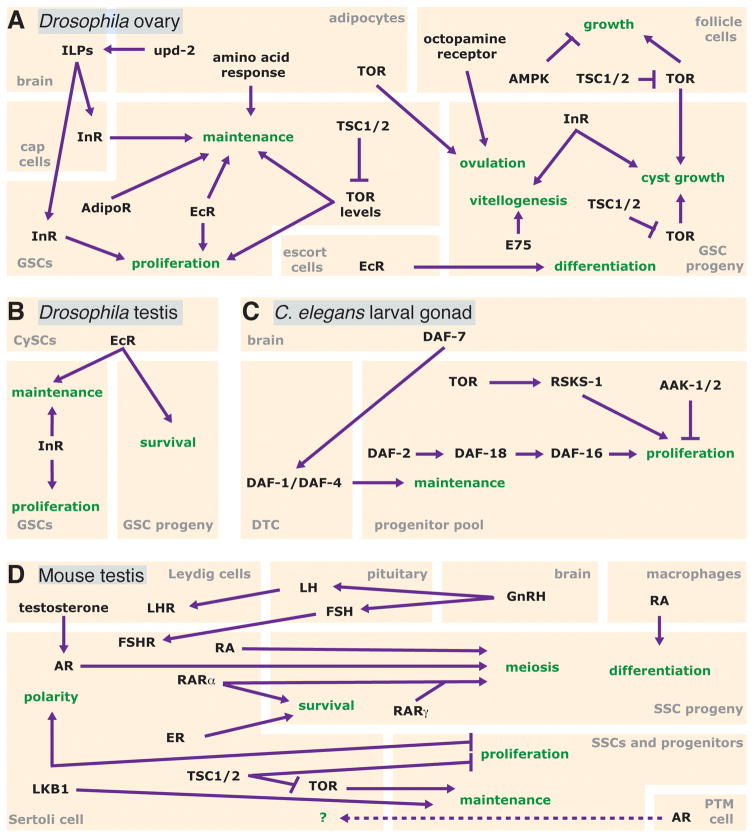
Figure 3.
Cell type-specific requirements for diet-dependent pathways involved in the control of GSCs and their progeny. (A) In the Drosophila ovary, intrinsic, local, and tissue non-autonomous signals coordinately regulate the GSC lineage. In addition to the niche, the brain, follicle cells, and adipocytes all communicate with the GSC lineage as part of the response to diet. (B) GSCs in the Drosophila testis require InR intrinsically for maintenance and proliferation. EcR in cyst stem cells (CySCs) promotes GSC maintenance and the survival of their progeny. (C) In the developing C. elegans gonad, but not in the adult, proliferation is regulated intrinsically by insulin, TOR, and AMPK (AAK-1/2). TGFβ ligand (DAF-7) promotes progenitor pool maintenance via the distal tip cell (DTC). (D) The mouse SSC pool is regulated by Leydig, Sertoli, and peritubular myeloid (PTM) cells, as well as macrophages. An additional layer of control is provided by the hypothalamic-pituitary-gonad axis, which controls the activity of these somatic support cells to influence spermatogenesis. Biological processes are indicated in green and cell types in gray. For details, see text and Table 1.
Not surprisingly, many other factors besides diet can impact organismal physiology. For example, changes in germline activity accompany aging in multiple organisms (Tatar 2010; Oatley and Brinster 2012). C. elegans and Drosophila fecundity is impaired in older females (Herndon et al. 2002; Partridge and Fowler 1992), sperm aneuploidy increases as mice age (Lowe et al. 1995), and sperm count declines in older men (Eskenazi et al. 2003). The germ line ages intrinsically and is also affected by the aging of the soma. In C. elegans, oocytes deteriorate as mated hermaphrodites age, and naturally occurring cell death protects oocyte quality in younger animals, as cell death mutants ced-3 and ced-4 have a premature drop in oocyte quality. This is a germline-autonomous effect because mutations that block only somatic cell death do not impair oocyte quality (Andux and Ellis 2008). In contrast, there is a systemic effect of aging in mouse SSCs, as transplantation of SSC from old donors to young recipients restores youthful function to the older SSCs (Ryu et al. 2006). In Drosophila males and females, GSCs proliferate more slowly as they age (Wallenfang et al. 2006; Cheng et al. 2008; Pan et al. 2007). By contrast, long-lived methuselah mutant males do not experience a drop-off in GSC proliferation as they age (Wallenfang et al. 2006). The number of niche cells and of GSCs decline with age in males and females (Wallenfang et al. 2006; Xie and Spradling 2000; Hsu and Drummond-Barbosa 2009; Zhao et al. 2008; Boyle et al. 2007). E-cadherin, and BMP and insulin signaling levels decline with age in the ovary (Pan et al. 2007; Hsu and Drummond-Barbosa 2009), and overexpression of E-cadherin or AdipoR in the germline (Pan et al. 2007; Laws et al. 2015) or Dpp or ILPs in the soma (Pan et al. 2007; Hsu and Drummond-Barbosa 2009) can reverse age-related GSC loss. Somatic and germline overexpression of superoxide dismutase (SOD), an antioxidant enzyme, rescues the age-associated decline of GSC function and cap cell number (Pan et al. 2007), further emphasizing the complexity of effects of aging on the germ line.
Disease states, including cancer, also disrupt organismal homeostasis. Tumors are their own dynamic signaling centers (Karagiannis et al. 2010) and can both hijack normal metabolism to support their growth and secrete factors that have pleiotropic effects (Patel et al. 2014). For example, tumors induced in the midgut of adult Drosophila or transplanted into the fly hemocoel secrete the ILP binding protein IMP-L2 (Kwon et al. 2015; Figueroa-Clarevega and Bilder 2015), causing systemic wasting, including that of ovaries. In humans, this wasting, called cachexia, is a hallmark of end-stage cancer and is uncoupled from tumor burden (Petruzzelli and Wagner 2016; Fearon et al. 2012), underscoring the role of systemic factors in cancer pathologies. While our understanding of the effect of cachexia on mammalian GSC lineages is limited, cachetic patients are often insulin resistant (Honors and Kinzig 2012), and chronic inflammation, found in many cancers (Crusz and Balkwill 2015), can lead to Interleukin-6 mediated hyperactivation of the hypothalamic-gonadal-pituitary axis (Raber et al. 1997). Therefore, although it is clear that many cancer treatments can impair fertility (Suhag et al. 2015; Vakalopoulos et al. 2015), it is also possible that the physiological changes caused by the tumors themselves may also have effects on the germ line of patients.
Finally, there is also evidence that diet can alter the epigenetic state of the germ line and thereby impact the next generation. For example, male mice fathered by fasted males have lower serum glucose concentrations than those fathered by normally fed animals (Anderson et al. 2006). Adult offspring of Drosophila males fed a high sugar diet have increased food intake, higher adiposity, and defects in lipid mobilization, a metabolic signature dependent on specific subtypes of heterochromatin (Ost et al. 2014). Notably, similar genomic derepression patterns are predictive for obesity in mice and humans, indicating a conserved pathway of diet-induced phenotypic variability (Ost et al. 2014). While the effects of paternal diet lasted a single generation in Drosophila (Ost et al. 2014), in certain mouse genetic backgrounds, paternal high fat diet causes offspring infertility in male and females for two generations (Fullston et al. 2012). Although the molecular mechanisms remain unknown, epidemiological studies have identified multi-generational ramifications for the children of males exposed to occupational and environmental toxins, including lead and pesticides, many of which could be mediated epigenetically (Soubry et al. 2014). As we learn more about how whole-body physiology control of GSC lineages, new light will be shed on how changes caused by diet, aging, diseases, infections, injuries or other stressors affect fertility and, potentially, future generations.
Table 1.
Physiological regulation of GSC lineages by diet-dependent pathways
| Pathway | Organism | Role |
|---|---|---|
| AMPK | C. elegans | Inhibits germline proliferation during larval starvation (Fukuyama et al. 2012; Narbonne and Roy 2006b) |
| Drosophila | Inhibits follicle cell growth in the ovary (Haack et al. 2013) | |
| mouse | Sertoli cell LKB1 promotes SSC proliferation and maintenance (Tanwar et al. 2012) | |
| TOR | C. elegans | Promotes larval progenitor proliferation (Korta et al. 2012) |
| Drosophila | Levels control GSC maintenance and proliferation (LaFever et al. 2010; Sun et al. 2010) and germline cyst survival (LaFever et al. 2010) in the ovary | |
| Regulates ovarian cyst growth intrinsically and via follicle cells (LaFever et al. 2010; Sun et al.) | ||
| A. aegypti | Elevated in ovary following blood meal (Hansen et al. 2005; Roy and Raikhel 2012) | |
| Required in the fat body for vitellogenesis (Hansen et al. 2004; Hansen et al. 2005; Roy and Raikhel 2012, 2011; Carpenter et al. 2012) | ||
| mouse (mTOR) | Promotes germline proliferation and meiosis during development (Busada et al. 2015) | |
| Global hyperactivation inhibits SSC maintenance (Hobbs et al. 2010) | ||
| Overactivation in Sertoli cells leads to intrinsic polarity defects, reduced SSC proliferation, and SSC loss (Tanwar et al. 2012) | ||
| Insulin | C. elegans | Promotes PGC proliferation (Michaelson et al. 2010) |
| Drosophila | Promotes ovarian GSC proliferation, cyst growth, and vitellogenesis (LaFever and Drummond-Barbosa 2005) | |
| Controls ovarian GSC maintenance via cap cells (Hsu and Drummond-Barbosa 2009) | ||
| Promotes GSC proliferation and maintenance in testis (Roth et al. 2012; McLeod et al. 2010) | ||
| A. aegypti | Required for progression through vitellogenesis (reviewed in Attardo et al. 2005) | |
| mouse | Global requirement for InR and Igf1r for testicular development (Pitetti et al. 2013a) | |
| InR and Igf1r promote developmental Sertoli cell proliferation (Pitetti et al. 2013b) | ||
| Global IRS2 mice have small testes and progressive germ cell loss as adults (Griffeth et al. 2013) | ||
| Global Igf1 mice have reduced spermatogenesis (Baker et al. 1996) | ||
| Ecdysone | Drosophila | Promotes ovarian GSC maintenance, proliferation (Ables and Drummond- Barbosa 2010), and vitellogenesis (Buszczak et al. 1999) |
| Required in escort cells for germline differentiation (Konig et al. 2011; Morris and Spradling 2012) | ||
| Required in CySCs for GSC maintenance and progeny survival in the testis (Li et al. 2014) | ||
| A. aegypti | After a blood meal, ecdysone response genes expressed in ovary (Pierceall et al. 1999) | |
| Required in the fat body for vitellogenesisa (Martin et al. 2001) | ||
| Retinoic acid | mouse | Germ cell RARα and Sertoli cell RARγ are together required for meiosis (Gely-Pernot et al. 2015) |
| Required in Sertoli cells for germ cell meiosis (Tong et al. 2013; Raverdeau et al. 2012) | ||
| Androgen receptor | mouse | Required in Sertoli cells for cell survival and meiosis (Abel et al. 2008; Hobbs et al. 2010; De Gendt et al. 2004) |
| Required intrinsically by PTMs for normal sperm counts (Zhang et al. 2006) | ||
| AdipoR | C. elegans | Global mutation reduces brood size (Svensson et al. 2011) |
| Drosophila | Required for ovarian GSC maintenance (Laws et al. 2015) | |
| mouse | AdipoR2 global mutants are aspermic (Bjursell et al. 2007) | |
| Leptin | Drosophila | Required in fat body to promote ILP secretion from the brain (Rajan and Perrimon 2012) |
| mouse | Promotes fertility (Mounzih et al. 1997) and germ cell survival (Bhat et al. 2006) |
References
- Abel MH, Baker PJ, Charlton HM, Monteiro A, Verhoeven G, De Gendt K, Guillou F, O’Shaughnessy PJ. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology. 2008;149(7):3279–3285. doi: 10.1210/en.2008-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ET, Bois KE, Garcia CA, Drummond-Barbosa D. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev Biol. 2015;400(1):33–42. doi: 10.1016/j.ydbio.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7(5):581–592. doi: 10.1016/j.stem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ET, Laws KM, Drummond-Barbosa D. Control of adult stem cells in vivo by a dynamic physiological environment: diet-dependent systemic factors in Drosophila and beyond. Wiley Interdiscip Rev Dev Biol. 2012;1(5):657–674. doi: 10.1002/wdev.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ETHGH, Finger DS, Hinnant TD, Drummond-Barbosa D. A genetic mosaic screen reveals ecdysone-responsive genes regulating Drosophila oogenesis. doi: 10.1534/g3.116.028951. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Qi Y, Singhal NS. Adipokines that link obesity and diabetes to the hypothalamus. Prog Brain Res. 2006;153:155–174. doi: 10.1016/S0079-6123(06)53009-2. [DOI] [PubMed] [Google Scholar]
- Al-Asmakh M, Stukenborg JB, Reda A, Anuar F, Strand ML, Hedin L, Pettersson S, Soder O. The gut microbiota and developmental programming of the testis in mice. PLoS One. 2014;9(8):e103809. doi: 10.1371/journal.pone.0103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LM, Riffle L, Wilson R, Travlos GS, Lubomirski MS, Alvord WG. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition. 2006;22(3):327–331. doi: 10.1016/j.nut.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Andux S, Ellis RE. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008;4(12):e1000295. doi: 10.1371/journal.pgen.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326(5955):954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Aquila S, Middea E, Catalano S, Marsico S, Lanzino M, Casaburi I, Barone I, Bruno R, Zupo S, Ando S. Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum Reprod. 2007;22(10):2594–2605. doi: 10.1093/humrep/dem243. [DOI] [PubMed] [Google Scholar]
- Armstrong AR, Laws KM, Drummond-Barbosa D. Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila. Development. 2014;141(23):4479–4488. doi: 10.1242/dev.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol. 2005;35(7):661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Baglietto L, English DR, Hopper JL, MacInnis RJ, Morris HA, Tilley WD, Krishnan K, Giles GG. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009;115(1):171–179. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10(7):903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24(7):400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, Ford BD, Mann DR. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27(2):302–310. doi: 10.2164/jandrol.05133. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Linden D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56(3):583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10(3):175–186. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66(7):606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- Boucheron-Houston C, Canterel-Thouennon L, Lee TL, Baxendale V, Nagrani S, Chan WY, Rennert OM. Long-term vitamin A deficiency induces alteration of adult mouse spermatogenesis and spermatogonial differentiation: direct effect on spermatogonial gene expression and indirect effects via somatic cells. J Nutr Biochem. 2013;24(6):1123–1135. doi: 10.1016/j.jnutbio.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1(4):470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2008;105(15):5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn KM, Shimada Y, Ayers K, Lu F, Hudson AM, Cooley L. Somatic insulin signaling regulates a germline starvation response in Drosophila egg chambers. Dev Biol. 2015;398(2):206–217. doi: 10.1016/j.ydbio.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis KC, Thummel CS, Jones CW, Karim FD, Hogness DS. The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell. 1990;61(1):85–99. doi: 10.1016/0092-8674(90)90217-3. [DOI] [PubMed] [Google Scholar]
- Busada JT, Geyer CB. The Role of Retinoic Acid (RA) in Spermatogonial Differentiation. Biol Reprod. 2016;94(1):10. doi: 10.1095/biolreprod.115.135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Niedenberger BA, Velte EK, Keiper BD, Geyer CB. Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo. Dev Biol. 2015;407(1):90–102. doi: 10.1016/j.ydbio.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126(20):4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Caminos JE, Nogueiras R, Gaytan F, Pineda R, Gonzalez CR, Barreiro ML, Castano JP, Malagon MM, Pinilla L, Toppari J, Dieguez C, Tena-Sempere M. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 2008;149(7):3390–3402. doi: 10.1210/en.2007-1582. [DOI] [PubMed] [Google Scholar]
- Campbell JM, Lane M, Owens JA, Bakos HW. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod Biomed Online. 2015;31(5):593–604. doi: 10.1016/j.rbmo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220(2):T47–59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154(3):1203–1211. doi: 10.1093/genetics/154.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter VK, Drake LL, Aguirre SE, Price DP, Rodriguez SD, Hansen IA. SLC7 amino acid transporters of the yellow fever mosquito Aedes aegypti and their role in fat body TOR signaling and reproduction. J Insect Physiol. 2012;58(4):513–522. doi: 10.1016/j.jinsphys.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55(9):2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456(7222):599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WL, Kapitskaya MZ, Raikhel AS. Mosquito ecdysteroid receptor: analysis of the cDNA and expression during vitellogenesis. Insect Biochem Mol Biol. 1995;25(1):19–27. doi: 10.1016/0965-1748(94)00045-j. [DOI] [PubMed] [Google Scholar]
- Chung SS, Choi C, Wang X, Hallock L, Wolgemuth DJ. Aberrant distribution of junctional complex components in retinoic acid receptor alpha-deficient mice. Microsc Res Tech. 2010;73(6):583–596. doi: 10.1002/jemt.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13(1):92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;29(2):82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (Oxf) 1999;50(2):229–235. doi: 10.1046/j.1365-2265.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- Croxford TP, McCormick NH, Kelleher SL. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr. 2011;141(3):359–365. doi: 10.3945/jn.110.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- Dalfo D, Michaelson D, Hubbard EJ. Sensory regulation of the C. elegans germline through TGF-beta-dependent signaling in the niche. Curr Biol. 2012;22(8):712–719. doi: 10.1016/j.cub.2012.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101(5):1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21(6):776–798. [PubMed] [Google Scholar]
- Deady LD, Sun J. A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Drosophila Ovulation. PLoS Genet. 2015;11(10):e1005604. doi: 10.1371/journal.pgen.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ, Capel B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015;12(7):1107–1119. doi: 10.1016/j.celrep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Patel VK, Swindell WR, Perrimon N. Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep. 2014;7(5):1481–1494. doi: 10.1016/j.celrep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Piccirillo R, Goldberg AL, Perrimon N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell. 2013;12(6):943–949. doi: 10.1111/acel.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P, Horwitz AR. Signaling networks that regulate cell migration. Cold Spring Harb Perspect Biol. 2015;7(8):a005959. doi: 10.1101/cshperspect.a005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298(5594):830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A. Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS One. 2015;10(3):e0119995. doi: 10.1371/journal.pone.0119995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231(1):265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Dupont J, Reverchon M, Bertoldo MJ, Froment P. Nutritional signals and reproduction. Mol Cell Endocrinol. 2014;382(1):527–537. doi: 10.1016/j.mce.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Endo T, Romer KA, Anderson EL, Baltus AE, de Rooij DG, Page DC. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc Natl Acad Sci U S A. 2015;112(18):E2347–2356. doi: 10.1073/pnas.1505683112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003;18(2):447–454. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- Evans MC, Rizwan M, Mayer C, Boehm U, Anderson GM. Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J Neuroendocrinol. 2014;26(7):468–479. doi: 10.1111/jne.12166. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu Y, Xue K, Gu G, Fan W, Xu Y, Ding Z. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 2015;10(4):e0120775. doi: 10.1371/journal.pone.0120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto AP, Nascimento AF, Cicogna AC, Kempinas WD. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32. doi: 10.1186/1477-7827-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Clarevega A, Bilder D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell. 2015;33(1):47–55. doi: 10.1016/j.devcel.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology. 2016;4(2):189–212. doi: 10.1111/andr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M, Sakuma K, Park R, Kasuga H, Nagaya R, Atsumi Y, Shimomura Y, Takahashi S, Kajiho H, Rougvie A, Kontani K, Katada T. C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol Open. 2012;1(10):929–936. doi: 10.1242/bio.2012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. Spermatogenesis. In: Bate MaA, AM, editors. The Development of Drosophila melanogaster. I. Cold Spring Harbor Laboratory Press; 1993. pp. 71–147. [Google Scholar]
- Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, Lane M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27(5):1391–1400. doi: 10.1093/humrep/des030. [DOI] [PubMed] [Google Scholar]
- Gely-Pernot A, Raverdeau M, Celebi C, Dennefeld C, Feret B, Klopfenstein M, Yoshida S, Ghyselinck NB, Mark M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology. 2012;153(1):438–449. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- Gely-Pernot A, Raverdeau M, Teletin M, Vernet N, Feret B, Klopfenstein M, Dennefeld C, Davidson I, Benoit G, Mark M, Ghyselinck NB. Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor. PLoS Genet. 2015;11(10):e1005501. doi: 10.1371/journal.pgen.1005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235(6):1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- Gourgari E, Saloustros E, Stratakis CA. Large-cell calcifying Sertoli cell tumors of the testes in pediatrics. Curr Opin Pediatr. 2012;24(4):518–522. doi: 10.1097/MOP.0b013e328355a279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan JA, Nadarajan S, Kim S, Starich TA, Greenstein D. Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans. Development. 2009;136(13):2211–2221. doi: 10.1242/dev.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracida X, Eckmann CR. Fertility and germline stem cell maintenance under different diets requires nhr-114/HNF4 in C. elegans. Curr Biol. 2013a;23(7):607–613. doi: 10.1016/j.cub.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Gracida X, Eckmann CR. Mind the gut: Dietary impact on germline stem cells and fertility. Commun Integr Biol. 2013b;6(6):e26004. doi: 10.4161/cib.26004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan LJ, de Cuevas M, Matunis E. Genetics of gonadal stem cell renewal. Annu Rev Cell Dev Biol. 2015;31:291–315. doi: 10.1146/annurev-cellbio-100913-013344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffeth RJ, Carretero J, Burks DJ. Insulin receptor substrate 2 is required for testicular development. PLoS One. 2013;8(5):e62103. doi: 10.1371/journal.pone.0062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group ECW. Nutrition and reproduction in women. Hum Reprod Update. 2006;12(3):193–207. doi: 10.1093/humupd/dmk003. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Reduced gonadotropin-releasing hormone gene expression with fasting in the male rat brain. Endocrinology. 1993;132(1):480–482. doi: 10.1210/endo.132.1.8419144. [DOI] [PubMed] [Google Scholar]
- Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One. 2011;6(5):e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445(7125):275–280. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- Haack T, Bergstralh DT, St Johnston D. Damage to the Drosophila follicle cell epithelium produces “false clones” with apparent polarity phenotypes. Biol Open. 2013;2(12):1313–1320. doi: 10.1242/bio.20134671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc Natl Acad Sci U S A. 2004;101(29):10626–10631. doi: 10.1073/pnas.0403460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Roy SG, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J Biol Chem. 2005;280(21):20565–20572. doi: 10.1074/jbc.M500712200. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29(2):99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26(3):190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Govindan JA, Yamamoto I, Schwartz J, Kaverina I, Greenstein D. Major sperm protein signaling promotes oocyte microtubule reorganization prior to fertilization in Caenorhabditis elegans. Dev Biol. 2006;299(1):105–121. doi: 10.1016/j.ydbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Hemminkl AMD, Tomlinson I, Avizlenyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelln K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hindupur SK, Gonzalez A, Hall MN. The Opposing Actions of Target of Rapamycin and AMP-Activated Protein Kinase in Cell Growth Control. Cold Spring Harb Perspect Med. 2015;5(7):a019141. doi: 10.1101/cshperspect.a019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell. 2010;142(3):468–479. doi: 10.1016/j.cell.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. Retinoic acid regulation of male meiosis. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):217–223. doi: 10.1097/MED.0b013e32836067cf. [DOI] [PubMed] [Google Scholar]
- Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle. 2012;3(1):5–11. doi: 10.1007/s13539-011-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci U S A. 2009;106(4):1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev Biol. 2011;350(2):290–300. doi: 10.1016/j.ydbio.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313(2):700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJ. Insulin and germline proliferation in Caenorhabditis elegans. Vitam Horm. 2011;87:61–77. doi: 10.1016/B978-0-12-386015-6.00024-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJ, Greenstein D. Introduction to the germ line. WormBook. 2005:1–4. doi: 10.1895/wormbook.1.18.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia. 2007;39(1):22–27. doi: 10.1111/j.1439-0272.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- Kannan K, Fridell YW. Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction. Front Physiol. 2013;4:288. doi: 10.3389/fphys.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis GS, Pavlou MP, Diamandis EP. Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol Oncol. 2010;4(6):496–510. doi: 10.1016/j.molonc.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim FD, Urness LD, Thummel CS, Klemsz MJ, McKercher SR, Celada A, Van Beveren C, Maki RA, Gunther CV, Nye JA, et al. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Kawwass JF, Summer R, Kallen CB. Direct effects of leptin and adiponectin on peripheral reproductive tissues: a critical review. Mol Hum Reprod. 2015;21(8):617–632. doi: 10.1093/molehr/gav025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- Kim S, Spike C, Greenstein D. Control of oocyte growth and meiotic maturation in Caenorhabditis elegans. Adv Exp Med Biol. 2013;757:277–320. doi: 10.1007/978-1-4614-4015-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Seidel H. StemBook. Cambridge (MA): 2008. C. elegans germline stem cells and their niche. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6(4):311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Wang S, Xie T. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 2011;138(23):5087–5097. doi: 10.1242/dev.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig A, Yatsenko AS, Weiss M, Shcherbata HR. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 2011;30(8):1549–1562. doi: 10.1038/emboj.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korta DZ, Tuck S, Hubbard EJ. S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Development. 2012;139(5):859–870. doi: 10.1242/dev.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs P, Parlow AF, Karkanias GB. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology. 2002;76(6):357–365. doi: 10.1159/000067585. 67585. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod. 2000;62(5):1146–1159. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci. 2003;60(8):1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Kwak SJ, Hong SH, Bajracharya R, Yang SY, Lee KS, Yu K. Drosophila adiponectin receptor in insulin producing cells regulates glucose and lipid metabolism by controlling insulin secretion. PLoS One. 2013;8(7):e68641. doi: 10.1371/journal.pone.0068641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell. 2015;33(1):36–46. doi: 10.1016/j.devcel.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault SW. The genetics and cell biology of spermatogenesis in the nematode C. elegans. Mol Cell Endocrinol. 2009;306(1–2):59–65. doi: 10.1016/j.mce.2009.01.008. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309(5737):1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137(13):2117–2126. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod Biol. 2013;13(1):1–14. doi: 10.1016/j.repbio.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Laws KM, Sampson LL, Drummond-Barbosa D. Insulin-independent role of adiponectin receptor signaling in Drosophila germline stem cell maintenance. Dev Biol. 2015;399(2):226–236. doi: 10.1016/j.ydbio.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Seong CS, Kim YC, Davis RL, Han KA. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264(1):179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Li Y, Ma Q, Cherry CM, Matunis EL. Steroid signaling promotes stem cell maintenance in the Drosophila testis. Dev Biol. 2014;394(1):129–141. doi: 10.1016/j.ydbio.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AL, 3rd, Chen J, Joo HJ, Drake M, Shidate M, Kseib C, Arur S. DAF-2 and ERK couple nutrient availability to meiotic progression during Caenorhabditis elegans oogenesis. Dev Cell. 2013;27(2):227–240. doi: 10.1016/j.devcel.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe X, Collins B, Allen J, Titenko-Holland N, Breneman J, van Beek M, Bishop J, Wyrobek AJ. Aneuploidies and micronuclei in the germ cells of male mice of advanced age. Mutat Res. 1995;338(1–6):59–76. doi: 10.1016/0921-8734(95)00012-u. [DOI] [PubMed] [Google Scholar]
- Mair W, McLeod CJ, Wang L, Jones DL. Dietary restriction enhances germline stem cell maintenance. Aging Cell. 2010;9(5):916–918. doi: 10.1111/j.1474-9726.2010.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R, Korenman SG. Estrogens and the human male. Annu Rev Med. 1976;27:457–370. doi: 10.1146/annurev.me.27.020176.002041. [DOI] [PubMed] [Google Scholar]
- Martin D, Wang SF, Raikhel AS. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol Cell Endocrinol. 2001;173(1–2):75–86. doi: 10.1016/s0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Martin LJ. Implications of adiponectin in linking metabolism to testicular function. Endocrine. 2014;46(1):16–28. doi: 10.1007/s12020-013-0102-0. [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20(23):2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137(4):671–680. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291(5511):2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264(1):38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Moran O, Phillip M. Leptin: obesity, diabetes and other peripheral effects--a review. Pediatr Diabetes. 2003;4(2):101–109. doi: 10.1034/j.1399-5448.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- Moret M, Stettler R, Rodieux F, Gaillard RC, Waeber G, Wirthner D, Giusti V, Tappy L, Pralong FP. Insulin modulation of luteinizing hormone secretion in normal female volunteers and lean polycystic ovary syndrome patients. Neuroendocrinology. 2009;89(2):131–139. doi: 10.1159/000160911. [DOI] [PubMed] [Google Scholar]
- Morris LX, Spradling AC. Steroid signaling within Drosophila ovarian epithelial cells sex-specifically modulates early germ cell development and meiotic entry. PLoS One. 2012;7(10):e46109. doi: 10.1371/journal.pone.0046109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138(3):1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- Mullaney BC, Ashrafi K. C. elegans fat storage and metabolic regulation. Biochim Biophys Acta. 2009;1791(6):474–478. doi: 10.1016/j.bbalip.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, Hu PJ. Insulin/insulin-like growth factor signaling in C. elegans. WormBook. 2013:1–43. doi: 10.1895/wormbook.1.164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development. 2006a;133(4):611–619. doi: 10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Regulation of germline stem cell proliferation downstream of nutrient sensing. Cell Div. 2006b;1:29. doi: 10.1186/1747-1028-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy PJ. Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol. 2014;29:55–65. doi: 10.1016/j.semcdb.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92(2):577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, Pospisilik JA. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159(6):1352–1364. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5):796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer NO, Bakos HW, Fullston T, Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012;2(4):253–263. doi: 10.4161/spmg.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1(4):458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Partridge L, Fowler K. Direct and Correlated Responses to Selection on Age at Reproduction in Drosophila melanogaster. Evolution. 1992;46(1):76–91. doi: 10.2307/2409806. [DOI] [PubMed] [Google Scholar]
- Patel S, Ngounou Wetie AG, Darie CC, Clarkson BD. Cancer secretomes and their place in supplementing other hallmarks of cancer. Adv Exp Med Biol. 2014;806:409–442. doi: 10.1007/978-3-319-06068-2_20. [DOI] [PubMed] [Google Scholar]
- Petruzzelli M, Wagner EF. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30(5):489–501. doi: 10.1101/gad.276733.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med. 1998;338(24):1729–1732. doi: 10.1056/NEJM199806113382404. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Li C, Biran A, Miura K, Raikhel AS, Segraves WA. E75 expression in mosquito ovary and fat body suggests reiterative use of ecdysone-regulated hierarchies in development and reproduction. Mol Cell Endocrinol. 1999;150(1–2):73–89. doi: 10.1016/s0303-7207(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Pitetti JL, Calvel P, Romero Y, Conne B, Truong V, Papaioannou MD, Schaad O, Docquier M, Herrera PL, Wilhelm D, Nef S. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013a;9(1):e1003160. doi: 10.1371/journal.pgen.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitetti JL, Calvel P, Zimmermann C, Conne B, Papaioannou MD, Aubry F, Cederroth CR, Urner F, Fumel B, Crausaz M, Docquier M, Herrera PL, Pralong F, Germond M, Guillou F, Jegou B, Nef S. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol Endocrinol. 2013b;27(5):814–827. doi: 10.1210/me.2012-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett TL, McCall K. Role of the insulin/Tor signaling network in starvation-induced programmed cell death in Drosophila oogenesis. Cell Death Differ. 2012;19(6):1069–1079. doi: 10.1038/cdd.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, O’Shea RD, Bloom FE, Campbell IL. Modulation of hypothalamic-pituitary-adrenal function by transgenic expression of interleukin-6 in the CNS of mice. J Neurosci. 1997;17(24):9473–9480. doi: 10.1523/JNEUROSCI.17-24-09473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151(1):123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109(41):16582–16587. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff T, Jacobson J, Cognigni P, Antonello Z, Ballesta E, Tan KJ, Yew JY, Dominguez M, Miguel-Aliaga I. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. Elife. 2015;4:e06930. doi: 10.7554/eLife.06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274(5291):1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci U S A. 1999;96(14):7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Bennett LD, RF, Moore JD, Vilchis LI. Response of red abalone reproduction ot warm water, starvation, and disease stressors: implications of ocean warming. Journal of Shellfish Research. 2010;29(3):599–611. doi: 10.2983/035.029.0308. [DOI] [Google Scholar]
- Rojas J, Chavez-Castillo M, Olivar LC, Calvo M, Mejias J, Rojas M, Morillo J, Bermudez V. Physiologic Course of Female Reproductive Function: A Molecular Look into the Prologue of Life. J Pregnancy. 2015;2015:715735. doi: 10.1155/2015/715735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TM, Chiang CY, Inaba M, Yuan H, Salzmann V, Roth CE, Yamashita YM. Centrosome misorientation mediates slowing of the cell cycle under limited nutrient conditions in Drosophila male germline stem cells. Mol Biol Cell. 2012;23(8):1524–1532. doi: 10.1091/mbc.E11-12-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SG, Raikhel AS. The small GTPase Rheb is a key component linking amino acid signaling and TOR in the nutritional pathway that controls mosquito egg development. Insect Biochem Mol Biol. 2011;41(1):62–69. doi: 10.1016/j.ibmb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SG, Raikhel AS. Nutritional and hormonal regulation of the TOR effector 4E-binding protein (4E-BP) in the mosquito Aedes aegypti. FASEB J. 2012;26(3):1334–1342. doi: 10.1096/fj.11-189969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24(6):1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127(6):3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Schackwitz WSIT, Thomas JH. Chemosensory neurons in parellel to mediate a phermone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster. J Insect Physiol. 2012;58(3):293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Seidel HS, Kimble J. Cell-cycle quiescence maintains Caenorhabditis elegans germline stem cells independent of GLP-1/Notch. Elife. 2015:4. doi: 10.7554/eLife.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47(1):R1–10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- Sieber MH, Spradling AC. Steroid Signaling Establishes a Female Metabolic State and Regulates SREBP to Control Oocyte Lipid Accumulation. Curr Biol. 2015;25(8):993–1004. doi: 10.1016/j.cub.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thomsen MB, Spradling AC. Electron Transport Chain Remodeling by GSK3 during Oogenesis Connects Nutrient State to Reproduction. Cell. 2016;164(3):420–432. doi: 10.1016/j.cell.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Davis S, Jones M. Local estrogen biosynthesis in males and females. Endocr Relat Cancer. 1999;6(2):131–137. doi: 10.1677/erc.0.0060131. [DOI] [PubMed] [Google Scholar]
- Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Jones M, Davis S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000;11(5):184–188. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149(6):2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkevicius KW, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL. Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology. 2009;150(6):2898–2905. doi: 10.1210/en.2008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur J Biochem. 1997;243(3):732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioessays. 2014;36(4):359–371. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, editor. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. [Google Scholar]
- Stone BL, Thummel CS. The Drosophila 78C early late puff contains E78, an ecdysone-inducible gene that encodes a novel member of the nuclear hormone receptor superfamily. Cell. 1993;75(2):307–320. doi: 10.1016/0092-8674(93)80072-m. [DOI] [PubMed] [Google Scholar]
- Suhag V, Sunita BS, Sarin A, Singh AK, Dashottar S. Fertility preservation in young patients with cancer. South Asian J Cancer. 2015;4(3):134–139. doi: 10.4103/2278-330X.173175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Quan Z, Zhang B, Wu T, Xi R. TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development. 2010;137(15):2461–2469. doi: 10.1242/dev.051466. [DOI] [PubMed] [Google Scholar]
- Sun W, Chen L, Zhang W, Wang R, Goltzman D, Miao D. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015;308(1):E51–62. doi: 10.1152/ajpendo.00076.2014. [DOI] [PubMed] [Google Scholar]
- Svensson E, Olsen L, Morck C, Brackmann C, Enejder A, Faergeman NJ, Pilon M. The adiponectin receptor homologs in C. elegans promote energy utilization and homeostasis. PLoS One. 2011;6(6):e21343. doi: 10.1371/journal.pone.0021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swevers LaI K. Ecdysteroids and ecdysteroid signaling pathways during insect oogenesis. 2009. [Google Scholar]
- Tanwar PS, Kaneko-Tarui T, Zhang L, Teixeira JM. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum Mol Genet. 2012;21(20):4394–4405. doi: 10.1093/hmg/dds272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Reproductive aging in invertebrate genetic models. Ann N Y Acad Sci. 2010;1204:149–155. doi: 10.1111/j.1749-6632.2010.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong MH, Yang QE, Davis JC, Griswold MD. Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc Natl Acad Sci U S A. 2013;110(2):543–548. doi: 10.1073/pnas.1214883110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Brindle E, Kupsik M, O’Connor KA. Responsiveness of the reproductive axis to a single missed evening meal in young adult males. Am J Hum Biol. 2010;22(6):775–781. doi: 10.1002/ajhb.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1(2):158–160. doi: 10.1046/j.1474-9728.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- Uryu O, Ameku T, Niwa R. Recent progress in understanding the role of ecdysteroids in adult insects: Germline development and circadian clock in the fruit fly Drosophila melanogaster. Zoological Lett. 2015;1:32. doi: 10.1186/s40851-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakalopoulos I, Dimou P, Anagnostou I, Zeginiadou T. Impact of cancer and cancer treatment on male fertility. Hormones (Athens) 2015;14(4):579–589. doi: 10.14310/horm.2002.1620. [DOI] [PubMed] [Google Scholar]
- Venara M, Rey R, Bergada I, Mendilaharzu H, Campo S, Chemes H. Sertoli cell proliferations of the infantile testis: an intratubular form of Sertoli cell tumor? Am J Surg Pathol. 2001;25(10):1237–1244. doi: 10.1097/00000478-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25(24):5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130(1):15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5(4):297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Jackson G, Jones TH, Matsumoto AM, Nehra A, Perelman MA, Swerdloff RS, Traish A, Zitzmann M, Cunningham G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34(7):1669–1675. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol. 2006;68:223–251. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- Wei Y, Lilly MA. The TORC1 inhibitors Nprl2 and Nprl3 mediate an adaptive response to amino-acid starvation in Drosophila. Cell Death Differ. 2014;21(9):1460–1468. doi: 10.1038/cdd.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Reveal B, Reich J, Laursen WJ, Senger S, Akbar T, Iida-Jones T, Cai W, Jarnik M, Lilly MA. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc Natl Acad Sci U S A. 2014;111(52):E5670–5677. doi: 10.1073/pnas.1419156112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. StemBook. Cambridge (MA): 2008. Germline stem cell niches. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290(5490):328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17(2):185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Yang H, Yamashita YM. The regulated elimination of transit-amplifying cells preserves tissue homeostasis during protein starvation in Drosophila testis. Development. 2015;142(10):1756–1766. doi: 10.1242/dev.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SA, Wang WD, Chen CT, Tseng CY, Chen YN, Hsu HJ. FOXO/Fringe is necessary for maintenance of the germline stem cell niche in response to insulin insufficiency. Dev Biol. 2013;382(1):124–135. doi: 10.1016/j.ydbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yu Y, Zhang V, Tian Y, Qi W, Wang L. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc Natl Acad Sci U S A. 2015;112(16):5219–5224. doi: 10.1073/pnas.1417838112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Yan X, Ye C, Zhao H, Chen X, Hu F, Li H. Bacterial Respiration and Growth Rates Affect the Feeding Preferences, Brood Size and Lifespan of Caenorhabditis elegans. PLoS One. 2015;10(7):e0134401. doi: 10.1371/journal.pone.0134401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci U S A. 2006;103(47):17718–17723. doi: 10.1073/pnas.0608556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7(3):344–354. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79(1):35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]