Abstract
Objectives
Despite clinical reports of poor outcomes, the degree to which REBOA exacerbates traumatic brain injury (TBI) is not known. We hypothesized that combined effects of increased proximal mean arterial pressure (pMAP), carotid blood flow (Qcarotid), and intracranial pressure (ICP) from REBOA would lead to TBI progression compared to partial aortic occlusion (PAO) or no intervention.
Methods
21 swine underwent a standardized TBI via computer Controlled cortical impact followed by 25% total blood volume rapid hemorrhage. After 30 minutes of hypotension, animals were randomized to 60 minutes of continued hypotension (Control), REBOA, or PAO. REBOA and PAO animals were then weaned from occlusion. All animals were resuscitated with shed blood via a rapid blood infuser. Physiologic parameters were recorded continuously and brain computed tomography obtained at specified intervals.
Results
There were no differences in baseline physiology or during the initial 30 minutes of hypotension. During the 60-minute intervention period, REBOA resulted in higher maximal pMAP (REBOA 105.3±8.8; PAO 92.7±9.2; Control 48.9±7.7, p=0.02) and higher Qcarotid (REBOA 673.1±57.9; PAO 464.2±53.0; Control 170.3±29.4, p<0.01). Increases in ICP were greatest during blood resuscitation, with Control animals demonstrating the largest peak ICP (Control 12.8±1.2; REBOA 5.1±0.6; PAO 9.4±1.1, p<0.01). There were no differences in the percentage of animals with hemorrhage progression on CT (Control 14.3%, 95%CI 3.6–57.9; REBOA 28.6%, 95%CI 3.7–71.0; and PAO 28.6%, 95%CI 3.7–71.0).
Conclusions
In an animal model of TBI and shock, REBOA increased carotid flow and pMAP, but did not exacerbate TBI progression. PAO resulted in physiology closer to baseline with smaller increases in ICP and pMAP. Rapid blood resuscitation, not REBOA, resulted in the largest increase in ICP after intervention, which occurred in Control animals. Continued studies of the cerebral hemodynamics of aortic occlusion and blood transfusion are required to determine optimal resuscitation strategies for multi-injured patients.
Keywords: Traumatic Brain Injury, Shock, Endovascular, Resuscitation, Intra-aortic balloon
Introduction
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a viable alternative to resuscitative thoracotomy for non-compressible torso hemorrhage (NCTH) by restoring perfusion to proximal vascular beds while arresting downstream hemorrhage. (1, 2) REBOA has gained popularity because its minimally invasive nature allows it to be proactively employed prior to hemodynamic collapse.(3) While adoption of REBOA is increasing, appropriate patient selection remains problematic because no consensus guidelines exist regarding its clinical use.(4, 5)
Physicians within the trauma community have hypothesized that REBOA can be detrimental to patients with non-compressible torso hemorrhage and concomitant traumatic brain injuries.(1, 4, 6–9) Supra-physiologic blood pressure and carotid blood flow created by aortic occlusion may worsen cerebral edema, increase intracranial pressure, or exacerbate intracranial hemorrhage. The mortality rate in brain-injured patients requiring REBOA as a resuscitative adjunct approaches 50% and case reports have demonstrated increased intracranial hemorrhage volumes after brief periods of REBOA.(7, 10–12) As such, alternative techniques of achieving partial aortic occlusion have been devised to off-load proximal pressure by permitting persistent low-volume distal blood flow through partial-REBOA (P-REBOA) or Endovascular Variable Aortic Control.(8, 13) In animal studies, P-REBOA improved proximal perfusion while minimizing the hypertension and excessive carotid flow seen with complete REBOA.(8, 9) Despite theoretical advantages of P-REBOA over complete REBOA for brain injured patients with NCTH, the impact of these techniques on brain injury has not been evaluated.(1, 8, 9) We hypothesized that the combined effects of increased proximal mean arterial pressure (pMAP), carotid blood flow (Qcarotid), and intracranial pressure (ICP) from REBOA would lead to TBI progression when compared to partial aortic occlusion or no intervention.
Materials and Methods
Overview
The Institutional Animal Care and Use Committee at David Grant Medical Center, Travis Air Force Base, California approved this study. All animal care and use was in strict compliance with the Guide for the Care and Use of Laboratory Animals in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Healthy adult, castrate male and non-pregnant female Yorkshire-cross swine (Sus scrofa), obtained from the University of California, Davis, were acclimated for a minimum of 7 days. At the time of experimentation, animals weighed between 50 and 75 kg.
Conduct of the protocol, including animal preparation, injury, intervention, and postoperative critical care is depicted in Figure 1. After creation of a severe TBI, animals underwent controlled hemorrhage of 25% of total blood volume. After 30 minutes of sustained hypotension (pre-hospital phase), animals were randomized to 60 minutes of treatment with Zone 1 REBOA, partial aortic occlusion (PAO) using a cable-driven automated aortic cross-clamp (AAC) positioned in Zone 1 allowing 300 mL/min of distal aortic flow, or no intervention (Control). Starting at 90 minutes, all animals were resuscitated with shed blood via a rapid blood infuser at a rate of 150 ml/min and graded restoration of systemic circulation was initiated, utilizing the AAC in the P-REBOA group and step-wise manual balloon deflation in the REBOA group. Subsequently, the animals entered an intensive care (ICU) phase during which all three groups were resuscitated to a defined goal (proximal MAP ≥70 mmHg) in an effort to create a consistent degree of cerebral perfusion following intervention.
Figure 1.
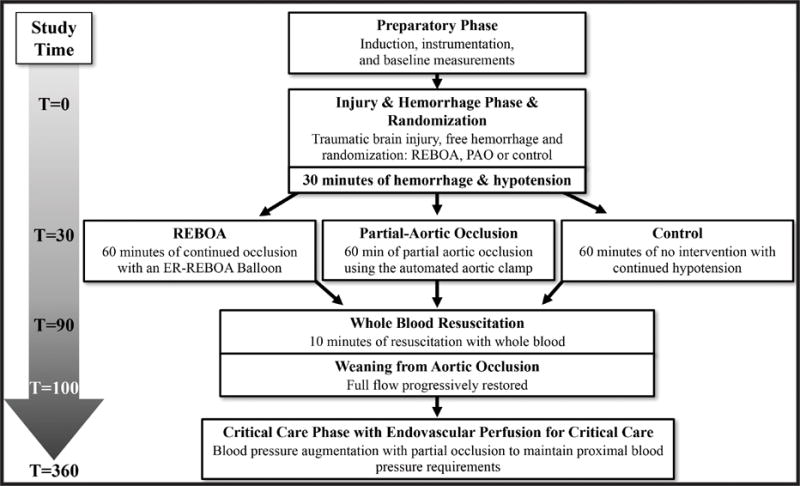
Study timeline
Animal Preparation
Animal preparation is described in detail in the supplemental methods content. Briefly, animals were sedated, intubated, and anesthesia was then maintained on 2% isoflurane. An intravenous infusion of norepinephrine (0.01 μg/kg/h) was titrated to achieve a target mean arterial pressure between 65 and 75 mmHg. Normal saline was administered at a rate of 5 mL/kg/hour to overcome insensible losses.
A 10 mm craniotomy was created adjacent to the left coronal suture for placement of an intracranial pressure-monitor (Codman Neuro, Raynham, MA). A second 20 mm craniotomy was performed in the right skull next to the sagittal and coronal sutures over the frontal lobe for preparation for controlled cortical impact. After cranial access was obtained, an arterial flow probe (Transonic Systems Inc., Ithaca, NY) was placed on the right carotid artery. Vascular access and monitoring has been previously described (14,15).
A laparotomy and splenectomy were performed. The left hemi-diaphragm was incised longitudinally and the supraceliac aorta was exposed. Two adjacent intercostal arteries were ligated, followed by placement of an aortic flow probe (Transonic Systems Inc., Ithaca, NY) as well as the variable automated aortic clamp (AAC).(15, 16, 17) A 32mm aortic occlusion balloon (ER-REBOA, Prytime Medical, Lakewood, CO) was positioned in the descending thoracic aorta through a 7F 13 cm introducer sheath (Cook Incorporated, Bloomington, IN) placed in the right femoral artery and confirmed via manual palpation.
Automated Aortic Clamp
To achieve tight control of distal aortic blood flow, our group devised a computer-controlled automated aortic clamp. This novel aortic occlusion apparatus represents an evolution from our previously described extracorporal variable aortic control device and was devised to facilitate animal preparation and the use of the CT scanner. The device regulates downstream blood flow by variably opening and closing, thus providing varying degrees of aortic occlusion across the spectrum of ranging from full flow to complete occlusion. The clamp is capable of automatically and dynamically responding to changes in animal physiology, moving in sub-millimeter increments to achieve prescribed pressure or flow endpoints, which varied based on the phase and randomization arm of the experiment. To achieve this, the system utilized custom-designed algorithms that integrated input from the aortic flow probe and proximal pressure to determine the movement of the clamp in a closed-loop feedback mechanism.
Data Collection
Physiologic parameters and aortic flow measurements were collected in real time using a Biopac MP150 multichannel data acquisition system. Parameters measured included heart rate, blood pressure proximal and distal to the AAC, central venous pressure, core temperature, pulse oxygenation, intracranial pressure, and carotid blood flow. Animals underwent serial CT scans using a BodyTom CT scanner (Neurologica, Danvers, MA).
Injury
The skulls of the pigs were attached to a custom stereotactic frame that secured the head at 90 degrees of forward flexion (Supplemental Figure 1, Supplemental Figure 2). Traumatic brain injury was created with a computer-controlled cortical impact device (Custom Design and Manufacturing, Richmond, VA) mounted to the stereotactic frame. A 10mm rounded polymer striking tip was used with a fixed velocity of 4.0m/s, depth of 12mm, and dwell time of 200μsec. A piezoelectric impact monitor (PCB Piezotronics, Depew, NY) embedded in the striking tip and connected to an oscilloscope (Silgent, Shenzhen, China) confirmed initial dural contact during alignment. After cortical impact, the nylon plug was repositioned into the craniotomy site to minimize leakage of CSF and eliminate artificial ICP readings.
Following the TBI, hemorrhagic shock was created by withdrawing 25% of estimated blood volume (Body Weight (g) × 0.06) through an arterial cannula into citrated blood collection bags over a 30-minute period.
Intervention
During the hemorrhagic shock phase, animals were randomized into one of three groups: Control, REBOA, and PAO. All animals that randomized to Control or PAO had their prepositioned REBOA catheters withdrawn prior to the onset of the intervention phase. Control animals were monitored without intervention throughout the subsequent 60-minutes. REBOA animals remained in complete occlusion for the 60-minute intervention phase. In the PAO arm, the ACC maintained a distal aortic flow rate between 150–300ml/min according to a pre-programmed algorithm to achieve a proximal aortic MAP > 65. After 50 minutes of intervention animals were pre-medicated with 30 mL of 23% calcium gluconate (Agri Laboratories, St. Joseph, MO) to counteract the effects of citrate present in the transfused blood and stabilize the myocardium during reperfusion. At the end of the 60-minute intervention period all animals underwent resuscitation with previously shed whole blood. The occlusion balloon in REBOA animals was deflated at a rate of 0.5ml/min until completely deflated. In the PAO group, the AAC autonomously weaned from occlusion as the proximal blood pressure allowed. All animals then entered the intensive care phase.
Intensive Care (ICU) Phase
To evaluate the delayed effects of the intervention itself on TBI progression without subsequent hemodynamic compromise confounding the study, the AAC was then activated in all 3 groups to maintain equivalent proximal blood pressures throughout the 4-hour ICU phase. To minimize investigator bias during this phase of care computerized decision support algorithms automatically prompted providers to administer crystalloids and titrate vasopressors. Animals received 500 mL fluid boluses if the central venous pressure (CVP) was <6 mmHg and norepinephrine was increased by 0.01 mcg/kg/hr every 15 minutes if the ACC was active. During refractory hypotensive episodes, the AAC maintained proximal pressure until intravenous fluids and/or vasopressors were effective in maintaining a proximal MAP >65 mmHg. Physiologic parameters were recorded continuously, laboratory analysis was obtained at predefined intervals, and brain CT imaging was obtained at T0, T40, T80, T180, and T360. Electrolyte abnormalities including hyper and hypoglycemia were corrected throughout the ICU phase. Animals were humanely euthanized at the end of study.
Data Analysis
Brain injury was quantified on non-contrasted CT of the area of injury. A neuroradiologist blinded to intervention provided volumetric analysis of the injury using the ABC/2 method.(18) The presence of subarachnoid hemorrhage, epidural hemorrhage, and or subdural hemorrhage was confirmed at necropsy immediately after euthanasia.
The primary outcome measure was the size of TBI observed on serial CT scans. This was analyzed using ANOVA for the discreet time points noted previously (the CT scan intervals and end of study). Post hoc pairwise comparisons were performed when indicated. Data was entered into Excel datasheets (Microsoft Corporation) and transferred to STATA version 14.0 (Stata Corporation, Bryan, TX) for analysis. Continuous variables are presented as means and standard errors of the means (SEMs) if normally distributed and as medians with interquartile ranges if not distributed normally. Statistical significance was set at p < 0.05.
Results
Baseline physiology was similar between the groups (Table 1). After 30 minutes of hypotension there were no differences in mean arterial pressure (MAP). During the 60-minute intervention period, REBOA resulted in higher maximal MAP when compared to Control (REBOA 105.3±8.8 mmHg; PAO 92.7±9.2 mmHg, Control 48.9±8.87.7 mmHg, p<0.05). REBOA resulted in the highest carotid blood flow when compared to PAO and Control (REBOA 673.1±57.9 mL/min; PAO 408.5±38.3; Control 170.3±29.4 mL/min, p<0.01). There were no differences in maximal change in ICP or average change in ICP between any of the groups during the intervention phase (maximal change p=0.49, average change p=0.26). Cerebral perfusion pressure was below critical thresholds (60 mmHg) during the intervention phase for Control animals (27.4±5.5mmHg), but was significantly increased in both REBOA animals (67.6±6.3mmHg) and PAO animals (66.2±5.7mmHg) when compared to the hypotensive phase (supplemental figure 3C). On average, PAO resulted in higher central venous pressure (CVP) during the intervention phase when compared to Control (PAO 4.7±0.3mmHg, Control 3.5±1.3mmHg) but there were no differences in CVP between PAO and REBOA and between REBOA and Control (Table 1, Figure 2D).
Table 1.
Hemodynamic and Cerebrovascular Physiology During Intervention and Resuscitation
| Control | REBOA | P-REBOA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | P | CxR | CxP | RxP | |
| Baseline | ||||||||||
| Aortic Flow | 2233.3 | 57.0 | 2331.7 | 112.3 | 2293.8 | 146.6 | 0.92 | |||
| Proximal MAP | 79.6 | 1.9 | 75.1 | 1.5 | 77.8 | 2.2 | 0.28 | |||
| Carotid Flow | 358.4 | 43.9 | 305.7 | 22.1 | 288.6 | 26.7 | 0.31 | |||
| ICP | 0.8 | 0.1 | 0.1 | 0.3 | −0.1 | 0.8 | 0.46 | |||
| CVP | 5.8 | 0.8 | 5.5 | 0.9 | 5.2 | 0.3 | 0.86 | |||
| T30 Proximal MAP | 43.6 | 1.3 | 48.6 | 3.4 | 52.5 | 9.3 | 0.58 | |||
| T30–90 Maximum | ||||||||||
| Aortic Flow | 1241.3 | 228.4 | 0.0 | 12.1 | 338.8 | 51.2 | <0.01 | <0.01 | <0.01 | 0.22 |
| Proximal MAP | 48.9 | 7.7 | 105.3 | 8.8 | 92.7 | 9.2 | <0.01 | <0.01 | <0.01 | 0.95 |
| Carotid Flow | 170.3 | 29.4 | 673.1 | 57.9 | 464.2 | 53.0 | <0.01 | <0.01 | <0.01 | 0.02 |
| Increase ICP | 3.1 | 0.8 | 5.7 | 2.3 | 4.6 | 1.0 | 0.49 | |||
| CVP | 4.6 | 0.3 | 5.0 | 0.3 | 5.7 | 0.4 | 0.03 | 0.60 | 0.03 | 0.41 |
| T30–90 Average | ||||||||||
| Aortic Flow | 1122 | 210 | 0.2 | 14 | 319 | 17.4 | <0.01 | <0.01 | <0.01 | 0.20 |
| Proximal MAP | 43.6 | 6.1 | 89.0 | 5.9 | 84.7 | 6.3 | <0.01 | <0.01 | <0.01 | 1.0 |
| Carotid Flow | 169.9 | 27.3 | 529.3 | 39.3 | 408.5 | 38.3 | <0.01 | <0.01 | <0.01 | 0.08 |
| Increase ICP | 1.9 | 0.7 | 3.4 | 0.8 | 2.3 | 0.5 | 0.26 | |||
| CVP | 3.5 | 1.3 | 3.8 | 0.4 | 4.7 | 0.3 | 0.04 | 1.0 | 0.05 | 0.16 |
| CPP | 27.4 | 5.5 | 67.6 | 6.3 | 66.2 | 5.7 | <0.01 | <0.01 | <0.01 | 1.0 |
| T90–120 Maximum | ||||||||||
| Aortic Flow | 3103 | 477.6 | 2288.5 | 324.7 | 2824.3 | 138.7 | 0.13 | |||
| Proximal MAP | 97.3 | 6.7 | 79.2 | 1.6 | 81.2 | 1.7 | 0.01 | 0.02 | 0.04 | 1.0 |
| Carotid Flow | 721.1 | 177.3 | 452.2 | 26.5 | 456.3 | 24.4 | 0.14 | |||
| Increase in ICP | 12.8 | 1.2 | 5.1 | 0.6 | 9.4 | 1.1 | <0.01 | <0.01 | 0.08 | 0.02 |
| CVP | 12.4 | 1.8 | 8.8 | 0.7 | 11.1 | 0.7 | 0.12 | |||
| T100–360 Average | ||||||||||
| Aortic Flow | 2540 | 173.1 | 1465 | 400.3 | 2407 | 127.4 | 0.02 | 0.04 | 1.0 | 0.07 |
| Proximal MAP | 79.3 | 0.6 | 74.8 | 1.7 | 80.2 | 2.1 | 0.06 | |||
| Carotid Flow | 477.3 | 126.6 | 422.7 | 21.1 | 362.5 | 31.6 | 0.58 | |||
| ICP | 8.8 | 0.6 | 7.4 | 0.6 | 8.1 | 0.5 | 0.29 | |||
| CVP | 6.0 | 0.2 | 5.2 | 1.8 | 6.4 | 0.4 | 0.10 | |||
| CPP | 56.5 | 1.5 | 50.4 | 2.6 | 58.6 | 2.0 | 0.03 | 0.15 | 1.0 | 0.03 |
|
End ICP End CPP |
10.7 53.0 |
1.0 1.5 |
9.9 44.6 |
1.1 3.5 |
9.7 54.2 |
0.8 1.3 |
0.73 0.02 |
0.07 | 1.0 | 0.03 |
CxR: p-value for post-hoc analysis of Control by REBOA; CxP: p-value for post-hoc analysis of Control by PAO; RxP: p-value for post-hoc analysis of REBOA by PAO
Figure 2.
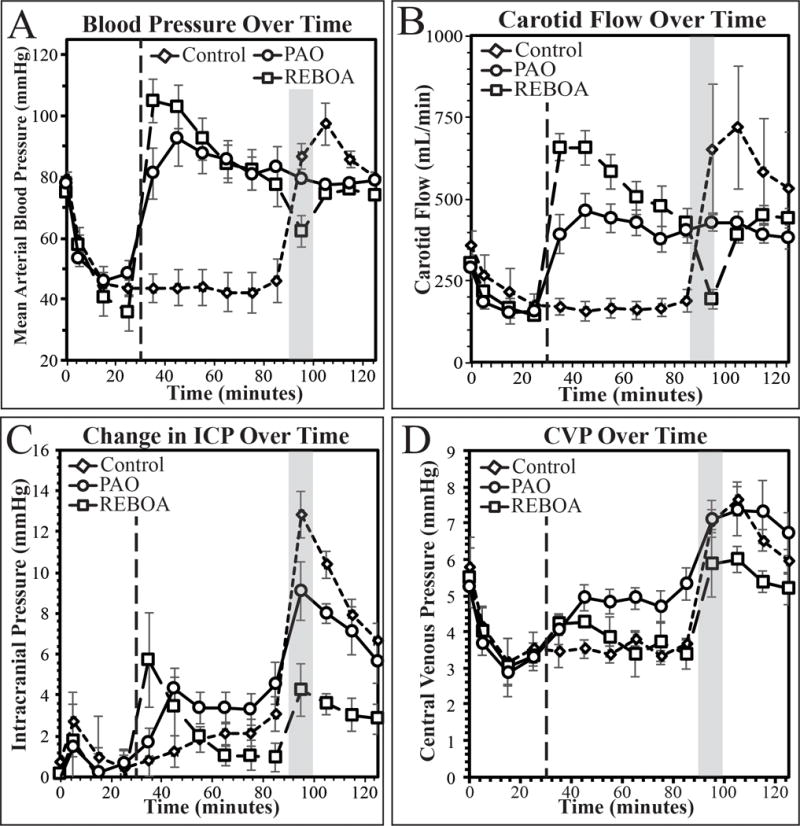
Mean arterial pressure (A), carotid blood flow (B), change in intracranial pressure (C), and central venous pressure (D) during the first 120 minutes of the experiment. Dashed line demarks beginning of intervention, shaded box demarks whole blood resuscitation.
During the resuscitation phase, Control animals had the highest maximal MAP when compared to REBOA (Control 97.3±6.7mmHg, REBOA 79.2±1.6mmHg, p=0.02) and PAO (PAO 81.2±1.7mmHg, p=0.04). REBOA animals had the lowest maximal increase in ICP when compared to Control animals (REBOA 5.1±0.6mmHg, Control 12.8±1.2mmHg, p<0.01) and PAO animals (PAO 9.4±1.1mmHg, p=0.02). Although CVP was increased in all animals when compared to baseline (Figure 2D), there were no statistical differences in CVP during the resuscitation phase (Table 1).
Within groups, Control and PAO animals experienced the highest ICP during the resuscitation phase when compared to the earlier intervention phase (Figure 2C, Control p<0.01, PAO p<0.01), while the REBOA animals had similar ICP increases during intervention and resuscitation (Figure 3C, Table 1).
Figure 3.
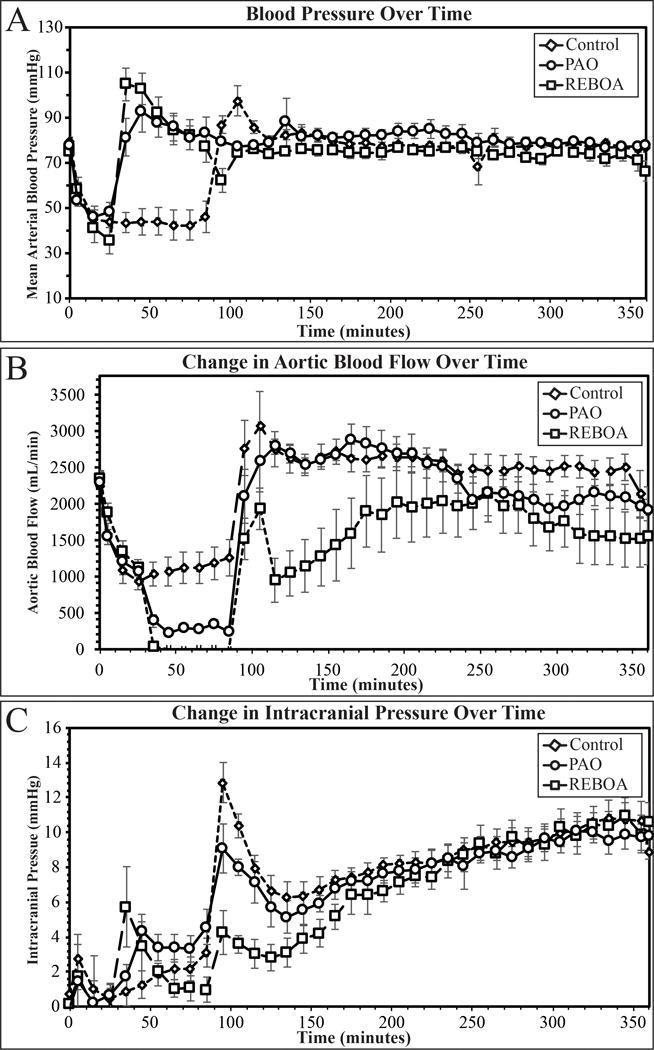
Mean arterial pressure (A), change in intracranial pressure (B), and aortic blood flow (C) over the entire time course of the experiment. Dashed line demarks beginning of intervention, shaded box demarks whole blood resuscitation.
The ICU phase of the experiment was designed to minimize proximal physiologic differences between groups by augmenting proximal aortic pressure to optimize flow to proximal vascular beds. From 90 minutes until the end of the experiment, the AAC ensured there were no differences in proximal MAP between the groups (Figure 3A, p=0.06). To achieve equivalent proximal MAPs, REBOA animals received more assistance from the AAC and thus, less distal aortic flow during the ICU phase when compared to Control and PAO animals (REBOA 1465±400mL.min, Control 2540±173mL/min, PAO 2407±127mL/min, figure 3B). Overall there were no differences in the change in ICP (Figure 3C, p=0.29) between all three groups throughout the critical care phase of the experiment. However, average CPP was significantly lower in REBOA animals when compared to PAO animals (Table 1, Supplemental Figure 3C), and all animals had CPP values below 60 during the critical care phase (Supplemental Figure 3C).
Laboratory values for lactate, pH, pCO2 and potassium are depicted in figure 4. Lactate peaked in the REBOA animals at 140 minutes, while the peak lactate for PAO animals occurred at 120 minutes. REBOA animal had increased lactate when compared to Control and PAO animals from 140 minutes until the end of the experiment. Lactate in PAO animals reached Control group levels starting at 240 minutes (figure 4A). pH had a trend similar to lactate, with REBOA animals remaining acidotic when compared to Control and PAO animals from 120 minutes until the end of the experiment (figure 4B). REBOA animals had a single period of hypocapnia at 50 minutes when compared to Control and PAO animals, with no other differences between groups for the remainder of the study (figure 4C). Immediately after balloon deflation (95 minutes), REBOA animals had a single episode of hyperkalemia when compared to Control and PAO animals, with no further hyperkalemic episodes in any of the remaining blood draws in any of the groups (Figure 4D).
Figure 4.
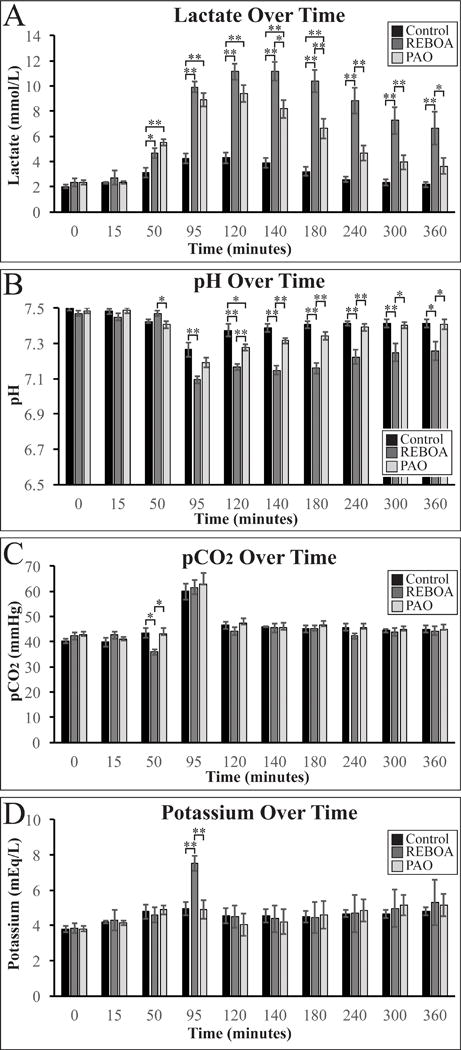
Lactate (A), pH (B), pCO2 (C), and potassium (D) levels measured by arterial blood gas during the experiment. * denotes p<0.05, ** denotes p<0.01
There were no statistically significant differences in the amount of fluids required by each group of animals (Control 56±17 mL/kg, REBOA 64±28 mL/kg, PAO 48±12 mL/kg, p=0.35). There were significant differences in the total cumulative dose of norepinephrine over the entirety of the experiment between groups (Control 1.4±0.6 mcg/kg, REBOA 3.1±0.9 mcg/kg, PAO 2.9±2.0 mcg/kg, p=0.05) but no significant differences within any group on post-hoc analysis. There were no differences in the size of TBI or in the percentage of animals with progression of hemorrhage on CT (Table 2, Supplemental Figure 4). There were no differences in the percentage of animals with evidence of extra-axial hemorrhage (Table 2).
Table 2.
Characteristics of Traumatic Brain Injury
| Control | REBOA | PAO | |||||
|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | P | |
| Extra-axial Hemorrhage* | 85.7 | 42.1–99.6 | 100 | 59.0–100.0 | 100 | 59.0–100.0 | 0.39 |
| Intraparenchymal Hemorrhage | 28.6 | 3.7–71.0 | 28.6 | 3.7–71.0 | 42.9 | 9.9–81.6 | 0.81 |
| Progression of Hemorrhage | 14.3 | 0.4–57.9 | 14.3 | 0.4–57.9 | 28.6 | 3.7–71.0 | 0.73 |
Extra-axial findings were confirmed at necropsy
Discussion
As use of REBOA increases, a better understanding of its effects in the multi-injured trauma patient is required. In the setting of TBI and shock, REBOA elevated carotid flow, proximal MAP, and ICP to supraphysiologic levels but did not cause radiographic evidence of TBI progression within this limited survival study. In contrast, PAO partially mitigated the development of intracranial hypertension and resulted in near-baseline carotid flow rates. Despite these findings, rapid blood resuscitation resulted in the largest increase in ICP for both Control animals and PAO animals.
Hypotension following TBI is known to be detrimental, yet there is increasing evidence that hypertension early after brain injury is also associated with poor outcomes.(19–25) Excessive blood pressure and blood flow to the brain may exacerbate a TBI by destabilizing intracerebral clots, promoting hemorrhage, and increasing cerebral edema.(24) In a propensity matched study from Japan, patients with head injury and low GCS were more likely to die if treated with REBOA.(7) Recent literature suggests a causal relationship, with at least one case report linking fatal TBI progression to REBOA.(12) Our findings support the many animal studies and clinical reports indicating that REBOA may result in supraphysiologic blood pressure and flow to proximal organs.(6, 8, 9, 26, 27) However, in this present study, the increased pMAP, carotid flow and ICP in the REBOA group did not correlate with radiographic evidence of TBI progression during intervention or by the end of the study.
There are many possible explanations for this finding. It is possible that the ability of REBOA to elevate pMAP was sufficient to overcome the corresponding elevation in ICP, thereby maintaining cerebral perfusion pressure (CPP) and minimizing any exacerbation of the injury. A likely alternative explanation is that the CT findings in this limited survival study are only representative of the early resuscitation period following TBI and did not have an opportunity to fully develop. In clinical scenarios, 35–50% of patients with severe TBIs demonstrate progression over the initial 24 hours, therefore it is plausible that the short time course for this study prevented observations of progression.(28, 29) Similarly, clinical studies of TBI victims emphasize that a lack of early radiographic progression is not predictive of outcomes.(30–32) Yet, elevated ICP is strongly associated with a fatal outcome following head trauma even when CPP is adequate.(33) Given the complex interplay of intracerebral pressure, blood flow, and hemorrhage volumes, future survival studies will be required to fully understand the effects of REBOA on TBI progression and mortality.
We also noted that supraphysiologic blood pressures were not maintained for the duration of the intervention in this TBI study. This finding stands in contrast to our prior work with a controlled hemorrhage model that demonstrated sustained increases in pMAP within the supraphysiologic range (>100 mmHg) for the duration of the intervention.(8) In this combined TBI and hemorrhage model, proximal blood pressures were only maintained above 100 mmHg for very brief periods, even though the degree of hemorrhage was the same between these experiments. This finding leads us to question whether the TBI had a direct, adverse effect on cardiac performance by effectively blunting the proximal hypertensive response previously demonstrated in other animal studies during sustained REBOA. Clinical studies of isolated, severe TBI have demonstrated that up to 25% of patients with isolated severe TBIs develop echocardiographic evidence of heart failure.(34, 35) Although it is difficult to directly compare the present experiment to clinical reports of heart failure in TBI, the relative inability of these brain-injured animals to produce the same supraphysiologic blood pressures seen in similar studies without TBI, does suggest the potential presence of TBI-induced cardiac dysfunction.(34, 35)
Depending on the degree of shock, CPP augmentation by REBOA can range from appropriate to extreme.(26, 27) Partial aortic occlusion delivered in a controlled manner may offer an alternative to decrease the risks associated with REBOA. Studies evaluating mild elevation of CPP by partial aortic occlusion in ischemic stroke patients found it to be safe even after treatment with thrombolytics.(36, 37) The baseline levels of carotid blood flow and smaller increases in ICP with PAO versus REBOA are consistent with other animal studies in NCTH models without TBI and in similar studies in euvolemic models.(8, 9, 38)
The most unexpected observation was that the largest physiologic fluctuations of the entire study occurred during massive transfusion rather than during the period of aortic intervention. Massive transfusion has been identified as a predictor of mortality in many trauma studies, but elucidating an etiology is confounded by injury severity, variations in transfusion ratios, coagulopathy, and transfusion reactions.(39, 40) A retrospective analysis at a level 1 trauma center observed that delivery of blood through a Rapid Infusion System resulted in greater than expected mortality, but was unable to discern an cause.(41) Our findings are consistent with other animal models that have shown worse outcomes with the combination of blood product administration and REBOA, more than either intervention in isolation.(26) The mechanism of this increase in ICP during blood resuscitation is likely multifactorial and may not be easily recognized during direct clinical care. Although an increase in MAP during blood resuscitation corresponded with an increase in ICP in the Control group, in both the REBOA and PAO groups, ICP increased during blood resuscitation despite an unchanged or decreasing MAP. This finding indicates that ICP increases may have resulted from rapid and dramatic increases in CVP brought on by the impaired venous drainage and venous return during rapid blood resuscitation While the ICP and CVP relationship is intriguing, future studies are required to identify the exact etiology of these ICP fluctuations. Nevertheless, the results from the resuscitation phase of the present study suggest that changes in ICP during a trauma resuscitation are not always readily apparent with current methods of hemodynamic monitoring. Appropriate resuscitation is reliant upon the astute physician to understand the se complex physiologic changes that can occur during massive transfusion. Further studies are needed to optimize fluid resuscitation and transfusion strategies in REBOA patients with respect to timing, rate, and volume of blood administered.
There are several limitations to our current model. First, there are inherent difficulties in studying TBI with an animal model. The term “traumatic brain injury” actually encompasses a wide array of injuries ranging from penetrating trauma, to intracranial hemorrhage, to diffuse axonal injury and more.(42) Blast injury and motor vehicle collisions represent the most common mechanisms of combined NCTH and TBI in military and civilian populations, respectively.(43, 44) While the controlled cortical impact model in this study created a reproducible injury that minimized variability and allowed for better scientific comparison within the study group, this injury is not necessarily analogous to the mechanisms of head injury seen in multi-system trauma patients. It is not known whether different types of brain injury respond differently to acute variations in cerebral perfusion. Unfortunately, this vital question remains unanswered by our study. Additionally, our study was limited to the immediate effects of intervention as we not able to perform prolonged survival studies. It is possible that more time is needed to observe quantifiable progression of brain injury on a CT scan.
Differentiating the effects of proximal hypertension during intervention from the effects of reperfusion can guide the development of new occlusion devices and resuscitation strategies. To isolate the variable of interest (proximal blood pressure during intervention), the proximal blood pressure was tightly regulated during the ICU phase by application of the AAC. Since the degree of blood pressure support needed to maintain equivalent proximal MAPs during the ICU phase varied between animals, each required a different degree of supportive AAC. The REBOA animals in particular required more blood pressure support than animals in other groups. This finding may indicate the presence of refractory hypotension or blood pressure variability in the absence of ACC in this group. Rebound hypotension, reperfusion injury, and myocardial depression following REBOA are well documented, particularly following intervention times in excess of 40 minutes. All of these variables may worsen TBI after REBOA. Currently REBOA is limited to resource rich environments capable of rapid surgical intervention to minimize occlusion times. We have previously demonstrated that partial aortic occlusion with low-volume permissive regional hypoperfusion distal to zone 1 allows for prolonged intervention times up to 90 minutes in a uniformly lethal liver injury model, while promoting lactate clearance after only several hours of critical care. This work has further strengthened the case for partial aortic occlusion as a viable alternative to complete occlusion. Continued refinements of PAO mechanisms, including techniques such as P-REBOA and Endovascular Variable Aortic Control (EVAC), are required to allow carefully titrated partial occlusion to advance beyond an experimental concept to a therapeutic reality. In that light, our group is designing future studies to examine the effects of REBOA and purpose-specific P-REBOA endovascular catheters on TBI as they would be used clinically, without subsequent mechanical blood pressure support to counter rebound hypotension.
These future TBI studies should include longer study durations, advanced neuroimaging and additional quantification of brain injury with histologic and chemical markers of neuronal damage.
Conclusion
In the setting of TBI and shock, REBOA elevated carotid flow, pMAP, and ICP but did not cause radiographic progression of TBI. Partial aortic occlusion resulted in baseline levels of blood flow to the head and systemic BP with smaller increases of ICP during intervention than REBOA. Moreover, rapid blood resuscitation, and not REBOA, resulted in the largest increase in ICP after intervention. Continued studies of the cerebral hemodynamics of aortic occlusion and blood transfusion are required to determine optimal resuscitation strategies for multi-injured patients.
Supplementary Material
Supplemental Figure 1: Experimental setup demonstrating automated aortic clamp with wireless transmission to computer controller and cortical impact device in place.
Supplemental Figure 2: External landmarks of craniotomy site (A), nylon sleeve for alignment of computer controlled cortical impactor in place (B), final placement of animal in cortical impactor frame prior to creation of a traumatic brain injury (C).
Supplemental Figure 3: Figure 2: Mean arterial pressure (A), intracranial pressure (B),, and cerebral perfusion pressure (C) during the entire time course of the experiment.
Supplemental Figure 3: Sagittal images (A,B) and coronal images (C,D) of hemorrhagic progression of a traumatic brain injury from baseline (A,C) to 360 minutes (B,D).
Acknowledgments
We thank SSG Kelly Caneen, Mr. Carl Gibbins, and Ms. Sally Knode, SSgt Elaine Spotts, SrA Geoffrey O’Hair, SSgt Vanessa Lang, and LtCol Robin Mitchell for their outstanding technical assistance, and the other staff of the Clinical Investigations Facility David Grant Medical Center for their support. We also extend thanks for Maj. Joel Dendulk, MSgt Timothy Fiol, and Mr. Jeffrey Sauerwein of the 60th Metals and Fabrication Shop, Travis AFB for their expertise in the design and fabrication of the stereotactic frame for the cortical impactor.
Sources of Funding: Dr. Johnson is the recipient of a training grant from the National Heart, Lung and Blood Institute: K12HL108964. This project was partly supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and TL1 TR000133. The Clinical Investigation Facility, David Grant Medical Center, Travis Air Force Base, California provided funding for this study.
Footnotes
Presented at the 30th Eastern Association for the Surgery of Trauma Annual Scientific Assembly, January 10–14, 2017 in Hollywood, FL.
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. Government, the Department of Defense, the Department of the Air Force, or the University of California Davis. The work reported herein was performed under United States Air Force Surgeon General approved Clinical Investigation No. FDG20160008A.
Conflicts of Interest: There are no personal conflicts of interest.
Level of Evidence: Level IV.
Author Contribution
MJ, TW, and LN conceived of the study, executed the study, and drafted and critically reviewed the manuscript. MJ and JG conducted the data analysis and statistical analysis. SF, AD, RR, TR, JG, and WO assisted in execution of the study and critically reviewed the manuscript.
Disclaimer:
The animals involved in this study were procured, maintained, and used in accordance with the Laboratory Animal Welfare Act of 1966, as amended, and NIH 80-23, Guide for the Care and Use of Laboratory Animals, National Research Council.
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. Government, the Department of Defense, the Department of the Air Force, or the University of California Davis. The work reported herein was performed under United States Air Force Surgeon General approved Clinical Investigation No. FDG20160008A.
References
- 1.Russo R, Neff LP, Johnson MA, Williams TK. Emerging Endovascular Therapies for Non-Compressible Torso Hemorrhage. Shock. 2016 doi: 10.1097/SHK.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400–9. doi: 10.1016/j.surg.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 3.DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, Moore L, Holcomb J, Turay D, Arbabi CN, et al. The AAST Prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) Registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA) J Trauma Acute Care Surg. :2016. doi: 10.1097/TA.0000000000001079. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 4.Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054–8. doi: 10.1097/TA.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, Fox EE, Scalea TM, Napolitano LM, Albarado R, Gill B, Dunkin BJ, Kirkpatrick AW, Cotton BA, Inaba K, et al. Current opinion on catheter-based hemorrhage control in trauma patients. J Trauma Acute Care Surg. 2014;76(3):888–93. doi: 10.1097/TA.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 6.Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, Rasmussen TE. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848–56. doi: 10.1016/j.surg.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721–8. doi: 10.1097/TA.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 8.Russo RM, Neff LP, Lamb CM, Cannon JW, Galante JM, Clement NF, Grayson JK, Williams TK. Partial Resuscitative Endovascular Balloon Occlusion of the Aorta in Swine Model of Hemorrhagic Shock. J Am Coll Surg. 2016 doi: 10.1016/j.jamcollsurg.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, Galante JM, Neff LP. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2016;80(3):372–80. doi: 10.1097/TA.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 10.Ogura T, Lefor AT, Nakano M, Izawa Y, Morita H. Nonoperative management of hemodynamically unstable abdominal trauma patients with angioembolization and resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78(1):132–5. doi: 10.1097/TA.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 11.Tsurukiri J, Akamine I, Sato T, Sakurai M, Okumura E, Moriya M, Yamanaka H, Ohta S. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24(1):13. doi: 10.1186/s13049-016-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchino H, Tamura N, Echigoya R, Ikegami T, Fukuoka T. “REBOA” - Is it Really Safe? A Case with Massive Intracranial Hemorrhage Possibly due to Endovascular Balloon Occlusion of the Aorta (REBOA) The American journal of case reports. 2016;17:810–3. doi: 10.12659/AJCR.900267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson MA, Neff LP, Williams TK, DuBose JJ, Group ES Partial Resuscitative Balloon Occlusion of the AORTA (P-REBOA): Clinical Technique and Rationale. J Trauma Acute Care Surg. 2016 doi: 10.1097/TA.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams TK, Neff LP, Johnson MA, Russo RM, Ferencz SA, Davidson AJ, Rasmussen TE, Extending REBOA. Endovascular Variable Aortic Control (EVAC) in a Lethal Model of Hemorrhagic Shock. J Trauma Acute Care Surg. 2016 doi: 10.1097/TA.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sondeen JL, Dubick MA, Holcomb JB, Wade CE. Uncontrolled hemorrhage differs from volume- or pressure-matched controlled hemorrhage in swine. Shock. 2007;28(4):426–33. doi: 10.1097/shk.0b013e31804a5791. [DOI] [PubMed] [Google Scholar]
- 16.Strauch JT, Spielvogel D, Lauten A, Zhang N, Shiang H, Weisz D, Bodian CA, Griepp RB. Importance of extrasegmental vessels for spinal cord blood supply in a chronic porcine model. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2003;24(5):817–24. doi: 10.1016/s1010-7940(03)00460-3. [DOI] [PubMed] [Google Scholar]
- 17.Yuan L, Ni GX, Luk KK, Cheung KM, Lu DS, Hu Y, Dai JX, Wong YW, Lu WW. Effect of segmental artery ligation on the blood supply of the thoracic spinal cord during anterior spinal surgery: a quantitative histomorphological fresh cadaver study. Spine (Phila Pa 1976) 2005;30(5):483–6. doi: 10.1097/01.brs.0000154622.49240.ff. [DOI] [PubMed] [Google Scholar]
- 18.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–5. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 19.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. The Journal of trauma. 1993;34(2):216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Chesnut RM, Marshall S, Piek J, Blunt B, Klauber M, Marshall L. Monitoring of Cerebral Blood Flow and Metabolism in Intensive Care. Springer; 1993. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank; pp. 121–5. [DOI] [PubMed] [Google Scholar]
- 21.Zafar SN, Millham FH, Chang Y, Fikry K, Alam HB, King DR, Velmahos GC, de Moya MA. Presenting blood pressure in traumatic brain injury: a bimodal distribution of death. J Trauma. 2011;71(5):1179–84. doi: 10.1097/TA.0b013e3182140d38. [DOI] [PubMed] [Google Scholar]
- 22.Fuller G, Hasler RM, Mealing N, Lawrence T, Woodford M, Juni P, Lecky F. The association between admission systolic blood pressure and mortality in significant traumatic brain injury: a multi-centre cohort study. Injury. 2014;45(3):612–7. doi: 10.1016/j.injury.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Butcher I, Maas AI, Lu J, Marmarou A, Murray GD, Mushkudiani NA, McHugh GS, Steyerberg EW. Prognostic value of admission blood pressure in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):294–302. doi: 10.1089/neu.2006.0032. [DOI] [PubMed] [Google Scholar]
- 24.Sellmann T, Miersch D, Kienbaum P, Flohe S, Schneppendahl J, Lefering R, Registry DGUT. The impact of arterial hypertension on polytrauma and traumatic brain injury. Dtsch Arztebl Int. 2012;109(49):849–56. doi: 10.3238/arztebl.2012.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barmparas G, Liou DZ, Lamb AW, Gangi A, Chin M, Ley EJ, Salim A, Bukur M. Prehospital hypertension is predictive of traumatic brain injury and is associated with higher mortality. J Trauma Acute Care Surg. 2014;77(4):592–8. doi: 10.1097/TA.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 26.Batchinsky A, Belenkiy S, Park T, Jordan B, Baker W, Necsoiu C, Aden J, Dubick M, Rasmussen T, Cancio L. Resuscitative endovascular balloon occlusion of the aorta restroes carotid blood flow faster than blood resuscitation during massive hemorrhage in swine. 45th Annual Meeting Western Trauma Association; March 6, 2015; Telluride, CO2015. [Google Scholar]
- 27.Park TS, Batchinsky AI, Belenkiy SM, Jordan BS, Baker WL, Necsoiu CN, Aden JK, Dubick MA, Cancio LC. Resuscitative endovascular balloon occlusion of the aorta (REBOA): Comparison with immediate transfusion following massive hemorrhage in swine. J Trauma Acute Care Surg. 2015;79(6):930–6. doi: 10.1097/TA.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 28.Juratli TA, Zang B, Litz RJ, Sitoci K-H, Aschenbrenner U, Gottschlich B, Daubner D, Schackert G, Sobottka SB. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. Journal of neurotrauma. 2014;31(17):1521–7. doi: 10.1089/neu.2013.3241. [DOI] [PubMed] [Google Scholar]
- 29.Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. Journal of neurotrauma. 2012;29(1):19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Symon L, Lassen N, Astrup J, Branston N. Oxygen Transport to Tissue—III. Springer; 1978. Thresholds of ischaemia in brain cortex; pp. 775–82. [DOI] [PubMed] [Google Scholar]
- 31.Miller JD, Stanek A, Langfitt TW. Concepts of cerebral perfusion pressure and vascular compression during intracranial hypertension. Progress in brain research. 1972;35:411–32. doi: 10.1016/S0079-6123(08)60102-8. [DOI] [PubMed] [Google Scholar]
- 32.Czosnyka M, Hutchinson P, Balestreri M, Hiler M, Smielewski P, Pickard J. Brain Edema XIII. Springer; 2006. Monitoring and interpretation of intracranial pressure after head injury; pp. 114–8. [DOI] [PubMed] [Google Scholar]
- 33.Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, Pickard JD. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocritical care. 2006;4(1):8–13. doi: 10.1385/NCC:4:1:008. [DOI] [PubMed] [Google Scholar]
- 34.Prathep S, Sharma D, Hallman M, Joffe A, Krishnamoorthy V, Mackensen GB, Vavilala MS. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Critical care medicine. 2014;42(1) doi: 10.1097/CCM.0b013e318298a890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slim J. Preliminary Report on Cardiac Dysfunction after Isolated Traumatic Brain Injury. Journal of Emergency Medicine. 2014;46(4):602. doi: 10.1097/CCM.0b013e318298a890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lylyk P, Vila JF, Miranda C, Ferrario A, Romero R, Cohen JE. Partial aortic obstruction improves cerebral perfusion and clinical symptoms in patients with symptomatic vasospasm. Neurol Res. 2005;27(Suppl 1):S129–35. doi: 10.1179/016164105X35512. [DOI] [PubMed] [Google Scholar]
- 37.Shuaib A, Bornstein NM, Diener HC, Dillon W, Fisher M, Hammer MD, Molina CA, Rutledge JN, Saver JL, Schellinger PD, et al. Partial aortic occlusion for cerebral perfusion augmentation: safety and efficacy of NeuroFlo in Acute Ischemic Stroke trial. Stroke. 2011;42(6):1680–90. doi: 10.1161/STROKEAHA.110.609933. [DOI] [PubMed] [Google Scholar]
- 38.Hammer M, Jovin T, Wahr JA, Heiss WD. Partial occlusion of the descending aorta increases cerebral blood flow in a nonstroke porcine model. Cerebrovasc Dis. 2009;28(4):406–10. doi: 10.1159/000235628. [DOI] [PubMed] [Google Scholar]
- 39.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. Journal of Trauma Injury Infection and Critical Care. 2003;54(5):898–907. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 40.Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, Spain DA, Brundage SI. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. Journal of the American College of Surgeons. 2009;209(2):198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Hambly PR, Dutton RP. Excess mortality associated with the use of a rapid infusion system at a level 1 trauma center. Resuscitation. 1996;31(2):127–33. doi: 10.1016/0300-9572(95)00910-8. [DOI] [PubMed] [Google Scholar]
- 42.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British journal of anaesthesia. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 43.Corrigan JD, Selassie AW, Orman JAL. The epidemiology of traumatic brain injury. The Journal of head trauma rehabilitation. 2010;25(2):72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 44.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Experimental setup demonstrating automated aortic clamp with wireless transmission to computer controller and cortical impact device in place.
Supplemental Figure 2: External landmarks of craniotomy site (A), nylon sleeve for alignment of computer controlled cortical impactor in place (B), final placement of animal in cortical impactor frame prior to creation of a traumatic brain injury (C).
Supplemental Figure 3: Figure 2: Mean arterial pressure (A), intracranial pressure (B),, and cerebral perfusion pressure (C) during the entire time course of the experiment.
Supplemental Figure 3: Sagittal images (A,B) and coronal images (C,D) of hemorrhagic progression of a traumatic brain injury from baseline (A,C) to 360 minutes (B,D).


