Abstract
Individuals with bulimia nervosa (BN) engage in episodes of binge eating, marked by loss of control and eating despite fullness. Does altered reward and metabolic state contribute to BN pathophysiology? Normally, hunger increases (and satiety decreases) reward salience to regulate eating. We investigated whether BN is associated with an abnormal response in a neural circuit involved in translating taste signals into motivated behavior, when hungry and fed. Twenty-six women remitted from BN (RBN) and 22 control women (CW) were administered water and sucrose during two counterbalanced fMRI visits, following a 16-hour fast or a standardized breakfast. Significant Group x Condition interactions were found in the left putamen, insula, and amygdala. Post-hoc analyses revealed CW were significantly more responsive to taste stimuli when hungry versus fed in the left putamen and amygdala. In contrast, RBN response did not differ between conditions. Further, RBN had greater activation in the left amygdala compared to CW when fed. Findings suggest that RBN neural response to rewarding stimuli may not be modulated by metabolic state. Data raise the possibility that disinhibited eating in BN could result from a failure to devalue food reward when fed, resulting in an exaggerated response.
Keywords: Bulimia Nervosa, reward, hunger
GENERAL SCIENTIFIC SUMMARY
This study suggests that adults remitted from bulimia nervosa may have elevated reward-related brain activation in response to taste after having eaten. This altered neural response may underlie the tendency to eat beyond satiety that characterizes binge eating behavior.
INTRODUCTION
Individuals with bulimia nervosa (BN) engage in regular binge eating episodes, in which they consume an objectively large amount of food, combined with a subjective feeling of loss of control. These episodes are typically followed by compensatory behavior such as self-induced vomiting and highly restricted intake to counteract weight gain.(American Psychiatric Association, 2013) What drives this extreme pattern of eating behavior? Metabolic (i.e., hunger or satiety) and hedonic (i.e., reward) mechanisms contribute to the regulation of eating,(Saper et al., 2002) raising the question of whether binge eating in BN may result from a disruption in one or both of these mechanisms, or their interaction.
In fact, behavioral studies suggest that metabolic state may contribute to binge eating in BN, but the mechanisms are not well-understood. For example, some studies show that fasting or dietary restriction may increase the likelihood of future binge eating,(Stice et al., 2008; Stice et al., 2005; Telch, 1996; Zunker et al., 2011) suggesting that restricting intake and prolonging hunger states may confer vulnerability for binge eating. Conversely, other findings indicate that although individuals with BN frequently fast between binge/purge episodes,(American Psychiatric Association, 2013) hunger does not necessarily trigger binge eating. In fact, lower dietary intake has been associated with a reduction in binge eating,(Stice et al., 2006) and a meta-analysis of ecological momentary assessment studies tracking the temporal relationship of hunger and binge eating determined that binge episodes were preceded by lower hunger ratings than were typical eating episodes (i.e. participants were less hungry before binge eating than before meals).(Haedt-Matt and Keel, 2011) This suggests bulimic episodes may, in fact, be related to an insensitivity to satiety. In support of this hypothesis, subjective ratings of hunger and fullness indicate that perception of metabolic state may be impaired in individuals ill with BN,(Halmi et al., 1989) and individuals with BN show blunted suppression of ghrelin (a hormone that signals hunger) in response to food consumption.(Monteleone et al., 2003) Together, findings suggest continued motivation to eat despite caloric repletion may contribute to binge eating. Understanding the influence of metabolic drives on brain-based responses to tastes and eating in BN is crucial for understanding the development of binge episodes and can inform novel interventions to address disinhibited eating.
In addition, BN is frequently associated with increased impulsivity, sensation-seeking, sensitivity to reward,(Chan et al., 2014; Harrison et al., 2010) and a lack of concern regarding future consequences,(Cassin and von Ranson, 2005; Rosval et al., 2006; Wagner et al., 2006) as well as an increased incidence of substance abuse.(Fouladi et al., 2015) This supports the hypothesis that increased sensitivity to reward may be a central characteristic of the disorder. Less is known about how metabolic state modulates reward response in BN to regulate eating, but it is possible that the influence of hunger or satiety on brain reward response is altered in BN. For example, in healthy individuals, hunger is associated with increased activation in the reward network in response to visual food images(Führer et al., 2008; Goldstone et al., 2009; Stice et al., 2013) and sweet taste stimuli,(Haase et al., 2009; Small, 2006) thereby motivating eating. Satiety may reduce the rewarding value of stimuli, perhaps through decreased responsivity of limbic circuitry or greater cognitive control.(Haase et al., 2009) In contrast, populations prone to overeating and weight gain(Cornier et al., 2008; Demos et al., 2011; Ely et al., 2014; Sun et al., 2015) show elevated activation compared to controls in reward-related brain regions in response to visual food stimuli after having eaten, suggesting that alterations in the modulatory effects of satiety on brain reward response may influence eating behavior.
This study explores gustatory and limbic neural circuits involved in processing highly palatable taste stimuli and integrating these signals with information about metabolic state. The primary gustatory cortex, including the frontal operculum and anterior insula(Fudge et al., 2005; Small, 2010) recognizes sweetness, water, and other tastes. These regions assess taste sensory experience in the context of motivational and internal drives (e.g. hunger, satiety), as well as the texture and reward value of food. The insula is structurally and functionally interconnected with the amygdala and the ventral striatum, including the nucleus accumbens and putamen,(Braun et al., 1982) which play a role in evaluating the salience of taste information and regulating consumption.(Fudge et al., 2005; Fudge et al., 2002; Hanlon et al., 2004; Williams et al., 1993) The subgenual cingulate and rostral anterior cingulate cortex (ACC), act as a visceromotor area(Devinsky et al., 1995; Freedman et al., 2000; Price and Drevets, 2012) through connections with the hypothalamus, amygdala and insula. The subgenual ACC has been shown to activate in response to highly palatable taste,(Small et al., 2001) suggesting its role in motivated behavior and the integration of metabolic states with appetitive drives.(Appelhans, 2009; Paus, 2001) Together, this circuitry integrates sensory taste information and reward valuation of food stimuli to drive eating behavior when hungry (see review).(Kaye et al., 2009)
Only a few studies have examined neural response to taste in BN, and findings are mixed. Ill adults with BN tend to show reduced activation in gustatory and limbic circuitry, including the amygdala, putamen, and insula when tasting palatable food, whether stimuli was expected(Bohon and Stice, 2011) or not.(Frank et al., 2011) In contrast, adults remitted from BN(Oberndorfer et al., 2013; Radeloff et al., 2014) show increased response to taste of sucrose in the insula and striatal regions. However, because these subjects were studied under variable levels of hunger and fullness and in different illness states, drawing conclusions from this limited literature is difficult. To date, the effects of hunger and satiety on gustatory and reward neural circuitry have not been assessed in BN, so how alterations in metabolic state may contribute to BN symptoms remains unclear.
The current study used functional magnetic resonance imaging (fMRI) to investigate the response of gustatory and limbic circuitry to sweet taste and water during hungry and fed states in RBN versus healthy control women (CW) in order to elucidate potential mechanisms contributing to binge eating. We hypothesized that CW would demonstrate a greater response to taste in these circuits when hungry versus fed, and in contrast, RBN would show an elevated response in the fed state, suggesting that satiety does not modulate reward response in BN. Further, we explored the relationship between the response to sweet taste in RBN and measures of symptom severity to clarify brain-behavior relationships.
METHODS
Subjects
Twenty-six women remitted from BN (RBN) (15 with a prior history of anorexia nervosa (AN), 11 without), were compared to 22 age- and weight-matched healthy comparison women (CW). BN remission was defined as having maintained weight above 85% of average body weight, regular menstrual cycles, and no eating disorder symptoms or behaviors for at least 1 year prior to the study. Exclusion criteria included: any psychoactive medication use in the 3 months before study; history of alcohol or drug abuse or dependence 3 months prior to study; medical or neurologic concerns; and contraindications to MRI. The study was approved by the Institutional Review Board of the University of California, San Diego, and all participants provided written informed consent.
Clinical Assessments
Beck Depression Inventory II (BDI(Beck et al., 1996)): The BDI-II is a widely used measure of depression, with high reliability, adequate convergent validity, and strong internal consistency (Cronbach’s alpha = 0.82 in this sample).(Beck et al., 1988; Beck et al., 1996) Twenty-one items are scored 0–3; higher scores indicate more severe depression.
State-Trait Anxiety Inventory (STAI(Spielberger, 1983)): This self-report questionnaire is a reliable and valid measure of anxiety severity,(Spielberger, 1983) which was fairly internally consistent in this sample (Cronbach’s alpha = 0.54). Each of the two subscales (State Anxiety and Trait Anxiety) contain 20 items on a 4-point rating scale, with higher scores indicating greater anxiety.
Diagnostic Assessments
Axis I diagnosis was assessed by doctorate-level clinicians using the full Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I(First et al., 1997)) for the first half of the study (7 CW, 7 RBN) and the Mini International Neuropsychiatric interview (M.I.N.I.(Sheehan et al., 1998)) in combination with Module H from the SCID for the second half (15 CW, 19 RBN). The MINI has been validated against the much longer SCID and is a more time-efficient alternative to the SCID,(Sheehan et al., 1998) which prompted the change in assessment. There were no differences on clinical self-report measures between participants assessed with the full SCID and those who completed the MINI, but those assessed with the full SCID were found to have significantly greater BMI (M = 23.06, SD = 2.34) than those assessed with the MINI (M = 21.76, SD = 1. 68; t (46) = 2.17, p = 0.035). Interrater reliability was not assessed as a part of this study, however all diagnostic assessments were completed by 1 of 4 Ph.D. or M.D. clinicians and assessments were discussed at weekly meetings to achieve a consensus diagnosis.
Hunger Assessments
Participants rated hunger and satiety using Likert-type scales ranging from 0 (not at all) to 7 (extreme) at 3:00 pm the day before a scan visit (baseline), and at 6:45 am (awakening), 8:45 am (pre-scan), and 11:00 am (post-scan) the day of a scan visit.
Experimental Design
Participants completed an fMRI scan on two counterbalanced visits 24 hours apart. For the “hungry” state, participants fasted for 16 hours prior to the scan session. During the “fed” state, participants consumed standardized meals on the day prior to study and a standardized breakfast (30% of overall daily caloric needs, calculated as 30kcal/kg body weight, approximating 450–500 kcal; 53% carbohydrates, 32% fat, and 15% protein) two hours prior to the 9 am scan session. Participants were housed and meals were provided by the UCSD Clinical & Translational Research Institute for 72 hours to ensure dietary compliance.
All scans were performed within the follicular phase (first 10 days) of each participant’s menstrual cycle. Blood samples were drawn at 1:30 pm on the day prior to the first scan to measure baseline levels of estradiol in order to confirm participants were in the follicular phase of their menstrual cycle. Estradiol levels were not obtained for 6 CW and 9 RBN due to errors in transporting blood samples. There were no differences on clinical variables between those with and without confirmed menstrual status.
Sucrose Taste Paradigm
The sucrose taste task was based on a paradigm developed by our group.(Frank et al., 2003) Water and sucrose solution were delivered with a programmable syringe pump (J-Kem Scientific, St. Louis MO). Two sterile silicone tubes were placed securely in the center of the tongue immediately adjacent to each other. Subjects received small samples (1.0 cc) of 10% sucrose solution or ionic water. The paradigm consists of four blocks of 20 trials each. Within blocks, stimuli were presented in the same pseudorandom order for all subjects. Sucrose and ionic water were delivered every 20 seconds for a total of 40 stimulus presentations for each condition.
MRI Acquisition Protocol
Functional images were acquired in a sagittal plane using T2* weighted echo planar imaging (EPI) with an 8-channel head coil. Imaging data were collected on one of two scanners: a 3T GE Signa HDx (GE Medical Systems, Milwaukee, WI) (TR = 2000 ms, TE = 30 ms, flip angle = 80°, 64 x 64 matrix, ASSET factor = 2, 40 2.6-mm ascending interleaved axial slices with a 0.4-mm gap, 256 volumes) or a 3T GE Discovery MR 750 (GE Medical Systems, Milwaukee, WI) (TR = 2000 ms, TE = 30 ms, flip angle = 80°, 64 x 64 matrix, ASSET factor = 2, 40 3.0-mm ascending interleaved axial slices, 256 volumes). Our use of two 3T scanners was due to a system upgrade. Multisite imaging studies suggest that inter-participant variance far outweighs that of site or magnet variance.(Brown et al., 2011; Gountouna et al.; Suckling et al., 2008) However, to control for potential differences due to magnet hardware, groups were balanced across magnets (Table 1), each participant was scanned on the same scanner for both imaging visits, and subject was nested within scanner and treated as a random effect in subsequent analyses. The first four volumes of each run were discarded to discount T1 saturation. EPI-based field maps were also acquired to correct for susceptibility-induced geometric distortions. High-resolution T1-weighted FSPGR anatomical images (Signa HDx: TR=7.7 ms, TE=2.98 ms, flip angle=8°, 192x256 matrix, 172 1.2 mm sagittal slices; MR 750: TR=8.1 ms, TE=3.17 ms, flip angle=8°, 192x256 matrix, 172 1.2 mm sagittal slices) were obtained for subsequent spatial normalization and activation localization.
TABLE 1.
Participant demographics and characteristics. Entries are of the form mean (standard deviation). CW: healthy comparison women; RBN: women remitted from bulimia nervosa; BMI: body mass index (kg/m2); WASI: Wechsler Abbreviated Scale of Intelligence; STAI: State-Trait Anxiety Inventory; EDI-2: Eating Disorder Inventory-2; BDI: Beck Depression Inventory. One CW and two RBN did not complete clinical assessments.
| Characteristic | CW (N = 22) | RBN (N = 26) | ||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | F | df | p | ||
| Demographics | Age | 25.95 | 6.32 | 28.24 | 5.40 | 1.79 | 45 | n.s. |
| BMI | 22.09 | 2.06 | 22.11 | 1.92 | 0.00 | 45 | n.s. | |
| Education | 15.77 | 1.31 | 16.54 | 2.35 | 1.84 | n.s. | ||
| Estradiol (pg/mL) | 12.88 | 7.91 | 13.52 | 5.52 | 0.08 | 32 | n.s. | |
| Scanner | Signa Excite | 10 | 14 | |||||
| MR 750 | 12 | 12 | ||||||
|
Lifetime Diagnosis (#) |
MDD | 0 | 15 | |||||
| Any anxiety disorder | 1 | 10 | ||||||
| OCD | 0 | 4 | ||||||
|
Past substance abuse/dependence (#) |
Alcohol | 0 | 3 | |||||
| Cannabis | 0 | 1 | ||||||
|
Self-Report Assessments |
STAI State Anxiety | 23.57 | 3.34 | 28.83 | 8.18 | 7.57 | 43 | 0.009 |
| STAI Trait Anxiety | 23.33 | 3.35 | 28.07 | 7.50 | 7.11 | 43 | 0.011 | |
| EDI-2 Bulimia | 0.00 | 0.00 | 0.17 | 0.64 | 1.43 | 43 | n.s. | |
| BDI | 0.29 | 0.46 | 2.00 | 2.75 | 7.94 | 43 | 0.007 | |
| Symptom Severity | Lowest BMI | 20.37 | 1.51 | 17.92 | 2.93 | 11.96 | 44 | 0.001 |
| Duration of BN (months) |
- | - | 69.76 | 47.11 | ||||
| Length of Recovery (months) |
- | - | 50.16 | 43.53 | ||||
| Worst Binge Frequency (per week) |
- | - | 19.08 | 13.63 | ||||
| Worst Purge Frequency (per week) |
- | - | 20.38 | 21.31 | ||||
MRI Preprocessing
Functional images were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software, and group analyses were performed using the nlme package in R (http://www.r-project.org). EPI images were motion-corrected and aligned to high-resolution anatomical images with align_epi_anat.py. Outliers were generated using AFNI’s 3dToutcount. Volumes with more than 10% of the voxels marked as outliers were censored from subsequent analyses. Approximately 5.1% of volumes were censored (for all subjects: M = 13 volumes; SD = 9.1; range = 3–27). Registration to the MNI-152 atlas was performed using FMRIB’s Non-linear Image Registration Tool (FNIRT), part of FSL (http://fsl.fmrib.ox.ac.uk/fsl/). The modeled hemodynamic responses were subsequently scaled so that beta weights would be equivalent to percent signal change (PSC). Data were spatially blurred with a 4.2 mm full-width, half maximum spatial filter.
Delineation of Search Region
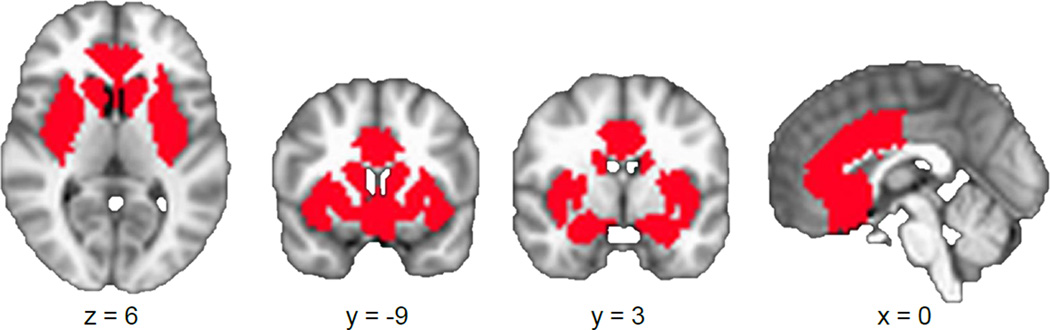
To limit multiple comparisons, a gustatory-reward circuit search region of interest was chosen based on previous neuroimaging research in BN of taste(Oberndorfer et al., 2013 ) and monetary(Wagner et al., 2010) rewards. Striatal regions included the bilateral putamen and the bilateral ventral striatum (comprising the nucleus accumbens extending into the rostroventral caudate and ventrolateral putamen).(Martinez et al., 2003; Mawlawi et al., 2001) The bilateral insula, anterior cingulate cortex, and amygdala masks from the Harvard-Oxford atlas(Desikan et al., 2006) were also used in their entirety. These regions were combined in a single circuit mask to reduce multiple comparisons and treated as a search region for subsequent analysis (Figure 1).
FIGURE 1.
Illustration of gustatory-limbic regions of interest, which included the amygdala, ventral striatum, anterior cingulate cortex, and insula.
Analysis
Statistical analyses were performed using a general linear model (GLM), with individual events modeled using AFNI’s SPMG3 function.(Cox, 1996) Six motion parameters (3 rotations and 3 translations) were used as nuisance regressors to account for motion artifact.
To test our hypothesis that hunger and satiety modulate response to sweet taste, we employed a Group (RBN, CW) x Condition (Hungry, Fed) x Taste (Sucrose, Water) linear mixed effects (LME) analysis within our search region mask. Subjects were embedded within scanner and were treated as a random effect with Group, Condition and Taste as fixed effects. This analysis was repeated separately for RBN with and without a history of AN to examine whether history of AN influenced the results. Exploratory whole-brain voxel-wise analyses using the same model with all subjects were also performed.
To guard against identifying false positive areas of activation, Monte-Carlo simulations (via AFNI’s 3dClustSim, May 2016 release) were conducted. At the ROI level using small volume correction, 3dClustSim identified a minimum cluster volume of 567 µL (21 contiguous voxels) for the bilateral insula, 270 µL (10 contiguous voxels) for the bilateral ventral striatum, 432 µL (16 contiguous voxels) for the bilateral putamen, 378 µL (14 contiguous voxels) for the bilateral amygdala, and 567 µL (21 contiguous voxels) for the bilateral anterior cingulate cortex. At the whole brain level, 3dClustSim identified a minimum cluster size of 1701 µL (63 contiguous voxels). All minimum cluster sizes corresponded to a cluster significance of p < 0.05 (one-sided) to result in a voxel-wise probability of p < 0.05 (one-sided) after correction for multiple comparisons.
To examine the relationship between clinical variables and group differences in activation in response to palatable taste, we tested the overlap between significant clusters identified in our task-related LME and those where percent signal change was significantly correlated with clinical variables, using Huber robust regressions. Since both analyses are individually corrected for multiple comparisons, and the clusters within are statistically significant, the resultant overlap is also considered statistically significant.(Nichols et al., 2005) Analyses were constrained to sucrose given that BN often binge on palatable foods.(Rosen et al., 1986) Clinical variables assessed included age, current BMI, lowest lifetime BMI duration of BN, and scores on the STAI, and BDI. Logistical regression was used to determine if percent signal change in clusters identified by the LME would differentiate those with and without lifetime histories of depression and anxiety disorders.
RESULTS
Demographics and Clinical Assessments
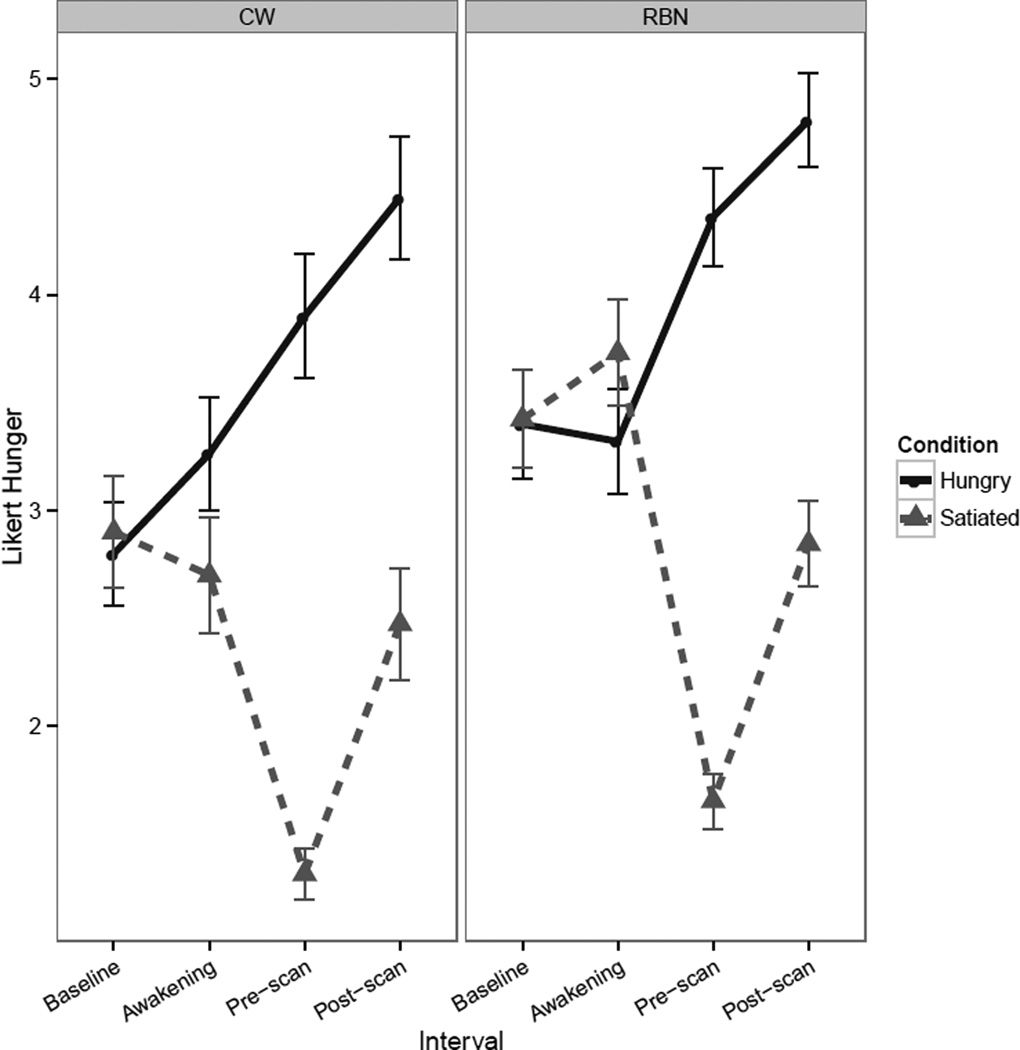
CW and RBN groups did not differ on BMI, age or years of education (Table 1). Both groups of participants reported significantly greater hunger during the hungry condition relative to the fed condition (Figure 2) but there were no group differences in hunger or fullness ratings. In either condition, there were no significant group differences in palatability ratings of water (fasted condition: F(43) = 0.06, p = 0.81; fed condition: F(42) = 0.61, p = 0.44) or sucrose solution (fasted condition: F(43) = 0.39, p = 0.54; fed condition: F(42) = 0.45, p = 0.51), nor was there a significant difference in palatability in the fasted versus fed state for water (t(43) = 0.53, p = 0.59) or for sucrose (t(43) = 1.49, p = 0.14). Participants rated water as significantly more palatable than the sucrose solution in both the fasted (t(44) = 3.52, p = 0.001) and fed (t(43) = 4.13, p < 0.001) conditions.
FIGURE 2.
Line graphs reflecting self-report Likert visual analog scale values. Line graph of pre- and post-scan self-report measures of hunger shows a Condition x Interval interaction [F(1,303)=11.35, p<0.001, with significant main effect of Condition [F(1,303)=458.39, p<0.001] and of interval [F(1,303)=64.97, p<0.001]. Error bars represent the standard error. CW: healthy comparison women; RBN: women remitted from bulimia nervosa.
Search Region Analysis
There was a main effect of Condition in the bilateral putamen, left amygdala and left subgenual ACC, but not the insula, with post-hoc analyses demonstrating significantly greater activation when hungry versus fed. However, this significant effect appeared to be driven by differences in response when hungry versus fed in CW. There was no main effect of Taste or of Group within our search region.
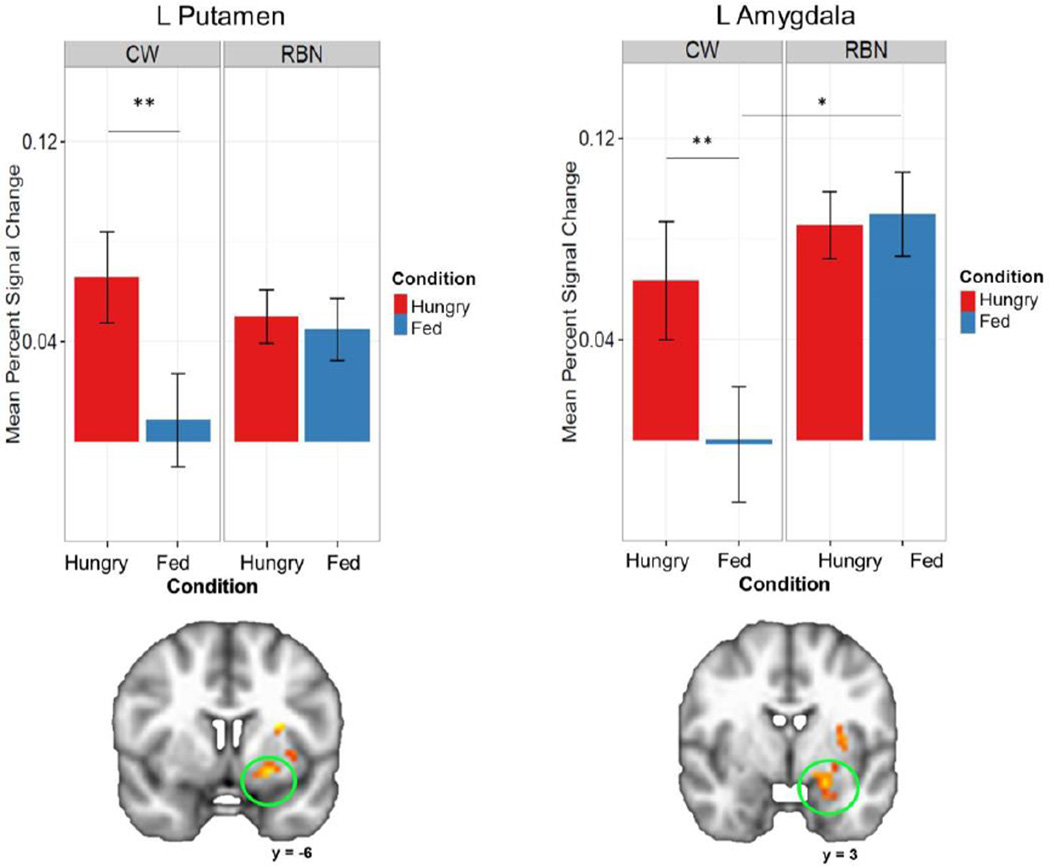
In areas involved in gustatory reward, the RBN and CW groups showed differing patterns of brain response to taste solutions when hungry versus fed (Table 2). A significant interaction of Group x Condition was found in the left ventral putamen, left anterior insula, and left amygdala. Post-hoc analyses (Figure 3) revealed that the CW group was significantly more responsive to taste when hungry versus fed in the left putamen and left amygdala, whereas there was no significant difference in the RBN group when hungry versus fed. In the left amygdala, CW were significantly less responsive to taste in the fed state compared with the hungry state, and significantly less responsive than the RBN group when fed. None of the post-hoc analyses revealed significant findings in the anterior insula.
TABLE 2.
Analysis of variance results within search region of interest demonstrating a main effect of Condition (A) and an interaction of Group (CW, RBN) by Condition (Hungry, Fed) in response to tastant (B). Coordinates are reported for the center of mass. L: left; R: right; CW: healthy comparison women; RBN: women remitted from bulimia nervosa. Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives. Post-hoc analyses were conducted using glht from the multcomp package in R to test general linear hypotheses using Tukey’s all-pair comparisons.
| (A) Main effect of Visit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | R/L | Volume (voxels) |
x | y | z | F | η2 | Post-hoc Comparisons | ||
| Contrast | z | p | ||||||||
| Putamen | L | 119 | 23 | −5 | −6 | 18.67 | 0.01 | Hungry > Fed | 4.10 | < 0.001 |
| Amygdala | L | 56 | 19 | 3 | −18 | 11.64 | 0.02 | Hungry > Fed | 4.65 | < 0.001 |
| Medial Frontal Gy | L | 54 | 2 | −26 | −19 | 13.99 | 0.01 | Fed > Hungry | 2.54 | 0.01 |
| Putamen | R | 48 | −22 | −9 | −1 | 10.03 | 0.01 | Hungry > Fed | 3.14 | < 0.001 |
|
(B) Group x Condition Interaction | ||||||||||
| ROI | R/L |
Volume (voxels) |
x | y | z | F | η2 | Post-hoc Comparisons | ||
| Contrast | z | p | ||||||||
| Putamen | L | 47.00 | 23 | −7 | −8 | 11.95 | 0.01 | CW Hungry > CW Fed | 4.60 | < 0.001 |
| Insula | L | 42.00 | 31 | −2 | 9 | 14.35 | 0.02 | ns | ||
| Amygdala | L | 34.00 | 18 | 4 | −16 | 10.84 | 0.02 | CW Hungry > CW Fed | 4.43 | < 0.001 |
| RBN Fed > CW Fed | 2.63 | 0.05 | ||||||||
FIGURE 3.
Plots depicting significant Group x Condition interaction and significant post-hoc differences within representative regions of interest. CW demonstrated elevated activation in response to taste in the left putamen and left amygdala in the hungry state compared to the fed state. In contrast, RBN did not show differentiation in response between conditions. However, RBN showed increased activation in response to taste compared to CW in the Fed condition in the left amygdala. CW: healthy comparison women; RBN: women remitted from bulimia nervosa; L: left; *p<0.05; **p<0.001, corrected. Right=Left.
There was no significant interaction of Group (CW, RBN) x Condition (Hungry, Fed) x Taste (Sucrose, Water). Similar to previous research,(Rolls, 2005; Wagner et al., 2008; Zald et al., 2002) there were no Group x Taste or Condition x Taste interactions (i.e., there were no differences between Sucrose and Water across conditions between groups or across groups between conditions). In our sample, individuals with a history of AN and BN did not differ in their BOLD response to taste compared to those with only a previous BN diagnosis.
Exploratory Whole-Brain Voxel-wise Analysis
There was a main effect of Condition, with greater response to taste in the left putamen, left cingulate gyrus and left precuneus when hungry versus fed, and greater activation in the right superior temporal gyrus (STG) when fed versus hungry. There were no main effects of Taste or Group.
A significant interaction of Group x Condition (Table 3) was found in the right STG, left precuneus, a limbic cluster including the left amygdala and parahippocampal gyrus, and right precentral gyrus. Post-hoc analyses revealed that the CW group was significantly more responsive to taste when hungry versus fed in the left precuneus, left amygdala/parahippocampal gyrus, and right precentral gyrus. The CW group showed greater activation when fed versus hungry in the right STG, whereas in the RBN group there was greater response when hungry versus fed in the right STG. The RBN group was significantly more responsive to taste than the CW in the right STG when hungry. In the fed state, CW were significantly less responsive to taste than the RBN group in the left parahippocampal gyrus. There were no interactions of Group x Taste or Condition x Taste.
TABLE 3.
Analysis of variance whole-brain results demonstrating (A) a main effect of Condition and (B) an interaction of Group (CW, RBN) by Condition (Hungry, Fed) in response to tastant. Coordinates are reported for the center of mass. L: left; R: right; CW: healthy comparison women; RBN: women remitted from bulimia nervosa; Sup: Superior. Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives. Post-hoc analyses were conducted using glht from the multcomp package in R to test general linear hypotheses using Tukey’s all-pair comparisons.
| (A) Main effect of Condition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | R/L | Volume (voxels) |
x | y | z | F | η2 | Post-hoc Comparisons | ||
| Contrast | z | p | ||||||||
| Lentiform Nucleus | L | 106 | 21 | −2 | −10 | 18.67 | 0.02 | Hungry > Fed | 5.25 | < 0.001 |
| Sup Temporal Gy | R | 82 | −50 | 49 | 16 | 14.69 | 0.002 | Fed > Hungry | 5.22 | < 0.001 |
| Cingulate Gy | L | 82 | 0 | 28 | 27 | 11.28 | 0.01 | Hungry > Fed | 3.08 | 0.002 |
| Precuneus | L | 69 | 21 | 63 | 36 | 15.11 | 0.01 | Hungry > Fed | 3.51 | < 0.001 |
|
(B) Group x Condition Interaction | ||||||||||
| Area | R/L |
Volume (voxels) |
x | y | z | F | η2 | Post-hoc Comparisons | ||
| Contrast | z | p | ||||||||
| Sup Temporal | R | 128 | −51 | 49 | 17 | 18.67 | 0.03 | CW Fed > CW Hungry | 2.59 | 0.047 |
| RBN Hungry > CW Hungry | 3.33 | 0.005 | ||||||||
| RBN Hungry > RBN Fed | 3.65 | 0.001 | ||||||||
| 63 | −49 | 23 | 6 | 18.19 | 0.03 | CW Fed > CW Hungry | 3.83 | 0.001 | ||
| Precuneus | L | 113 | 26 | 58 | 24 | 21.34 | 0.02 | CW Hungry > CW Fed | 3.56 | 0.002 |
| Amygdala/ Parahippocampal |
L | 94 | 14 | 0 | −10 | 16.06 | 0.03 | CW Hungry > CW Fed | 5.53 | < 0.001 |
| RBN Fed > CW Fed | 3.2 | 0.006 | ||||||||
| Precentral | R | 70 | −52 | 10 | 29 | 13.45 | 0.02 | CW Hungry > CW Fed | 4.03 | < 0.001 |
Exploratory Associations with Clinical Variables
BOLD response among RBN participants with a history of depression (N=15) or anxiety disorders (N=10) did not differ from those without. After correcting for multiple comparisons, there were no significant relationships between pre-scan hunger, age, lowest BMI or current BMI and percent signal change in response to sucrose in RBN or CW when hungry or fed for any ROI. Scores on the STAI, TCI and BDI were also unassociated with activation in response to sucrose in either group in either condition. In the RBN group, worst-ever binge eating frequency was unrelated to activation in response to sucrose in either condition.
DISCUSSION
As expected,(Goldstone et al., 2009; Tataranni et al., 1999) CW showed an increased response to taste stimuli when hungry versus fed in the left putamen and amygdala, brain regions implicated in translating sensory information about taste into motivated behavior.(Fudge et al., 2005) In contrast, brain response to taste in RBN did not differ when fed compared to when hungry in the left putamen and amygdala. In fact, activation was significantly greater in RBN than in CW in the left amygdala when fed, indicating that taste response in RBN may be insensitive to the effects of energy metabolism.
Surprisingly, we did not find a difference between brain response to sucrose and water in any of our ROIs. A recent review suggests that water has limitations as a comparison since it can activate taste regions.(Rolls, 2016) Participants in the current study rated water as more palatable than sucrose, suggesting water may not be a neutral stimulus. While some studies have shown discrimination between sugar and water in the insula(Schoenfeld et al., 2004) others have not,(Rolls, 2005; Wagner et al., 2008; Zald et al., 2002) potentially due to methodological differences. Some of these studies have also failed to show increased activation in response to sucrose relative to water in the amygdala and putamen(Wagner et al., 2008; Zald et al., 2002), consistent with the current findings. Thus, our results are based on the combined taste response to sucrose and water. The failure to detect a difference between response to sucrose and water suggests the lack of within-group differences in RBN when hungry versus fed may be related more to motivational rather than taste signals. It may be that RBN have altered integration of homeostatic, gustatory, and reward signals in regions (Figure 3) that are key for motivated behavior.
Increased amygdala response when fed in RBN, compared to CW, may provide new insight into understanding why these individuals tend to remain driven to eat when no longer hungry. The amygdala is well established as a primary region for processing both positive and negative emotional stimuli(Baxter and Murray, 2002; Bechara et al., 2003; Hamann and Mao, 2002) and forming conditioned stimulus-reward associations.(Baxter and Murray, 2002) Notably, a growing literature has implicated the amygdala in eating behavior and food cue responsivity. For example, abnormal amygdala connectivity with the ventral striatum and elevated activation elicited by food cues is linked with reward sensitivity(Beaver et al., 2006; Passamonti et al., 2009) and obesity.(Boutelle et al., 2015; Stoeckel et al., 2009) A recent study suggests that amygdala activation when sated may drive eating in the absence of hunger through projections to the hypothalamus, and is linked with future weight gain.(Sun et al., 2015) The putamen contributes to the assessment of the intensity and reward value of sweet tastes(Montague et al., 2004; Small et al., 2003; Spetter et al., 2010) and is both structurally and functionally connected with the insula and amygdala.(Fudge et al., 2005; Fudge et al., 2002; Hanlon et al., 2004; Williams et al., 1993) Our results support an important role for the putamen and amygdala in valuating reward in BN; exaggerated left amygdala activation in RBN in response to tastes when fed may contribute to a tendency to eat beyond metabolic need. In other words, insensitivity to hunger and satiety in RBN in this region suggests a failure of metabolic state to appropriately modulate brain circuitry important for motivated eating behavior. We hypothesize that this circuitry in BN may not exhibit attenuation of reward-related activation that typically accompanies satiety. Future studies that specifically assess the process of becoming full and related brain changes in this population are necessary to test this hypothesis.
Insensitivity to metabolic state may interact with dysfunction in reward networks in BN to contribute to binge eating. Ventral limbic brain regions, including the rostral anterior cingulate cortex, ventromedial prefrontal cortex, and anterior ventral striatum, have been shown to play a role in appraisal of rewarding or emotionally salient stimuli.(Delgado et al., 2000; Haber and Knutson, 2010; Kim et al., 2011; McClure et al., 2007; McClure, Laibson, et al., 2004; McClure, Li, et al., 2004; Onoda et al., 2011; Sripada et al., 2011; Wittman et al., 2010; Wittmann et al., 2007) There are structural differences in these regions in BN, with decreased gray matter volume of the putamen associated with increased self-reported sensitivity to reward in ill BN.(Frank et al., 2013) Functional alterations in striatal circuitry have also been demonstrated in BN using monetary rewards. While healthy individuals demonstrate greater response in striatal reward regions to positive feedback versus negative, RBN did not differentiate positive and negative feedback,(Wagner et al., 2010) suggesting deficits in modulating the evaluation of rewards. Difficulty distinguishing between positive and negative stimuli could contribute to an inability to use salience to guide behavior, and our results support the hypothesis that this may extend to the salience imbued by metabolic state. Dysregulated valuation of salient stimuli may mean individuals with BN struggle with adjusting response to rewards with satiety. The resulting reward response would thus remain static across hunger and sated states and potentially increase vulnerability to disinhibited eating behavior.
Previous research assessing response to actual food intake in BN has shown reduced neural response to taste in participants with active BN in the insula, putamen and amygdala.(Bohon and Stice, 2011; Frank et al., 2011) In contrast, individuals recovered from BN demonstrate increased response to taste receipt in the ventral striatum(Radeloff et al., 2014) and anterior insula.(Oberndorfer et al., 2013; Radeloff et al., 2014) It is unclear whether mixed findings have emerged because of differences in the state of illness (e.g., ill, subthreshold, or recovered), the type of taste administered (e.g., chocolate milkshake, cream, sucrose or sucralose solutions), metabolic state of the subjects prior to scanning (e.g., variation in size and timing of standardized meal) or statistical thresholding procedures. Additionally, given the clinical presentation of these patients, prior studies of ill BN likely include individuals who are actively restricting their intake in addition to engaging in binge eating, thus they may show a reduced response when hungry to the highly palatable taste stimuli.
Limitations and Future Directions
Several issues require further investigation. First, there was not a significant difference in brain response to water versus sucrose, as mentioned above. This may have been due to methodological reasons (such as the percent sucrose solution or averaging signal across entire ROI), the overlapping patterns of response to water and sucrose,(Rolls, 2016; Schoenfeld et al., 2004) or high palatability ratings for both sucrose and water. The lack of neural differentiation raises the possibility that the observed effect may also be related to oral somatosensory stimulation. Clinically, some patients with BN describe binges as containing odd combinations of flavors or foods of only moderate palatability. One study(Allison and Timmerman, 2007) reported that the most common items consumed during binges were breads and pastas, foods that are frequently bland. It is possible that, when full, individuals with BN may be more motivated to ingest substances regardless of palatability. Second, there were significantly higher palatability ratings of water versus sucrose solution in both conditions, which may have contributed to null results. Future research would benefit from tailoring experimental tastes to individual participant preferences to maximize appetitive differences to more clearly elucidate the contribution of taste and oral somatosensory response to the relationship between binge behavior, metabolic state, and reward response. Second, signal dropout prevented assessment of involvement of the orbitofrontal cortex, an additional part of the gustatory circuit.(Small, 2006) It is also possible that our sample size may have been too small to sufficiently power between-group comparisons. Post-hoc analyses of the relationship between clinical variables and response to tastant were exploratory, and should be interpreted with caution.
Results raise the intriguing hypothesis that a lack of sensitivity to the inhibiting effects of satiety, rather than reactivity to hunger, may underlie feelings of loss of control and the extreme consumption that characterize binge eating. This is in contrast to theories of bulimia nervosa that posit that short-term hunger increases binge eating vulnerability.(Stice et al., 2008; Stice et al., 2005; Telch, 1996; Zunker et al., 2011) Assessment of long-term restriction of intake below energy needs, dieting behavior, and ad libitum consumption in a laboratory setting in future research is necessary to clarify this effect. Physiological measures of hunger and satiety, such as ghrelin or leptin levels, would also be beneficial.
While it will be important to replicate our findings in ill BN, there are likely to be confounding effects of dysregulated eating patterns, as over- or under-feeding in the days prior to study visit have been shown to impact brain response to food stimuli.(Cornier et al., 2008) In order to control for this, we studied individuals remitted from BN. It is possible that group differences seen here may be the result of chronic binge eating or purging behavior, rather than premorbid alterations in neurobiology that persist after recovery. However, though empirically-supported treatments for BN lead to abstinence from binge eating and purging for 30–50% of treatment completers,(Keel et al., 1999; Mitchell et al., 2002; Wilson et al., 2007) certain characteristics commonly persist after remission.(Wagner et al., 2006) Individuals with BN tend to share stable temperament and personality traits, shown to be present in childhood, prior to the onset of the eating disorder, that may create or increase a vulnerability to develop an ED. It remains controversial whether neural findings reflect a trait contributing to BN, or a scar of having had an eating disorder. Notably, we did not find any relationship between duration of BN, a proxy of disease severity, and neural response to taste stimuli.
Clinical Implications
BN treatments are only marginally effective due to a limited understanding of the neurocognitive mechanisms of the disorder. Results of the current study suggest that individuals with BN are neurobiologically motivated to continue eating even when calorically replete, which may contribute to binge eating. These findings provide brain-based support for interventions immediately following a meal. For example, structured meal plans, stimulus control strategies, or incorporating social or other distracting activities after eating may reduce temptation or loss-of-control eating. These results may contribute to identifying neural mechanisms and thus open the door to the use of new medications, or other brain-based treatments.
Acknowledgments
This research was supported by funding from the National Institute of Mental Health (MH086017 and MH042984-17A1) and the Price Foundation to WHK.
References
- Allison S, Timmerman G. Anatomy of a binge: food environment and characteristics of nonpurge binge episodes. Eat Behav. 2007;8(1):31–38. doi: 10.1016/j.eatbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V. 5th. Washington, DC: 2013. [Google Scholar]
- Appelhans B. Neurobehavioral inhibition of reward-driven feeding: Implications for dieting and obesity. Obesity (Silver Springs) 2009;17(4):640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- Baxter M, Murray E. The amygdala and reward. Nat Rev Neurosci. 2002;5:563–573. doi: 10.1038/nrn875. 12094212. [DOI] [PubMed] [Google Scholar]
- Beaver J, Lawrence A, van Ditzhuijzen J, Davis M, Woods A, Calder A. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26(19):5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. 16687507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio A. Role of the amygdala in decision-making. Ann NY Acad Sci. 2003;985(1):356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. 12724171. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Carbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychology Rev. 1988;8(1):77–100. [Google Scholar]
- Beck A, Steer RBR, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;57(3):588–597. doi: 10.1207/s15327752jpa6703_13. 8991972. [DOI] [PubMed] [Google Scholar]
- Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord. 2011;44(7):585–595. doi: 10.1002/eat.20869. 21997421 PMCID: PMC3111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle K, Wierenga C, Bischoff-Grethe A, Melrose A, Grenesko-Stevens E, Paulus M, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes (Lond) 2015;39(4):620–628. doi: 10.1038/ijo.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J, Lasiter P, Kiefer S. The gustatory neocortex of the rat. Physiol Psychol. 1982;10:13–45. [Google Scholar]
- Brown G, Mathalon D, Stern H, Ford J, Mueller B, Greve D, et al. Multisite reliability of cognitive BOLD data. Neuroimage. 2011;54(3):2163–2175. doi: 10.1016/j.neuroimage.2010.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin S, von Ranson K. Personality and eating disorders: a decade in review. Clin Psycho Rev. 2005;25(7):895–916. doi: 10.1016/j.cpr.2005.04.012. 16099563. [DOI] [PubMed] [Google Scholar]
- Chan T, Ahn W, Bates J, Busemeyer J, Guillaume S, Redgrave G, et al. Differential impairments underlying decision making in anorexia nervosa and bulimia nervosa: a cognitive modeling analysis. Int J Eat Disord. 2014;47(2):157–267. doi: 10.1002/eat.22223. [DOI] [PubMed] [Google Scholar]
- Cornier M, Salzberg A, Endly D, Bessesen D, Rojas D, Tregellas J. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. Plos One. 2008;4(7):e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. 8812068. [DOI] [PubMed] [Google Scholar]
- Delgado M, Nystrom L, Fissel C, Noll D, Fiez J. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. 11110834. [DOI] [PubMed] [Google Scholar]
- Demos K, Kelley W, Heatherton T. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J Cogn Neurosci. 2011;23(8):1952–1963. doi: 10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Segonne FFB, Quinn B, Dickerson B, Blacker D, Buckner F, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. 7895011. [DOI] [PubMed] [Google Scholar]
- Ely A, Childress AJK, Lowe M. Differential reward response to palatable food cues in past and current dieters: an fMRI study. Obesity. 2014;22(5):E38–E45. doi: 10.1002/oby.20599. [DOI] [PubMed] [Google Scholar]
- First M, Biggon M, Spitzer R, Williams J, Benjamin L. User’s guide for the structured clinical interview for DSM-IV Axis II personal disorders (SCID-II) Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Fouladi F, Mitchell J, Crosby RESG, Crow S, Hill L, Le Grange D, et al. Prevalence of alcohol and other substance use in patients with eating disorders. Eur Eat Disord Rev. 2015;23(6):531–536. doi: 10.1002/erv.2410. [DOI] [PubMed] [Google Scholar]
- Frank G, Kaye W, Carter C, Brooks S, May C, Fissel K, et al. The evaluation of brain activity in response to taste stimuli--a pilot study and method for central taste activation as assessed by event related fMRI. J Neurosci Methods. 2003;131(1–2):99–105. doi: 10.1016/s0165-0270(03)00240-1. 14659829. [DOI] [PubMed] [Google Scholar]
- Frank G, Reynolds J, Shott M, O’Reilly R. Altered temporal difference learning in bulimia nervosa. Biol Psych. 2011;70(8):728–735. doi: 10.1016/j.biopsych.2011.05.011. 21718969 PMCID:PMC3186835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Shott M, Hagman J, Mittal V. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170(10):1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. The Journal of Comparative Neurology. 2000;421:172–188. 10813780. [PubMed] [Google Scholar]
- Fudge J, Breibart M, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490(2):101–118. doi: 10.1002/cne.20660. 16052493 PMCID: PMC2474655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J, Kunishio K, Walsh PRC, Haber S. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neurosci. 2002;110(2):257–275. doi: 10.1016/s0306-4522(01)00546-2. 11958868. [DOI] [PubMed] [Google Scholar]
- Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity. 2008;16(5):945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- Goldstone A, Prechl de Hernandez C, Beaver J, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. 19811532. [DOI] [PubMed] [Google Scholar]
- Gountouna V-E, Job D, McIntosh A, Moorhead T, Lymer G, Whalley H, et al. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage. 2010;49(1):552–560. doi: 10.1016/j.neuroimage.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44(3):1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. 19007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharm. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt A, Keel P. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol Bull. 2011;137(4):660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmi KA, Sunday S, Puglisi A, Marchi P. Hunger and satiety in anorexia and bulimia nervosa. Ann N Y Acad Sci. 1989;575:431–444. doi: 10.1111/j.1749-6632.1989.tb53264.x. 2633673. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13(1):15–19. doi: 10.1097/00001756-200201210-00008. 11924878. [DOI] [PubMed] [Google Scholar]
- Hanlon E, Baldo B, Sadeghian K, Kelley A. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacol (Berl) 2004;172(3):241–247. doi: 10.1007/s00213-003-1654-0. 14598017. [DOI] [PubMed] [Google Scholar]
- Harrison A, Sullivan S, Tchanturia K, Treasure J. Attentional bias, emotion recognition and emotion regulation in anorexia: state or trait? Biol Psych. 2010;68(8):755–761. doi: 10.1016/j.biopsych.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Kaye W, Fudge J, Paulus M. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. 19603056. [DOI] [PubMed] [Google Scholar]
- Keel PK, Mitchell JE, Miller KB, Davis TL, Crow SJ. Long-term outcome of bulimia nervosa. Arch Gen Psychiatry. 1999;56(1):63–69. doi: 10.1001/archpsyc.56.1.63. 9892257. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty J. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb Cortex. 2011;21:769–776. doi: 10.1093/cercor/bhq145. 20732900. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow and Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McClure S, Ericson K, Laibson D, Loewenstein G, Cohan J. Time discounting for primary rewards. J Neuroscience. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S, Laibson D, Loewenstein G, Cohen J. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure S, Li J, Tomlin D, Cypert K, Montague L, Montague P. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44(2):379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Halmi K, Wilson G, Agras W, Kraemer HC, Crow S. A randomized secondary treatment study of women with bulimia nervosa who fail to respond to CBT. Int J Eat Disord. 2002;32(3):271–281. doi: 10.1002/eat.10092. 12210641. [DOI] [PubMed] [Google Scholar]
- Montague R, Hyman S, Cohen J. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. 15483596. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Martidis V, Fabrazzo M, Serritella C, Maj M. Ghrelin and leptin responses to food ingestion in bulimia nervosa: implications for binge-eating and compensatory behaviours. Psychol Med. 2003;33(8):1387–1394. doi: 10.1017/s0033291703008316. 14672247. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Anderson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. 15808966. [DOI] [PubMed] [Google Scholar]
- Oberndorfer T, Frank G, Fudge J, Simmons A, Paulus M, Wagner A, et al. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry. 2013;214(2):132–141. doi: 10.1176/appi.ajp.2013.11111745. 23993362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, et al. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Res. 2013;214(2):132–141. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, unisato Y, Aoyama S, Shishida K, Okada G, et al. Inter-individual discount factor differences in reward prediction are topographically associated with caudate activation. Exp Brain Res. 2011 doi: 10.1007/s00221-011-2771-3. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rowe J, Schwartzbauer C, Ewbank M, von dem Hagen E, Calder A. Personality predicts the brain’s response to viewing appetizing foods: the neural basis of a risk factor for overeating. J Neuroscience. 2009;29(1):43–51. doi: 10.1523/JNEUROSCI.4966-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Price J, Drevets W. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Radeloff D, Willmann K, Otto L, Lindner M, Putnam K, van Leeuwen S, et al. High-fat taste challenge reveals altered striatal response in women recovered from bulimia nervosa - a pilot study. [Epub ahead of print] World J Biol Psychiatry. 2014;15(4):307–316. doi: 10.3109/15622975.2012.671958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. 15924905. [DOI] [PubMed] [Google Scholar]
- Rolls E. Reward systems in the brain and nutrition. Ann Rev Nutrition, Epub ahead of print. 2016:27146018. doi: 10.1146/annurev-nutr-071715-050725. [DOI] [PubMed] [Google Scholar]
- Rosen J, Leitenberg H, Fisher C, Khazam C. Binge-eating episodes in bulimia nervosa: The amount and type of food consumed. Int J Eat Disord. 1986;5(2):255–267. [Google Scholar]
- Rosval L, Steiger H, Bruce K, Israel M, Richardson J, Aubut M. Impulsivity in women with eating disorders: problem of response inhibition, planning, or attention? Int J Eat Disord. 2006;39(7):590–593. doi: 10.1002/eat.20296. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36(2):199–211. doi: 10.1016/s0896-6273(02)00969-8. 12383777. [DOI] [PubMed] [Google Scholar]
- Schoenfeld M, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf J, et al. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127(2):347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropspychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 34-57. 9881538. [PubMed] [Google Scholar]
- Small D. Central gustatory processing in humans. Adv Otorhinolaryngol. 2006;63:191–220. doi: 10.1159/000093761. [DOI] [PubMed] [Google Scholar]
- Small D. Taste representation in the human insula. Brain Structure and Function. 2010;214(5–6):551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Small D, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Small D, Zatorre R, Dagher A, Evans A, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(9):1720–1733. doi: 10.1093/brain/124.9.1720. 11522575. [DOI] [PubMed] [Google Scholar]
- Spetter M, Smeets P, de Graaf C, Viergever M. Representation of sweet and salty taste intensity in the brain. Chem Senses. 2010;35(9):831–840. doi: 10.1093/chemse/bjq093. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Sripada C, Gonzalez R, Phan K, Liberzon I. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Hum Brain Mapp. 2011;32(10):1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013;67:322–330. doi: 10.1016/j.neuroimage.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller N, Marti C. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol. 2008;117(4):941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Martinez E, Presnell K, Groesz L. Relation of successful dietary restriction to change in bulimic symptoms: a prospective study of adolescent girls. Health Psychology. 2006;25(3):274–281. doi: 10.1037/0278-6133.25.3.274. 16719598 PMCID: PMC1472292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Groesz L, Shaw H. Effects of a weight maintenance diet on bulimic symptoms in adolescent girls: an experimental test of the dietary restraint theory. Health Psychology. 2005;24(4):402–412. doi: 10.1037/0278-6133.24.4.402. 16045376 PMCID: PMC1196199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel L, Kim J, Weller R, Cox J, Cook E, III, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res Bull. 2009;79(6):388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J, Ohlssen D, Andrew C, Johnson G, Williams SGM, Chen C, et al. Components of variance in a multicentre functional MRI study and implications for calculation of statistical power. Hum Brain Mapp. 2008;29(10):1111–1122. doi: 10.1002/hbm.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kroemer N, Veldhuizen M, Babbs A, de Araujo I, Gitelman D, et al. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 2015;35(20):7964–7976. doi: 10.1523/JNEUROSCI.3884-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. 10200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch CAWS. The effects of short-term food deprivation on caloric intake in eating-disordered subject. Appetite. 1996;26(221–234):8800479. doi: 10.1006/appe.1996.0017. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizeinstein H, Venkatraman V, Bischoff-Grethe A, Fudge J, May J, et al. Altered striatal response to reward in bulimia nervosa after recovery. Int J Eat Disord. 2010;43(4):289–294. doi: 10.1002/eat.20699. 19434606 PMCID:PMC4286149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Frank GK, Figurski J, May JC, Putnam K, et al. Altered insula response to a taste stimulus in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33(3):513–523. doi: 10.1038/sj.npp.1301443. 17487228. [DOI] [PubMed] [Google Scholar]
- Wagner A, Barbarich N, Frank G, Bailer U, Weissfeld L, Henry S, et al. Personality traits after recovery from eating disorders: Do subtypes differ? Int J Eat Disord. 2006;39(4):276–284. doi: 10.1002/eat.20251. 16528697. [DOI] [PubMed] [Google Scholar]
- Williams G, Rolls E, Leonard C, Stern C. Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55(2):243–252. doi: 10.1016/0166-4328(93)90120-f. 8395182. [DOI] [PubMed] [Google Scholar]
- Wilson G, Grilo C, Vitousek K. Psychological treatment of eating disorders. Am Psychologist. 2007;62(3):199–216. doi: 10.1037/0003-066X.62.3.199. 17469898. [DOI] [PubMed] [Google Scholar]
- Wittman M, Movero K, Lane S, Paulus M. Now or later? Striatum and insula activation to immediate versus delayed rewards. J Neurosci, Psycology & Econ. 2010;3(1):15. doi: 10.1037/a0017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Leland D, Paulus M. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Zald D, Hagen M, Pardo J. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 2002;87:1068–1075. doi: 10.1152/jn.00358.2001. 11826070. [DOI] [PubMed] [Google Scholar]
- Zunker C, Peterson C, Crosby R, Cao L, Engel S, Mitchell J, et al. Ecological momentary assessment of bulimia nervosa: Does dietary restriction predict binge eating? Behav Brain Res & Therapy. 2011;49(10):714–717. doi: 10.1016/j.brat.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]