Abstract
Current T cell engineering approaches redirect patient T cells to tumors by transducing antigen–specific T cell receptors (TCRs) or chimeric antigen receptors (CARs) that target a single antigen.1–3 However, few tumor-specific antigens have been identified, and healthy tissues that express the targeted antigen may undergo T cell–mediated damage.4–7 Here we present a strategy to render T cells specific for a tumor in the absence of a truly tumor-restricted antigen. T cells are transduced with both a CAR that provides suboptimal activation upon binding of one antigen and a chimeric costimulatory receptor (CCR) that recognizes a second antigen. Using the prostate tumor antigens PSMA and PSCA, we show that co-transduced T cells destroy tumors that express both antigens but do not affect tumors expressing either antigen alone. This “tumor-sensing” strategy may help broaden the applicability and avoid some of the side effects of targeted T cell therapies.
Adoptive cell therapies utilizing genetically modified autologous T cells have shown efficacy for melanoma and indolent B cell malignancies.4–7 However, their broad applicability is limited by the paucity of truly tumor-specific target antigens. Extra-tumoral antigen expression may indeed result in “on-target, off-tumor” effects. These effects can be acceptable, as is the case with CD19, an antigen expressed in B cell malignancies and normal B lineage cells, resulting in B cell aplasia.5–7 In other instances, targeting for example carbonic anhydrase IX (CAIX) or human epidermal growth factor receptor 2 (HER2), these side effect may be intolerable and potentially life-threatening.8, 9
Here we present an approach to render engineered T cells specific for a tumor even in the absence of a truly tumor-restricted antigen. This approach integrates combinatorial antigen recognition, split signaling and, critically, balanced strength of T cell activation and costimulation. T cell activation requires TCR or CAR-mediated recognition of one antigen, here CD19 or prostate stem cell antigen (PSCA). T cell costimulation must be independently mediated by a CCR10 specific for a second antigen, here prostate-specific membrane antigen (PSMA). This dual-targeting approach facilitates augmented T cell reactivity against double-positive (DP) tumors compared to single-antigen positive (SP) tumors.10–12 However, this approach alone fails to prevent T cell reactivity to SP tumors, as we show here. To achieve tumor selectivity, we diminished the efficiency of T cell activation to a level where it is ineffective in the absence of simultaneous CCR recognition of the second antigen. We hypothesized and demonstrate below, that T cells expressing suboptimal activation receptors are functionally rescued at the tumor site by a CCR engaging a co-expressed tumor antigen.
To demonstrate that both T cell activation and costimulation signals can be supplied in vivo using two distinct antigen-specific receptors, we initially evaluated the combination of a CAR that provides a CD3ζ-mediated activation signal upon recognition of the B cell marker CD19 (19z1)13 and a CCR specific for PSMA.10, 14 Based on results showing synergy between CD28 and 4-1BB costimulation15, 16, including through their cytoplasmic domains arranged in tandem17–20, we added the 4-1BB cytoplasmic domain to the PSMA-specific CCR P2814 as described20 to generate P28BB (Fig. S1a). Primary human peripheral blood T cells were retrovirally transduced with 19z1 and/or P28BB, typically yielding expression of both receptors in 45–70% of T cells (Fig S1b). Four groups of T cells were analyzed in all subsequent studies, expressing 19z1, P28BB, 19z1+P28BB, or neither (mock).
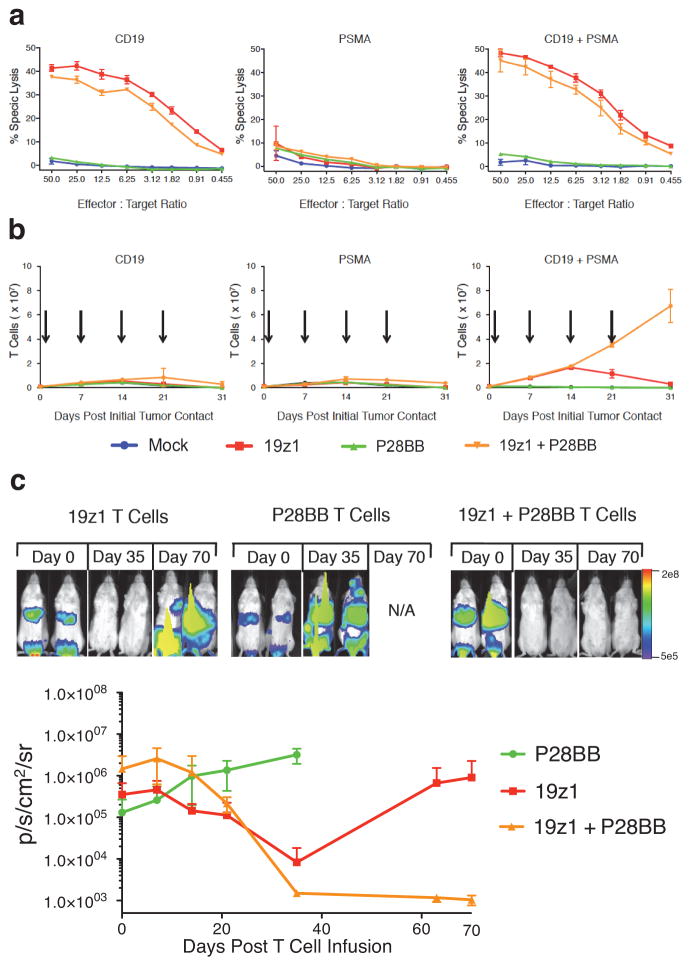
We first measured the in vitro cytotoxic and proliferative responses of transduced T cells exposed to EL4 target cells expressing CD19 and/or PSMA. Cytotoxicity against CD19-expressing target cells was, as expected, imparted by 19z1 expression and was unaltered in the presence of PSMA in all T cell groups (Fig. 1a). A quantitative comparison after normalizing to the fraction of 19z1-transduced T cells for the 19z1 and 19z1+P28BB groups and the P28BB-transduced fraction for the P28BB group showed that 19z1 and 19z1+P28BB T cells specifically lysed 40–47% CD19-expressing targets at the 50:1 E:T ratio while the P28BB-transduced T cells failed to lyse PSMA-expressing targets (Fig 1a). We next examined proliferation of T cells repeatedly exposed to artificial antigen-presenting cells (AAPCs) expressing CD19 and/or PSMA in the absence of exogenous cytokine. Over 4 weeks, only the 19z1+P28BB T cells underwent robust proliferation (58-fold expansion) when co-cultured on AAPCs expressing both antigens. In contrast, 19z1 or P28BB T cells underwent modest expansion over the first 14 days, as did the 19z1+P28BB T cells exposed to CD19+PSMA− AAPCs (Fig. 1b). Further evidence of stronger T cell activation in the presence of both antigens was provided by quantitative assessment of cytokine production and the induction of the anti-apoptotic molecule BclXL, which were maximal in 19z1+P28BB T cells (Fig. S2a,e).
Figure 1. Dual chimeric receptor-mediated activation and costimulation of human T cells facilitates robust cytotoxicity, proliferation, and tumor eradication.
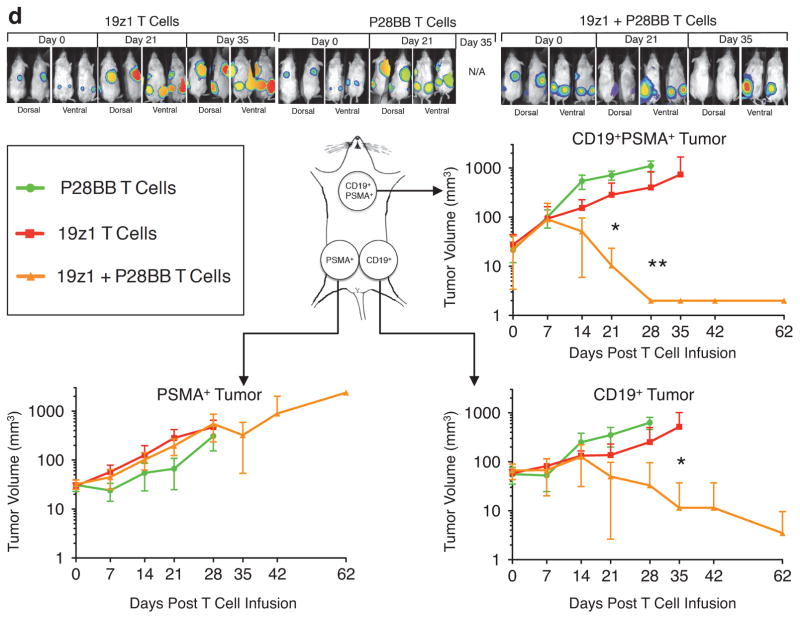
(a,b) T cells were mock transduced or were transduced with retroviruses encoding a CD19-specific CAR (19z1) and/or a PSMA-specific CCR (P28BB). (a) T cells were incubated at indicated effector:target ratios with 51Cr-loaded target cells expressing CD19 and/or PSMA, and target cell lysis (chromium release) was measured. Plots represent at least 4 experiments, with error bars representing standard deviation of the mean of 3 replicates. (b) T cells were co-cultured with PC3 tumor cell lines expressing CD19 and/or PSMA (arrows indicate re-stimulation of T cells using freshly irradiated tumor cells). T cell numbers were measured at indicated time intervals. Plots represent at least 4 experiments with error bars representing standard deviation of the mean of 3 replicates. (c) T cells (1.0 × 106) described in (a,b) were injected intravenously into NSG mice bearing green fluorescent protein/firefly-luciferase fusion protein (GFP/Luc) expressing CD19+PSMA+ PC3 human prostate tumors. Tumor burden was measured weekly by bioilluminescent imaging. Top, images of two representative mice from each group at each time point, with the pixel intensity represented in color. Bottom, average tumor burden as quantified by luminescence of the tumors using units of photons per second per square centimeter per steradian (p/sec/cm2/sr); error bars represent standard deviation from the mean. Values were generated from 6 mice per group. (d) 1 × 106 CD19+PSMA−, 1 × 106 CD19−PSMA+ and 1 × 106 CD19+PSMA+ PC3 cells were injected subcutaneously into the left flank, right flank and back, respectively, of NSG mice. T cells expressing 19z1 and/or P28BB were infused intravenously 7 days later. Top, representative images of 2 mice per time point per group as analyzed by bioluminescent imaging. Graphs, tumors were quantitatively measured using calipers and tumor volumes were plotted versus time for each tumor. Error bars represent standard deviation from the mean of 6 mice. Statistical significance was determined using two-tailed unpaired t tests to compare values obtained from 19z1 T cells and 19z1 + P28BB T cells and p values are represented as * for <0.05 or ** for <0.01.
We then tested the ability of these dual-receptor expressing T cells to eradicate established systemic human prostate tumors in immunocompromised NOD.SCID Il2rg−/−(NSG) mice. We intravenously infused 2.0 × 106 green fluorescent protein/firefly-luciferase (GFP/Luc) expressing PC3 tumor cells that expressed both CD19 and PSMA (Fig. S3) followed, 19 days thereafter, by a single intravenous infusion of 1.0 × 106 19z1, 19z1+P28BB, P28BB or mock transduced T cells. Thirty-five days later, mice that received P28BB T cells or mock T cells had to be sacrificed due to tumor burden. Mice treated with 19z1 T cells showed a marked reduction of tumor burden (Fig. 1c and data not shown). However, mice treated with 19z1+P28BB T cells had undetectable tumor burden. Over 70 days of post-infusion monitoring, the CD19+ tumors eventually relapsed in mice that received 19z1 T cells, while complete tumor remission persisted in all mice that received 19z1+P28BB T cell, suggesting that complete tumor eradication had been achieved.
To investigate T cell activity in a host bearing DP as well as SP tissues, we established subcutaneous CD19+PSMA− tumors into the left flanks of NSG mice, CD19−PSMA+ tumors in the right flank, and CD19+PSMA+ tumors in the back of the same mice. One week later, we administered 1.0 × 106 19z1, P28BB, or 19z1+P28BB T cells intravenously. All three tumors progressed in mice that received P28BB T cells, which had to be sacrificed within 35 days (Fig. 1d). In mice treated with 19z1 T cells, the CD19+PSMA− and CD19+PSMA+ tumors initially underwent substantial reduction in volume but eventually progressed. Mice treated with 19z1+P28BB T cells showed complete eradication of CD19+PSMA+ tumors. However, CD19+PSMA− tumors were also rejected in these mice more efficiently than in single tumor bearing mice (Fig. 1d). Thus, this combinatorial antigen approach failed to restrict T cell reactivity to dual expressing tumors.
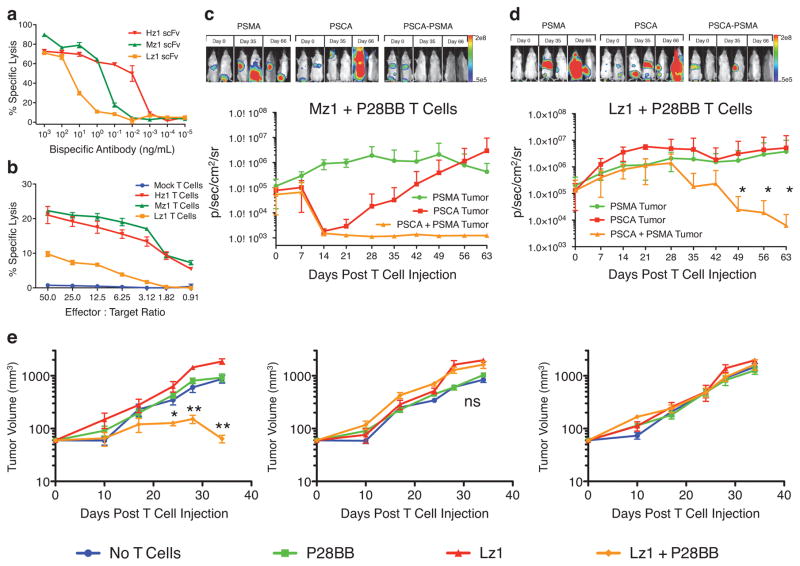
To remedy this failure, we reasoned that T cell activation would have to be minimized, almost to the point of extinction, only to be rescued by simultaneous engagement of the CCR. We thus searched for CARs with diminished activity, and while doing so switched from CD19 and PSMA to a combination of prostetic antigens, PSCA and PSMA. We evaluated three PSCA-specific scFvs with different binding affinities for PSCA. We incorporated each PSCA-specific scFv into bispecific PSCA/CD3 antibodies and incubated them with T cells and PSCA-expressing PC3 tumor cells to quantify tumor cell lysis (Fig. 2a). Picogram quantities of Hz1-containing bispecific antibody lysed tumor cells 1,000–10,000 fold more efficienty than Lz1-containing bispecific antibody. We then used these scFvs to construct CARs by linking them to CD3ζ cytoplasmic domains. As expected, T cells expressing the different CARs showed different activities in cytotoxicity assays (Fig. 2b). Hz1 and Mz1 CARs directed moderate lysis of PSCA+ targets (20% specific lysis at the 50:1 E:T ratio), while the Lz1 CAR only reached 10% specific lysis, qualifying it as a suboptimal antigen receptor. This hierarchy was further confirmed in cytokine release assays using T cells expressing each PSCA CAR together with the P28BB CCR (Supplementary Figure 2b–d). T cells transduced with 19z1 and Hz1 CARs produced relatively high amounts of T helper type 1 (TH1) and TH2 cytokines, whereas T cells expressing less efficient CARs such as Mz1 and Lz1 produced lower amounts of cytokines. The enhancement of cytokine levels in Lz1+P28BB T cells compared to Lz1 T cells was minimal except for IL-2 and IL-13. IL-2 induces proliferation and can promote either a TH1 or TH2 response21, while IL-13 is associated with a TH2 response specific to 4-1BB signaling22.
Figure 2. Reducing CAR mediated activation signals restricts T cell activity to tumors expressing both antigens upon combinatorial antigen recognition.
(a) Three different single chain variable fragments (scFvs) specific for PSCA were constructed and assembled into bispecific antibodies in which the other scFv binds to CD3. The bispecific antibodies were added in the indicated amounts to cocultures of T cells and 51Cr-labeled PSCA+ PC3 tumor cells (ratio of 20:1). Specific lysis was measured by chromium release. Graph is a representative of n > 4 experiments. (b) The anti-PSCA scFvs described in (a) were used to generate CARs. T cells were mock transduced or were transduced with retroviruses encoding these CARs, and were indubated with 51Cr-labeled PSCA+ PC3 tumor cells at indicated effector:target ratios. Specific lysis was measured by chromium release. Graph is a representative of n > 3 experiments. (c,d) Green fluorescent protein/firefly-luciferase fusion protein (GFP/Luc) expressing PC3 tumor cells expressing PSMA and/or PSCA were injected intravenously into the tail-vein of NSG mice. Fourteen days later, 1.0 × 106 Mz1 +P28BB (c) or Lz1 + P28BB (d) T cells were infused intravenously. Top, bioluminescent imaging of two representative mice from each group at each time point. Graphs, the average tumor burden was quantified by luminescence of the tumors using units of photons per second per square centimeter per steradian (p/sec/cm2/sr) and plotted with error bars representing standard deviation from the mean of values from 5 mice per group. Two mice that received PSMA+ tumors (green line) died after day 49 and therefore the mean value for luminescence was averaged from 3 values for days 56 and 63. (e) 0.5 × 106 CD19+PSMA−, 0.5 × 106 CD19−PSMA+ and 0.5 × 106 CD19+PSMA+ PC3 cells were injected subcutaneously into the left flank, right flank and back, respectively, of NSG mice. Where indicated 1.0 × 106 CAR+ T cells transduced with Lz1 and/or P28BB were infused intravenously 7 days later. Tumor volume was measured at indicated time points and plotted with error bars representing standard deviation from the mean of values from 5 mice per each group except for 10 mice for Lz1 + P28BB group). Statistical significance was determined using two-tailed unpaired t tests to compare values obtained from Lz1 T cells and Lz1 + P28BB T cells and p values are represented as * for <0.05 or ** for <0.01.
We next tested the anti-tumor activity of these T cells in animals bearing tumors expressing PSCA and/or PSMA. We inoculated mice intravenously with 2 × 106 GFP/Luc expressing PC3 cells expressing PSMA, PSCA, or both antigens (Fig. S3). Fourteen days later, we intravenously injected 1 × 106 Mz1+P28BB T or 1 × 106 Lz1+P28BB T cells into the mice. PSCA+PSMA− tumors decreased in size in recipients of Mz1+P28BB T cells but not in recipients of Lz1+P28BB T cells, confirming the inefficiency of the Lz1 CAR (Fig. 2c). Similar to the CD19 CAR experiment (Fig 1c), tumors responding to Mz1 eventually relapsed and increased in size. In mice bearing PSCA+PSMA+ tumor cells, however, Mz1+P28BB T cells induced robust and long-term tumor eradication. Most importantly, PSCA+PSMA+ tumor was eradicated in mice treated with Lz1+P28BB T cells, resulting in complete long-term survival of all treated mice (Fig. 2c). Tumor eradication was not induced in control mice bearing PSCA−PSMA+ tumors (Fig 2c).
To thoroughly evaluate the potential of Lz1+P28BB T cells on PSCA+PSMA− tumors in animals where these T cells could be costimulated, we analyzed the effect of Lz1+P28BB T cell infusion on PSCA+PSMA− tumors in animals also bearing PSCA+PSMA+ and PSCA−PSMA+ tumors. Strikingly, Lz1+P28BB T cells eradicated PSCA+PSMA+ tumors but not PSCA+PSMA− tumors. These results demonstrate the feasibility of decreasing T cell activation to the extent where dual CAR + CCR expressing T cells fail to react against tissues expressing either the CAR-targeted antigen or the CCR-targeted antigen and promoting T cell activation only on encounter with the two coexpressed antigens. These data also provide proof-of-principle for achieving two complementary outcomes that determine specificity and safety of T cell tumor therapy: first is the ability to harness combinatorial antigen recognition to design T cells specific for a tumor in the absence of a truly tumor-specific target antigen; second is the ability to protect cells that express only one of the targeted antigens by titrating activation and costimulatory signals so as to confine T cell activation to sites of target antigen coexpression.
This titration of CAR signals distinguishes our approach from studies that showed that two coexpressed antigen receptors boost T cell responsiveness against dual-antigen expressing cells beyond that induced by a single antigen receptor10–12 and addresses the central problem of abolishing or reducing T cell reactivity against single antigen-expressing tissues. Our approach ultimately parallels physiological antigen presentation and T cell priming23 wherein T cells are primed in lymph nodes where they receive TCR and costimulatory signals that are restricted to specialized antigen-presenting cells. T cells then migrate to peripheral sites where their activation does not depend on additional costimulation. Similarly, T cells stimulated through both a CAR and a CCR at one site may also recirculate to other peripheral sites and therein eliminate tumor cells without costimulutaory dependence. However, we showed that if these sites express either the CAR target antigen or the CCR target antigen, tumor-sensing T cells exhibiting balanced signaling will not eradicate the SP tumor, which we utilized here as a surrogate for normal SP tissue. Studies in a murine syngeneic model will be conducted to ascertain the tumor-sensing concept in a fully immune competent model. However, these studies would have the caveats of using a mouse system to develop a human therapy that may not share the same biological and technical aspects. Notably, as CARs and CCRs recognize cell surface antigens rather than HLA-peptide complexes, T cells engineered in this manner will recognize these antigens directly on the tumor but will not be costimulated by interacting with professional antigen-presenting cells that cross-present the targeted antigens in the context of HLA molecules. They also will not be activated by healthy tissues that may express a targeted antigen but do not express costimulatory ligands.
PSCA and PSMA are promising targets for the treatment of metastatic prostate cancer24, although neither antigen is absolutely prostate-specific. PSCA is expressed in prostate tumors, but also in the renal pelvis, ureter, urinary bladder, and urethra25. PSMA is highly expressed in metastatic prostate cancer, as well as in astrocytes type II, the kidney proximal tubule and the intestinal brush border.26 Combinatorial PSCA/PSMA targeting is thus expected to increase prostate cancer targeting and reduce reactivity against healthy tissues expressing either antigen alone.
The tumor-sensing approach can be extended to other tumor types for which a combination of antigens may confer enhanced tumor-specificity. For example, one may target breast cancer through combinatorial recognition of HER2, MUC1, CD44, CD49f, and/or EpCAM, none of which is truly cancer-specific27, 28. Likewise, one may selectively target ovarian cancer through a combination of mesothelin, folate receptor-α, CD44, and/or CD133.29, 30 When determining candidate sets of antigens for targeting a particular tumor type, an antigen should be selected based on higher cell expression in cancerous tissue, but not normal tissue or lymphocytes. A second antigen should be selected so that expression of both antigens is restricted to cancerous tissue. The antigen whose expression in normal tissue would have more detrimental off-target effects should be targeted using a CCR to minimize lysis of SP cells. For example, HER2, MUC1, and EpCAM are not highly expressed by normal tissues and coexpression of a pair should be limited to cancerous tissue. However since HER2 is lowly expressed by lung epithelial cells, targeting HER2+MUC1 would be attractive by directing the CAR to MUC1 and the CCR to HER2. As many breast cancers are HER2 negative, MUC1+EpCAM may represent an attractive alternative. The targeting of tumor initiating cells or cancer stem cells by tumor-sensing T cells represents another enticing application. Altogether, our proof-of-principle provides a path for restricting the selectivity and activity of engineered T cells in a manner that reconciles potency with safety.
Online Methods
Gammaretroviral vector construction and viral production
The gammaretroviral vector SFG-19z1 has been extensively described.13 This backbone construct was used to exchange scFvs to generate SFG-Hz1, SFG-Mz1, and SFG-Lz1 by directional cloning utilizing a NcoI site located 5′ of the scFv and a NotI site located 3′ of the scFv. To construct SFG-P28BB, the fused CD28 and 4-1BB domains were PCR amplified from a third-generation CAR vector (i.e. supplies two costimulatory signals and an activation signal upon scFv binding), SFG-P28BBz1 and ligated 3′ of the PSMA scFv using a 5′ NcoI site and a 3′ BamHI site to include a stop codon 3′ of the BB domain, while the CD3ζ domain was removed.20 We inserted an internal ribosomal entry site (IRES) to facilitate bicistronic expression of CARs and CCRs with dsRED and hrGFP, respectively. Vectors were used to transiently transfect cell lines to generate stable viral producing lines as previously described.31
Generation of anti-PSCA scFvs
Three PSCA specific scFvs, termed Hz1, Mz1, and Lz1 were generated by amplifying the variable heavy (VH) and variable light (VL) domains from hybridomas using degenerate primers as previously described32–34. These VH and VL domains were fused together using a glycine-serine linker and were used to replace the CD19 scFv in the SFG-19z1 backbone using 5′SphI to 3′NotI sites.
Isolation, retroviral transduction, and culture of primary human T cells
Peripheral blood leukocytes were isolated using Ficoll gradients and transduced as previously described.31 Briefly, after 48 hours of activation with 2 μg/mL phytohemagglutinin, cells were transduced twice via spinoculation for 1 hour on retronectin-coated plates over the next 48 hours and 20 U/mL of IL-2 was added. After allowing 3 days for vector expression, transduction efficiencies were determined via flow cytometry and bulk unsorted cells were used for various assays or adoptive transfers.
Generation of antigen expressing tumor cell lines
The PC3 human prostate tumor line was obtained from ATCC and retrovirally transduced to express the Green Fluorescent Protein/Firefly luciferase fusion protein (GFP/Luc) for future fluorescent/luminescent quantification to generate PC3-GFP/Luc.16 This line was subsequently transduced to create PC3-CD19, PC3-PSMA, PC3-CD19-PSMA, PC3-PSCA, and PC3-PSCA-PSMA via multiple retroviral transductions.
Cytotoxicity assays
Target cells expressing desired antigen were labeled with 51Cr and co-cultured with T cells at decreasing effector:target ratios. After 4 hours of culture, supernatant was removed and radioactivity released from chromium was measured. Specific lysis was determined by subtracting background radioactivity of target cells not cultured with T cells and dividing by the radioactivity measured from target cells completely lysed by treatment with 0.2% Triton X-100.
T cell proliferation assays
Tumor cells expressing desired antigen were irradiated with 30 Gy prior to co-culture with 1.0 × 106 T cells at a 5:1 effector:target ratio. T cells were counted weekly using an Invitrogen Countess cell counter and then re-stimulated with irradiated tumor cells. No exogenous cytokines were added to these co-cultures.
Mouse tumor models
NOD.SCID Il2rg−/− (NSG) mice were obtained from either Jackson Laboratories or from in-house breeding under the protocol 04-10-024 approved by the MSKCC Institutional Animal Care and Use Committee. For systemic tumor experiments, 2.0 × 106 tumor cells were infused intravenously via the tail-vein into mice, and 1.0 × 106 T cells were infused 14 days later. For subcutaneous tumor experiments, 1.0 × 106 tumor cells were injected subcutaneously per tumor site, and 7 days later 1.0 × 106 T cells were infused intravenously.
Quantification of tumor burden
For systemic tumor experiments, bioilluminescent imaging was used to quantitatively measure tumor burden by correlating the amount of tumor burden to luminescence using an IVIS 100 system (Caliper Life Sciences) as previously described.31 For subcutaneous tumors, calipers were used to measure tumor size. Tumor volume was calculated by multiplying the length, width, and height of each tumor.
Bispecific antibody-mediated tumor lysis
The VH and VL domains of an anti-PSCA antibody were PCR amplified and cloned in tandem with the VH and VL domains of an anti-CD3 antibody to create single chain bispecific Tandem Fragment variables (scBsTaFvs) binding to PSCA and CD3. Bispecific antibodies were added at various amounts to untransduced T cells co-cultured with 51Cr-labeled PSCA+ PC3 cells at a 20:1 ratio. Four hours later, chromium release was measured as described above.
Flow cytometry
Cells were analyzed using an LSRII flow cytometer or sorted using a FACSAria cell sorter (BD Biosciences) as previously described16. Detection of chimeric receptor at the cell surface could be achieved directly by using AF647 conjugated goat-anti-mouse antibody (Invitrogen). CD4-PE-Cy7, CD8-Pacific Blue, and CD19-APC antibodies were obtained from Invitrogen while PSCA antibodies were purified from hybridoma supernatants and PSMA antibodies were obtained from MBL International.
Cytokine analysis
Supernatants were harvested 48 hours after the second tumor stimulation (in T cell proliferation experiments). Cytokines were measured using a custom multiplex system HCYTMAG-60K (Millipore) and analyzed using a Luminex 100 instrument (Luminex) as previously described20.
Western blot analysis
Cells were harvested 24 hours after initial tumor stimulation (in T cell proliferation experiments). Western blots were performed as previously described20 using BclXL (Clone 54H6, #2764S) and Akt (Clone C67E7, #4691S) primary antibodies from Cell Signaling Technology with the PhosphoPlus(R) Akt (S473) Ab Kit (#9270S).
Supplementary Material
(a) CARs were generated by using first generation CAR construction (i.e. chimeric receptors that supplies solely an activation signal upon scFv binding) that fuses heavy and light chains of immunoglobulin variable domains to the CD8 transmembrane domain; this is fused to cytosolic signaling domains of CD3ζ. By using an Internal Ribosomal Entry Site (IRES) to enable bicistronic expression, CAR expression can be easily detected by correlation with dsRED fluorescence (data not shown). The CCR was generated by fusing an scFv to extracellular, transmembrane and signaling domains of CD2814; this was fused to a 4-1BB cytosolic signaling domain.20 By again using an IRES, CCR expression can be correlated with expression of hrGFP (data not shown). Abbreviations: LTR – Long Terminal Repeat, SD – Splice Donor site, SA – Splice Acceptor site, VH or VL – Variable Heavy or Light domains, respectively, EC – Extracellular domain, TM – Transmembrane domain, C – Cytosolic domain, IRES – Internal Ribosomal Entry Site, dsRED - Discosoma sp. Red fluorescent protein, hrGFP – Human Recombinant Green Fluorescent Protein (b) Representative transduction efficiencies of primary human T cells using these retroviral vectors (or mock transduction).
(a) Untransduced T cells or T cells transduced with 19z1 and/or P28BB were stimulated with either untransduced PC3 cells (Empty) or CD19+PSMA+ PC3 cells. Cytokine expression was analyzed using Luminex technology. Error bars represent standard deviation from the mean of 2 biological replicates. (b-d) Untransduced T cells or T cells transduced with Hz1 (b), Mz1(c), and Lz1(d) anti-PSCA CARs, and/or P28BB CCRs were stimulated with empty or PSCA+PSMA+ PC3 cells. Cytokine expression was analyzed using Luminex technology. Error bars represent standard deviation from the mean of 2 biological replicates. (e) Western blot analysis for BclXL expression in cellular lysates of untransduced T cells or T cells transduced with 19z1 and/or P28BB, after T cells were stimulated with CD19+PSMA+ target cells for 24 hours. Total amount of Akt was used as a loading control.
Untransduced PC3 cells (Empty) were transduced first with retrovirus encoding GFP/Luc and subsequently transduced with retroviruses encoding CD19, PSMA, PSCA, or a combination of two. Cells were purified by double sorting the cells with a BD FACSAria using high purity sort collection settings (low flow rate and high cell exclusion rate) for GFP/Luc, CD19, PSMA, and/or PSCA.
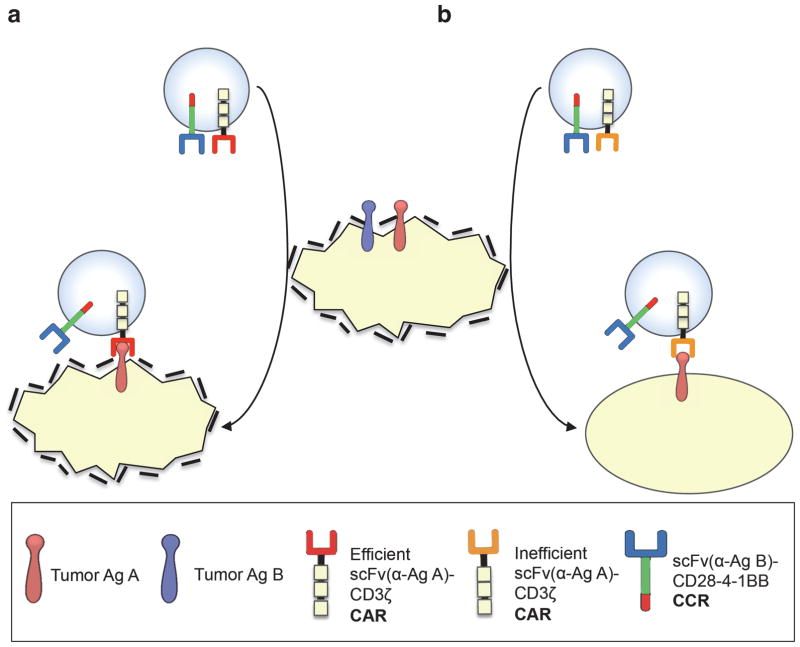
Figure 3. Concept of combinatorial antigen recognition and balanced signaling for selective tumor eradication.
(a) T cells expressing an efficient CAR (specific for antigen A) and a CCR (specific for antigen B), become potently activated and costimulated by A+B+ target cells, which are eliminated. If these T cells recirculate (indicated by arrows) and encounter A+B− target cells, they can eliminate these as well without requiring further costimulation, resulting in an “on-target off-tumor” effect. (b) In the tumor-sensing approach, T cells expressing a CCR and an inefficient activation receptor (e.g. low affinity TCR or suboptimal CAR) are sufficiently activated by A+B+ but not A+B− target cells.
Acknowledgments
We thank Isabelle Riviere for reviewing the manuscript and Jason Plotkin and Gertrude Gunset for outstanding technical assistance. Our work is supported by DOD PCTA PC101964 (CCK) and the Mr. and Ms. Goodwyn Commonwealth Center, the Major Family Foundation and Lewis Sanders (MS).
Footnotes
Explanation of author contributions:
C.C.K designed and performed experiments, analyzed data, and wrote the manuscript. M. Condomines contributed reagents, performed experiments, interpreted results, and reviewed the manuscript. M. Cartellieri contributed reagents and performed experiments. M.B. contributed reagents and designed experiments. M.S. designed experiments, analyzed data, interpreted results, and wrote the paper.
References
- 1.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nature reviews. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 2.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 3.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:e20–22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 9.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause A, et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. The Journal of experimental medicine. 1998;188:619–626. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong CP, Westwood JA, Berry LJ, Darcy PK, Kershaw MH. Enhancing the specificity of T-cell cultures for adoptive immunotherapy of cancer. Immunotherapy. 2011;3:33–48. doi: 10.2217/imt.10.81. [DOI] [PubMed] [Google Scholar]
- 12.Wilkie S, et al. Dual Targeting of ErbB2 and MUC1 in Breast Cancer Using Chimeric Antigen Receptors Engineered to Provide Complementary Signaling. Journal of clinical immunology. 2012 doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 13.Brentjens RJ, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 14.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 15.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 16.Stephan MT, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 17.Carpenito C, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tammana S, et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Human gene therapy. 2010;21:75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Human gene therapy. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 20.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin SM, et al. 4-1BB triggers IL-13 production from T cells to limit the polarized, Th1-mediated inflammation. Journal of leukocyte biology. 2007;81:1455–1465. doi: 10.1189/jlb.1006619. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RH. T cell anergy. Annual review of immunology. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 24.Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3533–3538. doi: 10.1158/1078-0432.CCR-09-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam JS, et al. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2591–2596. doi: 10.1158/1078-0432.CCR-04-1842. [DOI] [PubMed] [Google Scholar]
- 26.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clinical cancer research : an official journal of the American Association for Cancer Research. 1997;3:81–85. [PubMed] [Google Scholar]
- 27.Liu JC, et al. Seventeen-gene signature from enriched Her2/Neu mammary tumor-initiating cells predicts clinical outcome for human HER2+:ERalpha-breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5832–5837. doi: 10.1073/pnas.1201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer MJ, et al. CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer research. 2010;70:4624–4633. doi: 10.1158/0008-5472.CAN-09-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih Ie M, Davidson B. Pathogenesis of ovarian cancer: clues from selected overexpressed genes. Future Oncol. 2009;5:1641–1657. doi: 10.2217/fon.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strauss R, et al. Analysis of epithelial and mesenchymal markers in ovarian cancer reveals phenotypic heterogeneity and plasticity. PloS one. 2011;6:e16186. doi: 10.1371/journal.pone.0016186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gade TP, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer research. 2005;65:9080–9088. doi: 10.1158/0008-5472.CAN-05-0436. [DOI] [PubMed] [Google Scholar]
- 32.Feldmann A, et al. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: comparison of different antibody formats. The Prostate. 2011;71:998–1011. doi: 10.1002/pros.21315. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann A, et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J Immunol. 2012;189:3249–3259. doi: 10.4049/jimmunol.1200341. [DOI] [PubMed] [Google Scholar]
- 34.Orlandi R, Gussow DH, Jones PT, Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) CARs were generated by using first generation CAR construction (i.e. chimeric receptors that supplies solely an activation signal upon scFv binding) that fuses heavy and light chains of immunoglobulin variable domains to the CD8 transmembrane domain; this is fused to cytosolic signaling domains of CD3ζ. By using an Internal Ribosomal Entry Site (IRES) to enable bicistronic expression, CAR expression can be easily detected by correlation with dsRED fluorescence (data not shown). The CCR was generated by fusing an scFv to extracellular, transmembrane and signaling domains of CD2814; this was fused to a 4-1BB cytosolic signaling domain.20 By again using an IRES, CCR expression can be correlated with expression of hrGFP (data not shown). Abbreviations: LTR – Long Terminal Repeat, SD – Splice Donor site, SA – Splice Acceptor site, VH or VL – Variable Heavy or Light domains, respectively, EC – Extracellular domain, TM – Transmembrane domain, C – Cytosolic domain, IRES – Internal Ribosomal Entry Site, dsRED - Discosoma sp. Red fluorescent protein, hrGFP – Human Recombinant Green Fluorescent Protein (b) Representative transduction efficiencies of primary human T cells using these retroviral vectors (or mock transduction).
(a) Untransduced T cells or T cells transduced with 19z1 and/or P28BB were stimulated with either untransduced PC3 cells (Empty) or CD19+PSMA+ PC3 cells. Cytokine expression was analyzed using Luminex technology. Error bars represent standard deviation from the mean of 2 biological replicates. (b-d) Untransduced T cells or T cells transduced with Hz1 (b), Mz1(c), and Lz1(d) anti-PSCA CARs, and/or P28BB CCRs were stimulated with empty or PSCA+PSMA+ PC3 cells. Cytokine expression was analyzed using Luminex technology. Error bars represent standard deviation from the mean of 2 biological replicates. (e) Western blot analysis for BclXL expression in cellular lysates of untransduced T cells or T cells transduced with 19z1 and/or P28BB, after T cells were stimulated with CD19+PSMA+ target cells for 24 hours. Total amount of Akt was used as a loading control.
Untransduced PC3 cells (Empty) were transduced first with retrovirus encoding GFP/Luc and subsequently transduced with retroviruses encoding CD19, PSMA, PSCA, or a combination of two. Cells were purified by double sorting the cells with a BD FACSAria using high purity sort collection settings (low flow rate and high cell exclusion rate) for GFP/Luc, CD19, PSMA, and/or PSCA.