Abstract
Genetic influences on disordered eating (DE) increase across age and puberty in girls, an effect that is at least partially due to ovarian hormone activation. However, development shifts in genetic effects have not been detected in boys; genetic influences have been found to be relatively constant from pre-puberty to adulthood, suggesting that gonadal hormones may be less important. One caveat is that studies have examined males ages 10 or older. Genetic effects on DE may emerge earlier in boys, such as during adrenarche, when androgens begin to increase but the physical changes of puberty are not yet observable. The current study investigated this hypothesis in 1,212 male twins (ages 6-28) from the Michigan State University Twin Registry. Results supported a potential role of adrenarche, as genetic influences on DE increased during middle childhood, prior to the external physical changes of puberty. Specifically, genetic influences on DE were negligible (0%) in twins during pre-early adrenarche, but increased incrementally across advancing adrenarche (17% to 44%) and into early puberty (57%). Genetic effects then remained stable into mid-puberty and post-puberty (58%), suggesting that nearly all of the genetic effects on DE become prominent during adrenarche in males. Findings suggest that genetic effects on DE emerge sooner in boys than the mid-pubertal activation that is consistently found in girls. These data highlight a potentially important role for adrenarche in the genetic diathesis for DE in males and a need to examine younger ages in studies of developmental effects.
Keywords: disordered eating, adrenarche, males, eating disorders, puberty
Twin studies have consistently found developmental shifts in etiologic influences on disordered eating in girls, such that genetic effects on disordered eating increase across age and puberty. Specifically, genetic influences on disordered eating are negligible during pre-adolescence (Klump, Burt, McGue, & Iacono, 2007; Klump, McGue, & Iacono, 2000; Silberg & Bulik, 2005) and pre-puberty (Culbert, Burt, McGue, Iacono, & Klump, 2009; Klump, McGue, & Iacono, 2003; Klump, Perkins, Burt, McGue, & Iacono, 2007), as shared and non-shared environmental factors (i.e., environmental influences that make twins similar or dissimilar on disordered eating, respectively) predominate. In contrast, genetic effects account for over 50% of the variance in disordered eating during mid-to-late adolescence and after mid-pubertal onset; the remaining variance is largely accounted for by non-shared environmental influences (Culbert et al., 2009; Klump et al., 2007). Ovarian hormones have been hypothesized to be biological mechanisms that may drive developmental shifts in genetic effects on disordered eating in girls (Klump, Keel, Sisk, & Burt, 2010), as these hormones rise during puberty and are potent regulators (i.e., serving as activators or repressors) of gene transcription within several neurobiological systems (e.g., serotonin, dopamine; Östlund, Keller, & Hurd 2003) associated with eating disorders (for reviews, see Culbert, Racine, Klump, 2015; Klump, 2013). Consistent with this hypothesis, estradiol has been shown to moderate genetic influences on disordered eating. Genetic influences on disordered eating were minimal in adolescent female twins with low levels of estradiol, but substantial genetic influences were found in those with high levels of estradiol (Klump, Keel, Sisk, & Burt, 2010). Together, these findings highlight developmental shifts in genetic influences on disordered eating and begin to point to sex-specific biological factors (i.e., ovarian hormones) that may “activate” genetic vulnerability to disordered eating in girls during puberty.
Conversely, development shifts in genetic effects on disordered eating have not been detected in boys. Only one prior study has examined adolescent boys, but genetic effects accounted for ~50% of the variance in disordered eating, regardless of pubertal stage (Klump et al., 2012). The magnitude of genetic effects on disordered eating symptoms in males during late adolescence and young adulthood (~40-50%; for review, see Culbert et al., 2015) have also been found to be largely similar to those observed in boys during pre-to-early puberty and mid-to-late puberty (Klump et al., 2012). Consistent with post-pubertal females, non-shared environmental effects account for the remaining variance in disordered eating in males (Klump et al., 2012; Culbert et al., 2015). These data are indicative of no developmental differences in etiologic effects on disordered eating in males, and thus, indirectly suggest that pubertal changes in gonadal hormones (e.g., testosterone) may not impact the genetic diathesis of disordered eating in boys.
Nonetheless, one important caveat is that prior studies have only examined males that were age ten or older, yet genetic effects on disordered eating may emerge earlier in boys, such as during adrenarche – the first phase of puberty that begins during middle childhood (Dorn, Dahl, Woodward, & Biro, 2006; Guran et al., 2015). Adrenarche occurs in both boys and girls and is marked by the “awakening” of the adrenal glands and a rise in adrenal androgens, including androstenedione, dehydroepiandrosterone, and dehydroepiandrosterone-sulphate (Dorn et al., 2006; Patton & Viner, 2007; Styne & Grumbach, 2008). These adrenal androgens begin to rise around age 6, they typically hit the concentration threshold for adrenarche onset around age 8, and they steadily increase with advancing age until young adulthood (e.g., ~age 20; Guran et al., 2015; Ilondo et al., 1982; Kelner & Brook, 1983). Adrenarcheal changes precede the activation of gonadarche – a phase marked by dramatic increases in gonadal androgens in boys, predominantly testosterone (Dorn et al., 2006; Styne & Grumbach, 2008; Patton & Viner, 2007), that is traditionally conceptualized as puberty (Dorn et al., 2006; Dorn & Biro, 2011). Notably, children in the early stages of adrenarche are phenotypically categorized as pre-pubertal since hormonal increases occur in the absence of outward signs of physical changes (Dorn et al., 2006).
Given that adrenarche is characterized by increases in androgens, it may be a particularly important developmental stage for males. Androgens predominate in males, and prior research has shown that circulating androgens are inversely associated with psychological difficulties (e.g., mood; Goodyer et al., 1996), including disordered eating in boys (Culbert, Burt, Sisk, Nigg, & Klump, 2014). Moreover, androgens are an additional set of steroid hormones that regulate gene activity by stimulating or inhibiting gene transcription. Thus, in addition to the hypothesized ovarian hormone processes in girls (Klump et al., 2010; Klump et al., 2015), the rise in androgens during adrenarche may exert genomic effects (that could be protective) in boys by altering gene transcription (e.g., inhibiting risk genes; activating protective genes) within neurobiological systems (e.g., serotonin, dopamine; Fink, Sumner, Rosie, Wilson, & McQueen, 1999; Guivarch'h, Vernier, & Vincent, 1995) that are thought to be important for disordered eating. The onset of adrenarche and corresponding endocrine changes could therefore explain why prior research, which had not captured the adrenarcheal transition of hormonal changes, found no developmental shifts in genetic effects on disordered eating in boys. Importantly, if adrenal androgens contribute to changes in genetic effects on disordered eating in boys, we would expect incremental increases in estimates of heritability across advancing adrenarcheal development; increases in the contribution of genetic effects on disordered eating would be indicative of greater genetic similarity (e.g., similar inhibition or activation of gene transcription) in monozygotic versus dizygotic twins.
The current study aimed to investigate this possibility by examining whether the magnitude of genetic influences on disordered eating increases in male twins during adrenarche – before substantial physical changes in secondary sex characteristics are observed. We hypothesized that genetic influences would increase incrementally with advancing adrenarche and persist into early and mid-to-post puberty. Consistent with prior research, we hypothesized that the remaining variance in disordered eating would be largely accounted for by non-shared, rather than shared, environmental effects. The twin models used in this study cannot determine whether the genetic effects contributing to individual variation in disordered eating are risky or protective, but research (e.g., disordered eating risk in males < females; inverse androgen-disordered eating association) suggests that an adrenarcheal emergence of genetic influences on disordered eating may, at least in part, reflect protective genomic effects.
In this study, we did not have access to hormone measures of adrenarche; however, given that adrenarche is marked by predictable age-related increases in adrenal androgens in the absence of the physical signs of puberty, we were able to use age and measures of pubertal development as proxy measures of adrenarcheal development (see Methods). We also included groups of adolescent and young adult male twins in order to comprehensively test for differences in etiologic effects between adrenarche and other developmental stages (i.e., ranging from the onset to completion of puberty/gonadarche) that were examined in prior work (Klump et al., 2012). Similar to previous developmental studies (e.g., Culbert et al., 2009; Klump et al., 2007; Klump et al., 2010), disordered eating was measured continuously in order to increase statistical power and to capture a broad range of disordered eating symptoms that are common in eating disorders.
Methods
Participants
Participants were 1,212 same-sex male twins ages 6 to 28 years from the Michigan State University Twin Registry (MSUTR) (Burt & Klump, 2013; Klump & Burt, 2006). The majority of these MSUTR male twins have never been examined in disordered eating research (n = 838, 69.14% of total sample); however, a proportion of these male twins (n = 374, 30.86%; ages 10-28) were included in our prior report that found no differences in genetic effects on disordered eating during puberty (Klump et al., 2012). The inclusion of these previously examined twins allows us to test for significant developmental differences in etiologic effects across the full spectrum of interest – from adrenarcheal onset to gonadarcheal completion. The current sample primarily overlaps with the previous report in regards to mid-to-post pubertal twins (100% were included in prior report), whereas our key groups of interest (i.e., adrenarche-to-early pubertal twins) were largely comprised of a new sample. Specifically, 100% of twins ages 6-9 and 57% of twins ages 10-12 were newly assessed, resulting in a total of 84.93% new twins in our adrenarcheal and early pubertal status groups.
This study included data from the following MSUTR studies: Twin Study of Behavioral and Emotional Development in Children, Twin Study of Hormones and Disordered Eating across Puberty, or Adult Twin Study of Behavioral Adjustment and Development. The MSUTR is population-based as twins are recruited via birth records in collaboration with the Michigan Department of Health and Human Services (formerly the Michigan Department of Community Health) (see Burt & Klump, 2013); however, a subset of twins in this study were recruited using other methods (e.g., n = 168, 13.86% of total sample; advertisements, flyers, campus registrar). Twins from the MSUTR are demographically representative of the recruitment region (Culbert, Breedlove, Burt, & Klump, 2008). Indeed, in the current study, the majority of MSUTR twins were White (82.93%) and of non-Hispanic ancestry (98.8%), and the remaining twins were Black (8%), Asian/Pacific Islander (1%), Native American/Alaska Native (1%), or biracial/other (7%).
Measures
Zygosity Determination
Zygosity was determined using physical similarity questionnaires that have demonstrated over 95% accuracy (Lykken, Bouchard, McGue, & Tellegen, 1990; Peeters, Van Gestel, Vlietinck, Derom, & Derom, 1998) when compared with genotyping methods (Peeters et al., 1998). Zygosity questionnaires were completed by the twins and a parent (for child and adolescent twins only) as well as trained research assistants. Ratings from each reporter were scored separately, and any score discrepancies were resolved by the MSUTR principal investigators (KLK or SAB) via examination of item endorsements, photographs of the twins, and/or DNA (i.e., twin concordance across several single nucleotide polymorphisms; Burt & Klump, 2013; Klump & Burt, 2006).
Disordered Eating Symptoms
The total score from the Minnesota Eating Behavior Survey (MEBS1; von Ranson, Klump, Iacono, & McGue, 2005) was used to assess disordered eating symptoms. This total score is comprised of true/false items tapping body dissatisfaction (dissatisfaction with one's body size/shape), weight preoccupation (preoccupation with dieting, weight, and the pursuit of thinness), binge eating (thinking about and engaging in binge eating), and compensatory behaviors (using or contemplating the use of inappropriate compensatory behaviors for weight management). Higher scores represent elevated levels of disordered eating symptoms.2 The MEBS total score exhibits good internal consistency (e.g., α's ≥ .84; von Ranson et al., 2005; Culbert et al., 2014) and good convergent validity with other leading measures of disordered eating (e.g., Eating Disorder Examination Questionnaire, r's > .75; Culbert et al., 2013; Culbert et al., 2014; Klump et al., 2012; von Ranson et al., 2005) in pre-to-late adolescent males and females. The MEBS has also been shown to successfully discriminate between girls with and without an eating disorder (von Ranson et al., 2005). Finally, although there are sex and developmental differences in mean levels of disordered eating (i.e., females > males, after pubertal onset), the latent structure of the MEBS did not differ across sex or pubertal status (pre-pubertal vs. post-pubertal) subgroups and was similar to the latent structure observed in the overall sample of MSUTR twins (N = 3,032; ages 9-30; Luo, Donnellan, Burt, & Klump, 2016). Thus, it appears that sex and developmental differences between forms of disordered eating, as assessed via the MEBS, are quantitative, rather than qualitative, in nature (Luo et al., 2016).
The MEBS was developed for use in children (von Ranson et al., 2005), and thus, it has been used in previous developmental twin studies in females (Culbert et al., 2013; Klump et al., 2011; Suisman et al., 2014) and males (Culbert et al., 2014). The total score has been the most commonly used scale from this measure, as it exhibits the strongest psychometric properties, particularly in female and male youth (von Ranson et al., 2005; Culbert et al., 2013; Culbert et al., 2014). However, to our knowledge, the MEBS has not been used in children younger than age 8. Because our study included twins ages 6 and 7, we modified the administration procedures and had research assistants read the items to the young twins to ensure comprehension. For all other ages, research assistants were readily available to answer any questions about item content. Importantly, participant responses were confidential, in that the research assistant read the items across from the twin and did not see their responses.
Descriptive and psychometric data suggest that these modifications were successful (see Table 1). The MEBS total score showed good internal consistency in all groups (α's > .77) and expected correlations with body mass index (see Table 1). Indeed, similar to prior research (e.g., Gardner, Stark, Friedman, & Jackson, 2000), little-to-no BMI-disordered eating associations were observed in younger twins, whereas stronger positive associations were detected with advancing maturation. The MEBS total score was also positively associated with depression/anxiety symptoms3 in all developmental groups (r's = .17-.55, p's < .05), and boys who scored in the highest tertile on the MEBS total score showed elevated levels of depression/anxiety symptoms as compared to boys who scored in the lowest MEBS tertile (d's = .30-.68, across developmental groups). Finally, patterns of intercorrelations between the MEBS total score and MEBS subscale scores were similar across all developmental groups (r's = .41-.86; for additional details, see Footnote 2) and were on par with intercorrelations observed in previous samples of male and female youth and adults (r's = .41-.91; von Ranson et al., 2005).
Table 1.
Descriptive Statistics and Twin Correlations.
| Variables | Developmental Groups (N = 1,212) |
||||
|---|---|---|---|---|---|
| Adrenarche |
Early Puberty |
Mid-to-Post Puberty |
|||
| Ages 6-7 | Ages 8-9 | Ages 10-12 | |||
| Sample Sizes: | |||||
| MZ | 228 | 125 | 81 | 78 | 139 |
| DZ | 168 | 138 | 74 | 90 | 87 |
| Total (% of total sample) | 396 (32.67%) | 263 (21.70%) | 155 (12.79%) | 168 (13.86%) | 226 (18.64%) |
| Age: | |||||
| Range | 6-7 | 8-9 | 10-12 | 6-15 | 11-28 |
| Mean (SD) | 6.70 (0.3458) | 8.61 (0.54) | 10.41 (0.57) | 11.36 (2.17) | 19.40 (3.71) |
| PDS: | |||||
| Range | -- | -- | -- | 1.25-2.25 | 2.50-4.00 |
| Mean (SD) | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | 1.59 (0.34) | 3.75 (0.48) |
| BMI: | |||||
| Range | 7.96-30.62 | 8.51-30.70 | 10.40-31.55 | 13.02-35.28 | 16.15-36.76 |
| Mean (SD) | 16.42 (2.40) | 17.52 (3.43) | 18.16 (3.12) | 19.50 (4.24) | 23.49 (3.85) |
| MEBS Total Score: | |||||
| Range (max score = 30) | 0-22 | 0-18 | 0-16 | 0-18 | 0-19 |
| Mean (SD) | 5.54 (4.12) | 5.67 (4.02) | 4.42 (3.62) | 5.76 (4.63) | 3.48 (3.64) |
| Alphas | .78 | .78 | .77 | .84 | .82 |
| r with BMI | .10* | .36*** | .20** | .42*** | .30*** |
| Twin Correlations: | |||||
| MZ r | .07 | .33*** | .38** | .48*** | .53*** |
| DZ r | .12 | .15 | .18 | .28* | .22† |
| Test of Equality (z) | −0.47 | 1.53† | 1.33† | 1.49† | 2.64** |
Note: MEBS = Minnesota Eating Behaviors Survey; r = correlation; BMI = body mass index. Twin correlations were calculated using the MEBS total score adjusted for BMI (i.e., residual score after regressing out BMI). The “Test of Equality (z)” tests for differences between the MZ and DZ twin correlations, and a one-tailed p-value was used since MZ twin correlations were expected to be higher than DZ twin correlations. Notably, although the 6-7 year old group had the highest range of scores on the MEBS (i.e., score of 22), most (99.5%) of the 6-7 year old twins had scores between 0-17, similar to the other adrenarcheal groups.
p < .10
p<.05
p < .01
p < .001.
Pubertal Status
The Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988) was used to assess maturation of several secondary sex characteristics, and thus, served as an indicator of adrenarcheal (i.e., no secondary sex characteristics) and gonadarcheal (i.e., early, mid, or late/post puberty) status. Height spurts, body hair, skin, and voice changes were rated on a four-point continuous scale ranging from “development has not yet begun” to “development seems completed.” PDS ratings were summed and averaged, with higher scores indicating more advanced maturation. The PDS was completed by the twin (for twins ages 10-16) or a parent (for twins < age 10). Parent and child PDS ratings have been shown to correlate highly in MSUTR male twins (r's = .82-.87 for ages 10-16, including the current sample; Culbert et al., 2013; Culbert et al., 2014), and in the current study, parent reports of pubertal status were on par with normative data for boys ages 6-9 (i.e., pre-puberty = 95.23%, early puberty = 4.77%; see Herman-Giddens, Wang, & Koch, 2001; Patton & Viner, 2007). Previous research has demonstrated good psychometric properties for the PDS in preadolescent and adolescent males (Petersen et al., 1988), including high correlations with clinician ratings (Petersen et al., 1988). Internal consistency on the PDS was also good in the current sample (α = .90).
Twins ages 18 or older did not complete the PDS since nearly all twins (>99%) in this age range would be post-pubertal (Herman-Giddens et al., 2001, Lee, Guo, & Kulin, 2011). Thus, similar to prior studies (Suisman et al., 2014), these twins were assigned a maximum PDS score of 4 for statistical analyses.
Body Mass Index (BMI)
BMI (weight (in kilograms)/height (in meters)2) was calculated as a marker of adiposity and was adjusted, using BMI-for-age, for participants between the ages of 6 to 16 years old. Height and weight were measured using a digital scale and wall mounted ruler, respectively.
Statistical Analyses
Data Preparation
Missing data were minimal. MEBS total scores were prorated for 7 twins (0.58%) who were missing ratings for ≤10% of the items. Data were coded as missing for 12 twins (0.10%) who were missing ratings for ≥10% of the MEBS items. An additional subset of twins were missing PDS scores (n = 4; 0.33%) or BMI (n = 7; 0.58%) data. This study used the raw data option of the Mx statistical program (Neale, 1995), which treats missing data as missing-at-random and allows for the inclusion of all twin pairs even if one twin is missing data (Little & Rubin, 1987). Given the expected associations between disordered eating and BMI (see Table 1), BMI was regressed out of each twin's disordered eating score and the standardized residual disordered eating score was used in analyses to ensure that BMI could not account for any of the observed effects.
As noted previously (see Introduction), different adrenarche and puberty groups were created for analyses using age and pubertal status data. Boys were considered to be in adrenarche if they were in middle childhood or pre-adolescence (ages 6-12) and were of pre-pubertal status (i.e., no secondary sex characteristics; PDS score = 1). Rather than grouping all adrenarcheal boys together, these adrenarcheal twins were split into three age groups (i.e., ages 6-7 vs. 8-9 vs. 10-12) in order to capture the progression of adrenarcheal development and age-related increases in adrenal androgens (Guran et al., 2015). Indeed, extant data indicate substantial increases (of large effect sizes) in adrenal androgen concentrations across middle childhood; mean adrenal hormone levels are relatively similar from ages 4-5 versus age 6-7 (d = .11) and then substantially increase from ages 6-7 to ages 8-9 (d = 1.29), ages 9-11, (d = 1.57), ages 11-13 (d = 1.54), and ages 13-18 (d = 3.40) (see Guran et al., 2015). Moreover, by age 8, the vast majority of boys exhibit adrenal androgen levels at or above the “adrenarcheal threshold” (e.g., DHEA-S levels >108.4 nmol/l; Guran et al., 2015; Peretti, 1978; Reiter, Fuldauer, Root, 1977).
Twins that scored greater than a 1 on the PDS were considered to have begun puberty/gonadarche and were categorized into an early-pubertal (i.e., PDS scores = 1.25-2.25) or a mid-to-post pubertal (i.e., PDS scores = 2.5-4.0) group, consistent with previous studies (Culbert et al., 2014; Klump et al., 2012; Suisman et al., 2014).
Twin Correlations
Twin intraclass correlations were used as initial indications of genetic and environmental effects on disordered eating in each pubertal status group. Additive genetic influences are suggested when MZ twin correlations are approximately double the DZ twin correlations. Shared environmental influences (i.e., environmental effects that would act to make twins similar on disordered eating) are suggested when the MZ twin correlations are approximately equal to the DZ twin correlations. Nonshared environmental influences (i.e., environmental effects that would act to make twins dissimilar on disordered eating, including measurement error) are suggested when the MZ twin correlation is less than 1.00 and/or small and non-significant correlations are detected for both the MZ and DZ twin pairs. Twin pairs who were discordant on maturational status (e.g., twins were categorized in different adrenarche or pubertal status groups; n = 58 twin pairs; 9.57% of the full sample) had to be excluded from the twin correlations; however, these twins could be included in the formal tests of moderation (see Twin Moderation Models below).
Twin Moderation Models
We made use of extended univariate moderation models (van der Sluis, Posthuma, & Dolan, 2012) to examine developmental differences in genetic and environmental influences on disordered eating across adrenarcheal and pubertal groups. A strength of twin moderation models is that all twins, irrespective of concordance or discordance on adrenarche or pubertal status, can be included in analyses. The “extended” univariate models used herein (van der Sluis et al., 2012) are identical to “standard” univariate moderation models (Purcell, 2002) with the exception that the “extended” model accounts for co-twin covariance on the moderator (i.e., adrenarcheal or pubertal maturation group). In particular, the moderator values of both twins are entered into a means model of each twin's disordered eating, and moderation effects are modeled on the residual disordered eating variance (i.e., that which does not overlap with the moderator, see Figure 1; see van der Sluis et al., 2012). These model adjustments are important since simulation studies have demonstrated that false positive moderation can be found when co-twin covariance on the moderator is not accounted for (van der Sluis et al., 2012).
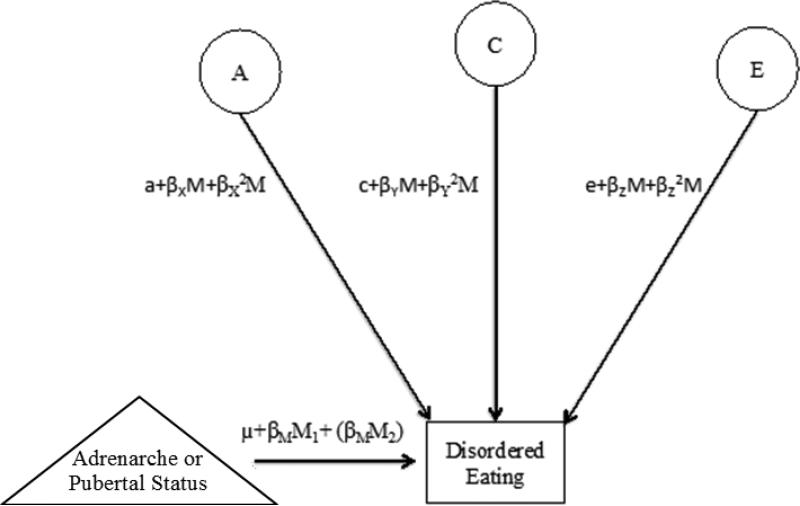
Figure 1.
Path diagram of the extended univariate moderation model representative of one twin only. Adrenarche or Pubertal Status = moderator; A = additive genetic effects; C= shared environmental effects; E= non-shared environmental effects; M1 and M2 =moderator in twin 1 and twin 2, respectively; βM =phenotypic regression coefficient; a, c, and e = paths or intercepts; βχ, βγ, and βΖ = linear moderators, βχ2, βγ2, and βΖ2 = quadratic moderators. Although the model is depicted for only one twin, parameters are fit separately for twin 1 and twin 2 and for MZ and DZ twins. Estimates denoted within parentheses (βMM2) are included in the extended univariate moderation model to account for co-twin covariance on the moderator (i.e., adrenarcheal or pubertal status groups).
The twin moderation models estimated three sets of parameters that indexed the extent to which genetic and environmental effects on disordered eating differed significantly across development (see Figure 1). The ‘paths’ or intercepts (a, c, and e) estimated genetic and environmental influences on disordered eating at the lowest level of the moderator (i.e., 0 since groups were “floored” for analyses). The linear moderators (βX, βY, and βZ) assessed the extent to which genetic and environmental influences on disordered eating linearly increased or decreased with adrenarcheal or pubertal maturation. The quadratic moderators (βX2, βY2, and βZ2) assessed the extent to which there were non-linear increases or decreases in genetic and environmental influences on disordered eating with adrenarcheal or pubertal maturation.
Because of the potentially large number of models that could be fit to the data, we initially fit the “full” moderation model (i.e., including all linear and quadratic moderators) to explore linear and nonlinear differences in genetic, shared environmental, and nonshared environmental influences on disordered eating across groups. We then examined moderation estimates to identify appropriate submodels that dropped different moderators from the model (e.g., a model that dropped the non-significant quadratic shared and nonshared environmental estimates only; for details, see Results for Twin Moderation Models). There were two goals with dropping moderation estimates: 1) obtain the most parsimonious model, and 2) test the significance of a parameter. This approach allowed us to test theoretically relevant submodels without unduly increasing the number of statistical tests. In addition to these submodels, we also fit the “no moderation” model that dropped all linear and quadratic moderators, as this model is the most parsimonious and directly tests the null hypothesis (i.e., no moderation of genetic or environmental influences on disordered eating across adrenarche or puberty).
The best fitting model was determined by comparing the fit between the full moderation model and the nested submodels via the difference in minus twice the log-likelihood (−2lnL) and Akaike's information criterion (AIC; Akaike, 1987). Specifically, the −2lnL values for the nested models were subtracted from the -2lnL value from the full moderation model to provide a likelihood-ratio chi-square test for the significance of the moderator effects. A significant chi-square value is indicative of significantly worse fit. The AIC (AIC=χ2-2*Δdf) balances model fit with parsimony, and models with the lower AIC were preferred.
All models were run a minimum of five times using multiple starting values to ensure stability of model fit and that the obtained estimates minimize the −2lnL value. Following previous recommendations (Purcell, 2002), the unstandardized parameter estimates (rather than proportional ACE estimates) were reported in tables and figures since these estimates more accurately depict absolute changes in etiologic influences. However, to facilitate the interpretation of unstandardized values, disordered eating scores were standardized prior to running analyses.
Results
Descriptive Statistics
Descriptive statistics are presented in Table 1. Importantly, in addition to good psychometric properties (e.g., internal consistency), there was ample variability in disordered eating symptoms (e.g., score range and standard deviations) in all adrenarche and puberty groups. A proportion of twins (n = 24; 1.92% of total sample) scored above the mean clinical cut-off (score ≥ 15.55; von Ranson et al., 2005), although as would be expected, this percentage is lower than rates observed in females (Culbert et al., 2009; Klump et al., 2012). Consistent with the age distribution of the sample (75% between ages 6-12) and typical pubertal development in males (Herman-Giddens et al., 2001), most twins were of pre-pubertal status (n = 814, 67.16% of total sample; raw PDS score = 1), and thus, were considered to be in adrenarche since they had experienced no external changes in secondary sex characteristics. The remaining twins were in early puberty (n = 168, 13.86% of total sample) or mid-to-post puberty (n = 226, 18.64% of total sample). Most twins (91%) were concordant on pubertal status, and thus, were coded within the same adrenarcheal or pubertal group.
Mean levels of disordered eating were relatively similar for twins in the adrenarche and early puberty groups (comparisons between adrenarche and early puberty groups: d's = .02-.26), but tended to be lower in the mid-to-post pubertal group (adrenarche and early puberty groups vs. mid-to-post puberty group: d's = .21-.46). The detection of lower levels of disordered eating after mid-puberty is consistent with some prior research on adolescent males (for review, see Klump, 2013) and may be due to the fact that mid-to-post pubertal males would have the full complement of androgens, resulting in greater protective effects on the phenotypic expression of disordered eating. Importantly, relatively similar mean levels of disordered eating across adrenarcheal and early pubertal groups does not preclude the presence of etiologic moderation effects, as significant genetic or environmental moderation could attenuate phenotypic effects across groups.
Twin Correlations
Twin intraclass correlations for the MEBS total score are presented in Table 1. The pattern of correlations is consistent with the notion that genetic effects likely become prominent during adrenarche. The MZ and DZ twin correlations were similar in magnitude in the andrenarche 6-7 year old group, yet genetic effects appeared to emerge in adrenarcheal twins in the 8-9 year old group. Specifically, the MZ twin correlations were nearly double the DZ twin correlations in the 8-9 year old and 10-12 year old adrenarcheal groups as well as in the early pubertal and mid-to-post pubertal status groups (see Table 1). However, these twin correlation results should be interpreted cautiously since, as noted above, twins that were discordant on adrenarche or puberty status had to be excluded. The formal tests of moderation (see Twin Moderation Models) would be most representative of the overall sample.
Twin Moderation Models
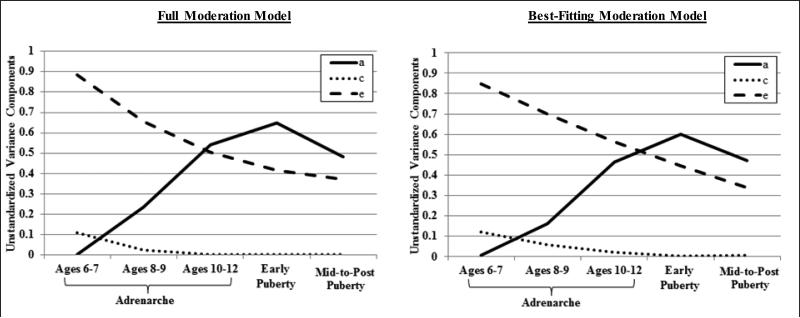
Results from the moderation analyses are presented in Tables 2 and 3 and Figure 2. The no moderation model provided a significantly worse fit to the data than the full moderation model (as indicated by the higher AIC and significant chi-square difference test - see Table 2), supporting the presence of significant etiologic moderation in our data. Estimates from the full moderation model were suggestive of significant moderation of additive genetic and nonshared environmental effects. Specifically, quadratic and linear moderation estimates were statistically significant for additive genetic effects (see Table 3), and plotted results showed that genetic influences appeared to increase linearly across advancing adrenarcheal and early puberty groups and then plateau from early puberty to mid-to-post puberty (see Figure 2). By contrast, the quadratic estimates for shared and non-shared environmental influences were non-significant (see Table 3), and plotted results were suggestive of linear declines only in these etiologic influences across groups (see Figure 2).
Table 2.
Model Fit Indices.
| Model Fit Statistics |
||||
|---|---|---|---|---|
| Model | −2lnL (df) | Δχ2 (Δdf) | p | AIC |
| Initial Moderation Analyses | ||||
| Full Moderation (Quadratic and Linear) | 3239.53 (1172) | -- | -- | 895.53 |
| No Moderation | 3262.18 (1178) | 22.65 (6) | <.001 | 906.18 |
| Alternative Moderation Models | ||||
| Drop Quadratic C and E Moderation | 3240.57 (1174) | 1.04 (21) | .31 | 892.57 |
| Linear Moderation | 3244.65 (1175) | 5.11 (3) | .16 | 894.64 |
| Post-Hoc Moderation Analyses | ||||
| Separating Mid-to-Late Puberty and Post-Puberty Groups | ||||
| Full Moderation (Quadratic and Linear) | 3225.98 (1172) | -- | -- | 881.98 |
| No Moderation | 3254.40 (1178) | 28.42 (6) | <.001 | 898.40 |
| Alternative Moderation Models | ||||
| Drop Quadratic C and E Moderation | 3226.22 (1174) | 0.24 (2) | .89 | 878.22 |
| Linear Moderation | 3235.12 (1175) | 9.14 (3) | .03 | 885.12 |
| Removal of Adrenarcheal Twins Ages 6-7 | ||||
| Full Moderation (Quadratic and Linear) | 2116.59 (778) | -- | -- | 560.59 |
| No Moderation | 2130.29 (784) | 13.70 (6) | .03 | 562.29 |
| Alternative Moderation Models | ||||
| Drop Quadratic E Moderation | 2117.01 (779) | 0.42 (1) | .52 | 559.01 |
| Drop Quadratic C and E Moderation | 2117.41 (780) | 0.82 (2) | .66 | 557.41 |
| Linear Moderation | 2127.09 (781) | 10.50 (3) | .01 | 565.09 |
Note: Full Moderation Model = allows genetic, shared environmental, and nonshared environmental estimates to vary both linearly and quadratically across levels of the moderator; No Moderation Model = constrains genetic, shared environmental, and nonshared environmental estimates to be equal across the moderator; Linear Moderation Model = allows genetic, shared environmental, and nonshared environmental estimates to only vary linearly across levels of the moderator; Drop Quadratic C and E Moderation Model = retained all linear moderation and the quadratic moderation of genetic influences, but dropped quadratic moderation of shared and nonshared environmental influences. Drop Quadratic E Moderation Model = retained all linear moderation and the quadratic moderation of genetic and shared environmental influences, but dropped quadratic moderation of nonshared environmental influences. A = additive genetic, C = shared environment, E = nonshared environment; −2lnL= −2 times the log likelihood; df= degrees of freedom; Δχ2 = change in chi-square, via differences in −2lnL, from the least restrictive moderation model (i.e., full moderation model); Δdf = change in degrees of freedom from the least restrictive moderation model (i.e., full moderation model). AIC =Akaike Information Criterion. To evaluate model fit, each nested model is compared to the full moderation model when calculating the change in −2lnL and degrees of freedom. The ‘Drop quadratic C and E moderation model’ was best-fitting in the initial moderation analyses and the post-hoc analyses. A significant p-value for Δχ2 indicates significantly worse fit of the more restrictive model.
Table 3.
Unstandardized Path and Moderation Estimates for the Full and Best-Fitting Moderation Models.
| Path |
Linear Moderation Estimates |
Quadratic Moderation Estimates |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | a | c | e | β X | β Y | β Z | β X 2 | β Y 2 | β Z 2 |
| Initial Moderation Analyses | |||||||||
| Full Moderation (Quadratic and Linear) | −.05 (−.48, .41) | .33 (−.51, .51) | −.94 (−1.04, −.84) | −.52 (−.93, −.11) | −.21 (−.70, .69) | .15 (.01, .28) | .09 (.01, .18) | .03 (−.17, .17) | −.02 (−.05, .01) |
| Drop Quadratic C & E Moderation | −.07 (−.39, .51) | .35 (−.51, .51) | −.92 (−1.01, −.84) | .56 (.08, .89) | −.11 (−.22, .22) | .08 (.05, .11) | −.09 (−.16, −.01) | -- | -- |
| Post-Hoc Moderation Analyses | |||||||||
| Separating Mid-to-Late Puberty and Post-Puberty Groups | |||||||||
| Full Moderation (Quadratic and Linear) | −.04 (−.48, .42) | .35 (−.52, .52) | −.92 (−1.02, −.84) | −.44 (−.75, −.11) | −.19 (−.55, .27) | .08 (−.02, .19) | .07 (.01, .12) | .02 (−.07, .11) | −.001 (−.02, .02) |
| Drop Quadratic C & E Moderation | .05 (−.48, .36) | −.34 (−.50, .50) | .93 (.85, 1.01) | −.49 (−.73, −.13) | .09 (−.04, .18) | −.08 (−.10, −.05) | .07 (.02, .11) | -- | -- |
| Removal of Adrenarcheal Twins Ages 6-7 | |||||||||
| Full Moderation (Quadratic and Linear) | .31 (−.52, .67) | .41 (−.63, .63) | −.81 (−.93, −.70) | −1.22 (−1.71, −.13) | −.61 (−1.26, 1.26) | .11 (−.14, .31) | .31 (.02, .47) | .19 (−.39, .39) | −.01 (−.08, .06) |
| Drop Quadratic C & E Moderation | .40 (−.38, .67) | .35 (−.58, .58) | .80 (.70, .90) | −1.24 (−1.64, −.46) | −.14 (−.33, .33) | −.07 (−.11, −.01) | .30 (.12, .43) | -- | -- |
Note. a = genetic path estimate; c = shared environmental path estimate; e = non-shared environmental path estimate; βX = linear moderation estimate of the genetic path estimate; βY = linear moderation estimate of shared environmental path estimate; βZ =linear moderation estimate of the non-shared environmental path estimates; βX2= quadratic moderation estimate of the genetic path estimate; βY2= quadratic moderation estimate of the shared environmental path estimate; βZ2= quadratic moderation estimate of the non-shared environmental path estimate. Estimates are followed by 95% confidence intervals in parentheses, and confidence intervals that do not overlap with zero indicate statistical significance at p <.05. Significant estimates are noted in bold text. The ‘Drop quadratic C and E moderation model’ was best-fitting in the initial moderation analyses and the post-hoc analyses. Since the lowest level of the moderator was coded as 0 in all models, the genetic and environmental contributions to disordered eating at this level can be obtained by squaring the path estimates (i.e., a, c, and e). For each subsequent level, linear (i.e., βX, βY, βZ) and non-linear (i.e., βX2, βY2, ζZ2) moderators were added to the genetic and environmental paths using the following equation: unstandardized variance total = (a + βX(moderator) + βX2(moderator2))2 + (c + βY(moderator) + βY2(moderator2))2 + (e + βZ(moderator) + βZ2(moderator2))2. The unstandardized variance estimates for genetic, nonshared, and shared environmental effects were divided by the total unstandardized variance and multiplied by 100 to obtain the standardized estimates, i.e., the proportion of variance in disordered eating accounted for by genetic and environmental effects at each level of the moderator.
Figure 2.
Unstandardized variance estimates for the full moderation model and the best-fitting model (i.e., drop quadratic shared and nonshared environmental moderators). a = additive genetic effects, c = shared environmental effects, e = non-shared environmental effects. Results indicated significant linear moderation of non-shared environmental effects and significant linear and quadratic moderation of genetic effects on disordered eating.
Based on these results, two additional submodels were fit to the data. First, we tested a moderation model that dropped the quadratic shared and nonshared environmental effects (i.e., the model retained all linear moderation estimates and only the quadratic genetic estimate) to determine if eliminating the non-significant quadratic moderation effects would improve overall model fit. Second, a linear moderation model that estimated only linear genetic, shared environmental, and nonshared environmental effects (i.e., the model dropped all quadratic moderation estimates) was tested to confirm that model fit would be worsened if the significant quadratic moderation effect was dropped, and thus, aimed to further ensure that the inclusion of the quadratic genetic estimate is important. Model fit comparisons indicated that the first submodel, i.e., the model that dropped quadratic moderation of shared and nonshared environment, was best-fitting. This model had the lowest AIC of all of the models examined (including the full and no moderation model), and the −2lnL difference between the full moderation model and this alternative model was non-significant (see Table 2).
The best-fitting model indicated significant linear moderation of nonshared environmental effects and significant linear and quadratic moderation of genetic effects on disordered eating, with advancing maturation (see Table 3 & Figure 2).4 Path and moderator estimates (see Table 3) were used to calculate the standardized estimates, i.e., the proportion of variance in disordered eating accounted for by genetic and environmental effects at each level of the moderator (for calculation details, see Table 3 note). The heritability (i.e., h2) of disordered eating substantially increased during adrenarche, across advancing age groups: 0% at ages 6-7, 17% at ages 8-9, and 44% at ages 10-12. Genetic effects also slightly increased during early puberty (h2 = 57%) and then persisted into mid-to-post puberty (h2 = 58%). The remaining variance in disordered eating was largely accounted for by nonshared environmental effects, which linearly decreased across groups: 87% at ages 6-7, 76% at ages 8-9, 54% at ages 10-12, and 43% or 42% in the early and mid-to-post pubertal groups, respectively. Shared environmental effects were non-significant and only accounted for a small proportion of the variance in disordered eating during adrenarche (ages 6-7: 12%; ages 8-9: 6%; ages 10-12: 2%; early and mid-to-post puberty: 0%). Overall, findings suggest that the genetic diathesis of disordered eating in boys likely emerges with the onset and progression of adrenarche, as genetic effects become prominent during middle childhood and before substantial physical changes in secondary sex characteristics arise.
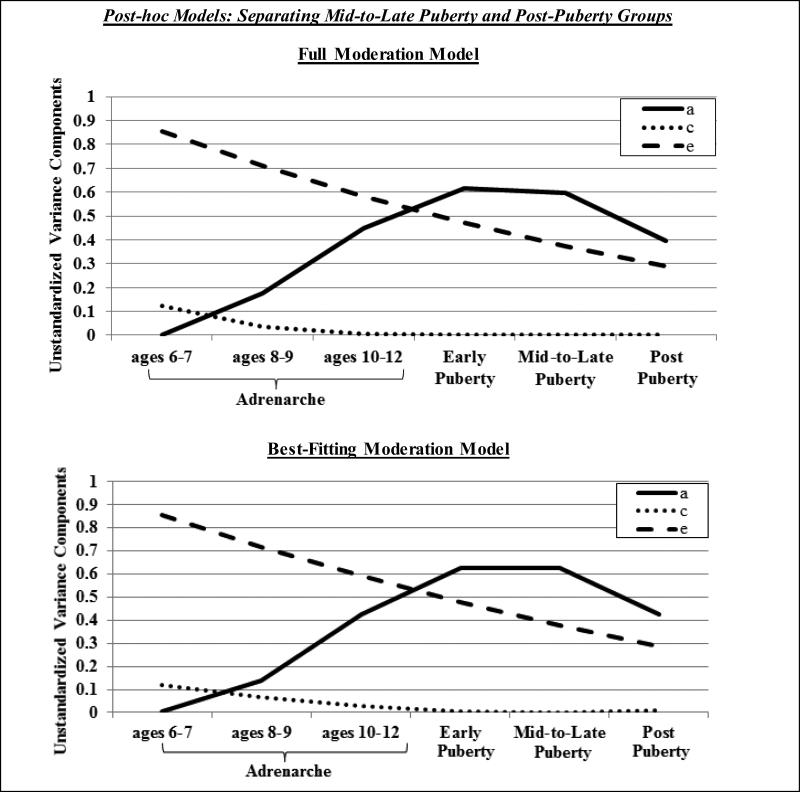
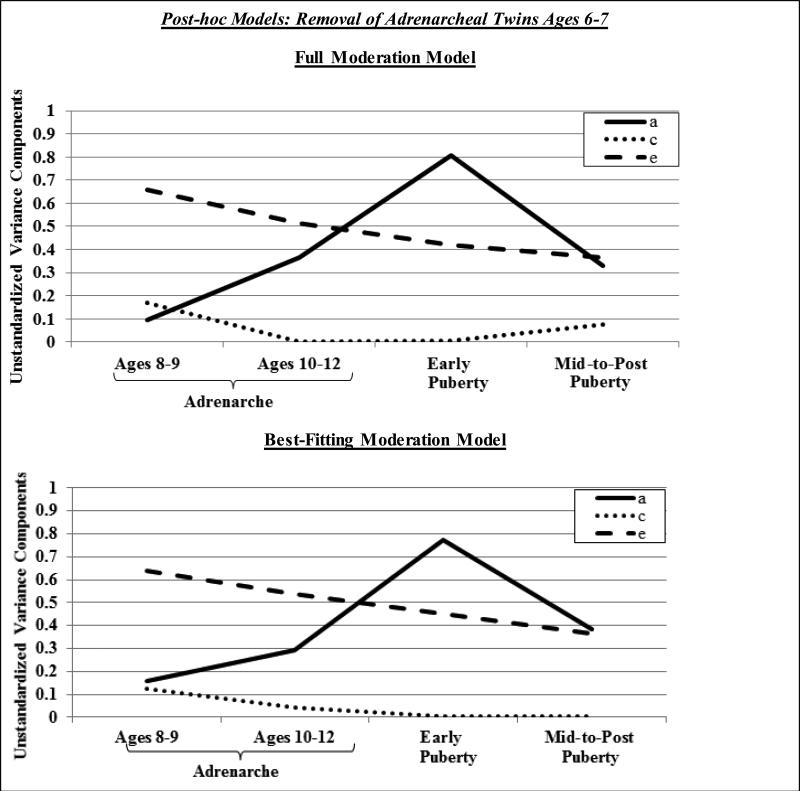
Notably, we conducted two sets of post-hoc models to confirm that our findings were not affected by some of our extreme age groups, i.e., combining all mid-to-post pubertal twins (ages 13-28) into one group or including twins who were very young (i.e., ages 6-7). First, we re-categorized our pubertal groups such that the mid-to-late pubertal group was comprised of adolescent twins (< age 16; MZ, n = 30; DZ, n = 24) and the post-pubertal group was comprised of young adult twins (ages 18-28; MZ, n = 108; DZ, n = 62). Second, we re-analyzed the data excluding the adrenarcheal 6-7 year-old age group from the models. We tested identical models as our initial analyses, with the exception of one additional submodel (i.e., dropping quadratic nonshared environment) for post-hoc analyses that excluded the 6-7 year old twins; this additional submodel was tested since the plotted results of the full moderation model pointed to possible quadratic shared environmental moderation. As shown in Table 2 and Figures 3 and 4, results were largely similar to our initial analyses. In both sets of post-hoc analyses, the submodel that dropped quadratic moderation of shared and nonshared environment was best-fitting (i.e., the lowest AIC and the other models showed significantly worse fit; see Table 2). Results indicated significant linear moderation of nonshared environmental effects and quadratic and linear moderation of genetic influences on disordered eating. Importantly, these models continued to demonstrate that genetic effects on disordered eating become prominent during adrenarche (h2 rise to 33-43%) and verified that the magnitude of genetic effects on disordered eating is similar during mid-to-late puberty (h2 = 62%) and post-puberty (h2 = 59%) (for all standardized heritability estimates, see figure notes).
Figure 3.
Unstandardized variance estimates for the full moderation model and best-fitting model (i.e., drop quadratic shared and nonshared environmental moderators) in post-hoc analyses that separated mid-to-late and post-pubertal groups. a = additive genetic effects, c = shared environmental effects, e = non-shared environmental effects. Results indicated significant linear moderation of non-shared environmental effects and significant linear and quadratic moderation of genetic effects on disordered eating. Standardized heritability estimates were 0% in ages 6-7, 15% in ages 8-9, 41% in ages 10-12, 56% in early puberty, 62% in mid-to-late puberty, and 59% in post-puberty.
Figure 4.
Unstandardized variance estimates for the full moderation model and best-fitting model (i.e., drop quadratic shared and nonshared environmental moderators) in post-hoc analyses that excluded andrenarcheal twins ages 6-7. a = additive genetic effects, c = shared environmental effects, e = non-shared environmental effects. Results indicated significant linear moderation of non-shared environmental effects and significant linear and quadratic moderation of genetic effects on disordered eating. Standardized heritability estimates were 17% in ages 8-9, 33% in ages 10-12, 63% in early puberty, and 51% in mid-to-post puberty.
Finally, we aimed to ensure that our findings were not unduly impacted by use of self-reported PDS ratings for twins ages 10-16 or by the inclusion of some young twins (ages 6-7) in our early pubertal group. Analyses were re-run after twins were categorized into adrenarche and pubertal status groups using parent-reported PDS scores. As expected (given the high parent-child PDS correlation, r = .83), results were nearly identical to those presented herein (data available upon request). We then reviewed the data from the young twins (ages 6-7; n = 10) who were classified in the early puberty group due to the initiation of body hair growth, as indicated via parent-reported PDS scores. Descriptive analyses confirmed that these cases were not outliers, but notably, our findings also remained unchanged when these twins were eliminated from analyses (data available upon request).
DISCUSSION
This study was the first to examine developmental differences in genetic contributions to disordered eating in a sample of male twins spanning the full spectrum of pubertal maturation, from adrenarche to mid-to-post puberty. Consistent with hypotheses, genetic influences on disordered eating showed significant increases during adrenarcheal development; genetic factors did not account for any of the variance (h2 = 0%) in disordered eating in twins ages 6-7, yet genetic influences on disordered eating incrementally increased in twins ages 8-9 (h2 = 17%) and ages 10-12 (h2 = 44%). Substantial genetic influences were also evident in the early puberty (h2 = 57%) and mid-to-post puberty (h2 = 58%) groups. These findings indicate that individual differences in genetic effects on disordered eating emerge during middle childhood and before the physical signs of pubertal maturation (i.e., external changes in secondary sex characteristics) ensue. Thus, in contrast to the robust mid-pubertal emergence of genetic effects in girls (e.g., Culbert et al., 2009; Klump et al., 2003; Klump et al., 2007), middle childhood may be a critical developmental period for the emergence of individual differences in genetic vulnerability to disordered eating in boys.
Moving forward, it will be important to understand the mechanisms underlying these developmental differences in genetic influences on disordered eating in males. Given that relevant factors come online during middle childhood and before substantial physical changes associated with pubertal maturation, our findings are consistent with the hypothesis that genetic influences on the phenotypic variation in disordered eating in boys may coincide with the onset and progression of adrenarche. Indeed, adrenarche is the earliest phase of puberty that is largely characterized by increases in adrenal androgens without the visible signs of pubertal development. Adrenal androgens rise during middle childhood, coinciding with the progression of adrenarcheal development, and continue to increase into gonadarche and young adulthood (Dorn et al., 2006; Guran et al., 2015; Kelner & Brook, 1983). One possibility is that the genetic diathesis for disordered eating in boys may be driven by the rise in adrenal androgens and subsequent regulation of genes for eating pathology. Indeed, like ovarian hormones, androgens regulate (i.e., activate or inhibit) gene transcription within several neural systems known to be disrupted in eating disorders (e.g., serotonin, dopamine; Fink et al., 1999; Guivarch'h et al., 1995; Simerly, Chang, Muramatsu, & Swanson, 1989). Such effects could explain the adrenarcheal emergence and incremental rise in genetic contributions with advancing childhood age (h2 = 0-44% from ages 6-7 to ages 10-12), and the additional increase and persistence of genetic influences with gonadarcheal development (h2 of 57-58% during early and mid-to-post puberty).
Although data highlight developmental moderation of genetic effects on disordered eating in boys that are consistent with a potential role of adrenarche, additional research will be necessary to confirm and elucidate these effects. As noted previously, shifts in the magnitude of genetic influences in twin designs means that the source of individual differences in disordered eating changes, such that the contribution of genetic effects increases; the twin models used in this study cannot delineate the underlying type or direction (e.g., risk or protective, including activation versus inhibition of gene transcription) of genetic effects that make monozygotic twins more similar to each other than dizygotic twins on a phenotype. However, given that phenotypic data indicate protective effects of circulating androgens (e.g., testosterone, dehydroepiandrosterone sulphate; androstenedione) on disordered eating (Culbert et al., 2014) and symptoms that co-occur with eating pathology (e.g., mood difficulties; Goodyer et al., 1996; Susman, Dorn, & Chrousos, 1991) during adrenarcheal or pubertal maturation, it may be that adrenal androgens serve to trigger protective genomic effects (e.g., inhibiting transcription of risk genes and/or activating transcription of protective genes) on disordered eating in boys. Future studies that directly explore these mechanisms will be a critical next step. Findings from such research would have the potential to reveal unique biological factors that, in combination with the genomic effects of ovarian hormones in females (Klump et al., 2010; Klump et al., 2015), could help explain sex-differentiated patterns of genetic risk for disordered eating.
While our findings fit nicely with age-related physiological changes associated with adrenarcheal development, we cannot rule-out the possible influence of other factors that may contribute to the moderation of genetic effects on disordered eating in boys. In order for factors to account for the moderating effects, they must serve to increase genetic influences and decrease environmental influences. Psychosocial risk (e.g., weight pressures, weight-based teasing; Gardner, Stark, Friedman, & Jackson, 2000) or protective (e.g., heightened self-esteem, positive perceptions of one's attractiveness; Kling, Hyde, Showers, & Buswell, 1999; Harter, 1993) factors could influence the heritability of disordered eating via gene-environment interactions or active gene-environment correlations - both types of processes would be partitioned into additive genetic estimates within twin models. That is, psychosocial effects might either enhance or buffer genetic vulnerability (gene-environment interaction; e.g., increases in weight pressures or self-esteem may potentiate or attenuate genetic susceptibility for disordered eating, respectively) or boys could actively create environments that are correlated with their genotype (gene-environment correlation; e.g., participation in weight-focused sports, thereby potentiating pre-existing genetic vulnerabilities). Nonetheless, in order to account for increases in genetic estimates during adrenarche, psychosocial factors would be expected to show developmental increases during middle childhood and to be uniquely influential in boys, given that girls show a mid-pubertal emergence in genetic risk for disordered eating (Culbert et al., 2009; Klump et al., 2003; Klump et al., 2007).
There is unfortunately a paucity of disordered eating research in early-to-mid childhood, particularly in males, and data to support psychosocial effects is currently limited. For example, weight-based teasing has been found to significantly predict disordered eating in boys at ages 12 and 14, but not from ages 7 to 11 (Gardner et al., 2000). Further, despite sex-differentiated and age-related changes in self-esteem (boys > girls, with increases across childhood-to-adolescence; Kling et al., 1999), studies have not explored whether these age-related changes in self-esteem are etiologically relevant for disordered eating. It will be important for future studies to explore possible developmental changes in psychosocial factors for disordered eating during middle childhood and to directly examine gene-environment interactions and correlations.
Several limitations of this study must be noted. First, data were cross-sectional, and thus, we were unable to examine longitudinal associations between advancing developmental status and changes in genetic effects on disordered eating. It will be important to utilize longitudinal designs to ensure that sample differences are not driving the observed etiologic shifts with advancing age/pubertal maturation. Second, age was used as a proxy for adrenarcheal development in pre-pubertal twins since hormone measures were not available. Future studies should aim to replicate and extend these effects by using other twin models (e.g., Choleskys) and direct measures of circulating androgens to confirm that differential androgen concentrations account for our observed effects and exert protective effects on disordered eating. Third, disordered eating was assessed via the MEBS, which was developed for children, but to our knowledge, has not been used in children less than age 8. To ensure comprehension of the items and to provide clarification of complex terms, trained research assistants read the questionnaire to young twins. Descriptive and psychometric data largely suggested that the MEBS was an adequate measure of disordered eating even in these younger boys. Across all developmental groups, the MEBS total score showed good psychometric properties (α's ≥ .77), expected associations with BMI and depression/anxiety symptoms, and expected intercorrelations with MEBS subscale scores. Similar mean levels of disordered eating were also observed across the adrenarcheal and early pubertal status groups, as would be expected (Klump et al., 2013), and post-hoc analyses confirmed that our general pattern of results (e.g., detection of adrenarcheal emergence of genetic effects) is largely unchanged even after excluding 6-7 year old twins. Taken together, it is unlikely that psychometric issues could account for the observed genetic increases in disordered eating during middle childhood; however, it will be important for future studies to replicate these effects.
Fourth, although our disordered eating measure assessed several core symptoms (e.g., weight preoccupation, body dissatisfaction, binge eating), it may be beneficial for future studies to examine whether our observed effects extend to specific symptoms (e.g., binge eating; body weight/shape concerns) and other disordered eating constructs (e.g., drive for muscularity) that are also known to be associated with eating pathology in males (McCreary & Sasse, 2000; Murray et al., 2012). Nonetheless, it is important to acknowledge that the examination of specific disordered eating constructs, particularly in children younger than age 8, may be difficult due to measurement limitations (e.g., lower internal consistency; readability of most questionnaires ≥ 5th grade-level). Finally, we examined disordered eating symptoms in a population-based sample of twins, rather than twins with clinical eating disorders. Unfortunately, this limitation is challenging to overcome given the low prevalence of eating disorders, particularly during pre-adolescence and in males (American Psychiatric Association, 2013), and the fact that twin moderation models have limited statistical power for categorical phenotypes.
Despite the aforementioned limitations, results from this study are novel and contribute to a growing literature on developmental changes in etiologic influences on disordered eating. In particular, our findings highlight middle childhood as a key developmental period for the emergence of genetic influences on disordered eating in boys, an effect that is in contrast to the well-documented mid-pubertal emergence of genetic effects in girls. Future research should begin to explore potential mechanisms (e.g., changes in androgen concentrations) that may underlie these observed developmental shifts. Studies that include multiple indicators of puberty, gonadal hormones, and psychosocial risk factors (e.g., teasing; participation in weight-focused sports) and that collect frequent assessments (e.g., developmental longitudinal design) would be best suited for elucidating the mechanisms driving developmental increases in genetic contributions to disordered eating in males.
Supplementary Material
General Scientific Summary.
Our findings show that there are developmental shifts in genetic influences on disordered eating in males, but notably, changes in genetic influences occur at earlier phases of development than prior studies have explored. In particular, genetic effects on disordered eating in boys become prominent during middle childhood and early puberty. Results underscore the fact that future developmental studies may benefit from exploring factors that contribute to disordered eating at younger ages.
Acknowledgements
Funding/Support: The research was supported by the National Institute of Mental Health: R21-MH070542-01, R01-MH081813, R01-MH092377. The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting agencies. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
The Minnesota Eating Behavior Survey (previously known as the Minnesota Eating Disorder Inventory) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Notably, we examined subscale-total score intercorrelations to determine if a particular subscale was driving the overall MEBS score in our sample. Within all of our developmental groups and consistent with prior research (e.g., von Ranson et al., 2005), the total score showed the highest correlations with the weight preoccupation subscale (r's = .80-.86), lowest correlations with the compensatory behavior subscale (r's = .41-.52), and intermediate correlations with the binge eating and body dissatisfaction subscales (r's = .54-.77). These moderate-to-large intercorrelations suggest that the total score is broadly representative of a range of disordered eating cognitions and behaviors, but is tapping weight preoccupation symptoms the most.
Depression and anxiety symptoms were assessed via the Semi-structured Clinical Interview for Children and Adolescents (SCICA; McConaughy & Achenbach, 2001) for twins ages 6-9, the Children's Depression Inventory (Kovacs, 1985) and Multidimensional Anxiety Scale for Children (March, Parker, Sullivan, Stallings, & Conners, 1997) for twins ages 10-16, and the Beck Depression Inventory (Beck, Steer, & Brown, 1996) and State-Trait Anxiety Inventory (Spielberger, 1983) for twins ages 18-28.
Following recommendations (van der Sluis et al., 2012), bivariate moderation models (Purcell, 2002) were conducted to confirm that results from the extended univariate moderation models were not unduly impacted by etiologic covariance between the moderator (i.e., adrenarche and pubertal status groups) and outcome (i.e., disordered eating). Moreover, extended univariate moderation models cannot distinguish between moderation of the covariance path and moderation of the residual, and thus, could produce false positive moderation (e.g., moderation of the covariance path may be picked up as moderation of the residual path; van der Sluis et al., 2012). Importantly, the follow-up bivariate moderation model indicated no significant genetic moderation of the covariance path and the unique genetic moderator estimate was identical to results from the extended univariate linear moderation model (see Supplemental Table 1). The shared (0.09) and nonshared (0.07) environmental moderators of the covariance path were statistically significant (p < .05); however, the unique shared and nonshared environmental moderator estimates were identical to those obtained from the extended univariate linear model (see Supplemental Table 1), and plotted effects were also strikingly similar between the two moderation models (see Supplemental Figure 1). These findings confirm that the results we obtained from our extended univariate moderation models are unlikely to have been unduly influenced by etiologic covariance.
None of the authors have financial conflicts of interest.
Parts of this manuscript were presented at the International Conference on Eating Disorders (ICED), San Francisco, California, May 5-7, 2016.
REFERENCES
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM 5. Arlington, VA.: 2013. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Burt SA, Klump KL. The Michigan state university twin registry (MSUTR): an update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Sisk CL, Burt SA, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: a role for prenatal testosterone exposure. Journal of Abnormal Psychology. 2013;122:420–432. doi: 10.1037/a0031791. doi: 10.1037/a0031791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. Journal of Abnormal Psychology. 2009;118:788–796. doi: 10.1037/a0017207. doi:10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, Sisk CL, Nigg JT, Klump KL. The effects of circulating testosterone and pubertal maturation on risk for disordered eating symptoms in adolescent males. Psychological Medicine. 2014;44:2271–2286. doi: 10.1017/S0033291713003073. doi: 10.1017/S0033291713003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Racine SE, Klump KL. Research review: what we have learned about the causes of eating disorders–a synthesis of sociocultural, psychological, and biological research. Journal of Child Psychology and Psychiatry. 2015;56:1141–1164. doi: 10.1111/jcpp.12441. doi: 10.1111/jcpp.12441. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and its measurement: A decade in review. Journal of Research on Adolescence. 2011;21:180–195. doi: 10.1111/j.1532-7795.2010.00722.x. [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. doi:10.1207/s1532480xads1001_3. [Google Scholar]
- Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behavioural Brain Research. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. doi: 10.1016/S0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Stark K, Friedman BN, Jackson NA. Predictors of eating disorder scores in children ages 6 through 14: A longitudinal study. Journal of Psychosomatic Research. 2000;49:199–205. doi: 10.1016/s0022-3999(00)00172-0. doi: 10.1016/S0022-3999(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PME, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8-to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Guivarc'h D, Vernier P, Vincent JD. Sex steroid hormones change the differential distribution of the isoforms of the D2 dopamine receptor messenger RNA in the rat brain. Neuroscience. 1995;69:159–166. doi: 10.1016/0306-4522(95)00228-b. doi: 10.1016/0306-4522(95)00228-B. [DOI] [PubMed] [Google Scholar]
- Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clinical endocrinology. 2015;82:712–718. doi: 10.1111/cen.12612. doi: 10.11.11/cen.12612. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988-1994. Archives of Pediatrics & Adolescent Medicine. 2001;155:1022–1028. doi: 10.1001/archpedi.155.9.1022. doi:10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- Ilondo MM, Vanderschueren-Lodeweyckx M, Vlietinck R, Pizarro M, Malvaux P, Eggermont E, Eeckels R. Plasma androgens in children and adolescents. Hormones. 1982;16:61–77. doi: 10.1159/000179486. doi: 10.1159/000179486. [DOI] [PubMed] [Google Scholar]
- Kelnar CJH, Brook CGD. A mixed longitudinal study of adrenal steroid excretion in childhood and the mechanism of adrenarche. Clinical endocrinology. 1983;19:117–129. doi: 10.1111/j.1365-2265.1983.tb00750.x. doi: 10.1111/j.1365-2265.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Klump KL. Puberty as a critical risk period for eating disorders: a review of human and animal studies. Hormones and behavior. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. doi:10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Archives of general psychiatry. 2007;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. doi:10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: evidence for a sex difference. Psychological Medicine. 2012;42:627–637. doi: 10.1017/S0033291711001541. doi:10.1017/S0033291711001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Hildebrandt BA, O'Connor SM, Keel PK, Neale M, Sisk CL, Burt SA. Changes in genetic risk for emotional eating across the menstrual cycle: a longitudinal study. Psychological Medicine. 2015;45:3227–3237. doi: 10.1017/S0033291715001221. doi: 10.1017/S0033291715001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010;40:1745–1753. doi: 10.1017/S0033291709992236. doi:10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. Journal of Abnormal Psychology. 2000;109:239. http://dx.doi.org/10.1037/0021-843X.109.2.239. [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33:287–292. doi: 10.1002/eat.10151. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump K, Perkins PS, Burt SA, McGue M, Iacono W. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. doi:10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI). Psycho-pharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lee PA, Guo SS, Kulin HE. Age of puberty: data from the United States of America. APMS. 2011;109:S156–S163. doi: 10.1034/j.1600-0463.2001.d01-107.x. doi: 10.1111/j.1600-0463.2001.tb05761.x. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. Wiley; New York: 1987. [Google Scholar]
- Luo X, Donnellan MB, Burt SA, Klump KL. The dimensional nature of eating pathology: evidence from a direct comparison of categorical, dimensional, and hybrid models. Journal of Abnormal Psychology. 2016;125:715–726. doi: 10.1037/abn0000174. doi: 10.1037/abn0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: some initial findings. Acta Geneticae Medicae et Gemellologiae: Twin Research. 39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- McConaughy SH, Achenbach TM. Manual for the Semistructured Interview for Children and Adolescents. University of Vermont. Research Center for Children, Youth, and Families; Burlington, VT.: 2001. [Google Scholar]
- McCreary DR, Sasse DK. An exploration of the drive for muscularity in adolescent boys and girls. Journal of American College Health. 2000;48:297–304. doi: 10.1080/07448480009596271. doi: 10.1080/07448480009596271. [DOI] [PubMed] [Google Scholar]
- Murray SB, Rieger E, Hildebrandt T, Karlov L, Russell J, Boon E, Touyz SW. A comparison of eating, exercise, shape, and weight related symptomatology in males with muscle dysmorphia and anorexia nervosa. Body Image. 2012;9:193–200. doi: 10.1016/j.bodyim.2012.01.008. 10.1016/j.bodyim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical Modeling. Department of Psychology; Richmond, VA: 1995. [Google Scholar]
- Östlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. The Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28:159–163. doi: 10.1023/a:1021416112215. doi: 10.1023/A:1021416112215. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. doi:10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rathouz PJ, van Hulle CA, Rodgers JL, Waldman ID, Lahey BB. Specification, testing, and interpretation of gene-by-measured-environment interaction models in the presence of gene–environment correlation. Behavior Genetics. 2008;38:301–315. doi: 10.1007/s10519-008-9193-4. doi: 10.1007/s10519-008-9193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JL, Bulik CM. The developmental association between eating disorders symptoms and symptoms of depression and anxiety in juvenile twin girls. Journal of Child Psychology and Psychiatry. 2005;46:1317–1326. doi: 10.1111/j.1469-7610.2005.01427.x. doi: 10.1111/j.1469-7610.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press, Inc.; Palo Alto: 1983. [Google Scholar]
- Styne DM, Grumbach MM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Kronenberg HM, Shlomo M, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology (11th edition) Saunders Elsevier; Philadelphia, PA: 2008. [Google Scholar]
- Suisman JL, Thompson JK, Keel PK, Burt SA, Neale M, Boker S, Klump KL. Genetic and environmental influences on thin-ideal internalization across puberty and preadolescent, adolescent, and young adult development. International Journal of Eating Disorders. 2014;47:773–783. doi: 10.1002/eat.22321. doi: 10.1002/eat.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Chrousos GP. Negative affect and hormone levels in young adolescents: Concurrent and predictive perspectives. Journal of Youth and Adolescence. 1991;20:167–190. doi: 10.1007/BF01537607. doi: 10.1007/BF01537607. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in G× E modelling of twin data. Behavior Genetics. 2012;42:170–186. doi: 10.1007/s10519-011-9480-3. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: a brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;4:373–392. doi: 10.1016/j.eatbeh.2004.12.002. doi:10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.