Abstract
Context:
Menstrual cycle hormone patterns in women approaching menopause are inadequately studied.
Objective:
To describe day-to-day menstrual cycle hormones in women as they approach menopause from the Study of Women's Health Across the Nation Daily Hormone Study (DHS).
Design:
DHS enrollees collected daily urine for one entire menstrual cycle or up to 50 days, whichever came first, annually, up to the final menstrual period (FMP) or for up to 10 years.
Setting:
Seven sites across the United States.
Participants:
A total of 511 premenopausal or early perimenopausal women at enrollment, within 10 years before menopause.
Intervention:
Time-to-FMP measurement.
Main Outcome Measures:
Evidence of luteal activity (ELA), determined using objective algorithms. Menstrual cycle/segment length; whole cycle, and segment integrated urinary luteinizing hormone, follicle-stimulating hormone, estrone conjugates, and pregnanediol glucuronide (Pdg) for each year, organized around the FMP.
Results:
Mean menstrual cycle length was remarkably preserved at 26 to 27 days in ELA cycles; non-ELA cycles had greater variability. The percentage of cycles that were ELA remained high until 5 years before the FMP (87.9%); only 22.8% of cycles within 1 year of the FMP were ELA. Whole cycle hormones remained relatively stable up to 3 years before the FMP, when gonadotropins began to increase. Pdg excretion declined slowly with progress to the FMP, but Pdg patterns of ELA cycles remained distinguishable from non-ELA.
Conclusions:
Menstrual cycle hormone patterns in perimenopausal women resemble those of midreproductive-aged women until 5 years before menopause, and presumably ovulatory cycles retain a potentially fertile pattern up to the end of reproductive life.
The proportion of cycles with a robust progesterone rise remains high until the final 5 years of reproductive life, with overall preserved reproductive hormone patterns as women approach menopause.
Menstrual cycle hormone changes across the menopausal transition have been only partially characterized in small, cross-sectional samples (1–3) and even smaller longitudinal studies (4). These studies have often lacked daily hormone sampling to allow for a full assessment of the day-to-day hormone changes (5–8). The Study of Women’s Health Across the Nation (SWAN) Daily Hormone Study (DHS) was developed to provide a definitive data set facilitating a description of the full panoply of changes in menstrual cycle hormones occurring as women traverse menopause in the context of menstrual cycle characteristics, race/ethnicity, body size, age at final menstrual period (FMP), and lifestyle. SWAN prospectively evaluated the menstrual cycle over time in women approaching menopause (9, 10) but has only recently had sufficient numbers of women who have completed the menopausal transition to anchor data to the FMP.
A rigorous analysis of the changes in menstrual cycle hormones that occur as women become menopausal is important for several reasons. Fertility declines with age, but whether there are direct hormonal correlates of this decline is not known. The presence of distinct hormonal changes that would predict irreversible infertility would allow women more certainty in decision-making about contraception in their latter reproductive years. The symptom and menstrual bleeding experience of women traversing menopause is also highly variable, and it is possible that day-to-day hormonal patterns may predict bleeding events that would allow women and their clinicians to make more precise predictions about the nature and timing of abnormal bleeding and the possible need for preventive treatment. The basic changes that can be expected in menstrual cycles and hormones as women traverse menopause can be used to provide physiologic, normative data against which an individual’s pattern may be compared.
In prior work, we have demonstrated differences in reproductive hormone patterns associated with body mass index (BMI) and with race/ethnicity (9) and over time (10) in women followed prospectively for up to 4 years from study onset. We report herein the results of up to 10 consecutive years of the SWAN DHS in a racially and ethnically diverse cohort of women who completed daily urine collection for one entire menstrual cycle or up to 50 days (in the absence of bleeding) annually to characterize the final stages of reproductive aging as fully as possible and to anchor data to the FMP. We hypothesized that progesterone production would decline, that evidence of less orderly folliculogenesis would be more common as women approached their FMP, and that these patterns would vary by smoking, race/ethnicity, and BMI.
Methods
Participants and eligibility criteria
SWAN is a multiethnic cohort study of middle-aged women from seven communities in the United States. Data from SWAN have been previously described, as has the assembly of the larger, community based cohort from which the DHS sample was derived (11). The DHS was initiated 2 years after the SWAN inception cohort was assembled. Criteria for entry into the DHS included (1) an intact uterus and at least one ovary, (2) at least one menstrual period in the previous 3 months (i.e., either premenopausal or early perimenopausal at the time of the first collection), (3) no use of sex steroid hormones in the previous 3 months, and (4) not pregnant. At the baseline DHS, or DHS1 collection, women collected their first morning voided urine samples daily for one complete menstrual cycle or 50 days (whichever came first). Collections were repeated once a year until the FMP or up to 10 years, the the final year of the DHS (DHS10 collection). Menopausal status was defined as follows: premenopausal, bleeding in previous 3 months with no past year change in cycle predictability; early perimenopausal, bleeding in the previous 3 months with a decrease in cycle predictability in the past year; late perimenopause, between 3 and 12 months of amenorrhea; postmenopause, at least 12 months of observed amenorrhea. Menstrual onset was used to initiate all DHS collections in women who were premenopausal or early perimenopausal. As women progressed to late perimenopause, after 2 months of an unsuccessful attempt to initiate with a menstrual period, random, 50-day (or sooner if a menstrual period intervened) collections were performed. Women from the New Jersey site had some discontinuity in their data collection but were not excluded if they met the minimum eligibility criteria (observed FMP and completion of at least one DHS collection).
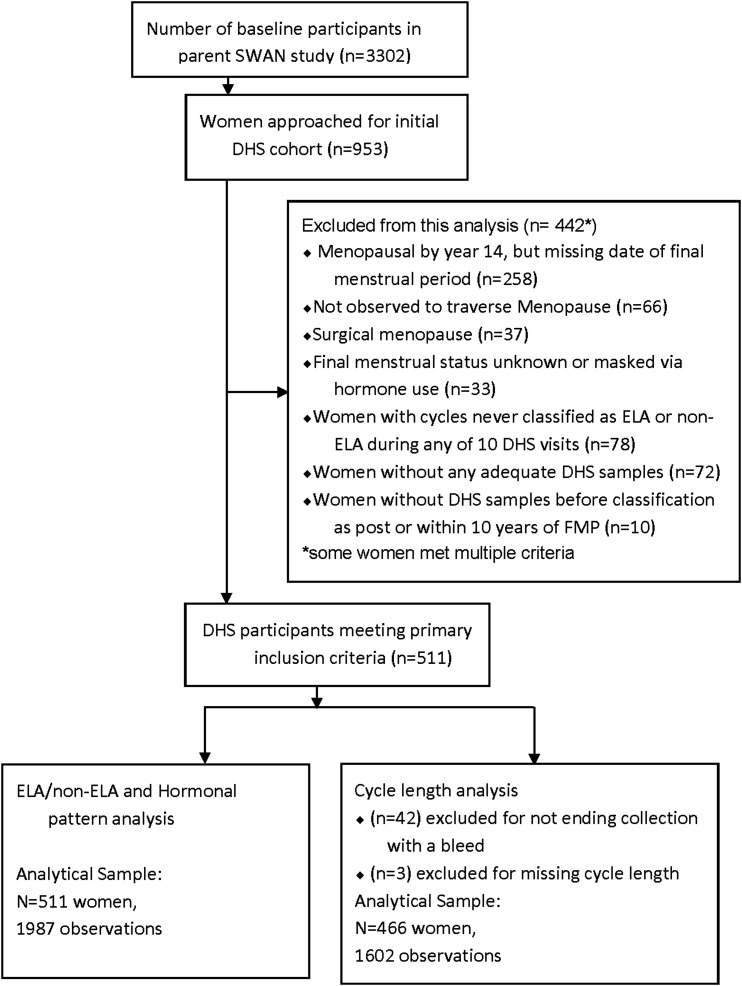
A total of 953 SWAN participants were initially enrolled in the DHS substudy. For these analyses, only women who completed at least one DHS collection and who had an observed date of FMP within 10 years after the DHS10 collection were included (n = 511; 1987 cycles). Cycles were included in hormone analysis regardless of whether the collection began with a bleed. Analyses of cycle length included only visits with known cycle length; that is, if urine collection began and ended with a bleed (n = 1584) or if cycle start and end could be determined from reported menstrual bleeding dates (n = 18); cycle length was truncated at 50 days for two women. Analyses excluded women with hysterectomy or bilateral oophorectomy, those who used reproductive hormones before their FMP, and those not yet postmenopausal by DHS10. Disposition of DHS participants is described in Fig. 1.
Figure 1.
Disposition of all participants in the DHS.
The study was approved by the Institutional Review Boards affiliated with all participating sites. Written informed consent was obtained from each participant.
Measures
Urinary follicle-stimulating hormone (FSH), luteinizing hormone (LH), estrone conjugates (E1c), and pregnanediol glucuronide (Pdg) were measured in the daily samples using prospective, validated methodologies that have been previously described in detail (12–14). Objective, validated algorithms were applied to detect hormonal patterns consistent with a preovulatory rise in urinary estrogen metabolites, a follicular-luteal phase shift in estrogen vs progesterone metabolite excretion, as well as a sustained and robust rise in progesterone metabolite excretion (15). Cycles were divided into those that qualified as having robust luteal functions (12) vs those that did not qualify as meeting this criterion. A change in the estrogen/progesterone metabolite excretion ratio was used to approximate the time of presumed ovulation in the cycles of midreproductive aged women (12–14). These algorithms have been adapted for use in older reproductive-aged women to include the identification of a midcycle surge of gonadotropins indicating the presence of a positive neuroendocrine feedback (i.e., an LH and FSH surge) (15). Cycles without evidence of luteal activity (non-ELA) did not meet the criteria set for ELA cycles.
Serum LH, FSH, estradiol, dehydroepiandrosterone sulfate, testosterone, and sex hormone binding globulin from annual sampling were measured using a previously described and validated chemiluminescent assays adapted for high-throughput (16). Whole-cycle hormones were derived using area-under-the curve methods as previously described (9, 15).
Covariates included self-reported race/ethnicity and geographic site as well as time-varying smoking and body mass index (BMI) coinciding with the annual blood draw, calculated from measured height and weight as weight/height (2).
Data analysis
Demographics and clinical characteristics of the DHS study sample were summarized using mean and standard deviation for continuous variables and using frequency and percent for categorical measures.
Descriptive statistics were generated for each of the outcomes (ELA cycles, whole-cycle hormones, follicular and luteal hormones, and the proportion of ELA to non-ELA cycles) as a function of years before the FMP.
Random effects binomial logistic regression (17) was used to model the proportion of ELA cycles, and linear mixed effects modeling was used to estimate cycle length for ELA/non-ELA cycles as a function of years before FMP (categorized into 1-year intervals) and adjusted for race/ethnicity, time-varying, three-category BMI (<25, 25 to 29.9, and ≥30 kg/m2) (18), and time-varying smoking status as well as for region (eastern United States, midwestern United States, and West Coast) to account for study design. To determine if the time course of the prevalence of ELA cycles was modified by a woman’s race/ethnicity, baseline BMI, or baseline smoking status, we tested the interactions of time before FMP with each of these variables, one at a time. Data were truncated at FMP-10 years or less due to very small cell sizes and corresponding high variability. For cycle length by ELA status figure, data were additionally truncated at FMP-8 years to avoid zero cells
Whole-cycle hormones were assessed at 1-year intervals approaching the FMP. LH, FSH, E1c, and Pdg were modeled in a linear mixed effects model; mean hormone level at each time point was categorized into 1-year intervals before the FMP. Right-skewed hormone values were analyzed on a log 10 scale, back-transformed, and reported as geometric mean and 95% confidence interval. To assess hormones within obesity categories, hormone patterns are presented by two-category BMI (<30 kg/m2 and >30 kg/m2).
Results
Study participants
Characteristics of the study participants at DHS1 are shown in Table 1. During the DHS1 collection, 953 women attempted a cycle collection and 848 cycles contained at least 80% complete samples and were evaluable (89% evaluable data) (9). By the DHS10, 69 women remained eligible and completed a urinary menstrual cycle collection (100% evaluable data). Women who participated in the DHS were more likely to be Chinese and less likely to be white than were nonparticipants, as has been previously reported. DHS participants did not differ from nonparticipants by BMI or smoking status (9). The numbers of women who completed a collection each year are shown in Supplemental Table 1 (79.4KB, docx) .
Table 1.
Baseline Characteristics of the DHS Study Sample
| Characteristic | Overall (n = 511) a |
|---|---|
| Age at first collection, y, mean (95% CI) | 47.3 (47.1–47.5) |
| Age at FMP, y; geometric mean ± SD | 52.5 ± 2.554 |
| Race/ethnicity demographics, n (%) | |
| White | 139 (27.31) |
| Japanese | 114 (22.40) |
| Black | 111 (21.81) |
| Chinese | 102 (20.04) |
| Hispanic | 43 (8.45) |
| BMI | 26.1 (25.6, 26.7) |
| BMI, n (%) | |
| <30 | 378 (74.70) |
| ≥30 | 128 (25.30) |
| Q32: Difficulty paying for basics, n (%) | |
| Very hard | 32 (6.31) |
| Somewhat | 161 (31.76) |
| Not very | 314 (61.93) |
| Q18: Live children, n (%) | |
| 0 | 84 (16.57) |
| ≥1 | 423 (83.43) |
| Site/region, n (%) | |
| East | 147 (28.77) |
| Midwest | 106 (20.74) |
| West | 258 (50.49) |
| Menopausal status at DHS 1, n (%) | |
| Early perimenopausal | 358 (70.20) |
| Premenopausal | 152 (29.80) |
| FSH, geometric mean (95% CI) | 20.2 (19.0–21.5) |
Abbreviations: CI, confidence interval; SD, standard deviation.
Some items have responses from a smaller subset than the n = 511 in the analytical DHS cohort; as such, some frequencies will add to a smaller total.
Luteal activity in relation to the FMP
The proportion of ELA cycles was near 100% at enrollment into the study (or 10 years before the FMP) and declined slowly until about 5 years before the FMP (87.9%); thereafter, %ELA cycles rapidly declined (Fig. 2). By the year before the FMP (FMP-1), only 22.8% of cycles were ELA. Adjustment for covariates had little impact on these proportions. Interactions of years prior to the FMP with race/ethnicity, smoking, and BMI at DHS1 were not statistically significant (P > 0.27 for all interactions).
Figure 2.
Percent of cycles with and without ELA by time FMP.
Cycle length changes with progress toward menopause
Menstrual cycle length remained stable up to 4 years before the FMP when they became longer (Fig. 3). Most of this change was related to non-ELA cycles, which at certain times were longer (FMP-4 to FMP-3) or shorter (FMP-8 to FMP-6 and FMP-3) than ELA cycles for the same premenopausal year. ELA cycles were overall remarkably preserved for 27 to 29 days. Interaction of years before the FMP with BMI categories at the first visit of DHS was significant overall (P = 0.01). In general, BMI >30 kg/m2 at DHS1 was associated with longer cycle length over 10 years before the FMP, except at FMP-1, when cycle length in obese women became shorter compared with women in the other two BMI categories. Additional adjustment for region and concurrent smoking had little impact, and baseline BMI remained an important predictor of cycle length. Interactions of years before the FMP with race/ethnicity and smoking at the DHS1 visit were not statistically significant in the overall sample.
Figure 3.
Predicted menstrual cycle length by luteal activity status. ELA cycles are shown in solid lines; non-ELA cycles are in dashed lines. Data are adjusted for race/ethnicity and region and concurrent BMI and smoking status.
Reproductive hormone patterns with progress toward menopause
Estrone conjugate excretion, FSH, LH, and Pdg remained relatively stable in ELA cycles as women approached menopause (Fig. 4). There was a slow decline in luteal Pdg across the years leading up to the FMP; however, the Pdg patterns of ELA cycles remained distinguishable from non-ELA throughout most of these years. On the other hand, non-ELA cycles exhibited substantially more year-to-year variability in mean gonadotropins and E1c, especially in the years FMP-10 to FMP-5 (Fig. 4). LH and FSH demonstrated small increases over time in non-ELA cycles, with the most striking rises in both hormones occurring at FMP-3 to FMP. Follicular and luteal phase hormones are shown in ELA cycles in Fig. 5(a), highlighting the change over time in luteal Pdg.
Figure 4.
Whole-cycle hormones by cycle type: ELA and non-ELA cycles are shown. Shaded area depicts the 95% confidence interval.
Figure 5.
(a) Whole-cycle hormones geometric mean in relation to FMP: ELA cycles only. Shaded area is ± 95% confidence interval. Follicular and luteal phase hormones as indicated. Note the follicular FSH rise in years FMP-3 onward and the progressive, slow decline in luteal Pdg. (b) Whole-cycle hormone patterns in relation to the FMP by two-category BMI (<30 kg/m2 and >30 kg/m2). Shaded areas indicate 95% confidence interval. Note the lower Pdg excretion in women with a BMI >30 kg/m2.
BMI related differences in cycles and hormones using two-category BMI
Women with BMI ≥30 kg/m2 excreted overall less LH, FSH, E1c, and Pdg than did women with BMI <30 kg/m2 [Fig. 5(b)]. The BMI related differences were most evident for Pdg and least for E1c and FSH. Pdg was overall lower in women with a BMI ≥30 kg/m2 and did not decline as much as was seen in women with a lower BMI as women approached FMP-1, making a distinction between the BMI groups more difficult as the FMP approached.
Racial/ethnic differences in cycles and hormones
Adjusting for years before FMP, the probability of having an ELA cycle was similar for white, Japanese, and Chinese women (80.6%, 82.9%, and 82.1%, respectively) and lower for African American and Hispanic women (72.6% and 66.3%, respectively; P = 0.019). Additional adjustment for region, concurrent smoking, and concurrent BMI reduced statistical significance (adjusted P = 0.11), but the pattern of racial/ethnic differences remained similar: adjusted % ELA was 62.2% for Hispanic subjects, 69.6% for African American subjects, 79.7% for white subjects, 83.7% for Japanese subjects, and 84.3% for Chinese subjects. The interaction of race/ethnicity and years before FMP was not statistically significant (P = 0.67) because ELA cycles were less prevalent for African American and Hispanic women consistently over the interval FMP-10 years to FMP. Although the interaction of race/ethnicity with categorized BMI was not statistically significant (P = 0.16), there was a suggestion of effect modification by BMI such that ethnic differences were largest in the obese (>30 kg/m2) category. Chinese and Japanese women were most likely and African American and Hispanic women were least likely to have an ELA cycle only in this BMI category.
Concurrent, three-category BMI-related differences in cycles and hormones
Adjusting for years before FMP, the probability of an ELA cycle was somewhat lower at visits when a participant was obese (74.0%) compared with visits when the participant was normal weight or overweight (81.2% and 80.1%, respectively; P = 0.06). Upon adjustment for race/ethnicity, however, the BMI-related differences were somewhat attenuated; the ethnicity-adjusted ELA ranged from 76.9% for obese, 79.9% for normal-weight, and 81.0% for overweight and were no longer statistically significant (P = 0.49). As noted above, the interaction of race/ethnicity and BMI was not statistically significant. Obesity was associated with a somewhat higher—rather than lower—proportion of cycles with ELA among Chinese and Japanese participants, although this difference was not statistically significant for either race/ethnic group. BMI was negatively associated with the proportion of cycles with ELA among the other three racial/ethnic groups, particularly African American women.
Concurrent smoking-related differences in cycles and hormones
Adjusting for years before FMP, the probability of an ELA cycle did not differ by time-varying smoking status (79.1% for nonsmoking and 76.5% for smoking; P = 0.56). Adjustment for covariates reduced this difference even further (79.4% and 78.6%; P = 0.86).
Discussion
Herein we demonstrate key changes in the pattern of the human menstrual cycle and reproductive hormones as women traverse the final stages of reproductive aging and approach their FMP. Regardless of whether a cycle had evidence of luteal activity or not, menstrual cycle length remained remarkably preserved in women up to the FMP, and length of ELA cycles was mostly within a range of 27 to 31 days. This is in keeping with the classic data of Treloar et al. (19), who reported on menstrual cycle length in women aged 11 to 51 years. In this study, women approaching age 51 demonstrated an overall well preserved lower bound of menstrual cycle lengths, with a 90th centile lower boundary of approximately 21 days, but a much longer 90th centile upper boundary (about 45 days) due to the inclusion of long intervals of amenorrhea. Cycle length data reported herein excluded cycles that did not end with a menstrual period (n = 42 women) (Fig. 1).
Hormone excretion remained remarkably preserved among cycles meeting the criteria for luteal activity, although Pdg demonstrated a small but significant steady decline over the years. Taken together, the data suggest that hormonally normal–appearing menstrual cycles, though they become rarer, can occur right up to the end of reproductive life. These findings suggest that fertility or risk for unintended pregnancy, at least based on urine metabolite hormone patterns, should not be minimized or dismissed on the basis of age or presumed menstrual cycle status until a woman has indeed had her FMP and is by definition menopausal (i.e., 12 consecutive months of amenorrhea) (20). Even this definition does not preclude subsequent ovulation (21).
Fertility potential and the menopausal transition
Natural fertility rates decline in women throughout their reproductive life span (22). After establishment of regular cycling shortly after menarche, women enter their peak reproductive years and maintain the highest levels of fecundity in their third decade of life. By the fourth decade, fertility rates decline, and by the fifth decade of life, a substantial proportion of women are unable to conceive (23). Reduced fertility potential occurs despite the presence of regular ovulatory menstrual cycles and is largely attributed to oocyte aging (24). Our data suggest that, beyond oocyte aging, infertility in the fifth decade of life may also be due, at least in part, to anovulation, luteal insufficiency, or both. Small-cohort studies of the menstrual cycles and follicle growth dynamics of women in their mid- to late 40s have revealed changes in follicular phase hormones (25), abnormal menstrual cycle hormone patterns, and aberrant follicle growth (7, 26–28). Some cycles demonstrate evidence of folliculogenesis yet a complete lack of luteal activity (26) and in some cases demonstrate a failure to mount an LH surge in response to appropriate preovulatory estrogen positive feedback conditions (29). Other women identify inappropriate development of new follicles during the luteal phase of the cycle, leading to abbreviated luteal phases, one atop the next (7). These mechanisms merit further exploration as potential endocrine causes of subfertility in reproductively aged women who wish to conceive because these women may be amenable to treatment.
Hormone synthesis is maintained throughout the menopausal transition
As women age, their follicles appear to be larger early in the cycle and to develop more rapidly (7, 26, 27), but their growth rate is overall slower than the growth rate in younger women (26). Peripheral (30, 31), though not local, production of key granulosa cell products, inhibin A and B, is impaired, often before women note anything more than subtle changes in their menstrual pattern (32). In older women, ovarian follicles contain fewer granulosa cells (33), but they produce proportionately more estradiol due to increased aromatase activity (34). Thus, the follicle appears to possess some compensatory mechanisms that preserve midreproductive aged hormone patterns. Progressively rising FSH levels maintain follicle growth, enhance aromatase, and shorten the follicular phase by several days (19), despite the smaller numbers of granulosa cells and follicles available. When ovulation occurs, overall Pdg production is not maintained, and relative luteal insufficiency is observed (35). Nonetheless, that the overall patterns of LH, FSH, estradiol, and progesterone in ELA cycles are maintained nearly to the very end of reproductive life in women is truly remarkable. However, because our measure of urinary estrone conjugates includes both estrone and estradiol, we may have underestimated the amount of change in estradiol across the DHS because estrone is a far more abundant hormone in the circulation of women.
Perimenopausal hormone patterns and risk for uterine pathology
The maintenance of E1c excretion and the decline or absence of significant Pdg excretion in many of the cycles examined indicates that perimenopausal women are more likely to be exposed to an “unopposed estrogen” environment, which could promote the development of endometrial proliferation, hyperplasia, or even cancer. The age-specific prevalence of endometrial hyperplasia without atypia peaks in women aged 50 to 54 years, with the peak prevalence of atypical hyperplasia appearing in their early 60s (36). Endometrial carcinoma prevalence is highest in women in their early 70s, well after the menopausal transition has occurred (37). Exposure to estrogen without concomitant progestin can increase the risk of endometrial hyperplasia (38) and, if prolonged, endometrial cancer (39). Modern menopausal hormone therapy takes these principles into account, and women with an intact uterus are not recommended to receive estrogen-only hormone therapy secondary to the excess risk of hyperplasia and carcinoma. It is therefore of great interest to see so many cycles in the late perimenopausal period with markedly low progesterone metabolite excretion. Successive cycles with low or absent progesterone production may be associated with increased endometrial abnormalities, such as polyps, unscheduled bleeding, or hyperplasia. Alternatively, although the small contribution of Pdg in non-ELA cycles does not meet the threshold for ELA, as defined by the algorithms used, Pdg may be still be sufficient to prevent excessive endometrial growth (40). The transient nature of this relatively unopposed estrogen exposure may be sufficient to cause an increase in endometrial hyperplasia in late perimenopausal women but not progression to cancer. In younger women exposed to unopposed estrogen during adolescence, endometrial abnormalities do not appear as prevalent; however, adolescents with polycystic ovary syndrome have been reported to develop hyperplasia and even carcinoma before the age of 25 (41).
Modifiers of the perimenopausal hormone milieu
Hormonal patterns of women traversing the menopause were modified by a number of demographic characteristics. BMI, which we previously reported was associated with lower overall menstrual cycle hormones (9), was again related to lower hormone output, but this association was completely attenuated after adjustment for race/ethnicity. BMI modifies perimenopausal hormone production in a complex way, with overall lower follicular phase estradiol before the FMP and higher estradiol thereafter, likely owing to the emergence of peripheral sources of estradiol production other than the ovary (42). It is possible that the data set available in the DHS, which does not include postmenopausal observations, was insufficient to clarify this change in the relationship of hormones to BMI. The likelihood of ELA cycles varied with race/ethnicity, with Hispanic and African American women being more likely to have non-ELA cycles. Again, after adjustment for covariates, these differences were no longer statistically significant, but a trend remained. Although we failed to detect a significant interaction, the observed nonsignificant BMI-related differences in the proportions of cycles with ELA by race/ethnicity include differences of clinical interest, with the largest obesity-related difference seen in African American women. Surprisingly, Chinese and Japanese women in this high BMI category did not demonstrate a decrease in the %ELA cycles. These findings await further exploration.
In the Penn Ovarian Aging Study cohort (n = 436), relative to white women, African American women demonstrated lower dehydroepiandrosterone sulfate and inconsistently lower follicular phase estradiol (43). Moreover, a more pronounced negative relationship between age and estradiol and BMI and estradiol was observed in African American women relative to white women (43). Mediation of hormone excretion by race/ethnicity and BMI have been reported in the SWAN cohort and will be explored further in subsequent examinations of these data; however, in this initial analysis a clear-cut relationships between race/ethnicity and hormone patterns and the likelihood of an ELA cycle were not obvious and did not persist in multivariable analyses. Other possible mediators of hormone excretion could include genetic variations in hormones or their receptors. For example, Sowers et al. (44) reported an association of the ESR1 rs3798557 genotype with more advanced ovarian aging, a factor that might influence the proportion of ELA to non-ELA cycles.
Further modeling of the data, including taking into account measures of financial strain, which differ widely among the racial ethnic groups in SWAN, did not explain the associations between race/ethnicity and ELA status. Hispanic women in the SWAN cohort experienced the greatest degree of financial strain (difficulty paying for basics), which may affect the function of the hypothalamic pituitary axis and is associated with greater risk of non-ELA cycles. Alternatively, the higher rate of metabolic syndrome observed in the Hispanic women in SWAN may contribute to dyslipidemia and perhaps a less healthy overall redox state, which might make them prone to less efficient ovarian function (45). However, the number of Hispanic women in the DHS is relatively small, and the numbers of participants included may not have provided enough statistical power to demonstrate these points.
Study strengths and limitations
The strengths of this study include the overall large sample size, the well-characterized cohort, and the inclusion of women from five different race/ethnic groups. The use of daily sampling to assess reproductive hormone patterns, rather than inferring the patterns from samples obtained monthly, weekly, or biweekly, allows for very precise resolution of the events that comprise a normal menstrual cycle. These strengths allow us to refine our conclusions on the basis of a number of participant-specific factors, such as racial/ethnic, BMI, and lifestyle factors, and to discover novel associations between them and hormone patterns.
There are several limitations of this study. Cycle collection for the DHS generally began with the onset of a menstrual period (about 80% of cycles in this report), which may have selected for relatively stable, midreproductive-type of hormone patterns than a truly random timing of the urine collection might have done. Because cycles that did not end with a bleed were excluded from this analysis, the cycle length data are not representative of the entire menopausal transition experience. The criteria for ELA include a relatively robust increment in Pdg consistent with fertile cycles of midreproductive aged women (12), and perimenopausal women may have more of a continuum of Pdg excretion patterns that are represented in the non-ELA cycles, which is a more heterogeneous group, even at DHS1 when all participants in the early stages of the transition (27). Currently these non-ELA cycles are being assessed in more detail. Additional data available on bleeding patterns (46), symptoms (47), and relationships of hormones to inflammatory and metabolic markers, which have been partially explored in prior DHS publications (48), are beyond the scope of this study, and a full synthesis of these data awaits subsequent analyses. We were not able to follow all women from enrollment through the FMP, which may introduce bias due to the differential loss to follow-up. Some women who provided annual specimens for multiple years were represented more than women who only completed a single DHS collection. Our multivariate analyses, however, adjusted for key factors related to attrition, including race/ethnicity, which should reduce nonresponse bias. Our use of mixed models, which are robust to intermittently missing data, also helps to minimize the introduction of bias due to differential loss to follow-up. Overall, women who did not continue participation in SWAN were of lower socioeconomic status and were more likely to have non-ELA collections at the DHS1 visit (10). Because these factors may have contributed to an increased likelihood of non-ELA cycles, this study may have underestimated the prevalence of disrupted hormone patterns in women leading up to the FMP.
Conclusion
This study provides evidence that relatively normal menstrual cycles, with hormone patterns resembling those of women of peak fertility, though rare, can occur up to the very end of reproductive life in women. The approach to the FMP is characterized by lower progesterone metabolite excretion, erratic estradiol excretion, and an eventual rise in gonadotropins as the ovary ultimately undergoes follicular exhaustion. The prevalence of cycles with low Pdg excretion (non-ELA) increases rapidly in the final 3 years before the FMP and is associated with more variability in cycle length but not with large changes in bleeding duration.
Acknowledgments
The authors thank the study staff at each site and all the women who participated in the SWAN.
Acknowledgments
The SWAN has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women’s Health (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Nursing Research, the National Institutes of Health Office of Research on Women’s Health, or the National Institutes of Health.
Clinical Centers: University of Michigan, Ann Arbor: Siobán Harlow, PI, 2011–present; MaryFran Sowers, PI, 1994–2011. Massachusetts General Hospital, Boston, MA: Joel Finkelstein, PI, 1999–present; Robert Neer, PI, 1994–999. Rush University, Rush University Medical Center, Chicago, IL: Howard Kravitz, PI, 2009–present; Lynda Powell, PI, 1994–2009. University of California, Davis/Kaiser: Ellen Gold, PI. University of California, Los Angeles: Gail Greendale, PI. Albert Einstein College of Medicine, Bronx, NY: Carol Derby, PI, 2011–present; Rachel Wildman, PI, 2010–2011; Nanette Santoro, PI, 2004–2010. University of Medicine and Dentistry, New Jersey Medical School, Newark, NJ: Gerson Weiss, PI, 1994–2004. University of Pittsburgh, Pittsburgh, PA: Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD: Chhanda Dutta, 2016–present; Winifred Rossi, 2012–2016; Sherry Sherman, 1994–2012; Marcia Ory, 1994–2001. National Institute of Nursing Research, Bethesda, MD Program Officers.
Central Laboratory: University of Michigan, Ann Arbor, MI: Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA: Maria Mori Brooks, PI, 2012–present; Kim Sutton-Tyrrell, PI, 2001–2012. New England Research Institutes, Watertown, MA: Sonja McKinlay, PI, 1995–01.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
Footnotes
- BMI
- body mass index
- DHS
- Daily Hormone Study
- W1c
- estrone conjugates
- ELA
- evidence of luteal activity
- FMP
- final menstrual period
- FSH
- follicle-stimulating hormone
- LH
- luteinizing hormone
- Pdg
- pregnanediol glucuronide
- SWAN
- Study of Women’s Health Across the Nation.
References
- 1.Metcalf MG. Incidence of ovulatory cycles in women approaching the menopause. J Biosoc Sci. 1979;11(1):39–48. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf MG. The approach of menopause: a New Zealand study. N Z Med J. 1988;101(841):103–106. [PubMed] [Google Scholar]
- 3.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–1501. [DOI] [PubMed] [Google Scholar]
- 4.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function before, during and after the menopause: a longitudinal study. Clin Endocrinol (Oxf). 1982;17(5):489–494. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf MG, Livesey JH. Gonadotrophin excretion in fertile women: effect of age and the onset of the menopausal transition. J Endocrinol. 1985;105(3):357–362. [DOI] [PubMed] [Google Scholar]
- 6.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function in normal women during the menopausal transition. Clin Endocrinol (Oxf). 1981;14(3):245–255. [DOI] [PubMed] [Google Scholar]
- 7.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16(1):50–59. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor KA, Ferrell RJ, Brindle E, Shofer J, Holman DJ, Miller RC, Schechter DE, Singer B, Weinstein M. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009;18(3):828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89(6):2622–2631. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Crawford SL, Lasley WL, Luborsky JL, Matthews KA, McConnell D, Randolph JF, Jr, Gold EB, Greendale GA, Korenman SG, Powell L, Sowers MF, Weiss G. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab. 2008;93(5):1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sowers MF, Crawford SL, Sternfeld B. SWAN: a multicenter, multiethnic community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, and Marcus R, eds. Menopause: Biology and Pathobiology. New York: Academic Press; 2000:175–188. [Google Scholar]
- 12.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 1996;104(4):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol. 1998;147(11):1071–1080. [DOI] [PubMed] [Google Scholar]
- 14.Baird DD, McConnaughey DR, Weinberg CR, Musey PI, Collins DC, Kesner JS, Knecht EA, Wilcox AJ. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995;6(5):547–550. [DOI] [PubMed] [Google Scholar]
- 15.Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, Lasley BL, McConnell D, McGaffigan P, Midgely R, Schocken M, Sowers M, Weiss G. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284(3):E521–E530. [DOI] [PubMed] [Google Scholar]
- 16.Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–1522. [DOI] [PubMed] [Google Scholar]
- 17.Molenberghs G, Verbeke G, Demétrio CG. An extended random-effects approach to modeling repeated, overdispersed count data. Lifetime Data Anal. 2007;13(4):513–531. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control . Healthy weight. Available at: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/. Accessed 4 April 2017.
- 19.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 20.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW + 10 Collaborative Group . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seungdamrong A, Weiss G. Ovulation in a postmenopausal woman. Fertil Steril. 2007;88:1438 e1-2. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor KA, Holman DJ, Wood JW. Declining fecundity and ovarian ageing in natural fertility populations. Maturitas. 1998;30(2):127–136. [DOI] [PubMed] [Google Scholar]
- 23.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW + 10 Collaborative Group . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Society for Assisted Reproductive Technology (SART) . What are my chances with ART? Available at: https://www.sartcorsonline.com/Predictor/Patient. Accessed 4 April 2017.
- 25.Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, Nelson DB. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83(2):383–392. [DOI] [PubMed] [Google Scholar]
- 26.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–5509. [DOI] [PubMed] [Google Scholar]
- 27.Vanden Brink H, Chizen D, Hale G, Baerwald A. Age-related changes in major ovarian follicular wave dynamics during the human menstrual cycle. Menopause. 2013;20(12):1243–1254. [DOI] [PubMed] [Google Scholar]
- 28.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81(3):1038–1045. [DOI] [PubMed] [Google Scholar]
- 29.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292(24):2991–2996. [DOI] [PubMed] [Google Scholar]
- 30.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84(1):105–111. [DOI] [PubMed] [Google Scholar]
- 31.Klein NA, Battaglia DE, Miller PB, Branigan EF, Giudice LC, Soules MR. Ovarian follicular development and the follicular fluid hormones and growth factors in normal women of advanced reproductive age. J Clin Endocrinol Metab. 1996;81(5):1946–1951. [DOI] [PubMed] [Google Scholar]
- 32.Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelson DB. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12(2):128–135. [DOI] [PubMed] [Google Scholar]
- 33.Seifer DB, Gardiner AC, Ferreira KA, Peluso JJ. Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril. 1996;66(4):593–598. [DOI] [PubMed] [Google Scholar]
- 34.Shaw ND, Srouji SS, Welt CK, Cox KH, Fox JH, Adams JA, Sluss PM, Hall JE. Compensatory increase in ovarian aromatase in older regularly cycling women. J Clin Endocrinol Metab. 2015;100(9):3539–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, Weinstein M. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16(6):1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed SD, Newton KM, Clinton WL, Epplein, M, Garcia, R, Allison, K, Voigt, LF, Weiss, NS. Incidence of endometrial hyperplasia. Am J Obstet Gynecol. 2009;200:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacey JV, Jr, Chia VM, Rush BB, Carreon DJ, Richesson DA, Ioffe OB, Ronnett BM, Chatterjee N, Langholz B, Sherman ME, Glass AG. Incidence rates of endometrial hyperplasia, endometrial cancer and hysterectomy from 1980 to 2003 within a large prepaid health plan. Int J Cancer. 2012;131(8):1921–1929. [DOI] [PubMed] [Google Scholar]
- 38.The Writing Group for the PEPI Trial . Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370–375. [DOI] [PubMed] [Google Scholar]
- 39.Weiss NS, Szekely DR, English DR, Schweid AI. Endometrial cancer in relation to patterns of menopausal estrogen use. JAMA. 1979;242(3):261–264. [PubMed] [Google Scholar]
- 40.Usadi RS, Groll JM, Lessey BA, Lininger RA, Zaino RJ, Fritz MA, Young SL. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93(10):4058–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farhi DC, Nosanchuk J, Silverberg SG. Endometrial adenocarcinoma in women under 25 years of age. Obstet Gynecol. 1986;68(6):741–745. [PubMed] [Google Scholar]
- 42.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manson JM, Sammel MD, Freeman EW, Grisso JA. Racial differences in sex hormone levels in women approaching the transition to menopause. Fertil Steril. 2001;75(2):297–304. [DOI] [PubMed] [Google Scholar]
- 44.Sowers MR, Jannausch ML, McConnell DS, Kardia SR, Randolph JF, Jr. Menstrual cycle markers of ovarian aging and sex steroid hormone genotypes. Am J Med. 2006; 119(9, Suppl 1)S31–S43. [DOI] [PubMed] [Google Scholar]
- 45.Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Voorhis BJ, Santoro N, Harlow S, Crawford SL, Randolph J. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol. 2008;112(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavlović JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, Lipton RB, Derby CA. Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology. 2016;87(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allshouse AA, Polotsky A, Crawford S, Chen HY, El Khoudary SR, Santoro N. Consistent ovulation may not be enough to make women healthy when approaching menopause: an update from the Study of Women’s Health Across the Nation. Menopause. 2015;22(3):267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]