Abstract
Context:
Traditional risk factors for type 2 diabetes mellitus are weak predictors of changes in glucose tolerance and insulin sensitivity in youth.
Objective:
To identify early metabolic features of insulin resistance (IR) in youth and whether they predict deterioration of glycemic control.
Design and Setting:
A cross-sectional and longitudinal study was conducted at the Yale Pediatric Obesity Clinic.
Patients and Intervention:
Concentrations of α-hydroxybutyrate, β-hydroxybutyrate, lactate, and branched-chain amino acids (BCAAs) were measured by nuclear magnetic resonance spectroscopy in 78 nondiabetic adolescents during an oral glucose tolerance test (OGTT). Associations between baseline metabolic alterations and longitudinal changes in glucose control were tested in 16 subjects after a mean follow-up of 2.3 years.
Main Outcome Measures:
The relationship between metabolite levels, parameters of IR, and glycemic control, and their progression over time.
Results:
Elevated fasting α-hydroxybutyrate levels were observed in adolescents with reduced insulin sensitivity after adjusting for age, sex, ethnicity, Tanner stage, and body mass index z-score (P = 0.014). Plasma α-hydroxybutyrate and BCAAs were increased throughout the course of the OGTT in this group (P < 0.03). Notably, borderline IR was associated with a progressive α-hydroxybutyrate decrease from elevated baseline concentrations to normal levels (P = 0.02). Increased baseline α-hydroxybutyrate concentrations were further associated with progressive worsening of glucose tolerance and disposition index.
Conclusion:
α-Hydroxybutyrate and BCAA concentrations during an OGTT characterize insulin-resistant youth and predict worsening of glycemic control. These findings provide potential biomarkers for risk assessment of type 2 diabetes and new insights into IR pathogenesis.
Elevated α-hydroxybutyrate and branched-chain amino acid levels characterize insulin resistance and predict deterioration of glycemic control in adolescents.
The rising prevalence of type 2 diabetes mellitus (T2DM) in adults and youth is associated with excess morbidity and mortality in affected individuals and places a large economic burden on society (1–4). Traditional risk factors for T2DM, particularly fasting plasma glucose, are weak predictors of changes in glucose tolerance and insulin sensitivity in youth (5). However, early alterations in several circulating nonglucose metabolites may be associated with reduced insulin sensitivity (6, 7) and the development of T2DM (8–11) as well. Elevated fasting concentrations of branched-chain amino acids (BCAAs; i.e., leucine, isoleucine, and valine) (8) and α-hydroxybutyrate (9–11) have been associated with insulin resistance (IR) and T2DM in adults. Furthermore, metabolomic studies using oral glucose-tolerance testing (OGTT) have found altered excursions of several metabolites in nondiabetic adults with IR (6, 7). Among these metabolites, BCAAs, β-hydroxybutyrate, and lactate are of particular interest because they reflect different axes of insulin action, namely, proteolysis, ketogenesis, and glycolysis (6, 7). These initial studies have provided the framework for further investigation of these small molecules as potential biomarkers for risk stratification and early recognition of T2DM, as well as for understanding mechanisms underlying IR.
Results obtained in adults may reflect the presence of possible confounders, such as smoking, alcohol consumption, age-related IR, and metabolic adaptive changes after long-standing disease. To exclude the known impact of these confounding factors on metabolism, metabolomic studies have been repeated in children and adolescents, leading sometimes to unexpected or contradictory findings (12–15). Conflicting results might be due to different study population characteristics as well as a low sensitivity of fasting metabolite analyses in detecting subtle alterations of insulin-induced fuel processing that mark incipient IR in youth. However, these alterations may be revealed by measuring plasma metabolites during an OGTT, a challenge that provides a dynamic view of biochemical changes produced by glucose-induced hyperinsulinemia. To our knowledge, no previous studies in youth have evaluated plasma profiles of relevant nonglucose metabolites during an OGTT or the putative association of these alterations with longitudinal changes in glucose control.
To characterize early metabolic changes induced by IR in youth, we applied a proton nuclear magnetic resonance spectroscopy (1H- NMR) procedure (16) to measure plasma concentrations of six metabolites at fasting and during an OGTT. These include BCAAs, α-hydroxybutyrate, β-hydroxybutyrate, and lactate, which reflect different axes of insulin action and have been implicated in the pathogenesis of T2DM in adults. We found that fasting levels of α-hydroxybutyrate and the time courses of α-hydroxybutyrate and BCAAs in response to a glucose challenge characterize IR in youth. We further determined that alterations in the processing of these metabolites might predict longitudinal deterioration in glucose tolerance and β-cell function relative to insulin sensitivity.
Material and Methods
Study cohort
Seventy-eight nondiabetic children and adolescents, age 8 to 18 years, were recruited from the Yale Pediatric Obesity Clinic. All participants had a detailed medical history, including information on absence of alcohol consumption and smoking, and a complete physical examination. Subjects with medical conditions or using medications that may affect glucose, amino acid, or lipid metabolism were excluded. A standard OGTT [1.75 g/kg body weight, up to 75 g (17)] was performed in all subjects at the Yale Clinical Center for Investigation after an overnight fast (10 to 12 hours) (18). After a follow-up of 2 years, a subgroup of 16 participants underwent a second OGTT to assess longitudinal changes in glucose control and indexes of insulin sensitivity and secretion. Insulin sensitivity was calculated using the OGTT-derived Whole-Body Insulin Sensitivity Index (WBISI) (19), and the acute insulin response was estimated using the Insulinogenic Index (IGI) (20). The oral Disposition Index (DI) was then calculated as the product of the WBISI and the IGI (21). Subjects were stratified into tertiles according to the individual WBISI. Before the formation of the three groups, we were unaware of potential differences in any of the outcomes measured.
The study was approved by the Yale University Human Investigation Committee. Written informed parental consent and written child assent were obtained from all participants. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Biochemical analyses
Plasma glucose levels at 0, 30, 60, 90, and 120 minutes during the OGTT were determined immediately using the YSI 2700 STAT glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin levels at 0, 30, 60, 90, and 120 minutes during the OGTT were measured using double antibody radioimmunoassay from Millipore (Billerica, MA).
Metabolite analyses were performed using a novel, targeted, quantitative 1H-NMR metabolomics technique that was recently developed and validated by our group (16). During the OGTT, venous blood samples were drawn at 0, 30, 60, 90, and 120 minutes into heparin-containing tubes. Tubes were chilled on ice and centrifuged within 1 hour. Plasma samples were stored at 193K before analysis, when a 300-µL plasma sample was taken and diluted with 300 µL of solution containing 250 mM phosphate buffer (pH 7.4), 5 mM formate, and 10% D2O. All 1H-NMR analyses were performed on a Bruker 11.74 T magnet (500.13 MHz for 1H) interfaced to an Avance III spectrometer equipped with triple-axis gradients (both Bruker Corp., Billerica, MA). The MR pulse sequence consisted of an adiabatic double-spin echo in which low and high diffusion-weighting was introduced with bipolar magnetic field gradients achieving diffusion weighting b factors of 0.01 and 15 ms/μm2, respectively. All samples were measured at 298K with 96 averages and a repetition time of 6000/3000 ms for low/high b value acquisitions. Data preprocessing included zero-filling to 32,000 points, fast Fourier transformation, zero-order phase correction, and chemical shift referencing of formate to 8.444 ppm. 1H-NMR spectra were quantified with a modified spectral fitting algorithm that simultaneously uses the lipoprotein and metabolite signals as previously described (16). Six metabolites were analyzed in the current study: α-hydroxybutyrate [Chemical Entities of Biological Interest (CHEBI):64552), β-hydroxybutyrate (CHEBI:37054), isoleucine (CHEBI:17191), lactate (CHEBI:75228), leucine (CHEBI:25017), and valine (CHEBI:27266) (22). Metabolite identifiers can be found through CHEBI (http://www.ebi.ac.uk/chebi). The spectral basis set was constructed through density matrix simulations in SpinWizard, a home-written program based in Matlab 8.0 (The Mathworks, Natick, MA).
Statistical analyses
Cross-sectional differences in patient characteristics were tested using one-way analysis of variance or χ2 tests for continuous and categorical data, respectively. If these tests revealed significant effects, post hoc pairwise comparisons between groups were performed using Dunnett tests. Areas under the curve (AUCs) were calculated by the trapezoidal rule. Correlations between variables were tested using Spearman rank correlations. General linear models were used to test for association between insulin sensitivity (WBISI tertile) and metabolite concentrations at fasting and throughout the OGTT (AUCs), adjusting for age, sex, ethnicity, and body mass index (BMI) z-score. To control for the potential confounding effect of different development stages in our pediatric population, the model was further corrected by including the Tanner stage as a covariate. Mixed-model repeated measures analysis was performed to compare OGTT profiles between WBISI tertiles by including time, WBISI tertile (WBISI), and time-WBISI tertile interaction (Time–WBISI) as fixed factors and subject as random effect, with unstructured covariance matrix to account for correlations between repeated measurements within the same individuals. The mixed model was further adjusted for potential confounding factors by including age, sex, ethnicity, and BMI z-score as covariates.
Based on these results, general linear regressions were used to test for correlations between longitudinal changes in indices of glucose control, insulin sensitivity, and insulin secretion [i.e., fasting and 2-hour glucose concentrations, hemoglobin A1c (HbA1c), WBISI, IGI, DI] and baseline fasting concentrations of α-hydroxybutyrate, its excursions throughout the OGTT (AUC), as well as the excursions of BCAAs, adjusting for changes in BMI z-score over time and follow-up duration. Multivariate linear regression analysis was also used to test for association between known metabolic risk factors (including fasting and 2-hour glucose levels, fasting insulin level, HbA1c, and BMI z-score) and changes in the DI, 2-hour glucose levels, and WBISI over time. We further assessed the effect of adding α-hydroxybutyrate AUC or its concentrations at either fasting, 90 minutes, and 120 minutes on the ability of the model to predict deteriorations of glucose control. Statistical tests were conducted using a two-sided α level of 0.05. Data are represented as mean ± standard deviation, unless otherwise specified. Statistical analyses were performed using JMP Pro 11.2.0 (SAS Institute, Cary, NC).
Results
Study cohort characteristics
Study participants were divided into three groups (tertiles) according to the individual WBISI (n = 26 for each group). The main anthropometric and metabolic characteristics of the three tertiles are shown in Table 1. Groups were matched for age, sex, and ethnicity. As expected, adolescents with lower insulin sensitivity (first WBISI tertile) had higher BMI, BMI z-score, fasting and 2-hour glucose levels, fasting insulin level, hemoglobin A1C, IGI, triglyceride levels, transaminase levels, and lower DI and HDL cholesterol levels than adolescents with higher insulin sensitivity (third WBISI tertile). Sixteen subjects underwent a second OGTT after a follow-up of ∼2 years. The main features of participants included in the longitudinal analyses were similar to those of the whole study population, including age (15.4 ± 3.1 years; P = 0.52), sex (nine males and seven females; P = 0.33), ethnicity (nine whites, two blacks, three Hispanics, two Asians; P = 0.83), BMI (30.5 ± 7.1 kg/m2; P = 0.50), BMI z-score (1.76 ± 1.03; P = 0.67), WBISI (2.23 ± 1.86; P = 0.28), IGI (3.15 ± 1.6; P = 0.75); DI (4.4 ± 1.5; P = 0.07), HbA1c (5.4% ± 0.3%; P = 0.91), and levels of fasting glucose (92.3 ± 6.9 mg/dL; P = 0.08) and 2-hour glucose (122.3 ± 27.5 mg/dL; P = 0.21).
Table 1.
Clinical and Metabolic Features of the Study Population Stratified by Tertiles of Insulin Sensitivity (WBISI)a
| First Tertile (n = 26) | Second Tertile (n = 26) | Third Tertile (n = 26) | P | |
|---|---|---|---|---|
| Clinical features | ||||
| Age, y | 14.4 ± 2.8 | 13.9 ± 3.7 | 15.5 ± 3.5 | 0.22 |
| Sex, M/F | 11/15 | 10/16 | 13/13 | 0.69 |
| Race, White/Black/Hispanic/Asian | 16/3/3/4 | 14/5/7/0 | 16/5/4/1 | 0.40 |
| BMI, kg/m2 | 34.1 ± 4.9 | 29.3 ± 7.4 | 24.3 ± 5.6 | <0.0001b |
| BMI z-score, cm | 2.24 ± 0.64 | 1.74 ± 0.76 | 0.76 ± 0.81 | <0.0001b |
| Glucose metabolism | ||||
| Fasting glucose, mg/dL | 92.0 ± 8.5 | 87.7 ± 6.3 | 86.6 ± 764 | 0.03b |
| Fasting insulin, µU/mL | 46.4 ± 14.4 | 18.3 ± 3.7 | 13.3 ± 6.4 | <0.0001b |
| 2-hour glucose level, mg/dL | 142.8 ± 31.0 | 109.8 ± 18.2 | 92.1 ± 22.3 | <0.0001b |
| NGT/IGT | 13/13 | 25/1 | 26/0 | <0.0001b |
| Hemoglobin A1C, % | 5.5 ± 0.4 | 5.4 ± 0.3 | 5.3 ± 0.3 | 0.015b |
| WBISI | 0.94 ± 0.41 | 2.65 ± 0.36 | 4.54 ± 1.00 | <0.0001b |
| IGI | 4.7 ± 3.0 | 3.4 ± 2.2 | 1.9 ± 1.1 | 0.0003b |
| DI | 4.3 ± 3.2 | 8.6 ± 5.4 | 7.7 ± 4.3 | 0.002b |
| Lipid profile | ||||
| Total cholesterol, mg/dL | 158.3 ± 32.8 | 155.3 ± 31.9 | 142.0 ± 24.3 | 0.14 |
| HDL cholesterol, mg/dL | 42.8 ± 13.0 | 46.9 ± 12.3 | 53.0 ± 9.9 | 0.01b |
| LDL cholesterol, mg/dL | 92.6 ± 24.7 | 89.7 ± 26.1 | 76.8 ± 24.3 | 0.07 |
| Triglycerides, mg/dL | 114.3 ± 57.8 | 94.5 ± 55.5 | 59.2 ± 24.2 | 0.0007b |
| Liver function | ||||
| Alanine transaminase, U/L | 36.2 ± 25.9 | 19.4 ± 12.2 | 15.1 ± 6.5 | 0.0001b |
| Aspartate transaminase, U/L | 28.0 ± 15.1 | 21.3 ± 5.9 | 20.9 ± 5.0 | 0.02b |
Abbreviations: IGT, impaired glucose tolerance; NGT, normal glucose tolerance.
Statistical comparisons were performed using either one-way analysis of variance for continuous variables or χ2 tests for categorical variables.
Statistically significant.
Association between fasting metabolite concentrations and insulin sensitivity in youth
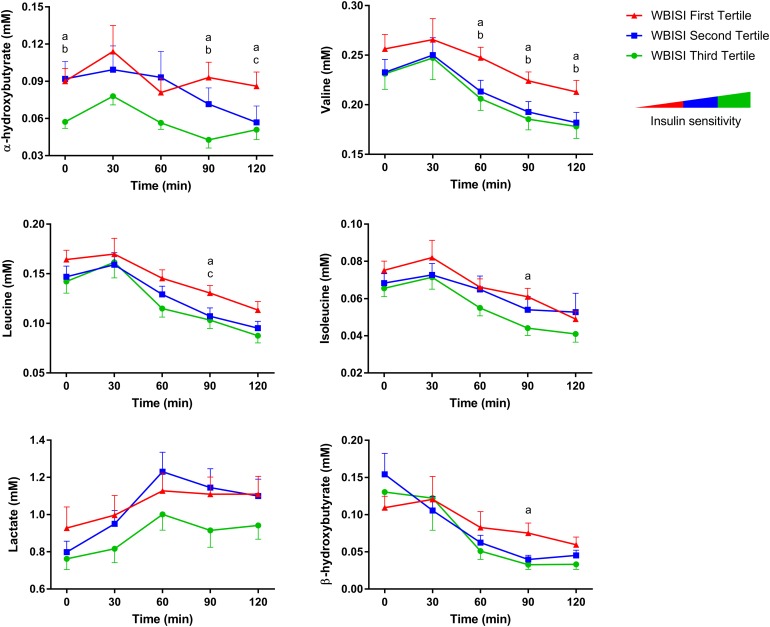
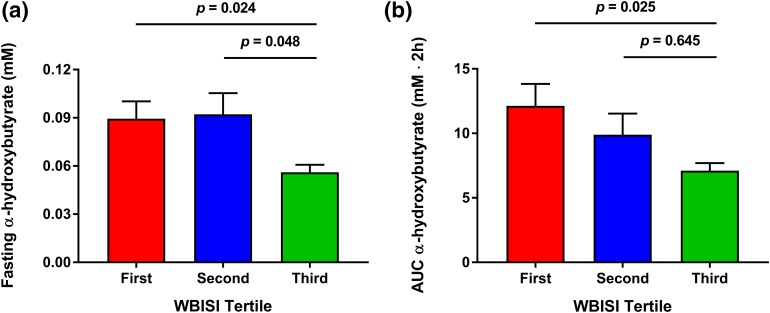
Fasting concentrations of α-hydroxybutyrate correlated with WBISI (r = −0.31; P = 0.010), IGI (r = 0.33; P = 0.006), and BMI z-score (r = 0.27; P = 0.027), and significantly differed among the three groups (P = 0.02; Figs. 1 and 2). In particular, higher levels were found in the first and the second WBISI tertiles compared with the third tertile [Fig. 2(a)]. This difference in fasting α-hydroxybutyrate concentrations remained statistically significant after correction for age, sex, ethnicity, and BMI z-score (P = 0.035) and when the model was further adjusted for individuals’ Tanner stage (P = 0.014). The same trend was observed for fasting concentrations of the BCAAs valine (P = 0.096), leucine (P = 0.092), and isoleucine (P = 0.115), but not for lactate and β-hydroxybutyrate after adjustment for confounding factors.
Figure 1.
Time courses of plasma metabolite concentrations during an OGTT in adolescent subjects stratified by tertiles of insulin sensitivity (▲, first tertile; ■, second tertile; ●, third tertile; n = 26 per tertile). Data are shown as mean ± SE of the mean. Post hoc pairwise comparisons were performed using Dunnett tests. a, P < 0.05 between first and third tertile; b, P < 0.05 between second and third tertile; c, P < 0.05 between first and second tertile.
Figure 2.
(a) Fasting plasma concentrations and (b) AUC of α-hydroxybutyrate during an OGTT in adolescent subjects stratified by tertiles of insulin sensitivity (n = 26 for each tertile). Data are shown as mean ± SE of the mean. Differences between tertiles of WBISI were tested using one-way analysis of variance followed by post hoc pairwise comparisons by using Dunnett test, where appropriate.
Effects of glucose-induced hyperinsulinemia and insulin sensitivity on metabolite processing
Plasma concentrations of all examined metabolites showed significant changes in response to an oral glucose challenge (time effect, P = 0.0001; Fig. 1; Table 2). α-Hydroxybutyrate and BCAA levels were consistently increased throughout the OGTT in adolescents with lower WBISI (WBISI effect, P < 0.03 for all). The AUC of α-hydroxybutyrate, in particular, was correlated with WBISI (r = −0.33; P = 0.013) as well as DI (r = −0.36; P = 0.010), BMI z-score (r = 0.26; P < 0.050), and 2-hour glucose (r = 0.46; P = 0.0004), and was significantly different between groups (P = 0.04), being higher in the first than in the third WBISI tertile [P = 0.025; Fig. 2(b)]. The difference in α-hydroxybutyrate AUC among WBISI tertiles remained statistically significant after correction for age, sex, ethnicity, Tanner stage, and BMI z-score (P = 0.034). In addition, the specific shape of its profiles also differed between tertiles (time–WBISI interaction effect, P = 0.02), underlining group differences in α-hydroxybutyrate metabolic processing in response to high glucose and insulin levels. Plasma α-hydroxybutyrate levels in the first and third WBISI tertiles were distinctly separated and remained relatively stable throughout the OGTT, whereas in the second tertile levels gradually declined, shifting from the high fasting values characteristic of adolescents with IR (first tertile) to the low postload concentrations typical of more insulin-sensitive adolescents (third tertile). This observation suggests that differences in α-hydroxybutyrate profiles during an OGTT can identify a subgroup of subjects in the earliest stages of metabolic decompensation.
Table 2.
Repeated-Measure Analysis of Plasma Metabolite Concentrations During an OGTT in an Adolescent Population (n = 78) Stratified by Tertiles of Insulin Sensitivitya
| Unadjusted P | Adjusted for Age, Sex, Ethnicity, and BMI z-score | |||||
|---|---|---|---|---|---|---|
| Time | WBISI | Time–WBISI | Time | WBISI | Time–WBISI | |
| α-Hydroxybutyrate | <0.0001b | 0.015b | 0.035b | <0.0001b | 0.03b | 0.02b |
| Isoleucine | <0.0001b | 0.02b | 0.69 | <0.0001b | 0.003b | 0.71 |
| Leucine | <0.0001b | 0.02b | 0.25 | <0.0001b | 0.01b | 0.21 |
| Valine | <0.0001b | 0.01b | 0.69 | <0.0001b | 0.01b | 0.66 |
| Lactate | <0.0001b | 0.10 | 0.79 | <0.0001b | 0.26 | 0.81 |
| β-Hydroxybutyrate | <0.0001b | 0.19 | 0.87 | <0.0001b | 0.63 | 0.86 |
Individual and combined effects of glucose-induced hyperinsulinemia and insulin sensitivity status on metabolite processing during an OGTT were tested by mixed-model repeated-measure analysis including time, WBISI tertile, and time–WBISI tertile interaction as fixed factors and subject as random effect, with an unstructured covariance matrix to account for correlations between repeated measurements. The model was further adjusted for potential confounding factors by including age, sex, ethnicity, and BMI z-score as fixed factors.
Statistically significant.
Correlation between metabolic features of IR in youth and longitudinal changes in glucose homeostasis
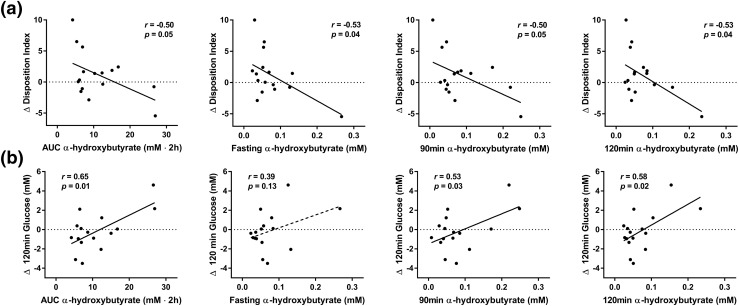
To examine the correlations between metabolic alterations identified in insulin-resistant youth and longitudinal changes of glucose control and indices of insulin secretion and sensitivity, a subgroup of adolescents was re-evaluated after a follow-up of 2.3 ± 0.6 years. In the univariate analysis, deterioration of the DI, a measure of β-cell function relative to insulin sensitivity, showed a robust correlation with α-hydroxybutyrate AUC (r = −0.50; P = 0.05) and its levels at fasting (r = −0.53; P = 0.036), 90 minutes (r = −0.50; P = 0.048), and 120 minutes (r = −0.53; P = 0.036) at the outset of our study [Fig. 3(a)]. Similarly, an impairment in glucose tolerance over time, as indicated by higher 2-hour glucose level, was associated with larger baseline α-hydroxybutyrate AUCs (r = 0.65; P = 0.006), as well as higher levels at 90 minutes (r = 0.53; P = 0.03) and 120 minutes (r = 0.58; P = 0.02; Fig. 3(b)].
Figure 3.
Correlations between α-hydroxybutyrate AUC and plasma concentration at fasting, 90 minutes, and 120 minutes during an OGTT at study onset and Change of (a) DI and (b) 2-hour glucose tolerance after 2.3 ± 0.6 years of follow-up in adolescent subjects (n = 16). Pearson correlation coefficients (r) and P values are shown. Δ, change in.
After adjustment for BMI changes and follow-up duration, the associations between changes in DI and α-hydroxybutyrate AUC [β, −7.89; standard error (SE), 3.34; P = 0.036] and 90- and 120-minute concentrations (β, −6.84; SE, 1.81; P = 0.003; β, −6.98; SE, 3.03; P = 0.04, respectively) remained statistically significant. The association between DI and fasting α-hydroxybutyrate came close to statistical significance (β, −6.44; SE, 3.12; P = 0.06). Similarly, the associations between changes in 2-hour glucose and baseline α-hydroxybutyrate AUC (β, 79.8; SE, 25.0; P = 0.01), and 90- and 120-minute concentrations (β, 41.3; SE, 19.1; P = 0.05; β 72.3; SE 22.3; P = 0.01, respectively) remained statistically significant after adjustment for confounding factors. Adding α-hydroxybutyrate levels to a model including most known metabolic risk factors (i.e., fasting and 2-hour glucose levels, fasting insulin level, HbA1C, and BMI z-score) significantly increased the ability to predict deteriorations in DI and glucose tolerance over time (Table 3).
Table 3.
Multivariate Linear Regression Analysis to Assess the Effect of Adding α-Hydroxybutyrate Concentrations to a Model Including Known Metabolic Risk Factors on the Ability to Predict Deteriorations in DI, Glucose Tolerance, and WBISI in an Adolescent Population (n = 16)a
| Metabolic Risk Factors | +Fasting α-Hydroxybutyrate | +90-Min α-Hydroxybutyrate | +120-Min α-Hydroxybutyrate | +AUC α-Hydroxybutyrate | |
|---|---|---|---|---|---|
| (r2) | [r2 (Δ)] | [r2 (Δ)] | [r2 (Δ)] | [r2 (Δ)] | |
| Δ DI | 0.298 | 0.567 (+0.269) | 0.504 (+0.206) | 0.605 (+0.307) | 0.463 (+0.165) |
| Δ 2-h glucose level | 0.577 | 0.721 (+0.144) | 0.879 (+0.302) | 0.771 (+0.194) | 0.895 (+0.318) |
| Δ WBISI | 0.656 | 0.686 (+0.030) | 0.657 (+0.001) | 0.753 (+0.097) | 0.686 (+0.030) |
Multivariate linear regression analysis was used to test for association between known metabolic risk factors, including fasting and 2-hour glucose, fasting insulin, HbA1c, and BMI z-score, and changes in the disposition index (Δ DI) and glucose tolerance (Δ 2-h glucose) over time. Plasma α-hydroxybutyrate concentrations at either fasting, 90 min, 120 min, or throughout the OGTT (AUC) were then added in four separate models.
Discussion
Using a 1H-NMR procedure, we measured plasma concentrations of six metabolites, including α-hydroxybutyrate, BCAAs, β-hydroxybutyrate, and lactate, in 78 nondiabetic adolescents. These metabolites reflect different axes of insulin action (i.e., proteolysis, ketogenesis, and glycolysis) and have been implicated in the pathogenesis of T2DM in adults. Metabolite concentrations at fasting and their time courses under glucose-induced hyperinsulinemia revealed early metabolic perturbations in subjects with impaired insulin sensitivity. In particular, we found higher fasting α-hydroxybutyrate concentrations and higher α-hydroxybutyrate and BCAA levels in response to an oral glucose challenge in insulin-resistant adolescents. Furthermore, we tested whether these newly identified alterations related to IR could predict deteriorations of glycemic control in youth. Our longitudinal data indicate that increased baseline α-hydroxybutyrate concentrations during an oral glucose challenge are associated with worsening of glucose tolerance and DI over time.
In this study, we have identified α-hydroxybutyrate as a key marker of insulin sensitivity in youth. Our results confirm that fasting α-hydroxybutyrate concentrations are negatively related to insulin sensitivity, as it has been reported in adults (9–11, 23, 24) and in children (13) [Figs. 1 and 2(a)]. Moreover, we found that early impairment of insulin sensitivity alters the minute-to-minute metabolic processing of α-hydroxybutyrate under glucose-induced hyperinsulinemia, as evident from different plasma time courses in response to a glucose challenge between individuals with different degrees of insulin sensitivity. Indeed, the two extreme WBISI tertiles (first and third) showed parallel but distinct plasma α-hydroxybutyrate profiles throughout the OGTT (Fig. 1). This is contrasted by subjects with a borderline WBISI (second tertile), in whom α-hydroxybutyrate levels gradually declined throughout the test, shifting from the high fasting values typical of adolescents with IR (first tertile) to the low postload concentrations characteristic of more insulin-sensitive adolescents (third tertile). This metabolite profile helped identify a subgroup of subjects in the earliest stages of untoward metabolic decompensation.
To our knowledge, this is the first study investigating the response of α-hydroxybutyrate concentrations during an OGTT in youth. In adults, a previous study showed a small reduction of α-hydroxybutyrate levels after a glucose challenge (−1.17-fold change) and a positive correlation between greater α-hydroxybutyrate excursions and higher 2-hour glucose concentrations (β, 23.4; standard deviation, 8.7; P = 0.008) (6). Several mechanisms linking the elevation of plasma α-hydroxybutyrate and IR have been suggested thus far (9, 10). It is known that α-hydroxybutyrate is derived from the conversion of α-ketobutyrate catalyzed by lactate dehydrogenase (25). α-Ketobutyrate, in turn, is a product of threonine and methionine catabolism, as well as of the anabolic pathway of glutathione (26), and is catabolized by pyruvate dehydrogenase (PDH) and branched-chain α-ketoacid dehydrogenase (BCKDH) complexes (27). Elevated oxidative stress in IR may increase the demand for glutathione synthesis, which, in turn, increases methionine catabolism via cystathionine and α-ketobutyrate levels (28). Furthermore, the activity of both PDH and BCKDH complexes is closely regulated by several common factors, including insulin (29). Therefore, decreased metabolic flux through PDH and BCKDH, as reported in T2DM (30, 31), may increase plasma α-ketobutyrate by decreasing its catabolic rate in insulin-resistant individuals. Of note, because the ratio between α-hydroxybutyrate and α-ketobutyrate typically ranges between 10:1 to 30:1 (32), small changes in α-ketobutyrate concentrations that reflect incipient IR may produce large elevations in α-hydroxybutyrate levels, as observed in our cohort of adolescents. From a pathophysiologic perspective, increased α-hydroxybutyrate levels that mark early IR may also play a role in the impairment of β-cell function, which usually characterizes a later phase of diabetes development (33). Indeed, in vitro studies have shown that both glucose- and arginine-mediated insulin release in β cells are inhibited by α-hydroxybutyrate in a dose-dependent manner (9).
Among the participants enrolled in this study, a subset of adolescents representative of the whole population underwent a second OGTT after a follow-up of ∼2 years. This interval length was chosen to evaluate early changes in glucose control over a relatively short time while minimizing biases introduced by environmental factors. Similar to the evidence in adults (9), our longitudinal data revealed that baseline alterations of α-hydroxybutyrate levels relate to IR and can predict further deteriorations of glucose tolerance, as reflected by increases of 2-hour glucose concentrations, even after correction for possible changes in BMI and for follow-up duration [Fig. 3(b)]. Furthermore, we found that α-hydroxybutyrate concentrations in response to a glucose challenge were associated with deterioration of the DI over time [Fig. 3(a)]. Remarkably, the association with OGTT concentrations of α-hydroxybutyrate (AUC, 90 and 120 minutes) was more robust than the one with fasting α-hydroxybutyrate levels alone. This highlights the benefits of using dynamic testing such as an oral glucose challenge to reveal subtle metabolic alterations of early IR. These exploratory observations from a relatively small subset of subjects provide a clear framework for confirmatory and mechanistic follow-up studies in larger cohorts of adolescents.
Although perturbations of BCAAs have been consistently implicated in the pathogenesis of IR in adults (8), studies in youth have produced conflicting results. In fact, previous reports showed either a positive (12, 15), a negative (13, 34), or no correlation (14) between plasma BCAAs and insulin sensitivity in adolescents and children. Conflicting results might be attributed to differences in study populations as well as to a low sensitivity of fasting metabolite analyses in detecting subtle metabolic alterations. Indeed, as suggested by our current data, mild alterations in BCAA metabolism that mark early IR may require challenge testing for identification (Fig. 1; Table 2), which might explain the lack of correlation reported by some investigators.
Although the exact mechanisms underlying the elevation of plasma BCAAs in IR are not fully understood, several metabolic alterations have been proposed (35, 36). These include an increase in the rate of appearance of BCAAs in the circulation, such as augmented dietary intake and tissue protein degradation, and factors that can reduce the rate of disappearance of BCAAs, such as lower protein synthesis and reduced BCAA tissue uptake, catabolism, and excretion (35, 36). Most of these mechanisms are strictly dependent on insulin release and action; thus, a dysregulation of BCAA metabolism already present at fasting might become more evident under glucose-induced hyperinsulinemia, as during the OGTT. By analyzing the decline of plasma BCAA concentrations in response to glucose ingestion in our study, we could show that BCAA levels were persistently higher during the OGTT in adolescents with IR (Fig. 1; Table 2). However, we did not find significant differences in the shape of BCAA profiles between insulin-sensitive and insulin-resistant subjects (i.e., a blunted suppression of BCAA levels during the glucose challenge in insulin-resistant individuals). This observation contrasts with previous data from adults (6, 7) and may reflect an earlier stage of metabolic perturbation in our population or the absence of protective mechanisms in older subjects. The same considerations hold true for two other metabolites measured in this study, β-hydroxybutyrate and lactate, which showed blunted excursions in response to the OGTT in insulin-resistant adults (6, 7) but not in our younger population (Table 2). Overall, because BCAAs, β-hydroxybutyrate, and lactate represent different axes of insulin action that appear to be impaired to varying degrees across the lifespan, these results reveal new insights into the progression of IR and support the need for further mechanistic studies.
In our cohort, we did not identify a relationship between altered BCAA concentrations and short-term longitudinal changes in insulin sensitivity, as had been previously reported in youth (14). In the previous study, however, baseline concentrations of BCAAs predicted a worsening in insulin sensitivity only in insulin-sensitive adolescents. Because adolescents included in the earlier investigation had overall higher insulin sensitivity (WBISI, 7.00 ± 4.02) than our subjects (WBISI, 2.23 ± 1.86), the different population characteristics may explain these contrasting results. Because both studies were conducted in relatively small, albeit carefully characterized, cohorts (n < 20 for both), conclusions based on failure to reject the null hypothesis will require confirmation in larger prospective studies.
A strength of the current study is that the quantitative analysis of human plasma samples was performed by using an innovative 1H-NMR method with several advantages: minimal sample preparation (limited to sample dilution), short NMR acquisition time (15 minutes per sample), and automated spectra analysis (16). Together, these favorable features make this new analytical technique suitable for semiautomated and high-throughput measurements of metabolic markers of IR, providing a great opportunity to apply this method to routine blood analysis in daily clinical practice.
In conclusion, fasting concentrations of α-hydroxybutyrate and the time courses of α-hydroxybutyrate and BCAAs in response to a glucose challenge characterize IR in youth. Furthermore, alterations of α-hydroxybutyrate metabolism predict incipient deterioration of β-cell function and longitudinal worsening of glycemic tolerance. These findings provide strategies for early risk stratification of adolescents with a high likelihood of developing impaired glucose metabolism and point toward mechanisms involved in the progression of IR that warrant further investigation in the future.
Acknowledgments
We thank the participating patients and their families, and the Yale Center for Clinical Investigation and Hospital Research Unit personnel.
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grants K08 DK082618 and R01 DK101984 to R.I.H, and R01-HD40787, R01-HD28016, and K24-HD01464 to S.C.; American Heart Association Grants 13SDG14640038, 11CRP5620013, and 16IRG27390002 to N.S.; the Yale Center for Clinical Investigation (2012 YCCI scholar award and YCCI Just-In-Time Grant), the Allen Foundation award to N.S.; Grant DK045735 to the Yale Diabetes Research Center and Clinical and Translational Science Awards Grant UL1-RR- 024139 from the National Center for Advancing Translational Sciences, a component of the NIH, and NIH Roadmap for Medical Research. This paper's contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Acknowledgments
Author contributions: C.G., S.C., N.S., and R.I.H. designed the study. D.T., C.G., S.C., N.S., and R.I.H. collected the data. H.P., C.G., R.d.G., and C.J. performed NMR metabolite measurements. H.P., R.d.G., and C.J. analyzed spectra. D.T. and F.L. analyzed the data. D.T. wrote the manuscript. F.L., S.C., N.S., and R.I.H. revised the manuscript. D.T., C.G., S.C., N.S., and R.I.H. are the guarantors of this work, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the analyses.
Clinical trial registry: ClinicalTrials.gov no. NCT00536250 (registered 25 September 2007).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BCKDH
- branched-chain α-ketoacid dehydrogenase
- BMI
- body mass index
- CHEBI
- Chemical Entities of Biological Interest
- DI
- Disposition Index
- 1H-NMR
- proton nuclear magnetic resonance spectroscopy
- IGI
- Insulinogenic Index
- IR
- insulin resistance
- OGTT
- oral glucose tolerance testing
- PDH
- pyruvate dehydrogenase
- SE
- standard error
- T2DM
- type 2 diabetes mellitus
- WBISI
- Whole-Body Insulin Sensitivity Index.
References
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12):1218–1226. [DOI] [PubMed] [Google Scholar]
- 3.D’Adamo E, Santoro N, Caprio S. Metabolic syndrome in pediatrics: old concepts revised, new concepts discussed. Pediatr Clin North Am. 2011;58(5):1241–1255, xi (xi). [DOI] [PubMed] [Google Scholar]
- 4.Santoro N, Amato A, Grandone A, Brienza C, Savarese P, Tartaglione N, Marzuillo P, Perrone L, Miraglia Del Giudice E. Predicting metabolic syndrome in obese children and adolescents: look, measure and ask. Obes Facts. 2013;6(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28(4):902–909. [DOI] [PubMed] [Google Scholar]
- 6.Ho JE, Larson MG, Vasan RS, Ghorbani A, Cheng S, Rhee EP, Florez JC, Clish CB, Gerszten RE, Wang TJ. Metabolite profiles during oral glucose challenge. Diabetes. 2013;62(8):2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62(5):1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. α-Hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care. 2016;39(6):988–995. [DOI] [PubMed] [Google Scholar]
- 11.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E; RISC Study Group . alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5):e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaliszyn SF, Sjaarda LA, Mihalik SJ, Lee S, Bacha F, Chace DH, De Jesus VR, Vockley J, Arslanian SA. Metabolomic profiling of amino acids and β-cell function relative to insulin sensitivity in youth. J Clin Endocrinol Metab. 2012;97(11):E2119–E2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, Göring H, Cole SA, Comuzzie AG. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr. 2015;102(2):256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK, Fleischman A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihalik SJ, Michaliszyn SF, de las Heras J, Bacha F, Lee S, Chace DH, DeJesus VR, Vockley J, Arslanian SA. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care. 2012;35(3):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Graaf RA, Prinsen H, Giannini C, Caprio S, Herzog RI. Quantification of (1)H NMR spectra from human plasma. Metabolomics. 2015;11(6):1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tricò D, Di Sessa A, Caprio S, Chalasani N, Liu W, Liang T, Graf J, Herzog RI, Johnson CD, Umano GR, Feldstein AE, Santoro N. Oxidized derivatives of linoleic acid in pediatric metabolic syndrome: is their pathogenic role modulated by the genetic background and the gut microbiota? [published online ahead of print April 7, 2017]. Antioxid Redox Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Malley G, Santoro N, Northrup V, D’Adamo E, Shaw M, Eldrich S, Caprio S. High normal fasting glucose level in obese youth: a marker for insulin resistance and beta cell dysregulation. Diabetologia. 2010;53(6):1199–1209. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286–292. [DOI] [PubMed] [Google Scholar]
- 21.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49(3):571–579. [DOI] [PubMed] [Google Scholar]
- 22.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6(2):e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab. 2013;98(6):E1060–E1065. [DOI] [PubMed] [Google Scholar]
- 24.Varvel SA, Pottala JV, Thiselton DL, Caffrey R, Dall T, Sasinowski M, McConnell JP, Warnick GR, Voros S, Graham TE. Serum α-hydroxybutyrate (α-HB) predicts elevated 1 h glucose levels and early-phase β-cell dysfunction during OGTT. BMJ Open Diabetes Res Care. 2014;2(1):e000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plummer DT, Elliott BA, Cooke KB, Wilkinson JH. Organ specificity and lactate-dehydrogenase activity. 1. The relative activities with pyruvate and 2-oxobutyrate of electrophoretically separated fractions. Biochem J. 1963;87:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landaas S. The formation of 2-hydroxybutyric acid in experimental animals. Clin Chim Acta. 1975;58(1):23–32. [DOI] [PubMed] [Google Scholar]
- 27.Paxton R, Scislowski PW, Davis EJ, Harris RA. Role of branched-chain 2-oxo acid dehydrogenase and pyruvate dehydrogenase in 2-oxobutyrate metabolism. Biochem J. 1986;234(2):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord RS, Bralley JA. Clinical applications of urinary organic acids. Part I: Detoxification markers. Altern Med Rev. 2008;13(3):205–215. [PubMed] [Google Scholar]
- 29.Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304(11):E1175–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999;48(8):1593–1599. [DOI] [PubMed] [Google Scholar]
- 31.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011;2(6):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson Legault J, Strittmatter L, Tardif J, Sharma R, Tremblay-Vaillancourt V, Aubut C, Boucher G, Clish CB, Cyr D, Daneault C, Waters PJ, Vachon L, Morin C, Laprise C, Rioux JD, Mootha VK, Des Rosiers C, Des Rosiers C; LSFC Consortium . A metabolic signature of mitochondrial dysfunction revealed through a monogenic form of Leigh Syndrome. Cell Reports. 2015;13(5):981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebovitz HE. Postprandial hyperglycaemic state: importance and consequences. Diabetes Res Clin Pract. 1998;40(Suppl):S27–S28. [PubMed] [Google Scholar]
- 34.Perng W, Gillman MW, Fleisch AF, Michalek RD, Watkins SM, Isganaitis E, Patti ME, Oken E. Metabolomic profiles and childhood obesity. Obesity (Silver Spring). 2014;22(12):2570–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]