Abstract
Context:
Central congenital hypothyroidism (CCH) is an underdiagnosed disorder characterized by deficient production and bioactivity of thyroid-stimulating hormone (TSH) leading to low thyroid hormone synthesis. Thyrotropin-releasing hormone (TRH) receptor (TRHR) defects are rare recessive disorders usually associated with incidentally identified CCH and short stature in childhood.
Objectives:
Clinical and genetic characterization of a consanguineous family of Roma origin with central hypothyroidism and identification of underlying molecular mechanisms.
Design:
All family members were phenotyped with thyroid hormone profiles, pituitary magnetic resonance imaging, TRH tests, and dynamic tests for other pituitary hormones. Candidate TRH, TRHR, TSHB, and IGSF1 genes were screened for mutations. A mutant TRHR was characterized in vitro and by molecular modeling.
Results:
A homozygous missense mutation in TRHR (c.392T > C; p.I131T) was identified in an 8-year-old boy with moderate hypothyroidism (TSH: 2.61 mIU/L, Normal: 0.27 to 4.2; free thyroxine: 9.52 pmol/L, Normal: 10.9 to 25.7) who was overweight (body mass index: 20.4 kg/m2, p91) but had normal stature (122 cm; –0.58 standard deviation). His mother, two brothers, and grandmother were heterozygous for the mutation with isolated hyperthyrotropinemia (TSH: 4.3 to 8 mIU/L). The I131T mutation, in TRHR intracellular loop 2, decreases TRH affinity and increases the half-maximal effective concentration for signaling. Modeling of TRHR-Gq complexes predicts that the mutation disrupts the interaction between receptor and a hydrophobic pocket formed by Gq.
Conclusions:
A unique missense TRHR defect identified in a consanguineous family is associated with central hypothyroidism in homozygotes and hyperthyrotropinemia in heterozygotes, suggesting compensatory elevation of TSH with reduced biopotency. The I131T mutation decreases TRH binding and TRHR-Gq coupling and signaling.
We identified and characterized a partially inactivating mutation in TRHR. The mutation caused central hypothyroidism in a homozygous child and hyperthyrotropinemia in heterozygous family members.
Central congenital hypothyroidism (CCH) is caused by deficient production of thyroid hormones (T4 and T3) due to low synthesis, secretion, or bioactivity of thyrotropin (thyroid-stimulating hormone [TSH]). CCH is an underdiagnosed disorder because CCH patients are not detected by TSH-based neonatal screening programs for congenital hypothyroidism (CH) implemented in most countries (1). However, uncommon T4-based CH screening programs in few countries recently estimated the prevalence of CCH as 1 in 16,000 to 30,000 newborns (2, 3). The paucity of clinical cases identified and the complexity of hypothalamic-pituitary regulation of the thyroid axis leaves the molecular mechanisms underlying CCH largely unknown (4). At present, genetic defects in only four genes have been identified in patients with isolated CCH: TSHB (encoding the B-subunit of the TSH glycoprotein hormone), TRHR (the specific 7-transmembrane domain receptor for hypothalamic thyrotropin-releasing hormone [TRH]), IGSF1 (a protein regulating the expression of TRHR in pituitary thyrotropes), and the recently identified TBL1X (a subunit of the NCoR-SMRT complex) (5). Most patients described with CCH harbored defects in TSHB and IGSF1, but three families with recessively inherited TRHR defects have been identified (6–8).
The TRH receptor (TRHR) is a G-protein–coupled receptor (GPCR) located at pituitary thyrotropes and activated by hypothalamic TRH. TRHR contains an extracellular N terminus, seven transmembrane domains (TMDs), three extracellular loops, three intracellular loops (ICLs), and a cytoplasmic tail. TRH interacts with amino acids of the extracellular loops and then moves into the TMD binding pocket (9, 10) triggering small, local structural changes near the binding site that are translated into larger-scale helix movements at the intracellular site, mainly TMDs 5 and 6, opening a cavity for the binding of the C-terminal α5 helix of the G-protein (11). The formation of the TRH-TRHR-Gq complex triggers activation of the phosphatidylinositol (IP3)-calcium-protein kinase C (PKC) pathway (12).
TRH-TRHR signaling promotes the synthesis, secretion, and bioactivity of TSH, all necessary for the proper synthesis of T4 and T3 in the thyroid gland (13, 14). The three unrelated patients with identified TRHR defects were missed in TSH-based neonatal screening programs (6–8). The first two patients described were referred to clinicians at the ages of 9 and 11 years with short stature and variable symptoms, consistent with hypothyroidism (lethargy, fatigue, poor school performance), whereas the third was diagnosed and treated for CH at 2 months of life (8). In all cases, thyroid hormone profiles revealed normal TSH (with suspected low bioactivity) and the presence of moderate hypothyroidism. Heterozygous carriers were reportedly euthyroid.
The first patient described was a compound heterozygote for an early stop codon in the TRHR (p.R17X) and an inframe deletion added to a missense change (p.S115-T117del + p.A118T) in the other allele (6). The same p.R17X mutation was found in the second patient in homozygous state (7), whereas the third had a homozygous missense mutation (p.P81R) (8). All TRHR mutations identified so far severely impaired TRHR signaling (6–8).
Here we present a unique missense mutation in TRHR located at a highly conserved hydrophobic position (Φ) at the (E/D)R3.50YX5PΦXY motif of GPCRs, which reduces affinity for TRH and impairs, but not fully abrogates, signal transduction by the receptor. Consistent with this residual function, the mutation causes moderate CH in the homozygous state and central hyperthyrotropinemia in heterozygotes, suggesting compensatory elevation of TSH with reduced biopotency.
Materials and Methods
Informed consent
Informed consent for genetic studies was obtained from the index patient and his family, according to protocols followed at the San Pedro de Alcántara Hospital (Cáceres, Spain), where the patient was clinically followed.
Hormonal determinations and TRH test
TSH, free thyroxine (FT4), luteinizing hormone (LH), follicle-stimulating hormone, and prolactin (PRL) were determined in serum by electrochemiluminescence with the Elecsys-170 platform (Roche, Basel, Switzerland). IGF-1, IGFBP-3, adrenocorticotropic hormone, and cortisol were measured by chemiluminescence with the Immulite 2000 system (Siemens, Munich, Germany). The TRH stimulation test was performed as previously reported (15). An indirect measure of TSH bioactivity was calculated through the percentage increase of serum FT4 180 minutes after TRH administration, as reported (16).
Mutation screening
All coding regions of TRH (the gene encoding the TRH), TRHR (encoding the TRH receptor), TSHB (encoding the specific TSH β subunit), and IGSF1 (coding for the immunoglobulin superfamily factor 1) were amplified by PCR using appropriate primers flanking each exon. PCR products were purified and directly sequenced on an automated DNA sequencer (3100 Genetic Analyzer; Applied Biosystems).
DNA samples of the index patient and his mother were used for next-generation sequencing (NextSeq-500). A panel of 320 thyroidal genes, of our own design, including TG, TPO, NIS, DUOX2, DUOXA2, DEHAL1, TSHR, and GNAS, was used.
Evaluation of TRHRs
In vitro activity of wild-type and mutant hemagglutinin-tagged TRHRs was evaluated using fixed-cell enzyme-linked immunosorbent assay to measure plasma membrane expression, [3H]Me-TRH binding to measure agonist affinity, and an AP1-luciferase reporter assay to measure signal transduction, as described previously (17–19) and in the Supplemental Methods (142.7KB, docx) .
Computational model of wild-type and I131T mutant TRH receptors in complex with TRH and Gq
The “active-like” state of human TRHR (UniProt entry P34981) in complex with TRH and Gq was built using a combination of structural templates. Crystal structures of active μ-opioid receptor [Protein Data Bank Identification (PDB ID): 5C1M] (20), the complex between β2-adrenergic receptor and Gs protein (PDB ID: 3SN6) (11) and Gq protein (PDB ID: 3AH8) (21), were used (Supplemental Methods (142.7KB, docx) ). TRH was docked into the “active-like” conformation of TRHR using MOE (Chemical Computing Group Inc., Montreal, QC, Canada) (Supplemental Methods (142.7KB, docx) ). To evaluate the effect of the I131ICL2T mutation in the TRHR-Gq interface, we performed molecular dynamics (MD) simulations of wild-type and mutant receptors (Supplemental Methods (142.7KB, docx) ).
Results
Clinical case
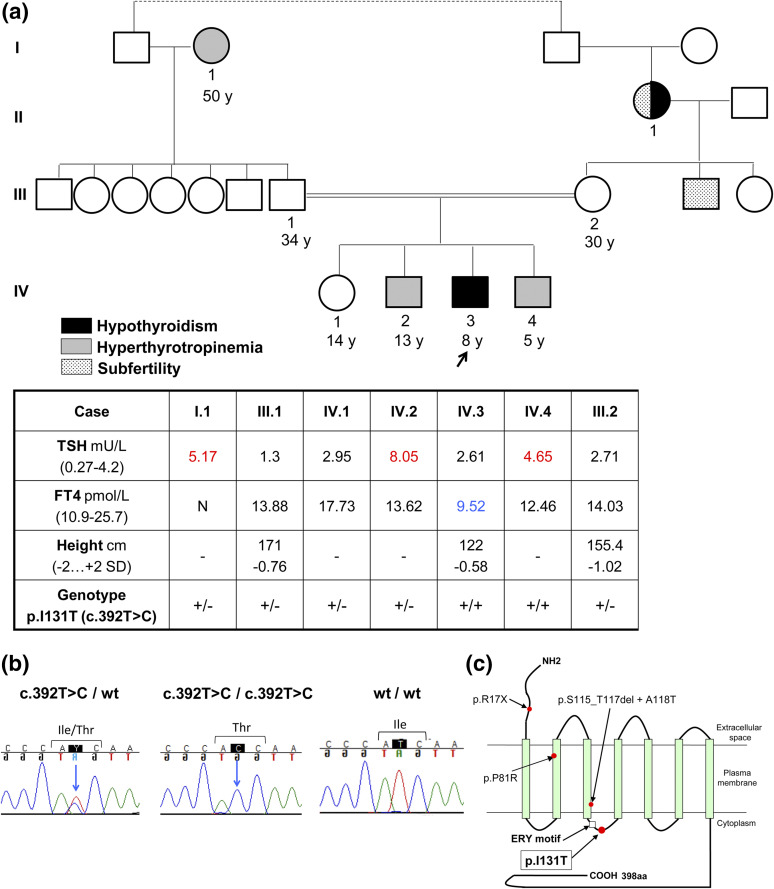
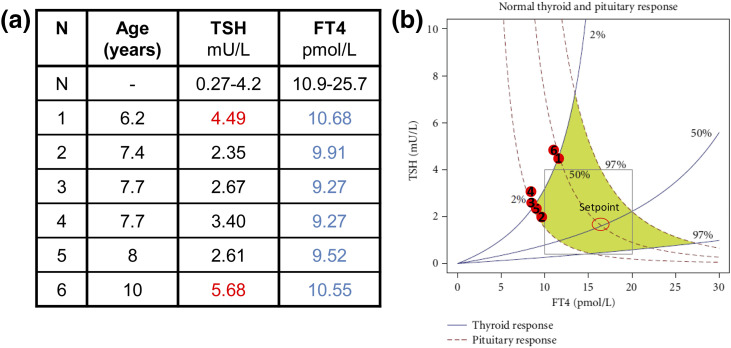
The index case is a male of Roma descent, the third sibling of a consanguineous kindred [Fig. 1(a)]. He was not detected by a TSH-based neonatal screening program (using TSH threshold >7 mIU/L). At the age of 8 years, he was referred to the pediatrician for evaluation of abnormal thyroid function. Hormonal tests revealed mild hypothyroidism (FT4: 9.52 pmol/L [Normal: 10.9 to 25.7 pmol/L] and TSH: 2.61 mIU/L [Normal: 0.27 to 4.2 mIU/L]) [Fig. 2(a)] in the absence of antithyroid antibodies. He was overweight (body mass index: 20.4 kg/m2, p91) but had normal stature (122 cm, –0.58 standard deviation) [Fig. 1(a)]. The patient did not present symptoms of hypothyroidism; however, all available TSH-FT4 paired determinations fell outside the area representing the normal dynamic relation between the two parameters, following the model of Dietrich et al. (22) [Fig. 2(b)]. The patient displayed normal TSH response to TRH (Supplemental Fig. 1 (142.7KB, docx) ). The adrenocorticotropic hormone dynamic test and basal PRL, follicle-stimulating hormone, LH, IGF-1, and IGFBP-3 determinations were normal. His testicular volume was 3 mL, consistent with a prepubertal stage. Brain magnetic resonance imaging showed normal size and shape of the pituitary and thyroid ultrasounds revealed normal thyroid size and structure (data not shown). He was started on levo-thyroxine (L-T4) replacement (50 μg/d), and 1 month later, his FT4 levels reached normal ranges (13.77 pmol/L) at the expense of suppressed TSH (0.07 mIU/L), a characteristic feature of treated central hypothyroidism (1). When the patient was 10 years of age, L-T4 treatment was withdrawn for 1 month, and his thyroid function was re-evaluated, showing elevated TSH levels and decreased FT4 but normal TSH and PRL responses to a second TRH test (Supplemental Fig. 2 (142.7KB, docx) ). As part of the test, FT4 was measured before (0 minutes) and 3 hours after (180 minutes) TRH administration, and the percentage FT4 increase was calculated as an indirect measure of TSH bioactivity, as reported (16). The FT4 increase was below the normal range (Supplemental Fig. 2 (142.7KB, docx) ).
Figure 1.
Clinical and biochemical features of a family with TRHR defect. (a) Phenotype of members of the pedigree showing consanguinity, expressed as a double line linking symbols for mother and father of the index patient (arrow). Thyroid hormone profile of several members of the family. Blue and red represent hormone values below and above normal ranges, respectively. (b) Representative chromatograms showing the wild type and the I131T TRHR mutation in heterozygous and homozygous state. (c) Scheme showing the location of the I131T mutation at the second ICL of the TRHR (highlighted) and other TRHR mutations previously described. Abbreviations: +/–, heterozygous mutation; +/+, homozygous mutation; wt, wild type; y, year.
Figure 2.
Historical thyroid hormone profiles of index patient from 6 to 10 years of age. (a) TSH and FT4 values during 4 years follow-up. All values were determined when the patient was not treated with L-T4. (b) Graphical correlation between TSH and FT4 values plotted in chart for normal thyroid and pituitary responses (adapted from 22). Blue and red represent hormonal values below and above normal ranges, respectively.
Remarkably, four members of the family (two siblings, mother, and paternal grandmother) showed mild hyperthyrotropinemia with normal FT4 [Figs. 1(a) and 3]. Retrospectively analyzed, the patient also displayed hyperthyrotropinemia at 6 years of age [Fig. 2(a)].
Figure 3.
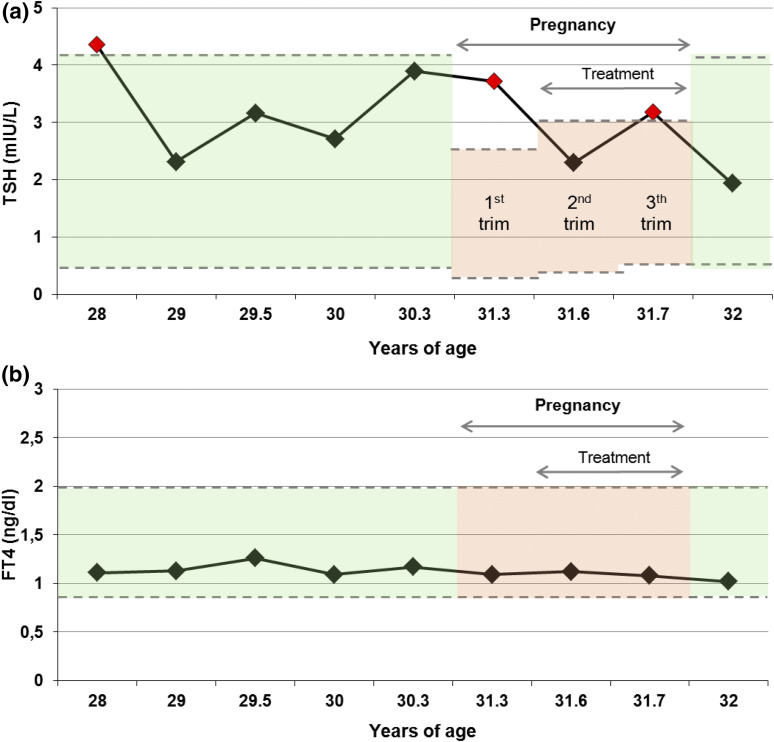
Longitudinal thyroid hormone profile of the patient’s mother over 4 years, including pregnancy. (a) TSH follow-up profile. Green area represents the normal ranges for healthy adult individuals (0.27 to 4.2 mU/L). Red area represents the normal ranges for healthy pregnant women (first trimester: 0.1 to 2.5 mU/L, second trimester: 0.2 to 3 mU/L, third trimester: 0.3 to 3 mU/L, following American Thyroid association recommendations) (23). (b) Free T4 follow-up profile. Green and red areas represent the normal ranges for healthy adult and pregnant women, respectively (0.85 to 2 ng/dL). Abbreviation: trim, trimester.
All siblings of the patient were hormonally re-evaluated on two occasions. In the first one, only one out of four showed hyperthyrotropinemia (Supplemental Fig. 2 (142.7KB, docx) ), whereas 6 months later, two out of four showed elevated TSH, indicating the intermittent nature of hyperthyrotropinemia in these individuals (Supplemental Table 1 (142.7KB, docx) ). Thyroid autoimmunity was negative in all family members. No goiter was present in the siblings at ultrasounds, according to age- and sex-specific reference intervals (24, 25) (Supplemental Table 1 (142.7KB, docx) ). Four of the five siblings, including the index patient, are currently obese (as defined as body mass index centile >95). Interestingly, the only child with normal weight also presented hyperthyrotropinemia, suggesting that TSH elevation in this family is not directly associated with obesity (Supplemental Table 1 (142.7KB, docx) ).
TRH tests were also performed in siblings and parents, showing normal TSH and PRL responses to TRH (Supplemental Fig. 2 (142.7KB, docx) ). TSH bioactivity was evaluated from the TRH test (Supplemental Fig. 2 (142.7KB, docx) ). FT4 increases were below the normal ranges, again suggesting low TSH biopotency in all serum samples available (16).
Two relatives of this consanguineous pedigree were treated with L-T4: the paternal grandmother and the patient’s mother during pregnancy with her fifth child when her TSH levels remained slightly elevated during treatment with 25-µg/d L-T4 (Fig. 3). No difficulties with lactation were reported. The maternal grandmother had hypothyroidism in her youth and difficulties becoming pregnant for more than 6 years after marriage. Thereafter, she experienced one spontaneous abortion and three fruitful pregnancies. One of the sons of the maternal grandmother has similar subfertility complaints [Fig. 1(a)].
Identification of the TRHR mutation
Direct sequencing of the coding exons of four candidate genes for central hypothyroidism (TRH, TRHR, TSHB, and IGSF1) revealed a homozygous missense mutation in the TRHR gene of the patient (c.392T > C), changing isoleucine 131 into threonine (p.I131T). The patient’s mutation was inherited from his parents, who are both heterozygous carriers. The paternal grandmother and three siblings of the patient are heterozygotes for the mutation, whereas one of his brothers is a homozygous carrier [Fig. 1(a–c); Supplemental Fig. 2 (142.7KB, docx) ].
Targeted next-generation sequencing in the index case and his mother showed no pathogenic mutations in genes involved in thyroid hormonosynthesis, including TG, TPO, NIS, DUOX2, DUOXA2, DEHAL1, TSHR, and GNAS (data not shown).
Functional characterization of the I131T-TRHR mutant
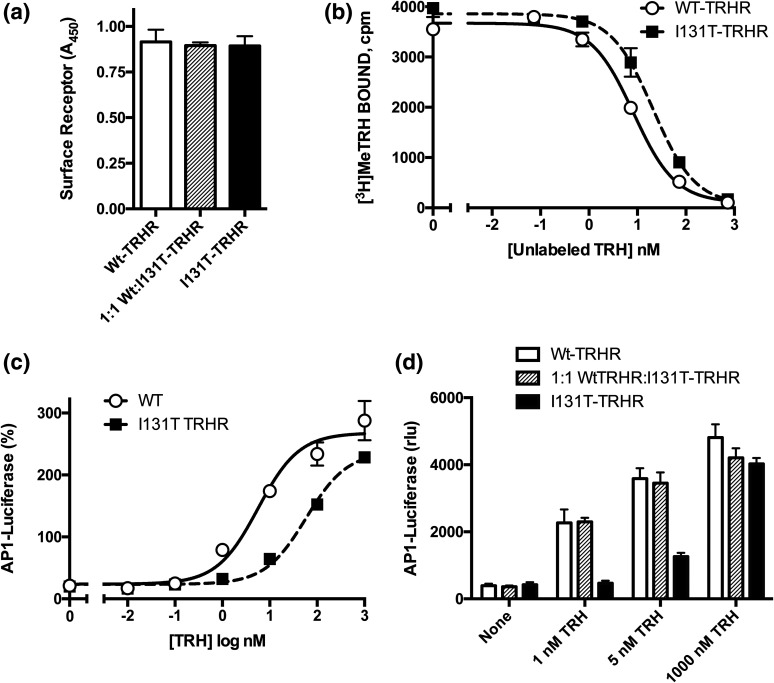
To determine whether the isoleucine-to-threonine mutation affected expression and trafficking of the receptor, the relative density of hemagglutinin-tagged receptors at the plasma membrane was quantified. Wild-type TRHR was strongly expressed on the plasma membrane, and there was no significant difference in receptor density when cells were transfected with equal amounts of complementary DNA encoding the I131T mutant or a 1:1 mixture of wild-type and mutant TRHR [Fig. 4(a)].
Figure 4.
I131T-TRHR functional studies. (a) I131T-TRH receptors are located at the cell membrane at the same density as the wild type. Hemagglutinin-tagged TRH receptors on the cell surface were measured by enzyme-linked immunosorbent assay in the experiment depicted in panel b. (b) I131T-TRHR has reduced affinity for [3H]Me-TRH. Cells transfected with control plasmid or TRH receptors were incubated with 2 nM [3H]methyl-TRH and concentrations of unlabeled TRH shown, and specific binding was measured after 1.5 hours. (c, d) Cells were transfected to express wild-type and/or mutant TRH receptors and an AP1-luciferase reporter. After 24 hours, cells were stimulated for 4 hours with the concentrations of TRH shown, and luciferase activity was quantified. (c) Response to different concentrations of TRH showing that the I131T-TRHR has a higher half-maximal effective concentration but similar maximum response compared with the wild-type receptor. The M3 muscarinic receptor was cotransfected as a control for downstream effects; results are expressed as percentage of the response to 10 µM carbachol. (d) I131T- and wild-type (WT)-TRHR were transfected alone or together using a 1:1 DNA ratio and stimulated with the concentrations of TRH shown; luciferase activity is not normalized. Abbreviations: A450, absorbance at 450 nm; rlu, relative light units.
The molecular basis for the signaling defect in the I131T-TRHR was investigated by measuring the affinity of wild-type and mutant TRHR for a high-affinity, radiolabeled agonist, [3H]Me-TRH. Cells were incubated with tracer [3H]Me-TRH and different concentrations of unlabeled TRH under equilibrium conditions. Significantly higher concentrations of unlabeled TRH were required to decrease [3H]Me-TRH binding to the I131T-TRHR, consistent with lower affinity for the natural ligand [Fig. 4(b)]. The relative affinity of the two receptors for TRH was 3.1 ± 0.3 and 9.1 ± 0.4 nM for wild-type and I131T mutant TRH receptors, respectively (P < 0.05).
The effect of the I131T mutation on signaling capacity was tested by expressing wild-type and mutant TRHR in HEK293 cells, a widely used system for evaluating GPCR signaling. TRHR signals through a classical Gq-coupled pathway, stimulating an increase in intracellular calcium and activation of PKC (26). Receptor activity was determined using an AP1-luciferase reporter containing a c-fos promoter sequence activated by the TRH-IP3-calcium-PKC pathway. TRH induced more than a 10-fold increase in AP1-luciferase activity in cells expressing the wild-type TRHR (average half-maximal effective concentration for TRH = 2.8 ± 0.9 nM [n = 7]). I131T-TRHR was capable of generating the same maximal response; however, significantly higher concentrations of TRH were required (average half-maximal effective concentration = 20.4 ± 0.8 nM [n = 6, P < 0.05 vs wild type]) [Fig. 4(c)].
Signaling via the TRH receptor was also tested in cells coexpressing wild-type and I131T-TRHR mutant receptors in an attempt to mimic the situation in individuals heterozygous for the mutation. Because activity of the two receptors differed more at low concentrations of TRH, responses were determined either without any stimulus (to monitor constitutive activity), low TRH (1 and 5 nM), or maximally effective TRH (1 μM) [Fig. 4(d)]. As expected, the I131T-TRHR signaled weakly compared with the wild type at 1 and 5 nM TRH [Fig. 4(d)]. At maximal TRH concentrations, no significant differences in activity between the mutant and wild type (P > 0.05) were present, consistent with the capacity of the mutant to generate maximal responses [Fig. 4(d)]. Cells coexpressing wild-type and mutant receptors responded as strongly as wild-type receptors. Constitutive activity was not affected by mutation of I131, indicating that functional impairment of I131T-TRHR involves ligand-activated signaling [Fig. 4(d)].
I131T-TRHR mutation disrupts the interaction with Gq in the active-like TRH-TRHR-Gq model
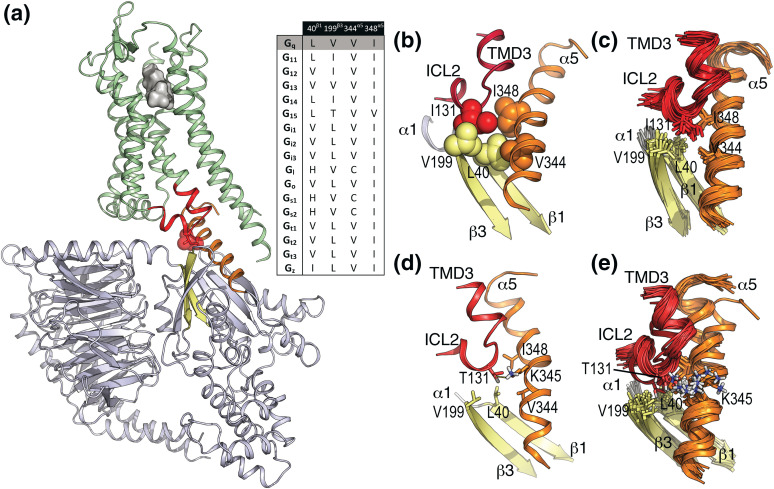
An “active-like” model of the TRHR in complex with Gq was built [Fig. 5(a); Supplemental Methods (142.7KB, docx) ]. I131ICL2 is located in ICL 2 and pointing toward Gq. The molecular interface between TRHR and Gq is mainly formed by the interaction of TMDs 3, 5, and 6 of TRHR with the C-terminal α5 helix of Gq and ICL 2 of TRHR with the N-terminal α1 helix and β1 and β3 strands of Gq. In detail, the hydrophobic side chain of I131ICL2 forms hydrophobic-hydrophobic interactions with L40β1 in the β1 strand, V199β3 in the β3 strand, and V344α5 and I348α5 in the α5 helix of Gq [Fig. 5(b)]. These interactions remain stable during MD simulation [see later and Fig. 5(c)]. Statistical analysis of sequence conservation at this I131ICL2 position, in the nonolfactory class A GPCR superfamily, was performed in a refined multiple sequence alignment. Among the 287 sequences reported in GPCRdb (27), we selected 247 sequences that contain at least one of the two characteristic signatures of ICL 2: a Pro residue at position (E/D)R3.50YX5P (28) that starts the two-turn α-helix conformation of ICL 2 and a Tyr residue at position (E/D)R3.50YX5PX2Y that interacts with the negative charge of the (E/D)RY motif in TMD3 (29). Clearly, this refined sequence alignment shows that most GPCRs contain a hydrophobic residue at the homologous I131ICL2 position (L, 39%; F, 14%; V, 11%; I, 9%). Similar analysis of sequence conservation in the G-protein family, at the homologous L40β1, V199β3, V344α5, and I348α5 positions [inset of Fig. 5(a)], shows that this hydrophobic pocket is conserved but with some variation.
Figure 5.
Computational model of the TRH-TRHR-Gq complex. (a) General view of the “active-like” model of TRHR in complex with Gq at the intracellular site and TRH at the extracellular site. TRH is shown as a gray surface, TRHR is shown as ribbons in green (intracellular TMD 3 and ICL 2 are shown in red), Gq is shown in blue-white (β1 and β3 strands in yellow and the α5 helix in orange), and I113 is shown in red spheres. Statistical analysis of sequence conservation in the G-protein family at the homologous L40β1, V199β3, V344α5, and I348α5 positions of Gq. (b) Detailed view of the interaction of I131ICL2 (wild-type) in ICL 2 of TRHR (in red) with L40β1 in the β1 strand (in yellow), V199β3 in the β3 strand (in yellow), and V344α5 and I348α5 in the α5 helix (in orange) of Gq. (c) Cartoon representation of 20 snapshots extracted from 500-ns MD trajectory of wild-type TRHR. The side chains of I113ICL2, L40β1, V199β3, V344α5, and I348α5 are shown as sticks. (d) Detailed view of the interaction of T131ICL2 (TRHR mutant) with L40β1, V199β3, V344α5, K345α5, and I348α5 of Gq. (e) Cartoon representation of 20 snapshots extracted from 500-ns MD trajectory of I131ICL2T mutant receptor.
To evaluate the effect of the I131ICL2T mutation in the TRHR-Gq interface, we performed MD simulations of wild-type [Fig. 5(c)] and mutant [Fig. 5(d) and 5(e)] receptors of the “active-like” model of TRHR in complex with Gq (Supplemental Methods (142.7KB, docx) ). Replacement of I131ICL2 by Thr adds a polar hydroxyl group at this TRHR-Gq interface. However, this small change in polarity causes a significant distortion. Whereas the hydrophobic I131ICL2 side chain maintains the interactions with L40β1, V199β3, V344α5, and I348α5 of Gq during the MD simulation [Fig. 5(c)], the polar side chain of T131ICL2 partly modifies the interaction of the α5 helix with TRHR [Fig. 5(e)] to interact with the polar side chain of K3445α5 [Fig. 5(d)]. Therefore, insertion of a polar Thr side chain into this hydrophobic pocket disrupts TRHR-Gq coupling.
Discussion
A unique missense TRHR mutation was identified in a consanguineous family causing central hypothyroidism in homozygotes and borderline and intermittent TSH elevation in heterozygous carriers of the defect. The study suggests that two affected TRHR alleles are necessary to develop the full hypothyroid phenotype and expands the scope of thyroid hormone derangements associated with TRHR mutations to include hyperthyrotropinemia, when one allele is affected.
Our index patient came to medical attention at the age of 8 for abnormal thyroid function tests in a routine checkup. Although the patient was overweight, features of hypothyroidism were not overt, but low FT4 and normal TSH suggested central hypothyroidism. Interestingly, 38% of children with central hypothyroidism due to IGSF1 defects are overweight or obese (30).
Retrospectively, it was evident that the patient already showed subtle hypothyroidism and hyperthyrotropinemia at 6 years of age. Strikingly, the youngest sibling of the proband, also homozygous for the defect, shows isolated hyperthyrotropinemia at age of 5. This suggests that TSH elevation may precede the development of overt hypothyroidism in homozygotes and represents a compensatory state that eventually fails, along with increased demands for thyroid hormones.
In pregnancy, thyroid hormone requirements physiologically increase and (mild) defects of the thyroid hormone axis are transiently detectable (31, 32). The mother of our patient, a heterozygous carrier of I131T, became pregnant during the course of the study. Following the guidelines of the American Thyroid Association for thyroid dysfunction in pregnancy, her TSH was elevated during the first trimester (TSH: 3.72 mIU/L; Normal: <2.5 mIU/L) (23). L-T4 was instituted, but TSH elevation recurred in the third trimester. After delivery, the mother’s TSH level returned to normal and L-T4 treatment was withdrawn. Therefore, gestational hyperthyrotropinemia in the pregnant mother may represent a compensatory attempt to meet increased demands for thyroid hormones during pregnancy.
The functional differences between the unique I131T mutation and the previously described TRHR mutations correlate well with the signaling capacity of the affected receptors (6–8). The R17X, S115-T117del+A118T, and P81R mutations all showed deleterious effects on receptor function. R17X results in a prematurely truncated protein, missing all seven TMDs. S115-T117del+A118T deeply alters the tertiary structure of the TMD 3 (essential to receptor function), and P81R allegedly alters the conformation of TMD 2 and therefore the TRH binding pocket. Unlike previous cases with biallelic TRHR mutations, our patient showed normal TSH and PRL responses to TRH (6, 7). The normal TSH responses of our patients to standard dynamic TRH tests (involving in vivo administration of high doses of TRH) are consistent with the finding that wild-type and I131T-TRHRs respond identically to saturating concentrations of TRH in vitro.
I131T is the first missense mutation identified in the ICL 2 of the TRHR and is located at a highly conserved hydrophobic position of the class-A GPCRs at the (E/D)R3.50YX5PΦXY motif, which is important for the essential conformational changes required for G-protein activation (29). Unlike the three previously identified mutations in TRHR that severely impair TRH binding and signal transduction (6–8), the unique I131T-TRHR mutant caused a threefold reduction in TRH affinity and a 7.3-fold shift in the concentration-response curve for activation of the Gq-IP3-PKC pathway yet showed normal responses to high concentrations of TRH, which might relate with the milder phenotype of hypothyroidism in the family. The model of TRHR in complex with Gq (Fig. 5) shows that the hydrophobic I131ICL2 side chain of TRHR interacts with a hydrophobic pocket formed by L40β1, V199β3, V344α5, and I348α5 of Gq. Thus, it seems reasonable to propose that insertion of a polar Thr side chain into this hydrophobic pocket of Gq disrupts TRHR-Gq coupling. Accordingly, higher concentrations of TRH are necessary to achieve the same maximal response in the I131T mutant [Fig. 4(c)]. Mutation of this hydrophobic residue in other GPCRs has similar effects but with a different extent of G-protein uncoupling (33–37).
The mechanism by which the I131ICL2T mutation at the intracellular site influences the binding of TRH to TRHR at the extracellular site is clearly indirect. Two related mechanisms have been proposed to explain this effect. First, the constitutive activity of the receptor (the equilibrium between inactive and active states in the absence of ligand) modulates ligand affinity and selectivity (38). Second, there is an allosteric coupling from G-protein to the agonist-binding pocket in GPCRs (39). Because the I131T mutation does not influence the constitutive activity of TRHR [Fig. 4(d)], we suggest that uncoupling of Gq from I131T-TRHR at the intracellular site causes the decrease of TRH binding to TRHR at the extracellular site [Fig. 4(b)]. This work provides a structural explanation for the G-protein–mediated enhancement of agonist affinity.
In addition to TSHB transcription, TRHR regulates glycosylation of TSH, which is required for the full biopotency of the TSH dimer (13, 14). Patients with central hypothyroidism, especially of hypothalamic origin, have been described with low bioactive TSH and sometimes slightly elevated immunoreactive TSH (1). The I131T-TRHR mutant seems to hinder the expected increase of FT4 after TRH stimulation in vivo, suggesting impaired TSH bioactivity. As reported in Trh–/– knockout mice (and also Trh–/+ mice to a lesser extent), decreased TRH-TRHR signaling in thyrotropes might be responsible for the development of hyperthyrotropinemia with low TSH biopotency in carriers of I131T (40). In the absence of TRH action, hyperthyrotropinemia exhibited by Trh-deficient mice is explained by decreased negative feedback of thyroid hormones at the pituitary, leading to increased synthesis of a TSH with low biological activity (40). We propose a similar situation in human pituitaries with a partially defective I131T-TRHR mutant.
The intrinsic mechanism of hyperthyrotropinemia in this family is not known, but it may relate to the uniquely milder functional behavior of the I131T-TRHR mutant itself, because a primary thyroidal defect was ruled out in this family and obesity in some (but not all) individuals of the pedigree is not linked to this feature. However, TSH elevation in this family contrasts with the normal TSH levels observed in knockout mice with deletion of the Trhr1 gene (Trhr1–/–) (41) and in individuals with previously described mutations in the TRHR gene (6–8). Two significant differences may exert a role in hyperthyrotropinemia detected in patients with I131T-TRHR mutation. First, as shown, the I131T mutant protein is stable and normally reaches the membrane. In contrast, previously described mutations in TRHR are reported to have no residual activity. Likewise, Trhr1 knockout mice are engineered to have complete deletion of the gene, leading to the absence of TRH receptors at the thyrotropic cell membrane. Second, the I131T mutant has a subtle effect on TRHR function, which can be rescued by increased TRH levels.
In summary, we identified a unique missense mutation (p.I131T) in TRHR associated with overt central hypothyroidism in the biallelic state. Although inheritance of TRHR defects is typically recessive, we describe the presence of central hyperthyrotropinemia in heterozygous carriers of this mutation. Hyperthyrotropinemia is proposed as a compensatory state preceding hypothyroidism in homozygotes. In individuals heterozygous for I131T-TRHR, central hyperthyrotropinemia is present with normal T4. However, development of hypothyroidism during pregnancy should be specifically ruled out. Undiagnosed central hypothyroidism in children calls for a higher degree of suspicion from pediatric endocrinologists dealing with patients with borderline-low T4 and normal TSH or with isolated hyperthyrotropinemia due to thyrotropic failure (22).
Acknowledgments
We thank the patient and his family for their close collaboration and Mercedes Tanarro for her expert technical assistance.
Acknowledgments
This work was supported in part by grants ENDOSCREEN S2010/BMD-2396 (to J.C.M.) from research funding of the Madrid Autonomous Region and the European Regional Development Fund and DK 48823 (to P.M.H.) from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CCH
- central congenital hypothyroidism
- CH
- congenital hypothyroidism
- FT4
- free thyroxine
- GPCR
- G-protein–coupled receptor
- ICL
- intracellular loop
- IP3
- phosphatidylinositol
- L-T4
- levo-thyroxine
- LH
- luteinizing hormone
- MD
- molecular dynamics
- PDB ID
- Protein Data Bank Identification
- PKC
- protein kinase C
- PRL
- prolactin
- TMD
- transmembrane domain
- TRH
- thyrotropin-releasing hormone
- TRHR
- thyrotropin-releasing hormone receptor
- TSH
- thyroid-stimulating hormone.
References
- 1.Persani L. Clinical review: central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97(9):3068–3078. [DOI] [PubMed] [Google Scholar]
- 2.van Tijn DA, de Vijlder JJ, Verbeeten B Jr, Verkerk PH, Vulsma T. Neonatal detection of congenital hypothyroidism of central origin. J Clin Endocrinol Metab. 2005;90(6):3350–3359. [DOI] [PubMed] [Google Scholar]
- 3.Adachi M, Soneda A, Asakura Y, Muroya K, Yamagami Y, Hirahara F. Mass screening of newborns for congenital hypothyroidism of central origin by free thyroxine measurement of blood samples on filter paper. Eur J Endocrinol. 2012;166(5):829–838. [DOI] [PubMed] [Google Scholar]
- 4.García M, Fernández A, Moreno JC. Central hypothyroidism in children. Endocr Dev. 2014;26:79–107. [DOI] [PubMed] [Google Scholar]
- 5.Heinen CA, Losekoot M, Sun Y, Watson PJ, Fairall L, Joustra SD, Zwaveling-Soonawala N, Oostdijk W, van den Akker EL, Alders M, Santen GW, van Rijn RR, Dreschler WA, Surovtseva OV, Biermasz NR, Hennekam RC, Wit JM, Schwabe JW, Boelen A, Fliers E, van Trotsenburg AS. Mutations in TBL1X are associated with central hypothyroidism. J Clin Endocrinol Metab. 2016;101(12):4564–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collu R, Tang J, Castagné J, Lagacé G, Masson N, Huot C, Deal C, Delvin E, Faccenda E, Eidne KA, Van Vliet G. A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab. 1997;82(5):1561–1565. [DOI] [PubMed] [Google Scholar]
- 7.Bonomi M, Busnelli M, Beck-Peccoz P, Costanzo D, Antonica F, Dolci C, Pilotta A, Buzi F, Persani L. A family with complete resistance to thyrotropin-releasing hormone. N Engl J Med. 2009;360(7):731–734. [DOI] [PubMed] [Google Scholar]
- 8.Koulouri O, Nicholas AK, Schoenmakers E, Mokrosinski J, Lane F, Cole T, Kirk J, Farooqi IS, Chatterjee VK, Gurnell M, Schoenmakers N. A novel thyrotropin-releasing hormone receptor missense mutation (P81R) in central congenital hypothyroidism. J Clin Endocrinol Metab. 2016;101(3):847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bílek R, Stárka L. The computer modelling of human TRH receptor, TRH and TRH-like peptides. Physiol Res. 2005;54(2):141–150. [PubMed] [Google Scholar]
- 10.Engel S, Gershengorn MC. Thyrotropin-releasing hormone and its receptors—a hypothesis for binding and receptor activation. Pharmacol Ther. 2007;113(2):410–419. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinkle PM, Gehret AU, Jones BW. Desensitization, trafficking, and resensitization of the pituitary thyrotropin-releasing hormone receptor. Front Neurosci. 2012;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fares F. The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: development of agonists and antagonists. Biochi Biophys Acta. 2006;1760(4):560–567. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub BD, Gesundheit N, Taylor T, Gyves PW. Effect of TRH on TSH glycosylation and biological action. Ann N Y Acad Sci. 1989;553:205–213. [DOI] [PubMed] [Google Scholar]
- 15.van Tijn DA, de Vijlder JJ, Vulsma T. Role of the thyrotropin-releasing hormone stimulation test in diagnosis of congenital central hypothyroidism in infants. J Clin Endocrinol Metab. 2008;93(2):410–419. [DOI] [PubMed] [Google Scholar]
- 16.Yamada M, Mori M. Mechanisms related to the pathophysiology and management of central hypothyroidism. Nat Clin Pract Endocrinol Metab. 2008;4(12):683–694. [DOI] [PubMed] [Google Scholar]
- 17.Zhu CC, Cook LB, Hinkle PM. Dimerization and phosphorylation of thyrotropin-releasing hormone receptors are modulated by agonist stimulation. J Biol Chem. 2002;277(31):28228–28237. [DOI] [PubMed] [Google Scholar]
- 18.Colson AO, Perlman JH, Jinsi-Parimoo A, Nussenzveig DR, Osman R, Gershengorn MC. A hydrophobic cluster between transmembrane helices 5 and 6 constrains the thyrotropin-releasing hormone receptor in an inactive conformation. Mol Pharmacol. 1998;54(6):968–978. [DOI] [PubMed] [Google Scholar]
- 19.Jones BW, Song GJ, Greuber EK, Hinkle PM. Phosphorylation of the endogenous thyrotropin-releasing hormone receptor in pituitary GH3 cells and pituitary tissue revealed by phosphosite-specific antibodies. J Biol Chem. 2007;282(17):12893–12906. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, Granier S, Gmeiner P, Husbands SM, Traynor JR, Weis WI, Steyaert J, Dror RO, Kobilka BK. Structural insights into µ-opioid receptor activation. Nature. 2015;524(7565):315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura A, Kitano K, Takasaki J, Taniguchi M, Mizuno N, Tago K, Hakoshima T, Itoh H. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc Natl Acad Sci USA. 2010;107(31):13666–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. J Thyroid Res. 2012;2012:351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum . Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez JM, Maravall FJ, Gómez N, Gumà A, Soler J. Determinants of thyroid volume as measured by ultrasonography in healthy adults randomly selected. Clin Endocrinol (Oxf). 2000;53(5):629–634. [DOI] [PubMed] [Google Scholar]
- 25.Zubiaur Cantalapiedra A, Zapico Alvarez-Cascos MD, Ruiz Pérez L, Sanguino López L, Sánchez Serrano FJ, Alfayate Guerra R, Sánchez-Paya J, Guirao Carratalá MD, Pico Alfonso A, Flores Serrano J. Iodine nutritional status in the school-aged population in Alicante (Spain). An Pediatr (Barc). 2007;66(3):260–266. [DOI] [PubMed] [Google Scholar]
- 26.Hinkle PM, Nelson EJ, Ashworth R. Characterization of the calcium response to thyrotropin-releasing hormone in lactotrophs and GH cells. Trends Endocrinol Metab. 1996;7(10):370–374. [DOI] [PubMed] [Google Scholar]
- 27.Vroling B, Sanders M, Baakman C, Borrmann A, Verhoeven S, Klomp J, Oliveira L, de Vlieg J, Vriend G. GPCRDB: information system for G protein-coupled receptors. Nucleic Acids Res. 2011;39(Database issue):D309–D319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 29.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494(7436):185–194. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Bak B, Schoenmakers N, van Trotsenburg AS, Oostdijk W, Voshol P, Cambridge E, White JK, le Tissier P, Gharavy SN, Martinez-Barbera JP, Stokvis-Brantsma WH, Vulsma T, Kempers MJ, Persani L, Campi I, Bonomi M, Beck-Peccoz P, Zhu H, Davis TM, Hokken-Koelega AC, Del Blanco DG, Rangasami JJ, Ruivenkamp CA, Laros JF, Kriek M, Kant SG, Bosch CA, Biermasz NR, Appelman-Dijkstra NM, Corssmit EP, Hovens GC, Pereira AM, den Dunnen JT, Wade MG, Breuning MH, Hennekam RC, Chatterjee K, Dattani MT, Wit JM, Bernard DJ. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet. 2012;44(12):1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkes IL, Schenker JG, Shufaro Y. Thyroid disorders during pregnancy. Gynecol Endocrinol. 2012;28(12):993–998. [DOI] [PubMed] [Google Scholar]
- 32.Pine-Twaddell E, Romero CJ, Radovick S. Vertical transmission of hypopituitarism: critical importance of appropriate interpretation of thyroid function tests and levothyroxine therapy during pregnancy. Thyroid. 2013;23(7):892–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moro O, Lameh J, Högger P, Sadée W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biol Chem. 1993;268(30):22273–22276. [PubMed] [Google Scholar]
- 34.Arora KK, Sakai A, Catt KJ. Effects of second intracellular loop mutations on signal transduction and internalization of the gonadotropin-releasing hormone receptor. J Biol Chem. 1995;270(39):22820–22826. [DOI] [PubMed] [Google Scholar]
- 35.Burstein ES, Spalding TA, Brann MR. The second intracellular loop of the m5 muscarinic receptor is the switch which enables G-protein coupling. J Biol Chem. 1998;273(38):24322–24327. [DOI] [PubMed] [Google Scholar]
- 36.Gáborik Z, Jagadeesh G, Zhang M, Spät A, Catt KJ, Hunyady L. The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology. 2003;144(6):2220–2228. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Tao YX. Functions of the DRY motif and intracellular loop 2 of human melanocortin 3 receptor. J Mol Endocrinol. 2014;53(3):319–330. [DOI] [PubMed] [Google Scholar]
- 38.Montanelli L, Van Durme JJ, Smits G, Bonomi M, Rodien P, Devor EJ, Moffat-Wilson K, Pardo L, Vassart G, Costagliola S. Modulation of ligand selectivity associated with activation of the transmembrane region of the human follitropin receptor. Mol Endocrinol. 2004;18(8):2061–2073. [DOI] [PubMed] [Google Scholar]
- 39.DeVree BT, Mahoney JP, Vélez-Ruiz GA, Rasmussen SG, Kuszak AJ, Edwald E, Fung JJ, Manglik A, Masureel M, Du Y, Matt RA, Pardon E, Steyaert J, Kobilka BK, Sunahara RK. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535(7610):182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada M, Saga Y, Shibusawa N, Hirato J, Murakami M, Iwasaki T, Hashimoto K, Satoh T, Wakabayashi K, Taketo MM, Mori M. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci USA. 1997;94(20):10862–10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabeler R, Mittag J, Geffers L, Rüther U, Leitges M, Parlow AF, Visser TJ, Bauer K. Generation of thyrotropin-releasing hormone receptor 1-deficient mice as an animal model of central hypothyroidism. Mol Endocrinol. 2004;18(6):1450–1460. [DOI] [PubMed] [Google Scholar]