Abstract
Context:
The increased use of diagnostic and therapeutic procedures that involve radiation raises concerns about radiation effects, particularly in children and the radiosensitive thyroid gland.
Objectives:
Evaluation of relative risk (RR) trends for thyroid radiation doses <0.2 gray (Gy); evidence of a threshold dose; and possible modifiers of the dose-response, e.g., sex, age at exposure, time since exposure.
Design and Setting:
Pooled data from nine cohort studies of childhood external radiation exposure and thyroid cancer with individualized dose estimates, ≥1000 irradiated subjects or ≥10 thyroid cancer cases, with data limited to individuals receiving doses <0.2 Gy.
Participants:
Cohorts included the following: childhood cancer survivors (n = 2); children treated for benign diseases (n = 6); and children who survived the atomic bombings in Japan (n = 1). There were 252 cases and 2,588,559 person-years in irradiated individuals and 142 cases and 1,865,957 person-years in nonirradiated individuals.
Intervention:
There were no interventions.
Main Outcome Measure:
Incident thyroid cancers.
Results:
For both <0.2 and <0.1 Gy, RRs increased with thyroid dose (P < 0.01), without significant departure from linearity (P = 0.77 and P = 0.66, respectively). Estimates of threshold dose ranged from 0.0 to 0.03 Gy, with an upper 95% confidence bound of 0.04 Gy. The increasing dose–response trend persisted >45 years after exposure, was greater at younger age at exposure and younger attained age, and was similar by sex and number of treatments.
Conclusions:
Our analyses reaffirmed linearity of the dose response as the most plausible relationship for “as low as reasonably achievable” assessments for pediatric low-dose radiation-associated thyroid cancer risk.
A pooling of nine cohort studies of childhood external radiation exposure revealed a linear increase in risk of thyroid cancer and reaffirmed the “as low as reasonably achievable” principal for pediatric low dose radiation.
The increasing use of diagnostic and therapeutic medical procedures that involve radiation raises concerns about the consequences of low-dose irradiation on cancer occurrence and in particular whether linear-based extrapolations represent the best estimate of low-dose cancer risk or whether a threshold dose, below which there is no risk of radiation-induced cancer, might exist (1–3) (see http://dels.nas.edu/Upcoming-Event/Planning-Towards-BEIR-VIII-Report/AUTO-0-14-84-B). With increased radiation exposures of children and the well-documented radiosensitivity of the thyroid gland, data on thyroid cancer incidence among low-dose thyroid-irradiated children provide an opportunity to address issues of pediatric radiation-associated thyroid cancer risk in relation to assessments of as low as reasonably achievable (ALARA), with implications for both clinical practice and radioepidemiology (3–7). With these considerations in mind, we pool epidemiologic studies on children exposed to external radiation and analyze thyroid cancer incidence for those exposed at low doses.
A recent pooling of 12 epidemiologic studies of thyroid cancer following radiation exposure in childhood included a broad range of thyroid radiation doses, from <0.1 gray (Gy) for treatment of benign diseases and exposures following the atomic bombings in Japan to >60 Gy resulting from radiation therapies for various childhood cancers (8). Analyses revealed a curvilinear relationship, with relative risks (RR) increasing supralinearly through about 2 to 4 Gy, leveling at doses between 10 and 30 Gy with RRs of 30 to 40 and declining thereafter, with RRs remaining 5 to 10 at 50 to 60 Gy. The leveling and downturn at higher doses may have reflected cell-killing effects, although compensatory accelerated stem cell repopulation may have partially mitigated the decline (9). The pooled analysis reported increased RRs for doses <0.1 Gy, but did not fully explore the result (8). Investigators also identified important modifiers of the dose–response association, namely, radiation dose–response trends emerged within 5 to 10 years of exposure, increased at younger ages at exposure, declined with increasing attained age, and persisted for 50 years and more after exposure (8, 10). Trends were comparable in strength for females and males. Notably, these analyses did not address whether patterns of effect modification derived exclusively from those exposed at moderate and high doses or whether there were similar patterns among those exposed only at lower doses.
Questions about thyroid cancer risks at low thyroid radiation doses in children are best addressed using data in children exposed at low doses. We therefore examined data in children exposed at low doses to consider the following: (1) RR patterns at doses <0.2 Gy and <0.1 Gy; (2) the possible existence of a threshold dose; and (3) modification of the dose response by sex, age at exposure, attained age, years since exposure, and radiation dose fractionation.
Methods
Study populations
We used MEDLINE to identify 12 epidemiologic (10 cohort and two case-control) studies of childhood external radiation exposure and thyroid cancer and approached the principal investigators to collaborate in a pooled data analysis. We required that studies had sufficient information for the calculation of individualized, quantitative, thyroid gland–specific dose estimates and enrolled at least 1000 irradiated subjects or 10 thyroid cancers (8). Given the highly specialized characteristics of the studies and, for cohorts, the need to accommodate continued follow-up, we were confident that these studies represented all eligible studies. After the pooling of data and harmonization of variables, the current analysis restricted data to doses <0.2 Gy, except where noted. This resulted in the exclusion of two case-control studies following treatment of childhood cancer (11, 12) and one cohort study of childhood lymphoid hyperplasia (13). Because there were no thyroid cancer cases <0.2 Gy, except for one nonexposed case for one study (11), and because analyses adjusted for study, the three omitted studies provided no information for the evaluation of RR patterns. Nine cohorts remained, including treatment of childhood cancer (n = 2), treatment of various benign diseases (e.g., tinea capitis, enlarged thymus, hemangioma, and enlarged tonsils and adenoids) (n = 6), and Japanese atomic bomb survivors (n = 1) (Supplemental Appendix (205.7KB, pdf) ). Detailed study-specific descriptions and study-specific analyses were reported previously (8 and its supplemental material). Cases were those who developed incident thyroid cancer during follow-up. Excluding nonmelanoma skin cancer, cases represented first primary cancers for studies of benign diseases and atomic bomb survivors and second primary cancers for studies of childhood cancer survivors. We censored autopsy-identified thyroid cancers at death, but did not include them as cases.
The institutional review boards approved each participating study and the data pooling.
Models for thyroid cancer risk
For seven cohorts, follow-up started at date of first radiation exposure or enrollment for nonexposed (Supplemental Appendix (205.7KB, pdf) ). For the Atomic Bomb Survivors Study, follow-up started in 1958, 13 years after exposure. For the Childhood Cancer Survivor Study (CCSS) cohorts, follow-up started 5 (CCSS-United States) or 3 years (CCSS-France/United Kingdom) after first cancer. Follow-up continued to the earliest date of death, loss to follow-up, incident cancer other than nonmelanoma skin cancer, or end of study.
Analyses used Poisson regression, with person-years of follow-up cross-tabulated by study, sex, age at exposure, calendar year of follow-up, time since exposure, attained age, treatment with chemotherapy, thyroid radiation dose, and number of radiation treatments, in which one treatment encompassed all doses within 6 months. Regressions adjusted for study, sex, and age and selected study-specific variables. For the Israel Tinea Capitis Study, variables included country of origin (North Africa/others) and comparison group (sibling/population). For the Rochester Thymus Study, variables included the presence of goiter (yes/no) and Jewish religion (yes/no). For the Atomic Bomb Survivors Study, variables included city of exposure (Hiroshima/Nagasaki), not in city at the bombing (yes/no), and enrollment in the Adult Health Study (yes/no), the latter variable accounting for possible surveillance-related differences in thyroid cancer rates (10). For CCSS cohorts, additional variables included type of first cancer (Hodgkin lymphoma or other) and chemotherapy treatment. Because most radiation-exposed cases had only one treatment (84%), we assumed one treatment of exposed patients who were missing number of treatments. We computed person-years weighted means within each cell of the cross-tabulation for continuous variables.
Supplemental Appendix (205.7KB, pdf) provides methodologic details. The regression model for thyroid cancer incidence rate, , included adjustment variables x, thyroid radiation dose d, and chemotherapy exposure c. We previously determined that an additive relationship best described the joint association of radiation exposure and chemotherapy treatment (c = 1 if yes and c = 0 if no). Given x, the incidence rate under an additive relationship for radiation dose and chemotherapy, termed nonsynergistic, is the sum of three terms: the incidence rate absent both factors, , and the excess rates for each exposure at the referent level of the other, and (14, 15), i.e.,
Factoring the nonexposed thyroid cancer rate, ,
| (1) |
where ERR(d) was the radiation-associated excess RR (ERR) and θ was the chemotherapy-associated ERR. We set with ϕ a vector of parameters. For <0.2 Gy, we fitted a simple linear model, , where β was the ERR/Gy, i.e., the slope. We examined departures from linearity with likelihood ratio tests and used a likelihood-based 95% confidence interval (CI) for estimates of β.
A threshold identifies a dose below which there is no radiation effect, whereas a linear threshold model specifies a linear relationship starting at the threshold dose, i.e., , where with η the unknown threshold. We compared deviances to identify the maximum likelihood estimate for the threshold. We also modeled the dose response using a 4-knot cubic spline (16, 17). Finally, we compared the full-dose range model (8), but fitted to the nine cohorts in this analysis.
We used the Epicure program for all modeling (18).
Results
For doses <0.2 and <0.1 Gy, there were 252 and 184 radiation-exposed thyroid cancer cases, respectively, and 2,588,559 and 2,114,683 person-years of follow-up (Supplemental Appendix (205.7KB, pdf) ). Among nonexposed individuals, there were 142 thyroid cancer cases and 1,865,957 person-years.
Radiation dose response
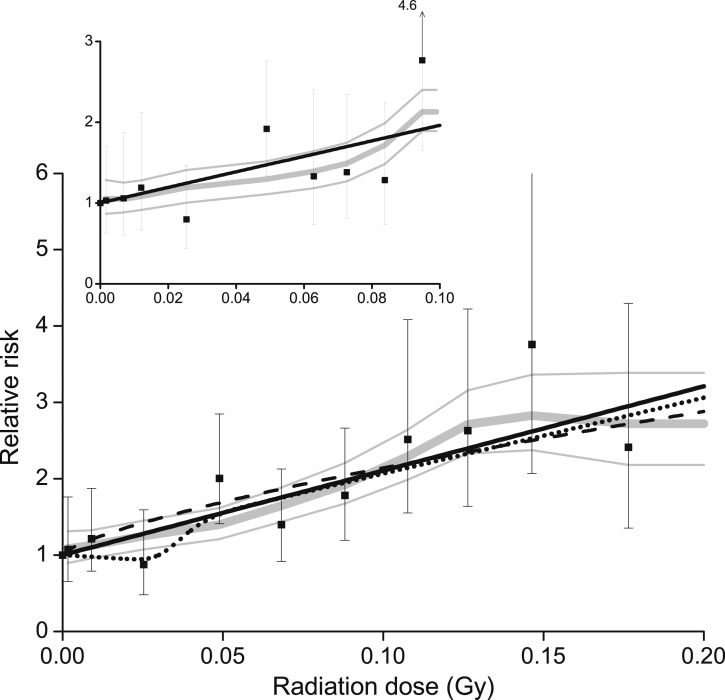
For doses <0.2 Gy, RRs increased significantly with radiation dose (Table 1; Fig. 1, main panel, solid black line) (P < 0.01), with no significant departure from linearity (P = 0.77). A moving-average smoothing of RRs (thick gray line) with ±1 standard deviation (thin gray line) highlighted the linear pattern. The downward extrapolation from the curvilinear model-fitted to the full-dose range closely approximated low-dose RRs (dash line). Results were similar for <0.1 Gy (inset panel), with no departure from linearity (P = 0.66).
Table 1.
Numbers of Thyroid Cancer Cases, Participants, Person-Years (P-yrs) of Follow-Up, RR, 95% CI, and Estimated Radiation-Associated Excess Cases
| Dose (Gy) |
Cases | Participants | P-yrs | RRa | 95% CI | Excessb | |
|---|---|---|---|---|---|---|---|
| Range | Mean | ||||||
| 0 | 0.000 | 142 | 46,439 | 1,865,957 | 1.00 | 0.0 | |
| 0.001–0.004 | 0.002 | 24 | 9464 | 367,606 | 1.07 | (0.7, 1.8) | 0.4 |
| 0.005–0.02 | 0.009 | 30 | 13,796 | 587,614 | 1.21 | (0.8, 1.9) | 2.7 |
| 0.02–0.03 | 0.025 | 13 | 8055 | 345,748 | 0.87 | (0.5, 1.6) | 4.6 |
| 0.04–0.06 | 0.049 | 54 | 7204 | 315,014 | 2.01 | (1.4, 2.8) | 15.0 |
| 0.06–0.08 | 0.068 | 31 | 5825 | 256,456 | 1.40 | (0.9, 2.1) | 16.9 |
| 0.08–0.09 | 0.088 | 32 | 5535 | 242,247 | 1.78 | (1.2, 2.7) | 17.3 |
| 0.10–0.12 | 0.107 | 20 | 3220 | 136,943 | 2.51 | (1.5, 4.1) | 9.4 |
| 0.12–0.14 | 0.126 | 21 | 3537 | 149,525 | 2.63 | (1.6, 4.2) | 11.0 |
| 0.14–0.16 | 0.146 | 13 | 1778 | 73,824 | 3.76 | (2.1, 6.8) | 5.5 |
| 0.16–0.19 | 0.177 | 14 | 2741 | 113,582 | 2.41 | (1.4, 4.3) | 11.1 |
| Total | 394 | 107,594 | 4,454,516 | 93.8 | |||
Pooled data for doses <0.2 Gy.
RRs adjusted for study, sex, age, other study-specific factors, and chemotherapy exposure.
Estimated excess number of thyroid cancer cases above fitted background of nonexposed participants.
Figure 1.
Category-specific RR of thyroid cancer by thyroid radiation dose (solid symbol) with 95% CI, a moving-average smoothing (gray line) and ±1 standard deviation (thin gray line), the fitted linear ERR model (solid black line), and a restricted cubic spline (dash-dot-dot line). Data pooled from nine cohort studies and limited to <0.2 Gy (main panel) or <0.1 Gy (inset). Also, the linear-exponential-linear model (Supplemental Appendix (205.7KB, pdf) ) fitted to all data with the full range of doses (dash line).
For <0.2 and <0.1 Gy, estimates of β were 11.1 (95% CI, 6.6, 19.7) and 9.6 (95% CI, 3.7, 17.0), respectively, with fitted RRs at 0.2 Gy of 3.2 (95% CI, 2.3, 4.3) and 2.9 (95% CI, 1.7, 4.5). Using the full-dose range model, the fitted RR at 0.2 Gy was 2.7 (95% CI, 2.1, 3.4).
The cubic spline was flat through 0.03 Gy (Fig. 1, dot line), largely influenced by the RR of 0.9 (95% CI, 0.5, 1.6) for the 0.0-2 to 0.03-Gy dose category with 13 cases (Table 1). Nonetheless, the simple linear model had the minimum Akaike Information Criterion (19), identifying it as the preferred model.
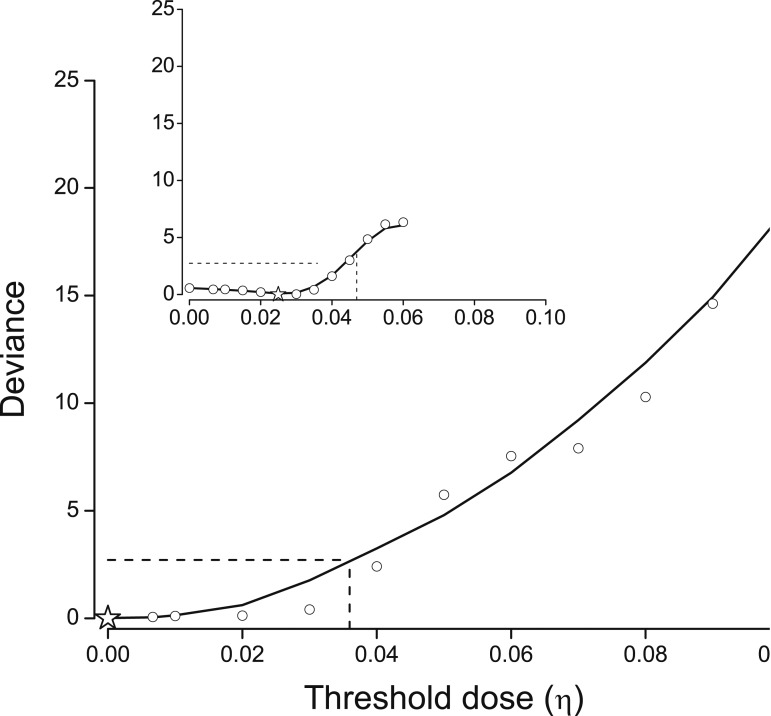
Estimates of a threshold dose
We examined deviances to estimate threshold dose (η). For <0.2 Gy, deviances (open circle) and a moving-average smoothing (solid line) increased (i.e., poorer fit) with possible threshold values, with minimum deviance (star symbol) at 0.00 Gy (Fig. 2, main panel). Deviances changed little through 0.02 Gy. For <0.1 Gy, the minimum deviance occurred at 0.025 Gy, with no change through 0.03 Gy (inset panel), indicating limited ability to identify a specific threshold. One-sided upper 95% CIs were 0.036 for <0.2 Gy and 0.044 for <0.01 Gy (dash line).
Figure 2.
Deviances for linear ERR models given a threshold dose (η) (open symbol) (see text for model), with deviances rescaled to zero at the minimum deviance (star symbol) and a moving average smoothing. Dash line identifies one-sided 95% confidence limit. Data pooled from nine cohort studies and limited to <0.2 Gy (main panel) and <0.1 Gy (inset).
Excess radiation-associated thyroid cancers
Using the equation for doses <0.2 Gy, we estimated 37.2% radiation-associated thyroid cases (252 observed with 93.8 excess exposed cases) (Table 1). This varied from 5.7% (54 observed with 3.1 excess cases) for doses <0.02 Gy to 54.4% (68 observed with 37.0 excess cases) for doses between 0.1 and 0.2 Gy.
Modification of the radiation dose response
The radiation dose response was similar by sex (P = 0.35) and number of treatments (P = 0.25) (Table 2). The dose response generally increased with younger age at exposure, except for those exposed under age 1 year (P = 0.01). Radiation dose responses decreased with greater attained age (P = 0.01) and decreased but remained elevated with years since exposure (P = 0.02), persisting 45 years and more after irradiation. Supplemental Appendix (205.7KB, pdf) displays fitted RRs at 0.2 Gy and models using continuous modifiers, illustrating the wide CI for the RR under age 1 year. These effect modification patterns were similar for doses <0.1 Gy (Table 2), although estimates were less stable. Results paralleled those in the complete data (8).
Table 2.
Evaluation of Effect Modification for the Thyroid Cancer Radiation Dose Response, Including Numbers of Radiation-Exposed Cases and Fitted RR at 0.2 Gy Under an Additive Adjustment for Treatment With Chemotherapy
| Modifier | Data With Doses <0.2 Gy |
Data With Doses <0.1 Gy |
|||||
|---|---|---|---|---|---|---|---|
| Cases | RR0.2 Gya | θ | Cases | RR0.2Gya | θ | ||
| None | 252 | 3.2 | 2.84 | 184 | 2.9 | 2.17 | |
| Sex | |||||||
| Male | 58 | 4.2 | 2.93 | 44 | 5.0 | 2.31 | |
| Female | 194 | 3.0 | 140 | 2.4 | |||
| P valueb | 0.35 | 0.15 | |||||
| Age at exposure (y) | |||||||
| <1 | 40 | 1.6 | 3.49 | 30 | 0.4 | 2.77 | |
| 1–4 | 64 | 4.2 | 31 | 2.9 | |||
| 5–9 | 84 | 3.9 | 71 | 3.9 | |||
| 10–14 | 42 | 3.5 | 35 | 3.7 | |||
| 15–19 | 22 | 1.6 | 17 | 0.6 | |||
| P valuec | 0.01 | 0.05 | |||||
| Attained age (y) | |||||||
| <20 | 17 | 9.4 | 3.72 | 10 | 7.5 | 2.49 | |
| 20–29 | 43 | 5.5 | 25 | 4.1 | |||
| 30–39 | 48 | 2.6 | 33 | 2.0 | |||
| 40–49 | 60 | 2.2 | 48 | 2.6 | |||
| 50–59 | 62 | 3.1 | 52 | 3.2 | |||
| 60+ | 22 | 2.5 | 16 | 1.9 | |||
| P valuec | 0.01 | 0.13 | |||||
| Time since exposure (y) | |||||||
| <20 | 37 | 5.3 | 3.16 | 22 | 4.2 | 2.41 | |
| 20–29 | 49 | 3.5 | 36 | 3.5 | |||
| 30–34 | 33 | 3.4 | 22 | 1.7 | |||
| 35–39 | 33 | 3.3 | 22 | 2.3 | |||
| 40–44 | 36 | 2.4 | 28 | 2.2 | |||
| 45+ | 64 | 2.3 | 54 | 3.2 | |||
| P valuec | 0.02 | 0.26 | |||||
| Number of treatmentsd | |||||||
| 1 | 226 | 3.5 | 2.80 | 170 | 3.4 | 2.13 | |
| ≥2 | 20 | 2.4 | 8 | 0.4 | |||
| P valueb | 0.25 | 0.25 | |||||
Pooled data limited to doses <0.2 Gy or <0.1 Gy.
Fitted RRs from a linear model in radiation dose with an additive effect for chemotherapy treatment, c, . Models adjusted for study, sex, age, and study-specific factors (see text). For modifiers, d) replaced βd where zj was an indicator variable for category j and βj was a linear parameter. There were 142 with nonexposed cases. For <0.2 Gy and <0.1 Gy, β estimates with 95% CI were 11.1 (6.6, 19.7) and 9.6 (3.7, 17.0), respectively.
Fitted RR at 0.2 Gy.
P value for likelihood ratio test of no variation based on a binary modifier.
P value for likelihood ratio test of no variation based on continuous modifier.
The definition of fractionation, which involved time between fractions, dose per fraction, and reason, varied by study. One treatment included all dose fractions received within 6 months in most studies and within 1 year for the Tinea Capitis Study.
Consistency of radiation effects across cohorts
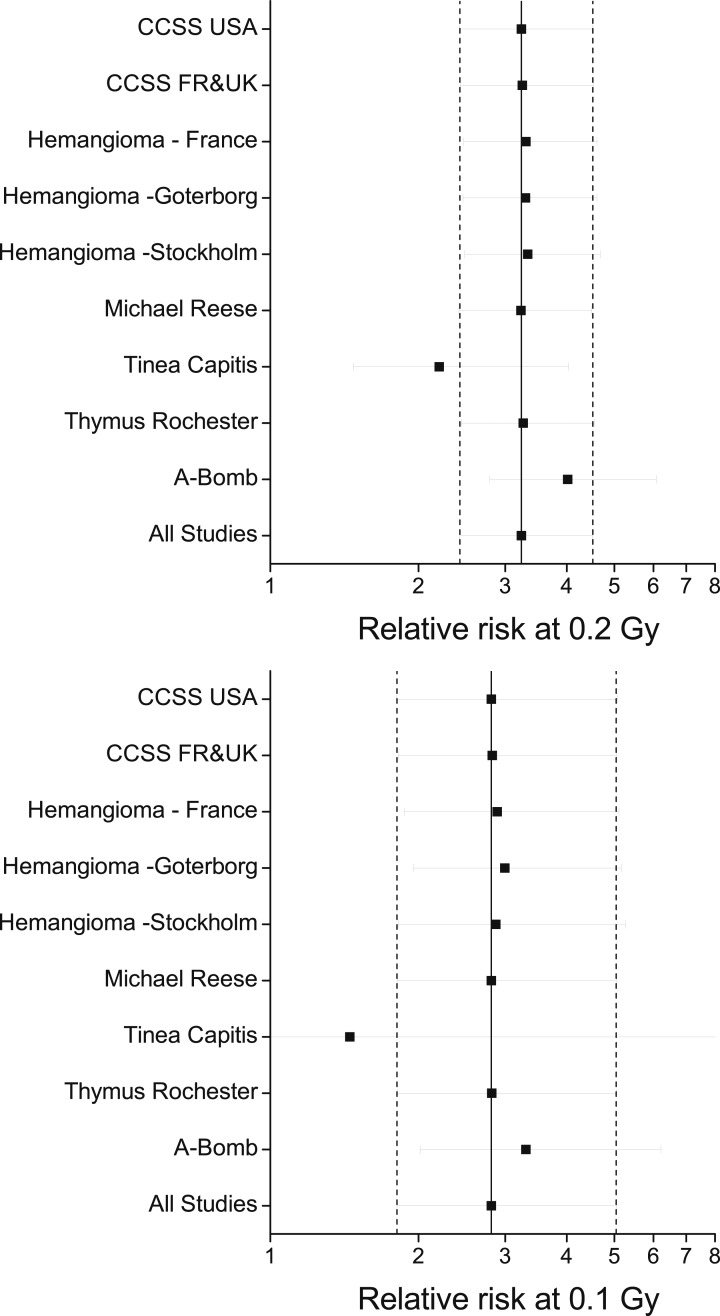
We evaluated the influence of each study, with particular attention to the Tinea Capitis Study and the Atomic Bomb Survivors Study, because they contributed 52% (132/252) and 28% (70/252) of exposed cases, respectively. For <0.2 Gy and with an adjustment to reflect age 5 years at exposure to account for cohort differences, the fitted RR at 0.2 Gy was 3.2 (95% CI, 2.4, 4.5) for all studies combined (Fig. 3, upper panel). With the Tinea Capitis Study omitted, the fitted RR estimate at 0.2 Gy decreased 31% to 2.2 (95% CI, 1.5, 4.0), and with the Atomic Bomb Survivors Study omitted, the estimate increased 25% to 4.0 (95% CI, 2.8, 6.1). Omitting other studies individually, as well as both CCSS cohorts, did not alter the summary estimate. For <0.1 Gy, the Tinea Capitis and Atomic Bomb Survivors Studies contributed 82% (150/282) of exposed cases, and omission of each study resulted in similar consequences.
Figure 3.
Fitted RRs at 0.2 and 0.1 Gy and 95% CIs under a linear ERR model with effect modification by the natural logarithm of age at exposure divided by 5 years overall and sequentially omitting one study at a time. RRs reflect age 5 years at exposure. Dash line represents lower and upper confidence limits for all data. FR, France; UK, United Kingdom; USA, United States.
We assumed the same ERR/Gy applied in all study populations. Allowing random effects for ERR/Gy (β) due to population differences and adjusting for age at exposure, Cochran Q-statistics were P = 0.89 for doses <0.2 Gy and P = 0.95 for doses <0.1 Gy, indicating homogeneity of the ERR/Gy across studies (20–22). The random effects-fitted RRs at 0.2 Gy were 3.0 (95% CI, 2.1, 4.9) for <0.2 Gy and 2.9 (95% CI, 1.7, 6.6) for <0.1 Gy.
Discussion
Given enhanced effects for childhood radiation exposures and the well-documented radiosensitivity of the thyroid gland, our pooling of all epidemiologic data with thyroid radiation doses <0.2 Gy enabled a direct assessment of a linear dose–response relationship for thyroid cancer risk and of a possible threshold dose. The analysis included 252 radiation-exposed thyroid cancer cases and was the most definitive to date of thyroid cancer following low-dose external radiation exposure.
For <0.2 and <0.1 Gy, RRs increased significantly with thyroid radiation dose, with trends consistent with linearity. Fitted estimates of RRs at these doses were also consistent with downward extrapolations with previous models based on all doses through 70 Gy and more. Consequently, our results refute suggestions of no excess radiation-related thyroid cancer risk below these a priori restriction levels and support linearity in ALARA assessments.
Estimates of a threshold dose were 0.0 Gy for <0.2 Gy and 0.03 Gy for <0.1 Gy. These estimates derived from the likelihood profile and therefore represented maximum likelihood estimates (23). One-sided upper 95% confidence limits were 0.036 and 0.044 Gy, respectively. Analysis did not account for dose measurement error, which may have introduced bias, typically an underestimation (24, 25). However, for the Atomic Bomb Survivors Study, we used measurement error-adjusted dose estimates (26, 27). For the Tinea Capitis Study, an analysis that accounted for measurement error estimated a similar dose response and assessment of effect modifiers (28, 29). With the Tinea Capitis and the Atomic Bomb Survivors Studies comprising 80% of exposed cases and 57% of exposed person-years, it was thus unlikely that measurement error substantially affected estimates of threshold dose, although a definitive conclusion was not possible.
A comprehensive accounting of dose measurement error for all studies was beyond the scope of this paper. In particular, we did not know to what extent dosimetric conditions of the Tinea Capitis Study applied to other studies. Nevertheless, we note one potential contribution to dose measurement error for some CCSS participants, namely, an underestimation of thyroid dose from additional radiological examinations associated with cancer diagnosis, treatment monitoring, and follow-up. This additional dose, more likely with head and neck cancers, was not captured in the dose assessments and may have amounted to 0.2 Gy and more. Because CCSS cohorts included 5% of cases and 3% of person-years and because influence analysis indicated minimal impact from the omission of CCSS, any underestimation of radiation doses in CCSS data most likely involved minimal bias.
The 12-study pooled analysis supported a minimum latency of 5 to 10 years (8). For 5 to 9, 10 to 14, and 15 to 19 years since exposure, there were 39, 65, and 128 exposed thyroid cancers, respectively, and fitted RRs at 0.2 Gy were 1.7, 1.9, and 3.3. For <5 years since exposure, there were three cases and the RR was not estimable. An important question was whether a similar latency applied for those exposed only at low doses. For <0.2 Gy, there were 3, 13, and 20 exposed cases for 5 to 9, 10 to 14, and 15 to 19 years since exposure, respectively, and fitted RRs at 0.2 Gy were 2.9 (95% CI, <0.0, 11.7), 6.2 (95% CI, 2.8, 13.5), and 5.5 (95% CI, 2.6, 10.6). For <0.1 Gy, there were 3, 10, and 8 exposed cases in the time since exposure categories, and estimated RRs at 0.2 Gy were 3.5 (95% CI, 0.7, 11.1), 4.8 (95% CI, 1.8, 10.5), and 1.6 (95% CI, 0.3, 4.1). Although data were limited, fitted RRs in the restricted data appeared compatible with a minimum latency of 5 to 10 years.
We previously identified an additive (nonsynergistic) relationship for the RRs of thyroid radiation dose and chemotherapy for childhood cancer and thyroid cancer incidence (8). We refitted models for two subsets of the data to remove any residual uncertainty about a nonsynergistic relationship chemotherapy: (1) participants who did not receive chemotherapy, and (2) participants in the seven non-CCSS. The estimated ERR/Gy was 11.0 for participants without chemotherapy and 11.1 for the seven cohorts, resulting in fitted RRs at 0.2 Gy of 3.2 for both subsets. This equaled the fitted RR of 3.2 for data with doses <0.2 Gy, indicating choice of risk model for chemotherapy did not impact results.
Vaccarella et al. (30) have suggested that increased medical surveillance and new diagnostic techniques are important factors in recent worldwide increases in the incidence rates for thyroid cancer. Although medical screening may have increased the ascertainment of thyroid cancer cases, there was little reason to presuppose that increased screening was also related to radiation dose and thus acted to confound our results. Information on thyroid cancer–screening practices was not explicitly available for all studies in our pooling; however, analytic evaluation was possible in two studies. Ron et al. (10) considered the effect of screening using surrogate information on the potential for heightened medical surveillance in the Atomic Bomb Survivors Study and the Michael Reese Hospital Study. For the former, Ron et al. (10) evaluated participation in the Adult Health Study, a companion study starting in 1958 that involved ∼20% of subjects who were invited to biennial clinical examinations. For the latter, Ron et al. (10) used calendar year 1974 as an approximate indicator year for the start of heightened public awareness of radiation effects and heightened surveillance. Analyses revealed the expected increase in the absolute rate of thyroid cancer incidence, but also found no significant variation in the radiation dose response for the RR of thyroid cancer (10). In the current analysis of restricted data, we similarly found no significant variation in the linear radiation dose–response parameter by participation in the Adult Health Study for the Atomic Bomb Survivor Study (P = 0.39). With one case, we could not assess the Michael Reese Hospital Study. We also evaluated variation of the radiation dose response across categories of calendar year of follow-up, reasoning that confounding from enhanced thyroid screening was more likely to have occurred in recent years. After adjustment for attained age, we found no significant variation of the radiation dose response by calendar year (P = 0.32).
With the nuclear releases from the 1986 Chernobyl power plant accident and from the Fukushima Daiichi power plant following the 2011 Great East Japan Earthquake and Tsunami, there are questions about comparative thyroid cancer risks for childhood exposure to low radiation doses from external and internal sources. External sources of exposure derive mainly from x- and γ-irradiation, whereas internal sources derive mainly from short-lived β-radiation decay of iodine-131 (1, 31–35). Studies of thyroid cancer in children exposed following the Chernobyl accident have reported a range of RRs. RR estimates at 0.2 Gy were 1.9 from a Russian Federation–Belarus study (36), 1.9 from a Ukraine study (37), with a more recent estimate of 1.2 (38), and 1.2 from a Belarus study (39). Our analysis of external thyroid radiation dose yielded an estimate of 3.2 (95% CI, 2.3, 4.3). For those aged <5 years at exposure, RR estimates at 0.2 Gy were 2.6 in the Ukraine study (37) and 1.9 in the Belarus study (39), whereas our estimate was 3.4 (95% CI, 2.0, 4.8). These results suggested the possibility of a slightly greater thyroid cancer risk from childhood external irradiation. Definitive conclusions are problematic due to the substantial differences between our populations of medically exposed individuals and atomic bomb survivors and the Chernobyl-exposed populations who were mildly to moderately iodine deficient and/or intensely screened (40).
The use of computed tomography (CT) scanning for medical diagnostic and therapeutic procedures has increased substantially in recent years in both adults and children, with children in the United States now receiving 5 to 9 million scans per year (41, 42) (http://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/pediatric-ct-scans). CT scans of children can result in thyroid radiation doses of 0.010 Gy for head CTs (38, 43), 0.027 Gy for chest CTs (43–45), 0.050 Gy for neck CTs (46, 47), 0.036 Gy for cervical spine CTs (48), and 0.008 Gy for abdominal CTs (43). With CT scans often repeated, multidetector row CT scans reducing scan times and enabling increased use of CT scans in pediatric medicine and the use of multiphase (repeat scanning before and after contrast injection) CT examinations, there is a high potential for pediatric patients to receive thyroid doses for which there is a demonstrably increased risk of thyroid cancer.
For a given total dose, the number of radiation treatments did not modify risks, although numbers of thyroid cancer cases with multiple treatments were limited (the Israel Tinea Study, Hemangioma–Stockholm Study, and Hemangioma–Göteborg Study contributed 10, 9, and 1 cases, respectively). Pooled analyses of seven studies, a subset of the current studies (10), 12 studies across the full range of doses including 144 cases with multiple treatments (8), and our current analysis do not support a significant dose fractionation effect. Notably, these analyses did observe a reduced strength of association with multiple radiation treatments, albeit not statistically significant. Harmonization of fractionation data was challenging due to diverse and calendar year varying treatment protocols and limited information on treatment specifics for the studies. Various definitions of dose fraction were possible. Due to harmonization necessities, we defined one treatment as all radiation doses received within 6 months. This resulted in the large majority of patients receiving one radiation treatment, diminishing our ability to evaluate fractionation.
In conclusion, these analyses reinforced the existence of an excess thyroid cancer risk at doses <0.2 and <0.1 Gy, and perhaps at even lower doses. The consistency of the linear radiation dose response at low doses and the apparent absence of a significant radiation fractionation effect reaffirm that the direct application of a linear relationship remains the most plausible approach for the extrapolation of radiation-associated thyroid cancer risk (49) and adds support to the use of a linear model for ALARA assessments (50).
Acknowledgments
We acknowledge members of the Nordic Countries Childhood Cancer Survivor Study Group, who have contributed to the Nordic Countries Childhood Cancer Survivor Study, including the following: H. Hertz and J. Olsen (Denmark); G. Jonmundsson and H. Tulinius (Iceland); M. Lanning and R. Sankila (Finland); and H. Døllner and F. Langmark (Norway). We also recognize the late Dr. Elaine Ron, who initiated and guided this pooling project.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The Childhood Cancer Survivor Study was supported by National Cancer Institute Grant U24 CA55727, Lance Armstrong Foundation Grant 147149, and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The enlarged thymus cohort was supported by National Heart, Lung, and Blood Institute Grant K-23 HL070930.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALARA
- as low as reasonably achievable
- CCSS
- Childhood Cancer Survivor Study
- CI
- confidence interval
- CT
- computed tomography
- ERR
- excess RR
- Gy
- gray
- RR
- relative risk.
References
- 1.United Nations Scientific Committee on the Effects of Atomic Radiation. Ionizing Radiation Exposure of the Population of the United States: 2006. Bethesda, MD: National Council on Radiation Protection and Measurements; 2009. NCRP report no. 160.
- 2.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100(24):13761–13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Scientific Committee on the Effects of Atomic Radiatio Sources and Effects of Ionizing Radiation, UNSCEAR 2013 Report to the General Assembly, with Scientific Annexes. New York, NY: United Nations Publications; 2013. [Google Scholar]
- 4.Belli M, Tabocchini MA, Jourdain J-R, Salomaa S, Repussard J. The European initiative on low-dose risk research: from the HLEG to MELODI. Radiat Prot Dosimetry. 2015;166(1-4):178–181. [DOI] [PubMed] [Google Scholar]
- 5.Salomaa S, Averbeck D, Ottolenghi A, Sabatier L, Bouffler S, Atkinson M, Jourdain JR. European low-dose radiation risk research strategy: future of research on biological effects at low doses. Radiat Prot Dosimetry. 2015;164(1-2):38–41. [DOI] [PubMed] [Google Scholar]
- 6.Kitahara CM, Linet MS, Rajaraman P, Ntowe E, Berrington de González A. A new era of low-dose radiation epidemiology. Curr Environ Health Rep. 2015;2(3):236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinnott B, Ron E, Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010;31(5):756–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veiga LH, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, Sakata R, Schneider AB, Inskip P, Bhatti P, Johansson R, Neta G, Shore R, de Vathaire F, Damber L, Kleinerman R, Hawkins MM, Tucker M, Lundell M, Lubin JH. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res. 2016;185(5):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci USA. 2005;102(37):13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD Jr. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141(3):259–277. [PubMed] [Google Scholar]
- 11.Svahn-Tapper G, Garwicz S, Anderson H, Shamsaldin A, De Vathaire F, Olsen JH, Døllner H, Hertz H, Jonmundsson G, Langmark F, Lanning M, Sankila R, Tulinius H, Möller T. Radiation dose and relapse are predictors for development of second malignant solid tumors after cancer in childhood and adolescence: a population-based case-control study in the five Nordic countries. Acta Oncol. 2006;45(4):438–448. [DOI] [PubMed] [Google Scholar]
- 12.Tucker MA, Jones PHM, Boice JD Jr, Robison LL, Stone BJ, Stovall M, Jenkin RD, Lubin JH, Baum ES, Siegel SE, et al. ; The Late Effects Study Group . Therapeutic radiation at a young age is linked to secondary thyroid cancer. Cancer Res. 1991;51(11):2885–2888. [PubMed] [Google Scholar]
- 13.Pottern LM, Kaplan MM, Larsen PR, Silva JE, Koenig RJ, Lubin JH, Stovall M, Boice JD Jr. Thyroid nodularity after childhood irradiation for lymphoid hyperplasia: a comparison of questionnaire and clinical findings. J Clin Epidemiol. 1990;43(5):449–460. [DOI] [PubMed] [Google Scholar]
- 14.Rothman KJ, Greenland S, Lash T L.Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 15.Weinberg C. Synergy of exposure effects. In: Armitage P, Colton T, eds. Encyclopedia of Biostatistics. 2nd ed. New York, NY: John Wiley & Sons; 2005. http://onlinelibrary.wiley.com/doi/10.1002/0470011815.b2a03124/pdf. Accessed 3 April 2017. [Google Scholar]
- 16.Korn EL, Graubard BI. Analysis of Health Surveys. New York, NY: John Wiley and Sons; 1999. [Google Scholar]
- 17.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 18.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure User’s Guide. Seattle, WA: HiroSoft International; 2008. [Google Scholar]
- 19.Akaike H. Information theory and an extension of the maximum likelihood principal. In: Petrov EB, Csaki F, eds. 2nd Annual Symposium on Information Theory and Control. Budapest, Hungary: Akademia Kiado; 1973:267–281. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 23.Ulm K. A statistical method for assessing a threshold in epidemiological studies. Stat Med. 1991;10(3):341–349. [DOI] [PubMed] [Google Scholar]
- 24.Gössl C, Küchenhoff H. Bayesian analysis of logistic regression with an unknown change point and covariate measurement error. Stat Med. 2001;20(20):3109–3121. [DOI] [PubMed] [Google Scholar]
- 25.Küchenhoff H, Carroll RJ. Segmented regression with errors in predictors: semi-parametric and parametric methods. Stat Med. 1997;16(1-3):169–188. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, Sugiyama H, Soda M, Ozasa K, Mabuchi K. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013;132(5):1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce DA, Stram DO, Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res. 1990;123(3):275–284. [PubMed] [Google Scholar]
- 28.Lubin JH, Schafer DW, Ron E, Stovall M, Carroll RJ. A reanalysis of thyroid neoplasms in the Israeli tinea capitis study accounting for dose uncertainties. Radiat Res. 2004;161(3):359–368. [DOI] [PubMed] [Google Scholar]
- 29.Schafer DW, Lubin JH, Ron E, Stovall M, Carroll RJ. Thyroid cancer following scalp irradiation: a reanalysis accounting for uncertainty in dosimetry. Biometrics. 2001;57(3):689–697. [PubMed] [Google Scholar]
- 30.Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-rsource countries. Thyroid. 2015;25(10):1127–1136. [DOI] [PubMed] [Google Scholar]
- 31.Bouville A, Likhtarev IA, Kovgan LN, Minenko VF, Shinkarev SM, Drozdovitch VV. Radiation dosimetry for highly contaminated Belarusian, Russian and Ukrainian populations, and for less contaminated populations in Europe. Health Phys. 2007;93(5):487–501. [DOI] [PubMed] [Google Scholar]
- 32.Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2016;27(3):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh L, Zhang W. Radiation risk models for all solid cancers other than those types of cancer requiring individual assessments after a nuclear accident. Radiat Environ Biophys. 2016;55(1):9–17. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Health Risk Assessment from the Nuclear Accident after the 2011 Great East Japan Earthquake and Tsunami Based on a Preliminary Dose Estimation. Geneva, Switzerland: World Health Organization; 2012.
- 35.United Nations Scientific Committee on the Effects of Atomic Radiation Sources, effects and risks of ionizing radiation UNSCEAR 2013 report. In: Report to the General Assembly Scientific Annex A: Levels and Effects of Radiation Exposure Due to the Nuclear Accident After the 2011 Great East-Japan Earthquake and Tsunami. Vol. I New York, NY: United Nations; 2014. http://www.unscear.org/unscear/en/publications/2013_1.html. Accessed 3 April 2017. [Google Scholar]
- 36.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, Drozdovitch V, Maceika E, Zvonova I, Vlassov O, Bouville A, Goulko G, Hoshi M, Abrosimov A, Anoshko J, Astakhova L, Chekin S, Demidchik E, Galanti R, Ito M, Korobova E, Lushnikov E, Maksioutov M, Masyakin V, Nerovnia A, Parshin V, Parshkov E, Piliptsevich N, Pinchera A, Polyakov S, Shabeka N, Suonio E, Tenet V, Tsyb A, Yamashita S, Williams D. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97(10):724–732. [DOI] [PubMed] [Google Scholar]
- 37.Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, Likhtarev IA, Fink DJ, Markov VV, Greenebaum E, Olijnyk VA, Masnyk IJ, Shpak VM, McConnell RJ, Tereshchenko VP, Robbins J, Zvinchuk OV, Zablotska LB, Hatch M, Luckyanov NK, Ron E, Thomas TL, Voillequé PG, Beebe GW. A cohort study of thyroid cancer and other thyroid diseases after the Chernobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98(13):897–903. [DOI] [PubMed] [Google Scholar]
- 38.Brenner AV, Tronko MD, Hatch M, Bogdanova TI, Oliynik VA, Lubin JH, Zablotska LB, Tereschenko VP, McConnell RJ, Zamotaeva GA, O’Kane P, Bouville AC, Chaykovskaya LV, Greenebaum E, Paster IP, Shpak VM, Ron E. I-131 dose response for incident thyroid cancers in Ukraine related to the Chernobyl accident. Environ Health Perspect. 2011;119(7):933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zablotska LB, Ron E, Rozhko AV, Hatch M, Polyanskaya ON, Brenner AV, Lubin J, Romanov GN, McConnell RJ, O’Kane P, Evseenko VV, Drozdovitch VV, Luckyanov N, Minenko VF, Bouville A, Masyakin VB. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chernobyl accident. Br J Cancer. 2011;104(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins J, Dunn JT, Bouville A, Kravchenko VI, Lubin J, Petrenko S, Sullivan KM, Vanmiddlesworth L, Wolff J. Iodine nutrition and the risk from radioactive iodine: a workshop report in the Chernobyl long-term follow-up study. Thyroid. 2001;11(5):487–491. [DOI] [PubMed] [Google Scholar]
- 41.Berrington de González A, Mahesh M, Kim K-P, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mettler FA Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009;253(2):520–531. [DOI] [PubMed] [Google Scholar]
- 43.Kim KP, Berrington de González A, Pearce MS, Salotti JA, Parker L, McHugh K, Craft AW, Lee C. Development of a database of organ doses for paediatric and young adult CT scans in the United Kingdom. Radiat Prot Dosimetry. 2012;150(4):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol). 2011;23(4):244–250. [DOI] [PubMed] [Google Scholar]
- 45.Brisse HJ, Robilliard M, Savignoni A, Pierrat N, Gaboriaud G, De Rycke Y, Neuenschwander S, Aubert B, Rosenwald JC. Assessment of organ absorbed doses and estimation of effective doses from pediatric anthropomorphic phantom measurements for multi-detector row CT with and without automatic exposure control. Health Phys. 2009;97(4):303–314. [DOI] [PubMed] [Google Scholar]
- 46.Spampinato MV, Tipnis S, Tavernier J, Huda W. Thyroid doses and risk to paediatric patients undergoing neck CT examinations. Eur Radiol. 2015;25(7):1883–1890. [DOI] [PubMed] [Google Scholar]
- 47.Mazonakis M, Tzedakis A, Damilakis J, Gourtsoyiannis N. Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiol. 2007;17(5):1352–1357. [DOI] [PubMed] [Google Scholar]
- 48.Journy N, Ancelet S, Rehel J-L, Mezzarobba M, Aubert B, Laurier D, Bernier MO. Predicted cancer risks induced by computed tomography examinations during childhood, by a quantitative risk assessment approach. Radiat Environ Biophys. 2014;53(1):39–54. [DOI] [PubMed] [Google Scholar]
- 49.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289–296. [DOI] [PubMed] [Google Scholar]
- 50.Strauss KJ, Kaste SC. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients--a white paper executive summary. Pediatr Radiol. 2006;36(Suppl 2):110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]