Abstract
Context:
Although radiation exposure is an important predictor of thyroid cancer on diagnosis of a thyroid nodule, the relationship between childhood radiation exposure and thyroid nodules has not been comprehensively evaluated.
Objective:
To examine the association between internal I-131 thyroid dose and thyroid nodules in young adults exposed during childhood.
Design, setting, and participants:
In this cross-sectional study, we screened residents of Belarus aged ≤18 years at the time of the Chernobyl nuclear accident for thyroid disease (median age, 21 years) with thyroid palpation, ultrasonography, blood/urine analysis, and medical follow-up when appropriate. Eligible participants (N = 11,421) had intact thyroid glands and doses based on direct individual thyroid activity measurements.
Main outcome measures:
Excess odds ratios per Gray (EOR/Gy, scaled at age 5 years at exposure) for any thyroid nodule and for nodules grouped by cytology/histology, diameter size, and singularity.
Results:
Risk of any thyroid nodule increased significantly with I-131 dose and, for a given dose, with younger age at exposure. The EOR/Gy (95% confidence intervals) for neoplastic nodules (3.82; 0.87 to 15.52) was significantly higher than for nonneoplastic nodules (0.32; <0.03 to 0.70) and did not vary by size; whereas the EOR/Gy for nonneoplastic nodules did vary by size (P = 0.02) and was 1.55 (0.36 to 5.46) for nodules ≥10 mm and 0.02 (<−0.02 to 0.70) for nodules <10 mm. EORs/Gy for single and multiple nodules were comparable.
Conclusions:
Childhood exposure to internal I-131 is associated with increased risk of neoplastic thyroid nodules of any size and nonneoplastic nodules ≥10 mm.
We screened 11,970 adults exposed to internal I-131 in childhood for thyroid nodules. Dose was associated with increased risk of neoplastic thyroid nodules of any size and large nonneoplastic nodules.
History of radiation exposure is an important factor guiding the clinical management of thyroid nodules (1). Although thyroid nodules are a common ultrasonography (US) finding, usually with few direct clinical implications, nodules larger than 10 mm in diameter or having suspicious features may require further evaluation to rule out a diagnosis of thyroid cancer (1). Although only 5 to 15% of nodules are found to be malignant (1–4), malignancy rates of over 30% have been reported among patients with a history of radiation exposure during childhood (5, 6), and radiation exposure is one of the most established risk factors for thyroid cancer (1).
Several epidemiological studies have evaluated the relationship between external or internal radiation dose during childhood and the risk of screen-detected thyroid nodules (7–13). Most studies report an increased risk of any thyroid nodule (7–11). Although nodule characteristics provide important information for clinical practice (4, 14, 15), few studies have evaluated the radiation dose-response relationship by nodule cytology/histology (e.g., nonneoplastic, neoplastic), by nodule size, or by single vs multiple nodule presentation. Radiation-related risk of thyroid nodules has also not been examined among individuals screened as adolescents and young adults, for whom the prevalence of thyroid nodules is generally low (16).
The 1986 Chernobyl Nuclear Power Plant accident and the 2011 core meltdown of the Daiichi Nuclear Power Plant in Fukushima, Japan, have reinforced concerns about the health effects of radiation exposure. The objective of this study was to assess the relationship between exposure to internal I-131 dose as a result of Chernobyl fallout and the risk of thyroid nodules prevalent at the time of the first thyroid disease screening. We conducted a population-based thyroid screening study of almost 12,000 residents of Belarus who were exposed as children and examined 10 to 18 years later. To our knowledge, this is the largest study of thyroid nodules in relation to individual thyroid dose. This study directly compared the radiation dose-response relationship for nodules with varying cytological/histological and ultrasound features.
Methods
Study population
Detailed descriptions of the cohort and methods have been previously described (17–19). Briefly, 11,970 individuals residing in Belarus who were aged 18 years or younger at the time of the Chernobyl accident (26 April 1986) with thyroid radioactivity measurements taken within 2 months of the accident were screened for thyroid diseases (n = 11,903 from 1996 to 2001, and n = 67 from 2002 to 2004). We serially excluded participants outside the age range of 0 to 18 years at the time of the accident (n = 114); those with unknown doses (n = 124), unknown or zero thyroid volume (n = 165), self-reported history of thyroid cancer (n = 2), aplasia (n = 4), and thyroid surgery (n = 21); and those who reported past or current use of thyroid hormones as part of their medical history (n = 119). The final study population included 11,421 subjects at risk for prevalent thyroid nodules at the time of screening.
Screening protocol
Study subjects were screened for thyroid disease at medical centers in the cities of Minsk and Gomel or at local medical clinics by visiting mobile screening teams according to a standardized protocol. Screening consisted of thyroid palpation and US examination by a physician certified in US (i.e., sonographer) and a clinical examination with independent palpation by an endocrinologist. Any discrepancies were resolved by a third, jointly conducted examination. Subjects were also administered standardized questionnaires to ascertain demographic information, location of residence, and dietary and medical history. Blood and spot urine samples were collected to measure thyroid hormone levels and urinary iodine concentration, respectively. This study was approved by institutional review boards in Belarus and the United States. Informed consent was provided by the study participants or by accompanying guardians for minors.
Ascertainment and classification of thyroid nodules
Prevalent thyroid nodules with maximum diameter of 3 mm or larger were ascertained by a certified sonographer using 7.5 MHz ultrasound probes. Participants with nodules that were (1) 10 mm or larger in diameter or (2) 5 to 9 mm with suspicious US features (hypoechoic, indistinct border, calcified inclusions, extension through the thyroid capsule, or suspicious lymphadenopathy) or with diffusely abnormal thyroid tissue accompanied by unexplained cervical lymphadenopathy were referred to the Minsk or Gomel study centers for further evaluation and US-guided fine-needle biopsy (FNB). All medical personnel were blinded to radiation doses.
Participants were considered cases if they had at least one US-detected nodule. We classified cases into neoplastic, suspicious/not otherwise specified (NOS), and nonneoplastic nodules. Neoplastic cases include people with thyroid cancer and/or follicular adenoma detected as a result of the first screening and histologically confirmed through surgery. Suspicious/NOS cases included people who were referred to FNB and/or surgery after ultrasound examination but did not comply or had a cytological conclusion of inadequate, indeterminate, suspicious for cancer or neoplasia FNB who never underwent surgery for histological confirmation. Nonneoplastic cases included people without suspicious nodules who were not referred to FNB or surgery and people whose nodules were confirmed to be benign (excluding follicular adenoma) through FNB and/or surgery. We also categorized participants into cases with single vs multiple nodules and large (≥10 mm) vs small (<10 mm) nodules based on ultrasound images. Among participants with multiple nodules, size designation was determined by the nodule with the largest maximum diameter. Similarly, among people with multiple nodules, the most serious cytological/histological finding or highest level of intervention was chosen. For example, if a person had a suspicious for neoplasia FNB conclusion for one nodule but not another, they were considered a suspicious/NOS case.
I-131 thyroid dose
Radiation exposure to the thyroid gland came primarily from intake of I-131 through inhalation or through consumption of contaminated milk, dairy products, and leafy vegetables (20). Thyroid dose estimation for each cohort member was based on I-131 activity in the thyroid derived from direct individual thyroid radioactivity measurements, a radio-ecological model used to assess the temporal variation of I-131 in the thyroid, and personal interviews, which provided information on residential history, individual diet, and administration of stable iodine to block intake of I-131. The following data sources were used to update the parameters of the dosimetry model: thyroid volume measurements by the Sasakawa Memorial Foundation to derive age-specific thyroid masses (21), measurements of I-131 in soil to verify the validity of calculated I-131 deposition density in settlements, and I-131 measurements in soil and grass samples to derive an interception factor of I-131 by vegetation (22). The arithmetic mean of 1000 individual stochastic doses due to I-131 exposure, calculated for each cohort member, was used as the best estimate of thyroid dose from I-131 (23).
Statistical analysis
All statistical analyses were conducted at the person level. To evaluate the association between I-131 thyroid dose and at least one prevalent thyroid nodule, we fit by maximum likelihood, a model of the prevalence probability, P(x,d,z), which had the following linear form on the odds scale:
in which exp(αx) is the background log-linear odds of thyroid nodules as a function of a vector of risk factors or confounding factors x at 0 Gray (Gy), d is radiation dose (Gy), and z is a vector of covariates representing effect modification variables. Parameters α, β, and γ are estimated by regression via likelihood maximization. The main parameter of interest is β, the excess odds ratio per Gray (EOR/Gy), or the odds ratio minus one. To test for departure from linearity, we also considered linear-quadratic and linear-exponential model forms.
Selection of models was conducted separately for background risk (at zero I-131 dose) and for examination of effect modification. Background models included sex, age at screening, oblast (administrative equivalent of a province or state) of residence (Gomel, Minsk, others), and urban status a priori because they are known to be strong risk factors for thyroid nodules or were used in previous studies of this population. The following potential confounders were also considered for background modeling because they could be significantly associated with both radiation dose and thyroid nodules but not believed to be on the causal pathway: current smoking, self-reported family history of thyroid disease, measures of iodine deficiency including urinary iodine concentration, and presence of diffuse goiter upon palpation. Nested models were compared using P values based on likelihood ratio tests. Non-nested models (e.g., linear-quadratic vs linear-exponential dose models) were compared using the Akaike information criterion. Significant factors that did not substantially change the radiation dose-parameter estimates were excluded from background models (i.e., smoking status).
The factors listed above, along with age at the time of the accident, were also tested as potential effect modifiers of the radiation dose-response relationship. Significance testing was used to compare dose-response relationships for different nodule groupings (e.g., large vs small) using the method of Pierce and Preston (24). Missing values were coded as separate categories and included as indicator variables in models. Statistical tests were two-sided, and findings at P < 0.05 were considered significant. Parameters and 95% confidence intervals (CIs) were estimated using the profile likelihood. Model fitting was carried out using Epicure (25).
Results
Characteristics of US-detected thyroid nodules
Thyroid nodules were detected at screening among 8% (n = 881 cases) of 11,421 eligible participants (Table 1). Among 881 participants with at least one thyroid nodule, 11% (n = 101) were neoplastic cases (66 with thyroid cancer only, 24 with follicular adenoma only, and 11 with both), 23% (n = 204) were suspicious/NOS cases, and 64% (n = 576) were categorized as nonneoplastic cases. A neoplasm was diagnosed in a greater proportion of people with large nodules (26%; 73/279) than small nodules (5%; 28/602) and with single (12%; 79/648) than multiple (9%; 22/233) nodules.
Table 1.
Classification of Thyroid Nodules Based on Histological/Cytological Results and Ultrasound Characteristics
| Cytology/Histology | Total |
Size
|
Singularity
|
||
|---|---|---|---|---|---|
| Small (<10 mm) | Large (≥10 mm) | Single | Multiple | ||
| All nodules | 881 | 602 | 279 | 648 | 233 |
| Neoplastic nodulesa | 101 | 28 | 73 | 79 | 22 |
| Thyroid cancer only | 66 | 19 | 47 | 52 | 14 |
| Follicular adenoma only | 24 | 6 | 18 | 21 | 3 |
| Thyroid cancer and follicular adenomab | 11 | 3 | 8 | 6 | 5 |
| Suspicious nodules/NOSc | 204 | 111 | 93 | 155 | 49 |
| Nonneoplastic nodulesd | 576 | 463 | 113 | 414 | 162 |
Neoplastic prevalent cases include thyroid cancer (malignant neoplasia) and follicular adenoma (benign neoplasia).
Incidental findings during surgery for six cases of both thyroid cancer and follicular adenoma, although only a single nodule was detected by ultrasound.
FNB and/or surgery recommended, but indeterminate or not completed (includes six cases who were suspected and went on to be diagnosed through surgery with a neoplasm > 3y from ultrasound).
Not referred to FNB and/or surgery (n = 450) or FNB and/or surgery completed with confirmation of no cancer or follicular adenoma (n = 126).
Background risk factors for all thyroid nodules
Background risk of having any nodule (at zero I-131 thyroid dose) for mutually adjusted factors is presented in Table 2. Risk was higher in participants who were older at screening (median age at screening was 24 years for participants with nodules vs 21 years in participants without nodules); in female vs male subjects; in participants with urban residence, diffuse goiter detected at screening, and family history of thyroid disease; and in current smokers.
Table 2.
Background Risk Factors for at Least One Thyroid Nodule Among Residents of Belarus Exposed to Chernobyl Fallout
| Characteristic |
Cases
a
|
Noncases
|
OR (95% CI) b | ||
|---|---|---|---|---|---|
| n | Percent | n | Percent | ||
| Total | 881 | 10,540 | |||
| Sex | |||||
| Male | 348 | 40 | 5232 | 50 | Ref. |
| Female | 533 | 60 | 5308 | 50 | 1.47 (1.26–1.73) |
| Age at screening, y | |||||
| 10–17 | 168 | 19 | 3343 | 32 | Ref. |
| 18–24 | 329 | 37 | 4178 | 40 | 2.47 (1.90–3.26) |
| 25–34 | 384 | 44 | 3019 | 29 | 4.58 (3.49–6.12) |
| Oblast of residence at screening | |||||
| Minsk | 208 | 24 | 2922 | 28 | Ref. |
| Gomel | 587 | 67 | 6440 | 61 | 1.05 (0.88–1.26) |
| Other | 86 | 10 | 1178 | 11 | 0.83 (0.63–1.10) |
| Urban status at screening | |||||
| Rural | 333 | 38 | 4172 | 40 | Ref. |
| Urban | 548 | 62 | 6368 | 60 | 1.24 (1.06–1.45) |
| Urinary iodine concentration,c μg/L | |||||
| 0–49 | 328 | 37 | 3903 | 37 | Ref. |
| 50–99 | 257 | 29 | 2954 | 28 | 1.00 (0.84–1.20) |
| 100–2120 | 288 | 33 | 3545 | 34 | 0.95 (0.80–1.13) |
| Unknown | 8 | 1 | 138 | 1 | 0.64 (0.28–1.26) |
| Diffuse goiter detected during screening | |||||
| No | 590 | 67 | 8940 | 85 | Ref. |
| Yes | 290 | 33 | 1575 | 15 | 3.14 (2.68–3.68) |
| Unknown | 1 | 0 | 25 | 0 | 0.62 (0.03–3.01) |
| Family history of thyroid disease | |||||
| No | 743 | 84 | 9513 | 90 | Ref. |
| Yes | 116 | 13 | 863 | 8 | 1.68 (1.34–2.07) |
| Unknown | 22 | 2 | 164 | 2 | 1.34 (0.82–2.09) |
| Current smoker | |||||
| No | 602 | 68 | 7391 | 70 | Ref. |
| Yes | 279 | 32 | 3148 | 30 | 1.22 (1.03–1.45) |
| Unknown | 0 | 0 | 1 | 0 | NE |
Abbreviations: NE, not estimated; OR, odds ratio; Ref., reference.
Person with any nodule identified through ultrasound at first screening.
ORs and 95% CIs based on maximum likelihood estimate and profile likelihood bounds and mutually adjusted for I-131 dose and all factors as presented with age at screening coded as continuous log age at screening.
Iodine categories: 0–49, severe/moderate deficiency; 50–99, mild deficiency; 100+, adequate/excessive.
Radiation-related risks of thyroid nodules
Thyroid radiation doses ranged from 0.001 to 39 Gy, with 98% of participants exposed to <5 Gy (Table 3). Although category-specific risk estimates were not significant for small nodule groupings (e.g., neoplastic nodules), the prevalence of each nodule grouping was associated with I-131 dose across the full dose range (P < 0.001) based on tests of linear trend, with dose categories coded 1 through 7 (Table 3). Using a continuous I-131 dose, the dose-response relationships were consistent with linearity for all major nodule groupings, except for nonneoplastic nodules, for which a linear-exponential dose-response model with upward curvature at high doses provided an improved fit to the data [linear term 0.14 (95% CI, <−0.11 to 0.41); linear-exponential term: 0.09 (95% CI, 0.02 to 0.21)].
Table 3.
Odds Ratios and 95% CIs for Thyroid Nodules Across Thyroid Radiation Dose Categories Among Residents of Belarus Exposed to Chernobyl Fallout a
| Nodule Grouping |
I-131 Thyroid Dose (Gy)
|
||||||
|---|---|---|---|---|---|---|---|
| 0–0.09 | 0.10–0.24 | 0.25–0.49 | 0.50–0.99 | 1.00–1.99 | 2.00–4.99 | 5.00–38.90 | |
| Total, n | 3203 | 2307 | 2119 | 1811 | 1186 | 620 | 175 |
| All nodules | |||||||
| OR | 1.00 | 1.12 | 1.37 | 1.46 | 2.4 | 2.38 | 5.1 |
| 95% CI | Ref. | 0.84 to 1.60 | 1.03 to 1.93 | 1.07 to 2.09 | 1.59 to 3.54 | 1.56 to 3.68 | 2.85 to 8.75 |
| Cases, n | 231 | 169 | 158 | 129 | 110 | 55 | 29 |
| Neoplastic | |||||||
| OR | 1.00 | 1.29 | 1.79 | 3.02 | 5.06 | 6.45 | 7.94 |
| 95% CI | Ref. | <−0.31 to 5.87 | <0.41 to 6.47 | <0.54 to 10.23 | <−0.94 to 17.98 | <0.94 to 25.96 | <−0.25 to 35.66 |
| Cases, n | 12 | 15 | 17 | 20 | 18 | 14 | 5 |
| Suspicious | |||||||
| OR | 1.00 | 2.65 | 3.69 | 3.36 | 5.48 | 7.22 | 15.91 |
| 95% CI | Ref. | 1.13 to 6.52 | 1.42 to 9.11 | 1.34 to 8.66 | 1.38 to 12.37 | 2.49 to 19.91 | 5.08 to 47.78 |
| Cases, n | 45 | 40 | 42 | 32 | 21 | 17 | 7 |
| Nonneoplastic | |||||||
| OR | 1.00 | 0.94 | 1.16 | 1.09 | 1.74 | 1.44 | 3.74 |
| 95% CI | Ref. | 0.67 to 1.40 | 0.84 to 1.65 | 0.77 to 1.65 | <0.83 to 2.83 | <1.12 to 2.36 | <1.59 to 7.20 |
| Cases, n | 174 | 114 | 99 | 77 | 71 | 24 | 17 |
| Large, ≥10 mm | |||||||
| OR | 1.00 | 1.24 | 2.31 | 1.61 | 4.57 | 6.36 | 10.8 |
| 95% CI | Ref. | 0.71 to 2.50 | 1.26 to 4.50 | 0.88 to 3.21 | 2.42 to 8.82 | 3.08 to 13.07 | 4.73 to 24.48 |
| Cases, n | 61 | 40 | 60 | 35 | 40 | 30 | 13 |
| Small, <10 mm | |||||||
| OR | 1.00 | 1.12 | 1.19 | 1.48 | 1.93 | 1.5 | 3.87 |
| 95% CI | Ref. | 0.80 to 1.72 | 0.87 to 1.75 | 1.03 to 2.26 | 1.19 to 3.05 | 0.99 to 2.48 | 1.65 to 7.49 |
| Cases, n | 170 | 129 | 98 | 94 | 70 | 25 | 16 |
| Multiple | |||||||
| OR | 1.00 | 0.86 | 1.45 | 1.27 | 2.21 | 2.19 | 5.27 |
| 95% CI | Ref. | <0.07 to 1.42 | <−1.18 to 2.52 | <0.60 to 2.52 | <0.49 to 4.94 | <0.83 to 5.30 | <1.62 to 15.05 |
| Cases, n | 64 | 44 | 46 | 28 | 29 | 14 | 8 |
| Single | |||||||
| OR | 1.00 | 1.25 | 1.33 | 1.53 | 2.43 | 2.4 | 4.95 |
| 95% CI | Ref. | 0.87 to 1.87 | 0.96 to 1.97 | 1.06 to 2.32 | 1.56 to 3.74 | 1.51 to 3.90 | 2.65 to 8.93 |
| Cases, n | 167 | 125 | 112 | 101 | 81 | 41 | 21 |
Abbreviation: OR, odds ratio.
Models for all nodule groupings adjust for sex, log age at screening, urban status at screening, oblast of residence at screening (Minsk, Gomel, other), diffuse goiter detected during screening, and any family history of thyroid disease and are scaled for participants aged 5 at time of the accident. P < 0.001 for test of linear trend with dose categories coded 1 through 7 for all nodule groupings. The odds ratio is equivalent to the excess odds ratio plus one (OR = EOR + 1).
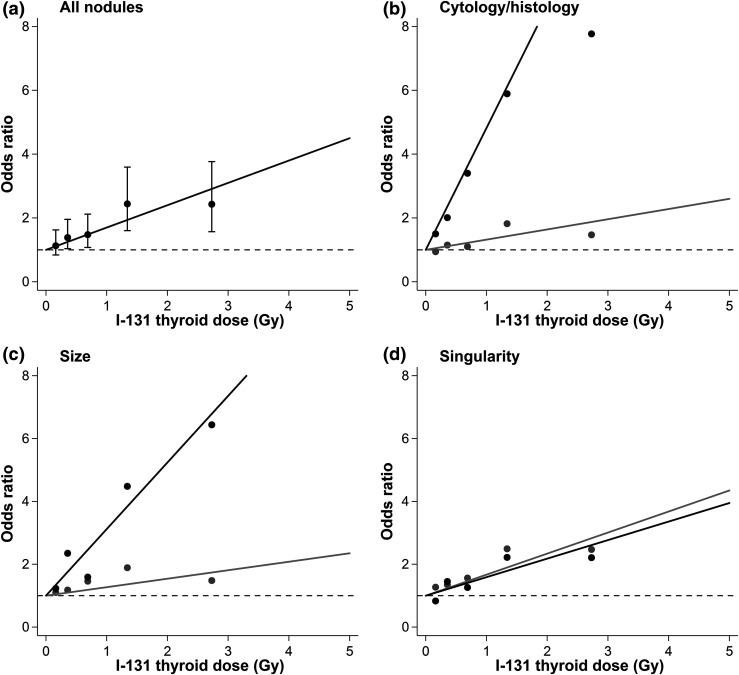
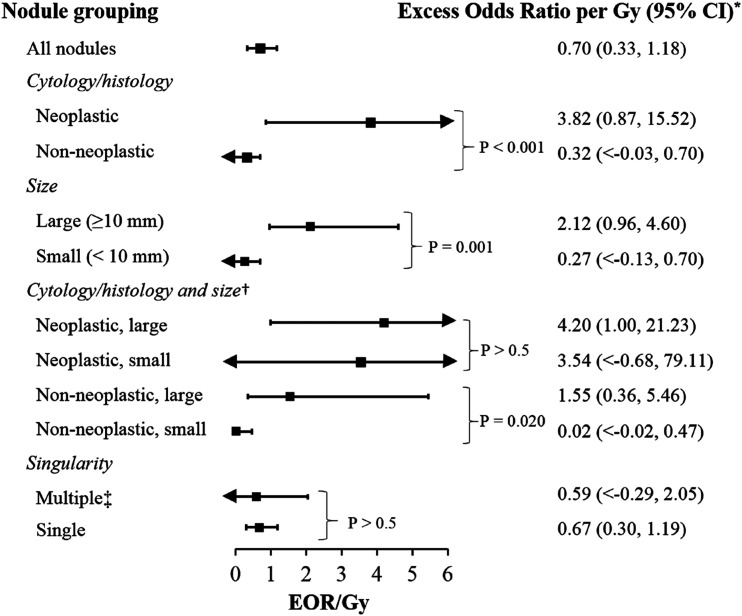
Dose-response relationships were linear for all nodule groupings in the 0- to 5-Gy dose range, so all further analyses are restricted to doses <5 Gy (Fig. 1). The EOR/Gy was 0.70 (95% CI, 0.33 to 1.18) for all nodules and 3.82 (95% CI, 0.87 to 15.52) for neoplastic, 0.32 (95% CI, <−0.03 to 0.70) for nonneoplastic, 2.12 (95% CI, 0.96 to 4.60) for large, 0.27 (95% CI, <−0.13 to 0.71) for small, 0.59 (95% CI, <−0.29 to 2.05) for multiple, and 0.67 (0.30 to 1.19) for single nodules scaled for a person aged 5 years at the time of the accident (Fig. 2).
Figure 1.
Radiation-related odds ratios and 95% CIs for thyroid nodules in Belarus. (a) Black line represents fitted dose-response relationship, and points represent categorical results with 95% CIs. (b) Black line and points represent neoplastic nodules, and gray line and points represent nonneoplastic nodules. (c) Black line and points represent large (≥10 mm) nodules, and gray line and points represent small (<10 mm) nodules. (d) Black line and points represent multiple nodules, and gray line and points represent single nodules. Models were adjusted for sex, log age at screening, urban status at screening, oblast of residence at screening (Minsk, Gomel, other), diffuse goiter detected at screening, and self-reported family history of thyroid disease and are scaled for a person 5 years old at time of exposure. Results are restricted to participants exposed to <5 Gy. The odds ratio is equivalent to the excess odds ratio plus one (OR = EOR + 1). The EOR is the vertical distance between the observed/fitted values and the dashed line.
Figure 2.
Radiation-related excess odds ratios and 95% CIs for thyroid nodules in Belarus. *Models were adjusted for sex, log age at screening, urban status at screening, oblast of residence at screening (Minsk, Gomel, other), diffuse goiter detected at screening, and any family history of thyroid disease and are scaled for participants 5 years old at time of exposure. P values based on likelihood ratio tests with models adjusted for all factors described above and an age at time of accident interaction term are shown. Results are restricted to participants exposed to <5 Gy. †Among small nodules, P = 0.023 for test of difference in dose-response between nonneoplastic and neoplastic nodules. Among large nodules, P = 0.381 for test of difference in dose-response between nonneoplastic and neoplastic nodules. ‡Sex-specific EORs for multiple nodules are 0.10 (95% CI, <−0.17 to 0.93) in men and 1.44 (95% CI, <−0.46 to 3.68) in women.
We also examined differences in the radiation dose-response relationship by various nodule characteristics (Fig. 2). The radiation dose-response relationship was higher for neoplastic vs nonneoplastic nodules (P < 0.001) and large vs small nodules (P = 0.001). When analyzed simultaneously by cytology/histology and size, the dose-response for neoplastic nodules did not vary by size (P > 0.5), whereas for nonneoplastic nodules the dose-response was higher for large than for small nodules (P = 0.020). There was little evidence for differences in the dose-response for single vs multiple nodules overall or when comparing single vs multiple nodules within sex strata (data not shown).
Effect modification of radiation dose-response
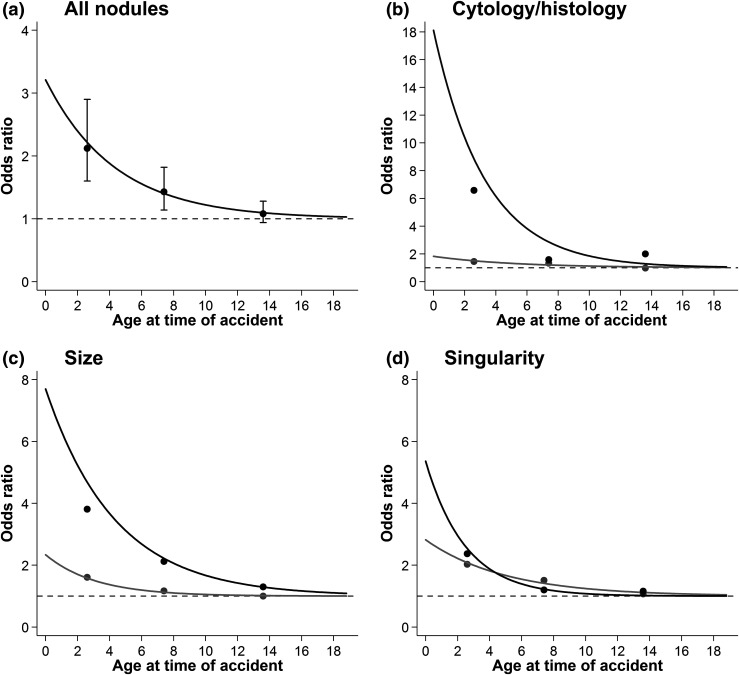
Age at the time of the accident (i.e., age at exposure) modified the dose-response associations for major nodule groupings so that younger age at the time of the accident was associated with a higher E/Gy (Fig. 3; Supplemental Table 1 (19.7KB, docx) ). For each 1-year increase in age at the time of the accident, the EOR/Gy changed by a factor of 0.79 (95% CI, 0.68 to 0.87) for all nodules and by 0.74 (95% CI, 0.60 to 0.89) for neoplastic, 0.82 (95% CI, 0.21 to 0.96) for nonneoplastic, 0.79 (95% CI, 0.70 to 0.88) for large, 0.73 (95% CI, 0.25 to 0.90) for small, 0.67 (95% CI, 0.21 to 0.88) for multiple, and 0.82 (95% CI, 0.70 to 0.90) for single nodules (Supplemental Table 1 (19.7KB, docx) ).
Figure 3.
Radiation-related odds ratios and 95% CIs for thyroid nodules by age at time of Chernobyl accident in Belarus. (a) Black line represents fitted dose-response realtionship; points represent categorical results with 95% CIs (dose-response P < 0.001). (b) Black line and points represent neoplastic nodules (dose-response P = 0.002), and gray line and points represent nonneoplastic nodules (dose-response P = 0.016). (c) Black line and points represent large (≥10 mm) nodules (dose-response P < 0.001), and gray line and points represent small (<10 mm) nodules (dose-response P = 0.002). (d) Black line and points represents multiple nodules (dose-response P = 0.001), and gray line and points represent single nodules (dose-response P < 0.001). Models were adjusted for sex, log age at screening, urban status at screening, oblast of residence at screening (Minsk, Gomel, other), diffuse goiter detected at screening, and self-reported family history of thyroid disease. Nonparametric point estimates use cut-offs of 5 and 10 years old. Results are restricted to participants exposed to <5 Gy. P values are based on likelihood ratio tests for the inclusion of an age at time of accident interaction term (coded continuously, 1 degree of freedom). The odds ratio is equivalent to the excess odds ratio plus one (OR = EOR + 1). The EOR is the vertical distance between the observed/fitted values and the dashed line.
We did not detect modification of radiation risk by urban status, oblast, urinary iodine, diffuse goiter, or family history of thyroid disease for any of the major nodule groupings (Supplemental Table 2 (19.7KB, docx) ). We observed a modification by sex (P = 0.03) of the dose-response relationship for multiple nodules so that the EOR/Gy was 0.06 (NE, 0.54) in men and 0.82 (95% CI, 0.29 to 1.73) in women.
Discussion
We assessed the radiation-related risks of various thyroid nodule types among residents of Belarus exposed to Chernobyl fallout during childhood. Internal I-131 thyroid dose was associated with increased risk of any thyroid nodule and the mutually exclusive nodule groupings of neoplastic/suspicious/nonneoplastic, large/small, and multiple/single, but the magnitude of radiation risk varied. The highest radiation risks were observed for neoplastic nodules irrespective of size and next for large nonneoplastic nodules. Radiation dose-response relationships for all nodule groupings were modified by age at time of the accident so that risks were highest for children exposed during infancy.
Our findings support previous research demonstrating an increased risk of thyroid nodules with both internal and external sources of radiation (7–11, 26). In people exposed to external gamma radiation from the Japanese atomic bombings, when restricting to nodules ≥10 mm, a significantly increased risk of all nodules (EOR/Gy = 1.65), benign nodules (EOR/Gy = 2.07), and malignant tumors (EOR/Gy = 4.40) was found (scaled for a person aged 5 years at exposure) (10). Our population was primarily exposed to internal I-131 and was younger at the time of examination, and our nodule groupings were not completely consistent. For example, we combined malignant thyroid cancer with follicular adenoma into a “neoplastic” group because both outcomes have shown similarly increased radiation-related risks in this cohort (18, 27) and are believed to usually be of monoclonal origin (28). Our “nonneoplastic” grouping includes both nodules that were confirmed through cytology/histology to be nonneoplastic and nodules that were not referred for FNB/surgery. Despite these differences, for nodules ≥10 mm, we found similar significantly increased risks for all nodules (EOR/Gy = 2.12), neoplastic nodules (EOR/Gy = 4.20), and nonneoplastic nodules (EOR/Gy = 1.55) in the 0- to 5-Gy dose range for a person aged 5 years at exposure (Fig. 2).
Our findings add evidence of variation in radiation risk according to nodule type defined by a combination of ultrasonographic, cytological, and histological characteristics. This is particularly important because thyroid nodules are etiologically and biologically heterogeneous (28). We formally evaluated differences in the radiation-related dose-response relationship for neoplastic vs nonneoplastic thyroid nodules, for large vs small nodules, and by nodule cytology/histology and size simultaneously (Fig. 2). We found a stronger dose-response relationship for neoplastic compared with nonneoplastic nodules. Interestingly, the dose-response relationship for neoplastic nodules did not vary by nodule size, but the dose-response relationship for nonneoplastic nodules did, being stronger and significantly elevated for large compared with small nonneoplastic nodules. Together, these findings raise the possibility that radiation exposure may act as an initiating event in the development of neoplastic nodules and as a promoting event in the development of nonneoplastic nodules. However, due to the cross-sectional design of this study, we do not know whether neoplastic nodules emerged from nonneoplastic nodules after radiation exposure, whether the behaviors of these nodules were apparent from their onset, or whether nodule growth was associated with radiation exposure. A prospective follow-up of this cohort to evaluate the dose-response relationship with incident thyroid nodules and the cohort members with prevalent nodules will be informative in this regard.
In contrast to findings among Japanese atomic bomb survivors, we observed a small but significant radiation-related risk of small nodules (<10 mm) as a group. This was mainly driven by a strong dose-response relationship for small neoplastic nodules. A partial explanation for this difference from the Japanese data may rest in the much younger age distribution of our study population or shorter time since exposure. In our study of adolescents and young adults evaluated for thyroid nodules 10 to 18 years after radiation exposure, small nodules were far less common (602/11,421 = 5%) than among the 62- to 75-year-old Japanese atomic bomb survivors (667/3,087 = 22%). It is unclear whether there were any thyroid cancers or adenomas among the small nodules in the Japanese study. Because the background prevalence of thyroid nodules and thyroid cancer increases with age (16), the radiation dose-response relationship in atomic bomb survivors may have been attenuated by a larger proportion of non–radiation-related small nodules that occurred sporadically during the 62 to 66 years between radiation exposure and screening.
This study evaluated the differences in the radiation dose-response relationship for single vs multiple nodules. A study of I-131 exposed vs unexposed residents living close to the Mayak Weapons facility in Ozyorsk, Russia, reported a higher adjusted relative risk with I-131 exposure for single vs multiple nodules detected at screening, particularly among women (26). In contrast, in our data for single nodules, men tended to have a higher EOR/Gy than women, whereas for multiple nodules we found a higher dose-response relationship among women (Supplemental Table 2 (19.7KB, docx) ). Other demographic and iodine deficiency–related factors did not significantly modify the dose-response relationship. However, nodule risk was nonsignificantly elevated for participants with iodine deficiency, as indicated by urinary iodine concentration and the presence of diffuse goiter at screening.
Our findings of increased radiation risk of thyroid nodules associated with younger age at exposure are consistent with findings from the Japanese atomic bomb survivors (9, 10) and for thyroid cancer and follicular adenoma in this cohort (18, 27). These results support the hypothesis that childhood constitutes a window of increased thyroid susceptibility to the effects of radiation, but these effects may not be limited to carcinogenesis. Although based on limited human data, this view attributes increased cancer susceptibility during childhood to more frequent cell division with inadequate time for DNA repair of mutations (29). However, it may also be explained by standard models of multistage carcinogenesis (30, 31). It remains largely unknown what other biological factors might be responsible for increased radiation sensitivity of the child’s thyroid to the effects of ionizing radiation more broadly, although these may include a higher metabolic rate and different hormonal profile than in adults.
Our findings should be interpreted in the context of several limitations. Although the categorization of neoplastic and nonneoplastic nodules was similar to other studies, true biological behavior and clonality of each thyroid nodule could not be confirmed. In addition, due to the cross-sectional design of this study, we do not know whether radiation exposure contributed to initiation of thyroid nodules or to growth or formation of suspicious features in preexisting nodules. The strengths of this study include a large population who underwent a standardized screening protocol, use of individual thyroid I-131 doses based on direct activity measurements, and the examination of dose-response relationships based on nodule cytology/histology, size, and presentation of single vs multiple nodules.
Conclusions
In this study of adolescents and young adults screened for thyroid disease 10 to 18 years after exposure, I-131 dose was significantly associated with increased risk of any thyroid nodule, although the magnitude of radiation risk varied substantially by nodule cytology/histology and size. The relationship of I-131 dose and all types of thyroid nodules was higher in participants who were exposed at a younger age. Future studies in this cohort assessing the radiation-dose response for more detailed nodule characteristics (e.g., nodule composition, shape, echogenicity, calcifications, and vascularization), progression of prevalent thyroid nodules, and incidence of thyroid nodules during subsequent screening cycles may shed further light on underlying biological mechanisms.
Acknowledgments
The authors thank the study participants, the BelAm medical and administrative staff for study management and data collection and Dr. Dale Preston (Hirosoft International) for statistical advice.
Acknowledgments
This research was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, US Department of Health and Human Services.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- EOR/Gy
- excess odds ratios per Gray
- FNB
- fine-needle biopsy
- Gy
- Gray
- NOS
- not otherwise specified
- US
- ultrasonography.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegedüs L. Clinical practice: the thyroid nodule. N Engl J Med. 2004;351(17):1764–1771. [DOI] [PubMed] [Google Scholar]
- 3.Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, Braverman LE, Clark OH, McDougall IR, Ain KV, Dorfman SG; American Thyroid Association . Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Intern Med. 1996;156(19):2165–2172. [PubMed] [Google Scholar]
- 4.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941–1946. [DOI] [PubMed] [Google Scholar]
- 5.Acharya S, Sarafoglou K, LaQuaglia M, Lindsley S, Gerald W, Wollner N, Tan C, Sklar C. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer. 2003;97(10):2397–2403. [DOI] [PubMed] [Google Scholar]
- 6.Favus MJ, Schneider AB, Stachura ME, Arnold JE, Ryo UY, Pinsky SM, Colman M, Arnold MJ, Frohman LA. Thyroid cancer occurring as a late consequence of head-and-neck irradiation. Evaluation of 1056 patients. N Engl J Med. 1976;294(19):1019–1025. [DOI] [PubMed] [Google Scholar]
- 7.Land CE, Kwon D, Hoffman FO, Moroz B, Drozdovitch V, Bouville A, Beck H, Luckyanov N, Weinstock RM, Simon SL. Accounting for shared and unshared dosimetric uncertainties in the dose response for ultrasound-detected thyroid nodules after exposure to radioactive fallout. Radiat Res. 2015;183(2):159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyon JL, Alder SC, Stone MB, Scholl A, Reading JC, Holubkov R, Sheng X, White GL, Jr, Hegmann KT, Anspaugh L, Hoffman FO, Simon SL, Thomas B, Carroll R, Meikle AW. Thyroid disease associated with exposure to the Nevada nuclear weapons test site radiation: a reevaluation based on corrected dosimetry and examination data. Epidemiology. 2006;17(6):604–614. [DOI] [PubMed] [Google Scholar]
- 9.Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E, Ashizawa K, Hida A, Soda M, Fujiwara S, Yamada M, Ejima E, Yokoyama N, Okubo M, Sugino K, Suzuki G, Maeda R, Nagataki S, Eguchi K. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55-58 years after radiation exposure. JAMA. 2006;295(9):1011–1022. [DOI] [PubMed] [Google Scholar]
- 10.Imaizumi M, Ohishi W, Nakashima E, Sera N, Neriishi K, Yamada M, Tatsukawa Y, Takahashi I, Fujiwara S, Sugino K, Ando T, Usa T, Kawakami A, Akahoshi M, Hida A. Association of radiation dose with prevalence of thyroid nodules among atomic bomb survivors exposed in childhood (2007-2011). JAMA Intern Med. 2015;175(2):228–236. [DOI] [PubMed] [Google Scholar]
- 11.Schneider AB, Ron E, Lubin J, Stovall M, Gierlowski TC. Dose-response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid. J Clin Endocrinol Metab. 1993;77(2):362–369. [DOI] [PubMed] [Google Scholar]
- 12.Hatch M, Brenner A, Bogdanova T, Derevyanko A, Kuptsova N, Likhtarev I, Bouville A, Tereshchenko V, Kovgan L, Shpak V, Ostroumova E, Greenebaum E, Zablotska L, Ron E, Tronko M. A screening study of thyroid cancer and other thyroid diseases among individuals exposed in utero to iodine-131 from Chernobyl fallout. J Clin Endocrinol Metab. 2009;94(3):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis S, Kopecky KJ, Hamilton TE, Onstad L; Hanford Thyroid Disease Study Team . Thyroid neoplasia, autoimmune thyroiditis, and hypothyroidism in persons exposed to iodine 131 from the Hanford nuclear site. JAMA. 2004;292(21):2600–2613. [DOI] [PubMed] [Google Scholar]
- 14.Kaliszewski K, Diakowska D, Wojtczak B, Strutyńska-Karpińska M, Domosławski P, Sutkowski K, Głód M, Balcerzak W, Forkasiewicz Z, Łukieńczuk T. Fine-needle aspiration biopsy as a preoperative procedure in patients with malignancy in solitary and multiple thyroid nodules. PLoS One. 2016;11(1):e0146883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barroeta JE, Wang H, Shiina N, Gupta PK, Livolsi VA, Baloch ZW. Is fine-needle aspiration (FNA) of multiple thyroid nodules justified? Endocr Pathol. 2006;17(1):61–65. [DOI] [PubMed] [Google Scholar]
- 16.Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22(6):901–911. [DOI] [PubMed] [Google Scholar]
- 17.Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, Minenko VF, Ostapenko VA, Petrenko SV, Polyanskaya ON, Rzheutski VA, Tronko MD, Bobylyova OO, Bogdanova TI, Ephstein OV, Kairo IA, Kostin OV, Likhtarev IA, Markov VV, Oliynik VA, Shpak VM, Tereshchenko VP, Zamotayeva GA, Beebe GW, Bouville AC, Brill AB, Burch JD, Fink DJ, Greenebaum E, Howe GR, Luckyanov NK, Masnyk IJ, McConnell RJ, Robbins J, Thomas TL, Voillequé PG, Zablotska LB; Chornobyl Thyroid Diseases Study Group of Belarus; Chornobyl Thyroid Diseases Study Group of Ukraine; Chornobyl Thyroid Diseases Study Group of the USA . A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res. 2004;161(4):481–492. [DOI] [PubMed] [Google Scholar]
- 18.Zablotska LB, Ron E, Rozhko AV, Hatch M, Polyanskaya ON, Brenner AV, Lubin J, Romanov GN, McConnell RJ, O’Kane P, Evseenko VV, Drozdovitch VV, Luckyanov N, Minenko VF, Bouville A, Masyakin VB. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer. 2011;104(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zablotska LB, Bogdanova TI, Ron E, Epstein OV, Robbins J, Likhtarev IA, Hatch M, Markov VV, Bouville AC, Olijnyk VA, McConnell RJ, Shpak VM, Brenner A, Terekhova GN, Greenebaum E, Tereshchenko VP, Fink DJ, Brill AB, Zamotayeva GA, Masnyk IJ, Howe GR, Tronko MD. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: dose-response analysis of thyroid follicular adenomas detected during first screening in Ukraine (1998-2000). Am J Epidemiol. 2008;167(3):305–312. [DOI] [PubMed] [Google Scholar]
- 20.Drozdovitch V, Minenko V, Khrouch V, Leshcheva S, Gavrilin Y, Khrutchinsky A, Kukhta T, Kutsen S, Luckyanov N, Shinkarev S, Tretyakevich S, Trofimik S, Voillequé P, Bouville A. Thyroid dose estimates for a cohort of Belarusian children exposed to radiation from the Chernobyl accident. Radiat Res. 2013;179(5):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skryabin AM, Drozdovitch V, Belsky Y, Leshcheva SV, Mirkhaidarov AK, Voillequé P, Luckyanov N, Bouville A. Thyroid mass in children and adolescents living in the most exposed areas to Chernobyl fallout in Belarus. Radiat Prot Dosimetry. 2010;142(2-4):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drozdovitch V, Zhukova O, Germenchuk M, Khrutchinsky A, Kukhta T, Luckyanov N, Minenko V, Podgaiskaya M, Savkin M, Vakulovsky S, Voillequé P, Bouville A. Database of meteorological and radiation measurements made in Belarus during the first three months following the Chernobyl accident. J Environ Radioact. 2013;116:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drozdovitch V, Minenko V, Golovanov I, Khrutchinsky A, Kukhta T, Kutsen S, Luckyanov N, Ostroumova E, Trofimik S, Voillequé P, Simon SL, Bouville A. Thyroid dose estimates for a cohort of Belarusian children exposed to (131)I from the Chernobyl accident: assessment of uncertainties. Radiat Res. 2015;184(2):203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res. 1993;134(2):134–142. [PubMed] [Google Scholar]
- 25.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure Risk Regression and Person-Year Computation Software: Command Summary and User Guide. Ottowa, ON, Canada: Risk Sciences International.
- 26.Mushkacheva G, Rabinovich E, Privalov V, Povolotskaya S, Shorokhova V, Sokolova S, Turdakova V, Ryzhova E, Hall P, Schneider AB, Preston DL, Ron E. Thyroid abnormalities associated with protracted childhood exposure to 131I from atmospheric emissions from the Mayak weapons facility in Russia. Radiat Res. 2006;166(5):715–722. [DOI] [PubMed] [Google Scholar]
- 27.Zablotska LB, Nadyrov EA, Polyanskaya ON, McConnell RJ, O’Kane P, Lubin J, Hatch M, Little MP, Brenner AV, Veyalkin IV, Yauseyenka VV, Bouville A, Drozdovitch VV, Minenko VF, Demidchik YE, Mabuchi K, Rozhko AV. Risk of thyroid follicular adenoma among children and adolescents in Belarus exposed to iodine-131 after the Chornobyl accident. Am J Epidemiol. 2015;182(9):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacini F, De Groot LJ. Thyroid nodules. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, and Vinik A, eds. Endotext. Available at: https://www.ncbi.nlm.nih.gov/books/NBK285543/. Accessed 31 March 2017. [Google Scholar]
- 29.Barton HA, Cogliano VJ, Flowers L, Valcovic L, Setzer RW, Woodruff TJ. Assessing susceptibility from early-life exposure to carcinogens. Environ Health Perspect. 2005;113(9):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little MP. Are two mutations sufficient to cause cancer? Some generalizations of the two-mutation model of carcinogenesis of Moolgavkar, Venzon, and Knudson, and of the multistage model of Armitage and Doll. Biometrics. 1995;51(4):1278–1291. [PubMed] [Google Scholar]
- 31.Little MP, Hawkins MM, Charles MW, Hildreth NG. Fitting the Armitage-Doll model to radiation-exposed cohorts and implications for population cancer risks. Radiat Res. 1992;132(2):207–221. [PubMed] [Google Scholar]