Abstract
Background:
Adrenocorticotropic hormone (ACTH) secretion is controlled by unobservable hypothalamic corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) pulses. Clamping exogenous CRH or AVP input could allow indirect quantification of the impact of the endogenous heterotypic hormone.
Methods:
We conducted a randomized, double-blind, placebo-controlled, crossover study in 28 healthy adults (16 men). Volunteers underwent a sex-steroid clamp and a cortisol clamp. ACTH was measured over 10 hours by 10-minute sampling during each of four randomized intravenous (IV) secretagogue clamps (i.e., continuous IV CRH, AVP, both peptides, or saline). Desensitization was tested by bolus injection of the noninfused peptide.
Results:
Mean ± standard error of the mean 10-hour ACTH concentrations (ng/L) in the sex-combined analysis were: saline, 32 ± 4.6; AVP, 29 ± 4.6; CRH, 67 ± 6.2; and CRH-AVP, 67 ± 8.8 (any CRH vs AVP or saline, P < 0.0001). CRH and AVP increased approximate entropy (relative randomness) of ACTH release (P < 0.0001). Bolus AVP injection after CRH infusion yielded a 2.5-hour ACTH concentration of 46 ± 4.3, exceeding that seen after bolus CRH or saline injection (26 ± 3.3 and 24 ± 3.6, respectively; P = 0.002 and 0.001). Sex hormone clamps did not influence ACTH levels.
Conclusions:
A CRH, but not AVP, clamp yields sustained pulsatile ACTH secretion with high ACTH secretory-burst mass and randomness. After 10-hour CRH infusion, bolus AVP but not CRH, evoked marked ACTH release, likely caused by heterotypic sensitization of corticotropes by CRH. Similar interactions might underlie chronic stress states.
Studies of ACTH secretion are hampered by unobservable hypothalamic CRH-AVP pulses. Clamping one of the secretagogues could allow indirect quantification of impact of endogenous heterotypic hormone.
The stress-responsive hypothalamo-pituitary-adrenal axis constitutes an interactive ensemble of central neural, anterior hypophysial, and adrenocortical regulatory loci (1–3). Neurotransmitter molecules and blood-borne hormones mediate adaptive responses via time-delayed (unobserved) dose-responsive signaling at the various regulatory interfaces (4). For example, neurotransmitter inputs from the cerebral cortex, hippocampo-limbic system, and brainstem stimulate or inhibit recurrent burst-like secretion of hypothalamic corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) into hypothalamo-pituitary portal blood (5–8). CRH and AVP both individually and synergistically trigger exocytotic discharge of adrenocorticotropic hormone (ACTH)-containing secretory granules and promote de novo biosynthesis and accumulation of ACTH in corticotrope cells (9, 10). Time-varying plasma concentrations of ACTH drive pulsatile adrenal production of cortisol, which associates with plasma proteins (e.g., albumin and cortisol-binding globulin) (11). In turn, cortisol feedback restrains neuronal secretion of AVP, CRH, and ACTH and antagonizes pituitary actions of AVP and CRH by activating selective signaling pathways, including nuclear (type I and II) glucocorticoid receptors (12). This complex set of neuroglandular interactions, rather than any single signal alone, maintains glucocorticoid homeostasis in health and mediates adaptive responses to stress and disease.
Various investigations have evaluated regulation of ACTH and cortisol outflow in normal puberty, aging, exercise, chronic alcoholism, polycystic ovarian syndrome, extended fasting, insulin-induced hypoglycemia, thyroidal failure, growth failure, sex, uremia, and Cushing’s disease and during glucocorticoid administration (1, 2, 13). However, more detailed study of the complex interrelations among AVP, CRH, ACTH, and cortisol is not straightforward because time-dependent changes in endogenous CRH and AVP have not been observed. To address these limitations, the current study implements a fourfold approach: (1) administration of ketoconazole to block cortisol secretion, with addback of cortisol intravenous (IV) to confine cortisol concentrations within a fixed physiological range; (2) imposition of reversible hypogonadism with leuprolide and addback with estradiol or testosterone to clamp sex steroids; (3) continuous IV infusion of individual and combined secretagogues, CRH, and/or AVP to fix one or both peptide inputs to the pituitary; and (4) blood sampling every 10 minutes concomitantly to allow quantification of the pulsatile and entropic (pattern-regularity) modes of ACTH secretion. The specific hypotheses were: (1) sex and sex steroids modulate AVP and testosterone (T) or CRH-clamped pulsatile and entropic ACTH secretion, (2) exogenous CRH and AVP drive unequal pulsatile and pattern-random ACTH secretion, and (3) the ACTH response to an acute peptide stimulus depends upon prior (10-hour) corticotrope exposure to exogenous peptide.
Materials and Methods
Subjects
The protocol was approved by the Mayo Institutional Review Board and reviewed by the Food and Drug Administration for off-label use of clinically marketed ketoconazole (KTCZ), CRH (Bachem Inc., Torrance, CA), AVP (Bachem Inc.), and IV cortisol. Witnessed voluntary written consent was obtained before study enrollment. Participants maintained conventional work and sleeping patterns and reported no recent (within 10 days) transmeridian travel, substantial weight loss or gain, intercurrent psychosocial stress, substance abuse, glucocorticoid exposure (within 3 months), neuropsychiatric illness, or systemic disease. A complete medical history, physical examination, and screening tests of hematological, renal, hepatic, metabolic, and endocrine function were normal. The demographic characteristics of the subjects are listed in Table 1.
Table 1.
Demographic Data of the Healthy Volunteers at the Baseline Visit
|
|
Women
|
Men
|
||
|---|---|---|---|---|
| Estradiol (+) | Estradiol (−) | Testosterone (+) | Testosterone (−) | |
| Number of subjects | 8 | 4 | 9 | 7 |
| Age, y | 59 ± 3.9 | 54 ± 2.8 | 56 ± 2.2 | 58 ± 4.0 |
| BMI, kg/m2 | 25.0 ± 1.0 | 24.3 ± 1.6 | 28.4 ± 0.6 | 26.7 ± 0.7 |
| TSH, mU/L | 1.6 ± 0.4 | 3.8 ± 1.4 | 2.3 ± 0.5 | 2.5 ± 0.4 |
| Estradiol, pg/mL | 104 ± 50 | 18.2 ± 5.9 | 28.0 ± 3.4 | 28.8 ± 3.4 |
| Testosterone, ng/dL | 21.0 ± 3.3 | 12.7 ± 1.4 | 408 ± 40 | 502 ± 76 |
| Cortisol, µg/dL | 12.4 ± 1.1 | 11.5 ± 1.1 | 12.0 ± 0.8 | 13.6 ± 1.4 |
Data are shown as means ± standard error of the mean (SEM).
No statistically significant differences in basal levels between subjects randomized to receive sex steroids and those who did not receive replacement were found with the unpaired Student’s t test except for estradiol in women of the placebo group, which consisted exclusively of postmenopausal women.
Study design
Volunteers were prospectively randomized in a prospective, double-blind, placebo-controlled partial within-subject crossover study comprising 16 men and 12 women with allowable ages of 45 to 75 years. Each subject received two doses 3.75 mg intramuscular leuprolide (Lupron) given 2 weeks apart to induce transient hypogonadism followed by either sex-relevant sex-steroid or no replacement starting on the day of the second leuprolide injection. In men, sex-steroid replacement schedules comprised three weekly injections of 200 mg testosterone enanthate or placebo. In women, sex-steroid replacement schedules comprised oral micronized estradiol-17β (Estrace) 1 mg or placebo twice daily for up to 26 days. Nine women were postmenopausal.
Infusion protocol
Studies were conducted in the Mayo Clinical Research Unit (CRU) (a US Center for Translational Science Activities). Admissions to the CRU for infusion/sampling sessions were scheduled 14 days after beginning sex-steroid addback and 4 to 6 days apart in a within-subject, randomized, crossover design so that each subject completed four study sessions. Volunteers were admitted the evening before the study for repetitive blood sampling overnight (described later). The adrenal-blocking regimen comprised oral administration of clinically available KTCZ in all four of the CRU sessions (14). Male subjects received 800 mg at 10:00 pm and 300 mg at 6:00 am, and female subjects received 600 mg at 10:00 pm and 200 mg at 6:00 am. To maintain cortisol levels, soluble hydrocortisone hemisuccinate (Solucortef) was infused intravenously over 14 hours (10:00 am to 12:00 pm) at an estimated midphysiological cortisol production rate of 0.25 mg/m2/h (or 3.5 mg/m2/14 h total dose).
The four randomly ordered CRU peptide/saline infusion protocols in each of the 28 subjects were conducted between 11:30 pm and 9:30 am with (1) IV saline 300 mL/10 h, (2) human corticotropin-releasing hormone (hCRH) 1 µg/kg/h over 10 hours, (3) AVP 10.5 IU/m2 over 10 hours, and (4) combined hCRH 1 µg/kg/h and AVP 10.5 IU/m2 over 10 hours based upon literature estimates of near-maximal CRH and AVP stimulation (10, 15–17). At 9:30 the next morning, subjects received an IV bolus of the heterotypic (noninfused) peptide. Men received bolus 1.0 µg/kg hCRH after AVP infusion, 0.67 IU/m2 AVP after hCRH infusion, the same doses of combined hCRH and AVP after saline infusion, and saline after combined hCRH and AVP infusion. Women received bolus 0.67 µg/kg hCRH and 0.5 IU/m2 AVP in an otherwise identical injection schema. Between 10:00 pm and 12:00 pm, blood (2 mL) was sampled at 10-minute intervals for plasma ACTH and serum cortisol measurements. The 10-minute sampling frequency of ACTH is required for estimating secretion accurately given the plasma ACTH half-life of 15 to 30 minutes and the fact that 50% of ACTH released within a burst occurs over 10 to 12 minutes (4) (see Analytical Methods later).
Materials
Human CRH was obtained from Bachem Pharmaceuticals (Bachem Americas Inc., Torrance, CA). AVP (as aqueous Pitressin 20, units/mL) was obtained from Parke-Davis Co. (Morris Plains, NJ). Leuprolide was from Abbott Laboratories (Abbott Park, IL); ketoconazole from Teva Pharmaceuticals (North Wales, PA); and soluble hydrocortisone succinate for infusion from Pharmacia and Upjohn (New York, NY).
Exclusion criteria
Exclusion criteria were acute or chronic systemic diseases; HIV positivity by medical history; anemia; endocrine disorders (except hypothyroid subjects, who were biochemically euthyroid on replacement); psychiatric illness; alcohol or drug abuse; prostatic disease; deep venous or arterial thromboses; cancer of any type (except localized basal or squamous cell cancer treated surgically without recurrence); allergy to medications used in the study; substantial recent weight change (loss/gain of ≥6 lb over 6 weeks); transmeridian travel (exceeding three time zones within the preceding week); systemic drugs (including angiotensin-converting enzyme inhibitors, angiotensin II receptor blocking agents, and diuretics); abnormal renal, hepatic, or hematologic function; concomitant sex-hormone replacement; pregnancy or lactation; and unwillingness to provide written informed consent.
Assays
Plasma ACTH was assayed in each 10-minute sample via sensitive and specific solid-phase immunochemiluminescent assay (Siemens Health Care Diagnostics, Deerfield, IL). The detection threshold was 5 ng/L (divide by 4.5 for pmol/L). Intra-assay coefficients of variation were 6.4, 2.7, and 3.2% at 5.7, 28, and 402 ng/L, respectively. Cortisol was assayed via competitive binding immunoenzymatic assay (Beckman Coulter, Fullerton, CA). The detection limit was 0.4 μg/dL, and intra-assay coefficients of variations were 13, 9.4, and 6.6% at 1.6, 2.8, and 30 μg/dL, respectively. Testosterone, estradiol, and estrone were assayed by mass spectrometry as described (18, 19). Screening safety tests were performed by the Mayo Medical Laboratory.
Secondary analyses: deconvolution
Ten-minute ACTH concentration profiles variable-waveform deconvolution analysis was used to reconstruct into underlying trains of secretory bursts, superimposed upon basal (time-invariant) secretion, allowing biexponential elimination (fixed fast half-life for rapid diffusion and advection and estimated slow half-life for delayed metabolic elimination) (4). The analysis implements a Matlab-based adaptive-mesh multiparameter-search algorithm that was cross-validated previously (20).
Approximate entropy
Secretory regularity was appraised via the approximate entropy (ApEn) statistic (21). ApEn provides a sensitive (>90%), model-free, and scale-invariant measure of relative randomness due to loss of feedback control within a network. Cross-ApEn was not used because of the partial KTCZ clamp of cortisol secretion.
Statistics
The primary endpoint was the 10-hour mean ACTH concentration, determined from 60 serial measurements between 11:30 pm and 9:30 am (continuous saline or peptide infusion interval). The last 2.5 hours (between 9:30 am and 12:00 pm) after AVP-CRH bolus injections were used to evaluate pituitary ACTH responsiveness. Secondary mechanistic outcomes were deconvolution estimates of ACTH secretory-burst mass (analytical integral of reconstructed waveform), secretory-burst shape (mode of generalized Gamma density), bursting regularity (gamma of Weibull), and hormone-concentration ApEn.
A priori clinical hypotheses were that sex and/or exposure to the hypogonadal versus the clamped T (in men) or estradiol (E2) (in women) milieus determines mean ACTH concentrations under the cortisolemic clamp during (1) continuous saline or peptide-secretagogue infusions over 10 hours and (2) bolus injection of saline, CRH, and/or AVP thereafter over 2.5 hours (22).
Data were analyzed on the logarithmic scale to accommodate asymptotic ACTH secretory responses to secretagogues using a generalized linear model (GLM) comprising three-way analysis of covariance with partially repeated measures. The categorical variables were sex (two factors), sex-steroid or placebo administration (two factors), and infused peptides or saline (four factors), with cortisol concentration as a covariate. Calculations were performed with Systat 13 (Systat Software, Inc., San Jose, CA) and Matlab 8.6 (The MathWorks, Inc., Natick, MA). P < 0.01 was construed as significant for the overall study. Post hoc multiple comparisons were performed with Sidak’s correction.
Results
All subjects completed the studies, and no adverse events were noted. Estradiol concentrations were mean (range) 12.9 (5.9 to 19.0) pg/mL in sex-steroid deplete women and 681 (112 to 1700) pg/mL in E2-replete women. T concentrations were 17.2 (7.4 to 44.0) ng/dL in T-deplete men and 1670 (1208 to 2138) ng/dL in T-replete men Table 1.
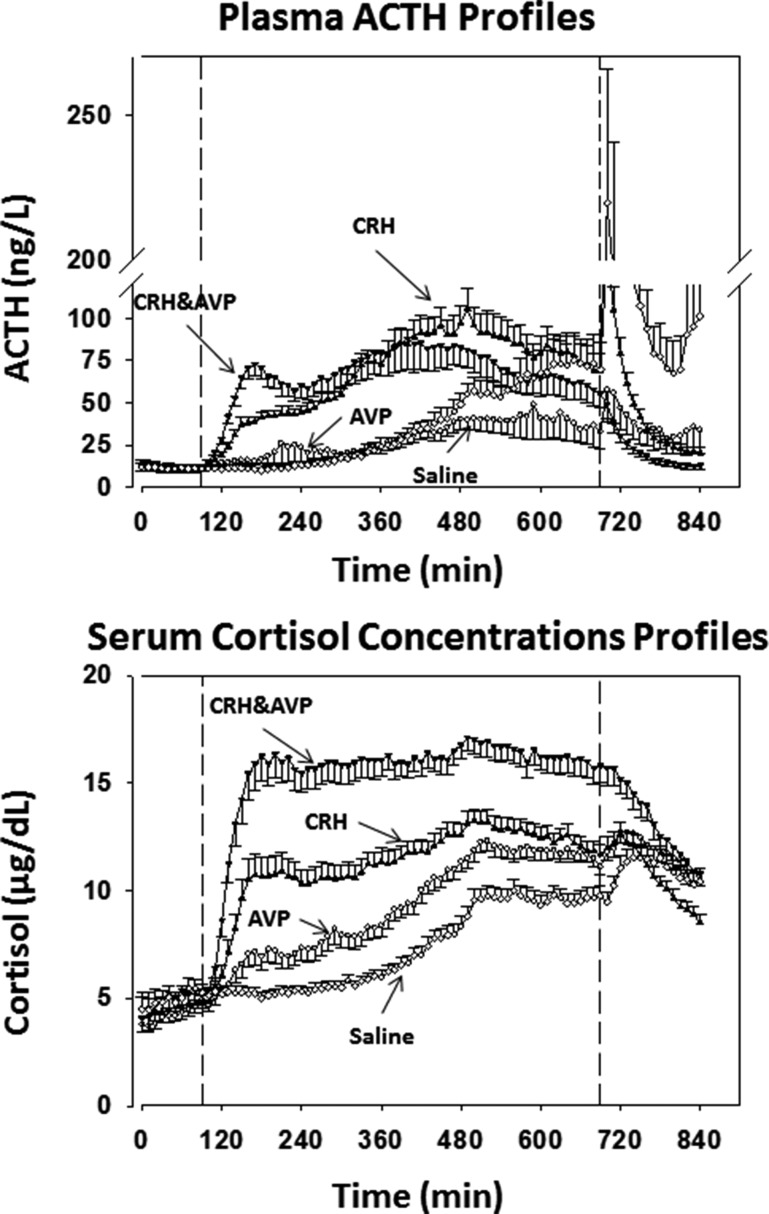
Mean ACTH and cortisol concentration-time profiles are displayed in Fig. 1. The 10-hour continuous IV saline, hCRH, and/or AVP infusion interval is shown between the two vertical lines. Distinct ACTH secretion patterns emerged during the infusions, along with a gradual circadian rise in ACTH and cortisol during saline (placebo) infusion. Prompt ACTH release occurred over 2.5 hours after bolus injection of peptidyl secretagogues (right extreme of curves).
Figure 1.
ACTH and cortisol concentration-time plots in healthy subjects exposed to different exogenous ACTH secretagogues (CRH, AVP, CRH and AVP, saline). Peptides were infused between 90 and 690 min after the start of blood sampling, as indicated by the dashed vertical lines. Arrows indicate the hormone or combination infused continuously. After 690 min, the heterologous peptide was injected by bolus. During saline infusion, both ACTH and cortisol show the expected circadian rise overnight.
Statistical analysis revealed that the response of 10-hour mean ACTH concentrations to hCRH and to the combination of hCRH and AVP averaged 2.3-fold greater than that the response to AVP infusion (P < 0.0001) (Table 2). Neither sex nor sex-steroid addback (E2 or T) altered 10-hour mean ACTH concentration responses to the infused secretagogues. There was a detectable cortisol rise in response to 10-hour hCRH or combined hCRH and AVP infusion (but not saline or AVP), indicating some adrenal secretory breakthrough (P < 0.05) (Table 2). Cortisol concentrations during the infusion sessions were consistently higher in women than in men (P < 0.0001).
Table 2.
Ten-h Mean ACTH and Cortisol Concentrations During Continuous IV Infusion of CRH and/or AVP or Saline
| CRH | CRH-AVP | AVP | Saline | GLM P Value | |
|---|---|---|---|---|---|
| ACTH, ng/L | |||||
| Men (n = 16) | 81.4 ± 9.8A | 63.2 ± 11.9A | 34.5 ± 7.5B | 40.3 ± 7.5B | <0.0001 |
| Women (n = 12) | 53.5 ± 5.0A | 78.4 ± 14.5A | 24.0 ± 5.0B | 31.3 ± 5.9B | |
| Cortisol, µg/dL | |||||
| Men (n = 16) | 10.3 ± 0.52A | 14.1 ± 0.64B | 8.6 ± 0.49C | 6.5 ± 0.34D | <0.0001 |
| Women (n = 12) | 12.9 ± 0.67A | 18.2 ± 0.92B | 10.6 ± 0.51C | 8.2 ± 0.36B |
Data are shown as means ± SEM. Statistical calculations were done on logarithmically transformed ACTH data. Infusions: P < 0.001; sex: P = 0.88; sex steroids: P = 0.46.
Post hoc contrasts are denoted by different letters within any given row, assessed by Sidak correction for multiple comparisons at P < 0.01.
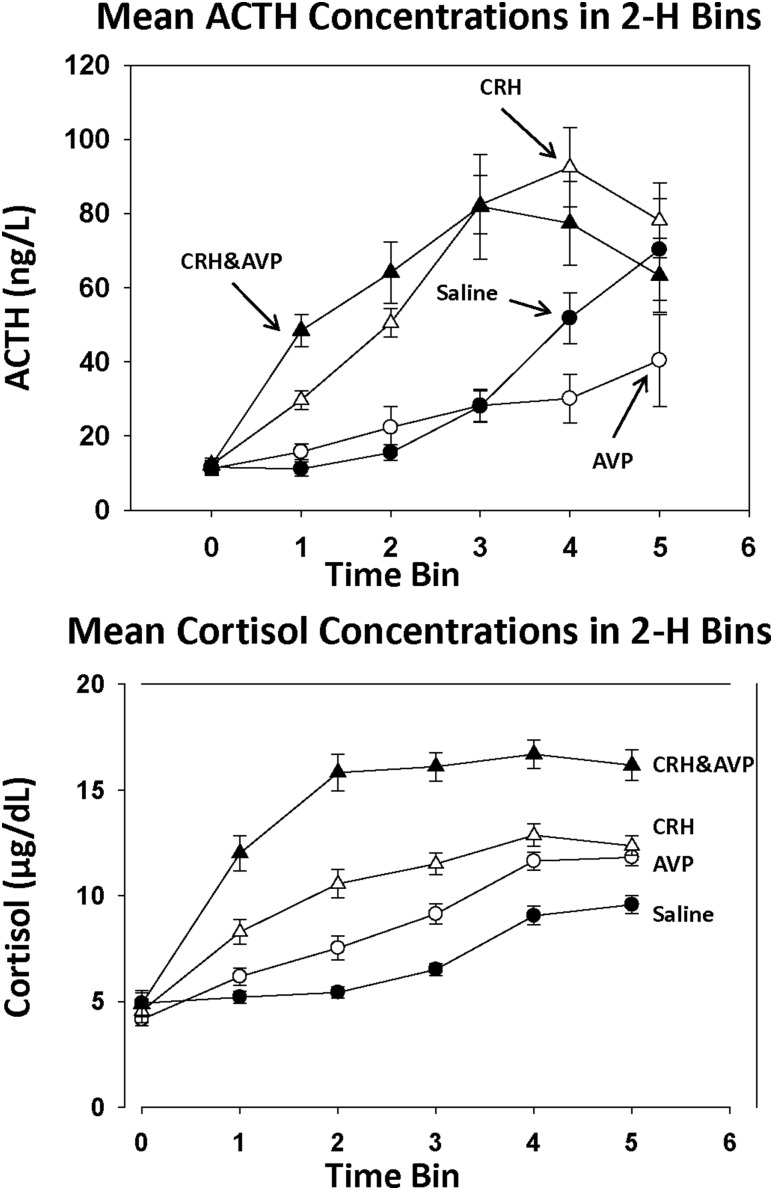
To minimize the impact of circadian drift (especially on the saline day), mean ACTH concentrations were quantified also across six consecutive 2-hour blocks, excluding the first 90 minutes, before any peptide was infused, and the last 0.5 hours after bolus peptide injections, as displayed in Fig. 2. In the baseline 90 minutes, ACTH means were similar in the four groups (P = 0.98). During infusions, successive 2-hour mean ACTH concentrations rose significantly (P < 0.0001) under distinct secretagogues (P < 0.0001). However, there was no effect of sex (P = 0.90) or addback of sex hormones (P = 0.50). Maximal ACTH concentrations occurred 4 to 6 hours after the start of continuous 10-hour secretagogue infusions.
Figure 2.
Mean ACTH (upper panel) and cortisol (lower panel) concentrations before peptide infusion (bin 0) and during 10 h of secretagogue infusion. Duration of bins 1 through 5 was 2 h each, and that of bin 0 was 90 min. Data are shown as means ± SEM.
During the first 2 hours of the peptide infusions, synergy AVP and CRH was verified (Fig. 2). The mean ACTH concentration during combined CRH and AVP infusion was 48.4 ± 4.3 ng/L, which exceeded that during CRH alone (29.7 ± 2.5 ng/L; P = 0.005) and AVP alone (P < 0.001). Beyond this initial 2-hour window, no ACTH differences were found between CRH and combined AVP-CRH infusions. Additionally, ACTH levels during AVP and saline infusion were similar, except during the first (P = 0.023) and the last (P = 0.001) 2 hours of infusion. In all time bins, CRH and CRH combined with AVP infusions stimulated higher ACTH concentrations than AVP alone (P < 0.0001).
Cortisol concentrations in 2-hour blocks depended on time (P < 0.0001), infused secretagogue type (CRH infusions > AVP or saline alone) (P < 0.0001), and sex (women > men) (P < 0.0001) (Fig. 2). The interaction term time × infusion type was significant for cortisol (P < 0.0001). An initial difference between AVP (less) and CRH (greater) associated cortisol concentrations disappeared over the last 4 hours.
Secretion of ACTH was estimated by deconvolution analysis (Table 3). Pulsatile, but not basal, ACTH secretion differed among the four infusion types, being 1.5- to 2.1-fold greater during continuous IV infusion of CRH-AVP and CRH, respectively (analysis of variance P < 0.0001 versus saline or AVP). This was due principally to amplification of ACTH secretory-burst mass (P < 0.0001), with a small increase in burst frequency during CRH infusion (P = 0.001). Secretory-burst mode and interburst regularity were not influenced by infused secretagogue type (P = 0.74). Sex (P ≥ 0.45) and the addback of sex steroids (P > 0.11) were not significant categorical variables under the GLM analysis, using cortisol concentrations as the covariate. The latter was negative, reflecting negative feedback on ACTH.
Table 3.
Deconvolution Analysis of 10-h Plasma ACTH Profiles During Secretagogue Infusions and Saline
| Infusion | CRH | CRH-AVP | AVP | Saline | GLM P Value |
|---|---|---|---|---|---|
| Burst number | 11.7 ± 0.3 | 9.9 ± 0.4 | 9.4 ± 0.5 | 10.3 ± 0.4 | 0.001 |
| Slow half-life, min | 19.3 ± 0.3 | 17.5 ± 0.6 | 17.3 ± 0.9 | 19.7 ± 0.2 | 0.001 |
| Burst mode, min | 11.3 ± 1.0 | 13.5 ± 0.8 | 10.6 ± 0.9 | 10.7 ± 1.0 | 0.08 |
| Basal secretion, ng/L/10 h | 734 ± 109 | 1243 ± 124 | 363 ± 42 | 279 ± 49 | 0.11 |
| Pulsatile secretion, ng/L/10 h | 1287 ± 167 | 931 ± 171 | 583 ± 123 | 814 ± 145 | <0.0001 |
| Total secretion, ng/L/10 h | 2021 ± 186 | 2174 ± 258 | 946 ± 137 | 1093 ± 157 | <0.0001 |
| Mean burst mass, ng/L | 110 ± 14 | 93 ± 17 | 64 ± 14 | 82 ± 15 | <0.0001 |
| Bursting regularity | 2.57 ± 0.16 | 2.25 ± 0.18 | 2.36 ± 0.23 | 2.44 ± 0.13 | 0.74 |
Data are shown as means ± SEM. Data were logarithmically transformed before GLM analysis. Sex and sex steroid addback were not significant variables in any of the parameters.
Contrasts with Sidak correction for multiple comparisons: Pulsatile secretion: CRH vs AVP, saline and CRH-AVP: P < 0.0001, 0.027, and 0.97, respectively. AVP vs CRH-AVP and saline: 0.011 and 0.032. Total secretion: CRH vs AVP, saline, and CRH-AVP: P < 0.0001, < 0.0001, and 0.52; AVP vs CRH-AVP and saline: P < 0.0001 and 0.92. Mean pulse mass: CRH vs AVP, saline, and CRH-AVP: P = 0.001, 0.09, and 0.99, respectively; AVP vs CRH-AVP and saline: P = 0.014 and 0.60.
The results of deconvolution analysis of the cortisol profiles are displayed in Table 4. Basal (and total) secretion was amplified, but pulsatile secretion was not.
Table 4.
Deconvolution Analysis of 10-h Serum Cortisol Profiles During Secretagogue Infusions and Saline
| Infusion | CRH | CRH-AVP | AVP | Saline | GLM |
|---|---|---|---|---|---|
| Burst number | 10.1 ± 0.43 | 10.1 ± 0.41 | 8.6 ± 0.47 | 8.4 ± 0.47 | 0.002 |
| Slow half-life, min | 54 ± 2.7 | 49 ± 2.3 | 60 ± 2.4 | 60 ± 1.9 | 0.003 |
| Burst mode, min | 9.3 ± 1.0 | 9.0 ± 1.1 | 8.2 ± 1.2 | 9.2 ± 1.2 | |
| Basal secretion, µg/L/10 h | 97 ± 10.4 | 161 ± 11.6 | 61 ± 7.4 | 44 ± 4.7 | <0.0001 |
| Pulsatile secretion, µg/L/10 h | 50 ± 4.2 | 51 ± 4.9 | 48 ± 3.6 | 36 ± 4.0 | 0.06 |
| Total secretion, µg/L/10 h | 147 ± 10.2 | 212 ± 12.3 | 109 ± 8.0 | 80 ± 3.6 | 0.002 |
| Mean burst mass, µg/L | 5.0 ± 0.38 | 5.1 ± 0.46 | 5.8 ± 0.47 | 4.2 ± 0.41 | 0.10 |
| Bursting regularity | 2.84 ± 0.23 | 2.64 ± 0.18 | 2.06 ± 0.12 | 2.37 ± 0.26 | 0.08 |
Data are shown as means ± SEM. Data were logarithmically transformed before GLM analysis. Sex and sex-steroid addback were not significant variables for any of the deconvolution measures.
Contrasts with Sidak correction for multiple comparisons: basal secretion: AVP vs CRH, saline and CRH-AVP: P = 0.026, 0.016, and < 0.0001, respectively. CRH vs CRH-AVP and saline: P < 0.0001. Total secretion: AVP vs CRH, saline, and CRH-AVP: P = 0.019, < 0.0001, and 0.001, respectively. CRH vs CRH-AVP and saline: P < 0.0001. Mean pulse mass: CRH vs AVP, saline, and CRH-AVP: P = 0.001, 0.09, and 0.99, respectively; AVP vs CRH-AVP and saline: P = 0.014 and 0.60. Burst number: AVP vs CRH: P = 0.005. Slow half-life: AVP vs CRH-AVP: P = 0.006; CRH-AVP vs saline: P = 0.02.
Ninety-minute prestimulation mean ACTH concentrations correlated significantly with serum estradiol in women (R2 = 0.15; P = 0.007; β = 0.009 ± 0.003). During the first 4 hours of peptide infusions (divided into blocks of 2 hours), linear regressions of mean ACTH on serum E2 were significant only during AVP administration (R2 = 0.52 and 0.64 for the first 2 hours and for the second 2 hours respectively; P = 0.008 and 0.002, respectively; and β = 0.019 ± 0.006 and 0.067 ± 0.016, respectively). No correlations were present during continuation of the peptide infusions, and no relations between T and mean ACTH were found in men.
After the 10-hour continuous infusion of CRH and/or AVP (or saline), subjects received a submaximal bolus of the peptide not infused and before blood sampling was continued for another 150 minutes. The ACTH response was evaluated by three different approaches: (1) the mean ACTH concentration in this period, (2) the maximal ACTH concentration increase (or decrease), and (3) secretory-burst mass calculated by deconvolution analysis. The results are shown in Supplemental Table 1 (18KB, docx) . The ACTH-stimulating effect of bolus combined CRH-AVP injection, after 10-hour saline infusions, was greater than that of bolus saline, CRH, and AVP, in all three calculations (P < 0.01). The administration of bolus AVP induced a larger ACTH response after continuous CRH infusion than bolus CRH injection did after continuous AVP infusion. ACTH decreased gradually after bolus saline injection despite prior infusion of combined CRH and AVP.
The ApEn of ACTH concentration-time series rose during infusion of CRH and/or AVP, denoting altered feedback/feedforward compared with saline. Neither sex nor the administration of sex hormones altered ACTH ApEn (Supplemental Table 2 (21.7KB, docx) ).
Discussion
This study was designed to explore the individual and joint contributions of CRH and AVP to pulsatile ACTH secretion under nominal pharmacologically imposed cortisol-feedback and sex-steroid clamps in men and women. The paradigm comprised exogenously constrained CRH or AVP (or both) stimulation, along with partial cortisol feedback restraint imposed by blocking cortisol synthesis with ketoconazole, and adding back a fixed amount of cortisol intravenously. Deliberate differences in sex-hormone milieu were achieved by inducing hypogonadism with leuprolide and adding back E2 or T versus placebo in women and men, respectively. Sex hormone addback resulted in pharmacologic to high-normal young-adult values in hormone-replete subjects and very low levels in the leuprolide-suppressed nonreplaced subjects. However, cortisol levels, although strictly within normal levels (highest absolute 2-hour mean, 13.4 ± 0.6 µg/dL), depended on the infused ACTH secretagogue and on sex, being consistently higher in women than men. Therefore, in the statistical analyses of ACTH secretion, the mean cortisol concentration of each subject in each time period was used as a covariate. The covariate was significantly negative, consistent with negative feedback.
In the sex-combined analysis (allowable statistically in the absence of a sex effect), mean 10-hour ACTH concentrations and ACTH secretion rates rose significantly and similarly during CRH and combined CRH-AVP infusions over AVP or saline infusion. However, during the first 2 hours of the peptide-secretagogue infusions, AVP and CRH achieved prominent synergy over either peptide alone, confirming the expected two-peptide potentiation of ACTH secretion, at least acutely. The present data show that this clear-cut synergy is not maintained during 10 hours of combined CRH and AVP infusions. Moreover, the initial ACTH-stimulating effects of AVP over saline infusion were not maintained. The reason that sustained AVP input fails to augment endogenous CRH’s drive of ACTH secretion in this setting is not known, but desensitization to AVP action or feedback of cortisol onto endogenous CRH is possible (9). Desensitization of CRH stimulation by sustained AVP action might also explain why delayed acute bolus injection of CRH after 10 hours of AVP stimulation evoked much less ACTH secretion than that caused by delayed acute bolus injection of AVP after 10 hours of CRH stimulation. In vitro studies of corticotrope secretion also allow for both homologous and heterologous desensitization (9, 23). Whether similar peptide-specific mechanisms operate in pathological states of sustained endogenous CRH or AVP drive is not known. However, if so confirmed, heterologous peptide-regulating mechanisms may also mediate altered ACTH secretion patterns in certain chronic disease settings.
ACTH secretion remained pulsatile during continuous single- and dual-peptide infusions. As well known for many decades, pituitary hormones are secreted in a pulsatile fashion, under signaling by stimulatory and inhibitory hypothalamic peptides and by systemic feedback signals (positive or negative). For instance, the pulsatile secretion of growth hormone is generated by the interplay between growth hormone–releasing hormone and somatostatin, whereas luteinizing hormone and follicle-stimulating hormone pulsatility is controlled by the kisspeptin-GnRH interaction. The classical view of ACTH pulsatility requires an interplay between CRH and AVP, which are synthesized in central nervous system neurons and transported via the portal vessels to the pituitary gland. The consideration that ACTH pulsatility requires pulsatile feedback by cortisol has been illustrated mathematical modeling (24). Our data showing low-level cortisol pulsatility under partial adrenal biosynthetic blockade are consistent with these ideas. For our experiments, it was crucial that pulsatile cortisol feedback was maintained. Persistence of cortisol pulsatility in the study setting is physiologically important because cortisol pulsatility in brain and subcutaneous tissue follows intravascular cortisol pulsatility (25, 26). Furthermore, ultradian corticosterone pulsatility regulates directly the transcription of period 1 gene in the hippocampus of the rat (27).
The question whether our volunteers exhibited centrally or peripherally mediated ACTH oscillations cannot be answered with certainty from our data. Walker et al. (24), using a theoretical model of the hypothalamic-pituitary-adrenal axis, proposed that cortisol oscillations per se can maintain pulsatile ACTH secretion. In their model, ACTH pulsatility and cortisol pulsatility emerged when the CRH signal was large and unchanged when the CRH signal strength was small. How hypothalamic factors interact with pulsatility in men is not known. Hypothetically it could be that pulsatile AVP and CRH patterns are both required for the generation of ultradian ACTH rhythms. However, pulsatile ACTH secretion was preserved during infusion of both ACTH secretagogues at maximally clinically acceptable concentrations. This outcome favors the notion of cortisol-driven pulsatility but does not exclude unknown pulsatility inputs from other hypothalamic sources.
Essentially the same ACTH secretory-burst frequency was present in the absence and presence of infused secretagogues, consistent with the concept of a preserved endogenous hypothalamic-pituitary-adrenal pulsing system.
Basal (nonpulsatile), rather than pulsatile, cortisol secretion was stimulated weakly by infused AVP/CRH and inferably inhibited by ketoconazole. Alternatively, continuous IV delivery of cortisol may have suppressed any rise in cortisol pulsatility. Whatever the mechanism, mean cortisol concentrations remained a major negative covariate of ACTH secretion. Indeed, by ANCOVA, cortisol concentrations also explained the sex differences of lower ACTH in women. Physiological (free) glucocorticoid levels in the brain are extremely important in negative feedback signal on hypothalamic neurons but appear to be comparable by sex in animal models (12). In the present clinical study, mean unstimulated and stimulated total cortisol concentrations were higher in women than in men, although they were still strictly within the physiological range, as observed earlier by some investigators in humans and other species. On the other hand, in an earlier analysis using a comparable protocol of ketoconazole blockade and cortisol addback, measured cortisol-binding globulin concentrations remained higher, and calculated free cortisol concentrations remained lower, in women than in men (14). The current study did not repeat such measurements, using covariate analysis instead to parse the contribution of unequal total cortisol concentrations by sex.
The addback of sex hormones, evaluated as a categorical variable in the GLM analysis, had little or no effect on peptide-stimulated ACTH concentrations or secretion rates. However, E2 concentrations correlated positively with ACTH secretion before continuous peptide infusions and during the first 4 hours of AVP infusion. This relationship was strong (R2 > 0.5) for AVP but was not evident during saline, CRH, or CRH-AVP administration. These results indicate a potentiating effect of E2 on AVP-stimulated ACTH secretion, whether directly or indirectly. Estrogenic regulation of the glucocorticoid receptor and/or endogenous CRH release would be major mechanistic considerations for these outcomes (28–30).
Approximate entropy, a metric of secretory randomness, increased significantly during the three peptide infusions. Higher ApEn denotes increased autonomy of feedforward over feedback control, as reflected here by strong exogenous stimulation of corticotropes (feedforward) and weak inhibition by cortisol feedback. Under these conditions, no influences of sex, sex hormone addback, or sex hormone concentrations were present. Indeed, in healthy subjects ACTH ApEn is age, body mass index, and sex independent, unless the glucocorticoid receptor is blocked experimentally (31).
Caveats of the study in the current study include the relatively short duration; the pharmacologic nature of the peptide, cortisol, E2, and T clamps; and the partial escape of adrenal cortisol secretion.
Conclusions
The present mechanistic investigation of regulated ACTH secretion in men and women under partially controlled cortisol feedback reveals an inhibitory effect of sustained AVP exposure on acute CRH drive and a potentiating effect of sustained CRH exposure on acute AVP stimulation. In this setting, the impact of sex hormones on ACTH secretion was explained by total cortisol concentrations and inferable potentiation of endogenously driven and exogenous AVP-stimulated ACTH secretion by systemic E2 concentrations. The complex interactions between AVP and CRH inferred here both acutely (2 hours) and over a longer interval (10 hours) provide an possible framework for developing hypotheses to explain the potentially puzzling effects of different stressor environments and medications on ACTH secretion.
Acknowledgments
The authors thank Jill Smith for help with manuscript preparation, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Current Affiliation: P. Aoun’s current affiliation is Palm Beach Diabetes & Endocrine Specialists, West Palm Beach, Florida 33401.
Acknowledgments
This work was supported in part by Grants R01 DK073148 and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health. The project described was supported by Grant UL1 TR000135 from the National Center for Advancing Translational Sciences. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- ApEn
- approximate entropy
- AVP
- arginine vasopressin
- CRH
- corticotropin-releasing hormone
- CRU
- Mayo Clinical Research Unit
- E2
- estradiol
- GLM
- generalized linear model
- hCRH
- human corticotropin-releasing hormone
- IV
- intravenous
- KTCZ
- ketoconazole
- SEM
- standard error of the mean
- T
- testosterone.
References
- 1.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34(2):271–292, vii. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. [DOI] [PubMed] [Google Scholar]
- 3.Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98(7):4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan DM, Veldhuis JD. Cortisol feedback state governs adrenocorticotropin secretory-burst shape, frequency, and mass in a dual-waveform construct: time of day-dependent regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R950–R961. [DOI] [PubMed] [Google Scholar]
- 5.Liu JP, Clarke IJ, Funder JW, Engler D. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep: II. The central noradrenergic and neuropeptide Y pathways cause immediate and prolonged hypothalamic-pituitary-adrenal activation. Potential involvement in the pseudo-Cushing’s syndrome of endogenous depression and anorexia nervosa. J Clin Invest. 1994;93(4):1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caraty A, Grino M, Locatelli A, Guillaume V, Boudouresque F, Conte-Devolx B, Oliver C. Insulin-induced hypoglycemia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophysial portal blood of conscious, unrestrained rams. J Clin Invest. 1990;85(6):1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink G, Robinson IC, Tannahill LA. Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J Physiol. 1988;401:329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander SL, Irvine CH, Ellis MJ, Donald RA. The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous blood from the horse. Endocrinology. 1991;128(1):65–72. [DOI] [PubMed] [Google Scholar]
- 9.Vale W, Vaughan J, Smith M, Yamamoto G, Rivier J, Rivier C. Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophysial peptides, and other substances on cultured corticotropic cells. Endocrinology. 1983;113(3):1121–1131. [DOI] [PubMed] [Google Scholar]
- 10.Watabe T, Tanaka K, Kumagae M, Itoh S, Kogure M, Hasegawa M, Horiuchi T, Morio K, Takeda F, Ubukata E, et al. . Role of endogenous arginine vasopressin in potentiating corticotropin-releasing hormone-stimulated corticotropin secretion in man. J Clin Endocrinol Metab. 1988;66(6):1132–1137. [DOI] [PubMed] [Google Scholar]
- 11.Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89(1):281–287. [DOI] [PubMed] [Google Scholar]
- 12.Droste SK, de Groote L, Lightman SL, Reul JM, Linthorst AC. The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: an in vivo microdialysis study in Wistar rats. J Neuroendocrinol. 2009;21(2):132–140. [DOI] [PubMed] [Google Scholar]
- 13.Keenan DM, Roelfsema F, Carroll BJ, Iranmanesh A, Veldhuis JD. Sex defines the age dependence of endogenous ACTH-cortisol dose responsiveness. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R515–R523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma AN, Aoun P, Wigham JR, Weist SM, Veldhuis JD. Estradiol, but not testosterone, heightens cortisol-mediated negative feedback on pulsatile ACTH secretion and ACTH approximate entropy in unstressed older men and women. Am J Physiol Regul Integr Comp Physiol. 2014;306(9):R627–R635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salata RA, Jarrett DB, Verbalis JG, Robinson AG. Vasopressin stimulation of adrenocorticotropin hormone (ACTH) in humans. In vivo bioassay of corticotropin-releasing factor (CRF) which provides evidence for CRF mediation of the diurnal rhythm of ACTH. J Clin Invest. 1988;81(3):766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulte HM, Chrousos GP, Gold PW, Booth JD, Oldfield EH, Cutler GB Jr, Loriaux DL. Continuous administration of synthetic ovine corticotropin-releasing factor in man. Physiological and pathophysiological implications. J Clin Invest. 1985;75(6):1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBold CR, Sheldon WR, DeCherney GS, Jackson RV, Alexander AN, Vale W, Rivier J, Orth DN. Arginine vasopressin potentiates adrenocorticotropin release induced by ovine corticotropin-releasing factor. J Clin Invest. 1984;73(2):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–384. [DOI] [PubMed] [Google Scholar]
- 19.Singh RJ. Validation of a high throughput method for serum/plasma testosterone using liquid chromatography tandem mass spectrometry (LC-MS/MS). Steroids. 2008;73(13):1339–1344. [DOI] [PubMed] [Google Scholar]
- 20.Liu PY, Keenan DM, Kok P, Padmanabhan V, O’Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297(2):E538–E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe C, Barkan A, Johnson ML, Pincus S. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R721–R729. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39(3):787–794. [PubMed] [Google Scholar]
- 23.Watanabe T, Orth DN. Detailed kinetic analysis of adrenocorticotropin secretion by dispersed rat anterior pituitary cells in a microperifusion system: effects of ovine corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1987;121(3):1133–1145. [DOI] [PubMed] [Google Scholar]
- 24.Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277(1688):1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian X, Droste SK, Lightman SL, Reul JM, Linthorst AC. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153(9):4346–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149(7):3244–3253. [DOI] [PubMed] [Google Scholar]
- 27.Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22(10):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144(2):311–321. [DOI] [PubMed] [Google Scholar]
- 29.Kirschbaum C, Schommer N, Federenko I, Gaab J, Neumann O, Oellers M, Rohleder N, Untiedt A, Hanker J, Pirke KM, Hellhammer DH. Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. J Clin Endocrinol Metab. 1996;81(10):3639–3643. [DOI] [PubMed] [Google Scholar]
- 30.Altemus M, Redwine L, Leong YM, Yoshikawa T, Yehuda R, Detera-Wadleigh S, Murphy DL. Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor mRNA expression in the luteal phase of the menstrual cycle. Neuropsychopharmacology. 1997;17(2):100–109. [DOI] [PubMed] [Google Scholar]
- 31.Roelfsema F, Aoun P, Veldhuis JD. Pulsatile cortisol feedback on ACTH secretion is mediated by the glucocorticoid receptor and modulated by gender. J Clin Endocrinol Metab. 2016;101(11):4094–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]