Abstract
Context:
The effectiveness of pulsatile gonadotropin-releasing hormone (GnRH) therapy in patients with congenital combined pituitary hormone deficiency (CCPHD) has not been investigated because of the limited number of patients, as well as these patients’ presumed pituitary hypoplasia, poor gonadotrophic cell reserve, and impaired gonadotrophic response to GnRH.
Objective:
To assess the pituitary response to pulsatile GnRH therapy in men with CCPHD.
Design:
Prospective, self-controlled, 3-month clinical trial.
Settings:
University endocrine clinic.
Patients:
Men with hypogonadotropic hypogonadism caused by CCPHD.
Intervention:
Pulsatile GnRH was administered subcutaneously for 3 months.
Main outcome measures:
Primary endpoints were total serum testosterone, testicular volume, and luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels. Secondary endpoints included occurrence of spermatogenesis.
Results:
A total of 40 men with CCPHD completed the study. Of these, 60% (24 of 40) showed a good response to pulsatile GnRH treatment (response group). At 3 months, their LH and FSH levels increased to within the normal range and their testosterone levels increased to 8.67 ± 4.83 nmol/L. Of the patients in the response group, 33.3% (8 of 24) of them achieved spermatogenesis. The remaining 40% (16 of 40) of patients had a poor response to pulsatile GnRH treatment. Magnetic resonance imaging (MRI) did not reveal any correlation between pituitary response and pituitary height and/or integrity of the pituitary stalk.
Conclusions:
This study suggests that gonadotrophs in patients with CCPHD can exist and be functional—even with MRI evidence of pituitary hypoplasia or dysplasia. Pulsatile GnRH therapy restored pituitary–testis axis function in 60% of patients with CCPHD. These results may directly guide the clinical therapeutic choice.
This study found pulsatile gonadotropin-releasing hormone therapy restored pituitary-testis axis function in 60% of male patients with congenital combined pituitary hormone deficiency.
Congenital combined pituitary hormone deficiency (CCPHD) is a disease that features short stature and absence of puberty (1–3). Many patients with CCPHD also show absence of the pituitary stalk, also referred to as pituitary stalk interruption syndrome (PSIS). PSIS is a developmental defect characterized by a triad of morphologic anomalies that can be visualized by pituitary magnetic resonance imaging (MRI), including an absent or thin pituitary stalk, pituitary hypoplasia, and/or ectopic neurohypophysis (4, 5). Furthermore, CCPHD has been associated with other midline malformations, such as a cleft lip and palate, short cervical spine, and/or limited neck rotation (2, 6).
Despite its clinical presence, the mechanisms underlying CCPHD have yet to be fully determined. Several environmental and genetic factors have been implicated. For instance, a high incidence of adverse perinatal events (e.g., breech presentation) in patients with CCPHD suggested that mechanical injury may contribute to breakage of the pituitary stalk and subsequent CCPHD (4). Currently, data derived predominantly from familial cases and patients with specific morphologic anomalies associated with PSIS support the view that PSIS may be caused by genetic aberrations (7–10). However, genetic mutations have been identified in only <5% of patients with PSIS (1, 2, 9, 11, 12), leaving the cause for most patients with this disease unresolved.
Given the underlying pituitary abnormalities, it should come as no surprise that patients with CCPHD often present with multiple pituitary hormone deficiencies. Gonadotropin deficiency occurs in approximately 76.9% to 100% of patients (1, 13, 14), and sex hormone replacement is the primary form of therapy. If a male patient desires fertility, combined gonadotropin therapy is required for induction of spermatogenesis (15).
Pulsatile administration of gonadotropin-releasing hormone (GnRH) is effective in activating the pituitary–gonadal axis. This approach can result in induction of spermatogenesis in approximately 75% of patients with congenital hypogonadotropic hypogonadism who have isolated GnRH and gonadotropin deficiencies (16–18). The precondition for GnRH therapy is the existence of a normal gonadotrophic cell reservoir in the anterior pituitary gland. Given this, pulsatile GnRH therapy has been presumed to be ineffective in patients with CCPHD; given the hypoplastic or dysplastic pituitary glands in these patients, it has been assumed that no or few gonadotrophs are present.
In this prospective study, we sought to formally evaluate the effectiveness of pulsatile GnRH therapy in male patients with CCPHD. The primary end points were serum gonadotropins, total testosterone levels, and testicular volumes after 3 months of pulsatile GnRH treatment. A secondary end point was the occurrence of spermatogenesis. In contrast to conventional expectations, our study showed that 60% of patients with CCPHD responded well to pulsatile GnRH infusion.
Patients and Methods
This longitudinal, prospective, self-controlled, investigator-initiated clinical trial included 40 male patients with CCPHD. They were recruited from an existing CCPHD population at Peking Union Medical College Hospital between November 2015 and June 2016. Patients were selected according to the following criteria: (1) absent or incomplete pubertal development by 18 years of age; (2) multiple (at least two) anterior pituitary hormone deficiencies, including gonadotropin deficiencies; and (3) sellar MRI showing (i) an absent, thin, or interrupted pituitary stalk, (ii) pituitary hypoplasia, and/or (iii) ectopic posterior pituitary.
Exclusion criteria were (1) tumor, surgery, and/or radiation in the sellar region; (2) traumatic brain injury; (3) severe systemic disease; (4) testosterone replacement therapy within the past 6 weeks or gonadotropin injection within the past 6 months; and (5) history of cryptorchidism.
Baseline clinical and biochemical evaluation
A detailed medical history was taken upon each patient’s first visit to the hospital. Information gathered included labor and delivery conditions, gestational age, neonatal hypoxemia, micropenis, cryptorchidism, and presence of other malformations.
Complete evaluation of the anterior pituitary function was performed in all patients at the time of initial diagnosis and was repeated during follow-up as indicated.
Growth hormone (GH) deficiency was defined as peak GH level less than 10 µg/L after two pharmacologic stimulation tests (insulin-induced hypoglycemic GH stimulation test and levo-dopa GH stimulation test) (19). Thyroid-stimulating hormone (TSH) deficiency was defined as a low serum free thyroxine level (<1.04 ng/dL) associated with an inappropriately low or normal serum TSH level (normal range, 0.38 to 4.34 mIU/L). Adrenocorticotropic hormone (ACTH) deficiency was defined as a morning basal cortisol (8 am) value of less than 138 nmol/L associated with an inappropriately low or normal ACTH level (20). Gonadotropin deficiency was defined as delayed or absent pubertal development with a low serum testosterone level (<3.47 nmol/L) associated with inappropriately low or normal luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels. CCPHD was defined as the presence of hormone deficiency affecting at least two anterior pituitary hormone lineages. The absence of polyuria (urinary volume < 3 L) and a urinary osmolality above 600 mOsm/L in a morning urine sample defined normal posterior pituitary function (21).
MRI
Pituitary MRI was performed with sagittal and coronal T1-weighted sequences and axial T2-weighted sequences. The pituitary height was the perpendicular measurement giving the greatest distance between the base and top of the gland on a sagittal T1-weighted image. The pituitary stalk was considered "interrupted" when it was not visible over its entire length and "thin" when its diameter was below 2 mm with a very spindly appearance. The pituitary stalk was considered "absent" when it was not visible at all. The precise location and presence of the posterior pituitary bright spot were also established by using MRI. Any other malformations and/or midline abnormalities present were also recorded.
Interventions
Pulsatile GnRH (Fengyuan Pharmaceutical Co., Anhui Province, China) was administered subcutaneously via a portable infusion pump (Weichuang Medical Science Co., Shanghai, China) for at least 3 months. The GnRH dose was chosen on the basis of the results of previous studies among patients with congenital hypogonadotropic hypogonadism (22). Participants were evaluated at baseline at Peking Union Medical College Hospital, with follow-up assessments at 1 and 3 months. Adherence was assessed at each visit by participant report. In each patient, the GnRH dose was initiated at 10 µg/90 minutes and was progressively adjusted to maintain serum testosterone levels of 6.94 to 17.35 nmol/L (200 to 500 ng/dL). The maximum GnRH dose was set at 15 µg/90 minutes. The dose of glucocorticoids and levothyroxine was adjusted according to hormonal levels and clinical response.
Outcome measurements
At each visit, stage of pubertal development was evaluated according to Tanner stage criteria (23). Testicular volume was measured by using a Prader orchidometer, and the mean value of bilateral testicular volumes was used in all subsequent data analysis. A morning fasting blood sample was taken to measure total serum testosterone, thyroid function, and LH and FSH levels. For patients who were able to ejaculate, a standard seminal fluid analysis was conducted (24). Sperm appearance was defined as at least one sperm in the semen.
Laboratory analysis
GH, insulin-like growth factor 1, plasma free triiodothyronine, free thyroxine, TSH, prolactin, cortisol (at 8:00 am), and ACTH (at 8:00 am) were measured by chemiluminescent methods. FSH, LH, and total testosterone were measured with commercial chemiluminescence kits (ACS:180; Automatic Chemiluminescence Systems, Bayer, Germany). The intra- and interassay variation coefficients were 3.9% and 4.5% for FSH, 2.3% and 2.8% for LH, and 5.6% and 6.6% for total testosterone, respectively.
Ethical statement
The study protocol was reviewed and approved by the ethics committee of the Peking Union Medical College Hospital in China. Written, informed consent was obtained from all participants.
Statistical analysis
SPSS software, version 19.0 (SPSS Inc., Chicago, IL), was used for data analysis. Normally distributed data were described as the mean ± standard deviation, and data not normally distributed were expressed as median (quartiles). Wilcoxon matched-paired signed-ranks test was performed to analyze differences in testicular volume and serum FSH, LH, and testosterone level in the response group and poor-response group before and after therapy. The independent sample t test and Mann–Whitney test were used to compare values between two subgroups (response and poor-response groups). The rates of each pituitary hormone deficiency and pituitary stalk visibility between the different groups were compared by using a Fisher exact test. Binary logistic regression analysis was used to determine significant factors in affecting the hormonal response to pulsatile GnRH therapy. For all comparisons, P values < 0.05 were considered to indicate statistically significant differences.
Results
Characteristics of patients with CCPHD
In total, 44 patients were eligible as per our inclusion criteria and enrolled in the study. Four patients withdrew from the study for personal reasons (two were unable to return for follow-up visits and two felt that using the pump at work would be an inconvenience). Data from these patients were not included for analysis.
During the study period, the average age was 25.5 ± 4.5 years (n = 40). All patients were initially referred for growth retardation during childhood and diagnosed with GH deficiency, and thus previous GH treatment had been administered to all the patients to stimulate growth. In addition, no patients had spontaneous pubertal development. Testosterone or gonadotropins were previously administered intermittently to 29 of 40 patients, before this study. Furthermore, all cases were sporadic. Neither consanguineous parents nor a family history of CCPHD was documented.
On physical examination, all had no or incomplete pubertal development. Other extrapituitary anatomic anomalies, such as retinal dystrophy, anophthalmia or microphthalmia, short cervical spine, or limited neck rotation, were not present in any patients.
Anterior pituitary hormone deficiency
At first assessment, both GH and gonadotropin deficiencies were present in 100% of cases; TSH deficiency, in 85%; ACTH deficiency, in 50%; and diabetes insipidus, in 5% (Table 1; Supplemental Table 1 (33.1KB, docx) ).
Table 1.
Baseline Clinical Characteristics and Radiologic Features in 40 Patients With CCPHD
| Baseline Characteristics | Total Patients With CCPHD (n = 40) | Response Group (n = 24) | Poor-Response Group (n = 16) | P Value |
|---|---|---|---|---|
| Age (y) | 25.5 ± 4.5 | 25.5 ± 4.8 | 25.5 ± 4.1 | 0.977 |
| Height (cm) | 170.4 ± 5.8 | 169.6 ± 5.4 | 171.5 ± 6.5 | 0.322 |
| Weight (kg) | 66.9 ± 11.5 | 64.2 ± 10.6 | 71.0 ± 11.9 | 0.065 |
| BMI (kg/m2) | 23.1 ± 3.9 | 22.4 ± 3.6 | 24.2 ± 4.3 | 0.168 |
| Perinatal complications (%) | 100 | 100 | 100 | |
| Pituitary hormone deficiency | ||||
| GH deficiency | 100 (40/40) | 100 | 100 | |
| Gonadotropin deficiency | 100 (40/40) | 100 | 100 | |
| TSH deficiency | 85 (34/40) | 75 (18/24) | 100 (16/16) | 0.064 |
| ACTH deficiency | 50 (20/40) | 37.5 (9/24) | 68.8 (11/16) | 0.105 |
| ADH deficiency | 5 (2/40) | 4.2 (1/24) | 6.3 (1/16) | 1.0 |
| MRI findings | ||||
| Pituitary stalk | ||||
| Not visible | 45 (18/40) | 37.5(9/24) | 56.3 (9/16) | 0.335 |
| Slim stalk | 37.5 (15/40) | 41.7(10/24) | 31.2 (5/16) | 0.740 |
| Interrupted | 17.5 (7/40) | 20.8(5/24) | 12.5 (2/16) | 0.681 |
| Pituitary height (mm) | 2.3 ± 0.9 | 2.2 ± 0.8 | 2.4 ± 1.0 | 0.334 |
| Posterior pituitary | ||||
| Ectopic | 90 (36/40) | 91.7 (22/24) | 87.5 (14/16) | 1.0 |
| Not visible | 10 (4/40) | 8.3 (2/24) | 12.5 (2/16) | 1.0 |
Values expressed with a plus/minus sign are the mean ± standard deviation. Unless otherwise noted, values are the percentage of patients, with numbers of patients in parentheses. The difference between response and poor-response groups was compared; P < 0.05 indicates statistically significant differences.
Abbreviation: ADH, antidiuretic hormone.
After 3 months of treatment, the mean pulsatile GnRH dose was 11.4 ± 1.7 µg/90 minutes (n = 40). The mean levothyroxine dose was 86.0 ± 25.5 µg/d (n = 34). For glucocorticoid substitution, the mean hydrocortisone dose was 13.5 ± 2.4 mg/d (n = 20). During the study, 18 of 40 patients (8 patients in the response group and 10 in the poor-response group) were administered subcutaneous recombinant human GH (0.4 mg nightly).
Perinatal complications
All patients had perinatal complications, including breech delivery in 38 cases, cesarean delivery due to prolonged labor duration in 1 case, and premature delivery in 1 case. In addition, neonatal asphyxia was reported in 8 cases and intracranial hemorrhage in three cases.
Sellar MRI
Pituitary MRI findings in all patients were consistent with PSIS (Supplemental Fig. 1 (7.5MB, tif) ). MRI revealed that 100% had anterior pituitary hypoplasia, 45% (18 of 40) had complete absence of the pituitary stalk, 37.5% (15 of 40) had a thin pituitary stalk, and 17.5% (7 of 40) had an interrupted pituitary stalk. Of these patients, 90% (36 of 40) had an ectopic posterior pituitary bright spot and 10% (4 of 40) had a complete absence of the posterior pituitary bright spot on T1 phase MRI images. Moreover, extrapituitary abnormalities were revealed in 2 patients: One had Dyke–Davidoff–Masson syndrome (patient 20; Supplemental Table 1 (33.1KB, docx) ) and the other had corpus callosum dysplasia (patient 29; Supplemental Table 1 (33.1KB, docx) ).
Hormonal response to pulsatile GnRH therapy
According to serum gonadotropin and testosterone levels at 3 months, patients were divided into two groups: response group (n = 24) and poor-response group (n = 16). The response group was defined as patients having a substantial increase in testosterone levels compared with baseline and LH levels within the normal range (1.24 to 8.62 IU/L). Patients in the poor-response group were defined as having no substantial increase in testosterone levels and LH below or within the normal range (Table 2).
Table 2.
Comparison of Hormone Levels, Testicular Volume, and Spermatogenesis Between the Response Group and Poor-Response Group
| Variable | Response Group (n = 24) | Poor-Response Group (n = 16) | P Value |
|---|---|---|---|
| Baseline | |||
| LH (IU/L) | 0.48 ± 0.62 | 0.19 ± 0.29 | 0.199 |
| FSH (IU/L) | 1.38 ± 1.61 | 0.53 ± 0.50 | 0.032a |
| T (nmol/L) | 0.29 ± 0.45 | 0 (0.0) | 0.018a |
| TV (mL) | 3.3 ± 2.9 | 1.9 ± 0.7 | 0.032a |
| At 3 mo | |||
| LH (IU/L) | 6.01 ± 2.61 | 1.09 ± 0.77 | 0.000a |
| FSH (IU/L) | 9.33 ± 5.76 | 3.19 ± 1.75 | 0.000a |
| T (nmol/L) | 8.67 ± 4.83 | 0 (0.0) | 0.000a |
| TV (mL) | 6.0 ± 2.9 | 2.9 ± 2.0 | 0.001a |
| Spermatogenesis, % (n/n) | 33.3 (8/24) | 0 | |
| GnRH dose (µg/90 min) | 10.3 ± 0.8 | 12.9 ± 1.4 | 0.000a |
Values expressed with a plus/minus sign are the mean ± standard deviation. P < 0.05 indicates statistically significant differences.
Abbreviations: T, testosterone; TV, testicular volume.
Subgroup analysis
Response group (n = 24)
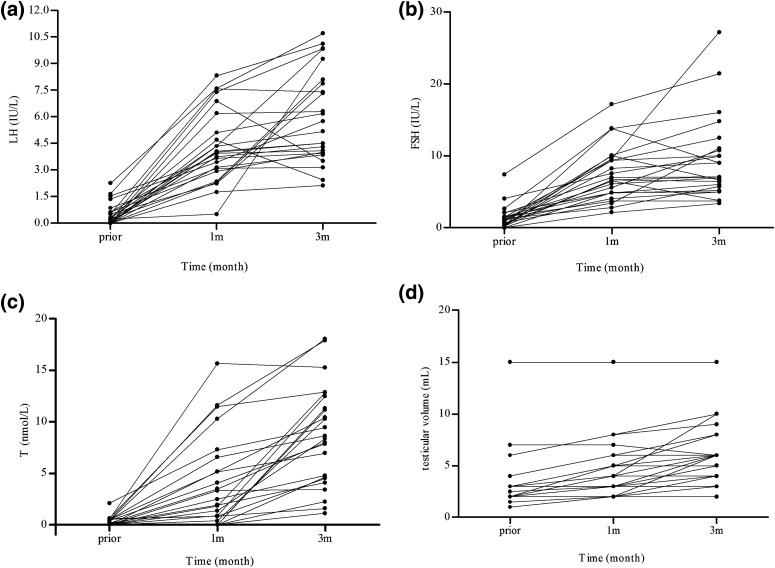
In this group, serum LH and FSH levels increased to within the normal range (Fig. 1). At 3 months, LH levels increased from 0.48 ± 0.62 IU/L to 6.01 ± 2.61 IU/L (P = 0.00002), FSH levels increased from 1.38 ± 1.61 IU/L to 9.33 ± 5.76 IU/L (P = 0.00002), total serum testosterone increased from 0.29 ± 0.45 nmol/L to 8.67 ± 4.83 nmol/L (P = 0.00002), and testicular volume significantly increased from 3.3 ± 2.9 mL to 6.0 ± 2.9 mL (P = 0.00005). During follow-up, 8 of 24 (33.3%) patients had achieved the presence of sperm in seminal fluid samples, and the mean sperm concentration was 5.51 ± 3.36 million/mL at 3 months. The mean pulsatile GnRH dose throughout this period was 10.3 ± 0.8 µg/90 minutes (n = 24).
Figure 1.
(a, b) Serum gonadotropin levels, (c) total testosterone levels, and (d) testicular volume increased during 1 to 3 months of pulsatile GnRH treatment in the response group (n = 24). T, testosterone.
At 1 month, serum LH levels increased to within the normal range in 23 of 24 cases and testosterone levels were significantly increased in 18 of 23 patients. In 5 of 23 patients (patients 6, 25, 34, 37, and 40; Supplemental Table 1 (33.1KB, docx) ), LH levels were 4.34 IU/L, 2.35 IU/L, 3.15 IU/L, 3.44 IU/L, and 4.06 IU/L, and FSH levels were 10.09 IU/L, 5.62 IU/L, 6.71 IU/L, 6.14 IU/L, and 6.64 IU/L, respectively, whereas their testosterone levels were very low and undetectable, unchanged from pretherapy baseline levels. At 3 months, LH and FSH levels were maintained within the normal range and serum testosterone levels increased to 2.22 nmol/L, 4.65 nmol/L, 1.11 nmol/L, 11.14 nmol/L, and 11.3 nmol/L, respectively.
Notably, one patient (patient 4) had very low LH, FSH, and testosterone levels of 0.24 IU/L, 1.26 IU/L, and 0 nmol/L at baseline. During follow-up, his LH, FSH, and testosterone levels had increased to 0.5 IU/L, 2.84 IU/L, and 0.87 nmol/L, respectively, by 1 month and further increased to 9.25 IU/L, 5.79 IU/L, and 12.49 nmol/L by 3 months.
In the response group, 8 of 24 patients received current GH therapy. The spermatogenic rate was 50% (4 of 8) and 25% (4 of 16), respectively (P = 0.363) in the GH and non-GH therapy subgroups.
Poor-response group (n = 16)
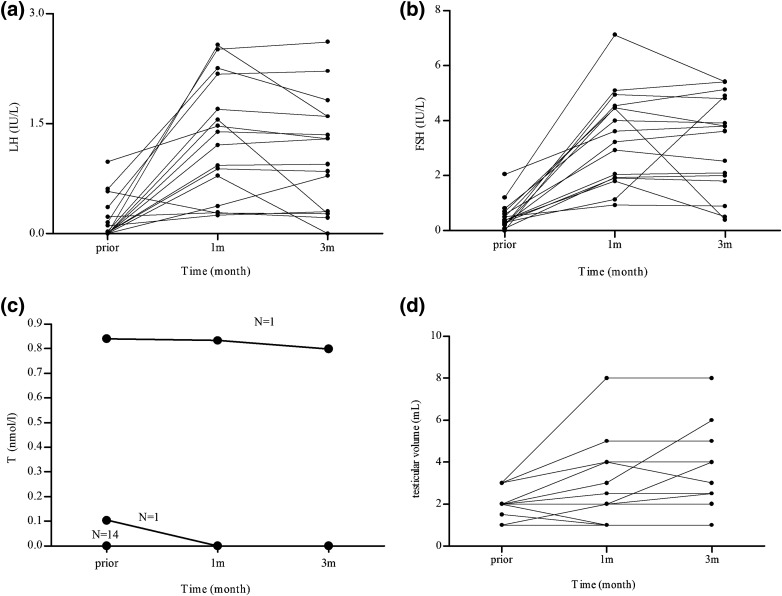
After 3 months, this group showed an increase in LH levels from 0.19 ± 0.29 IU/L to 1.09 ± 0.77 IU/L (P = 0.002) and FSH levels increased from 0.53 ± 0.50 IU/L to 3.19 ± 1.75 IU/L (P = 0.001) (Fig. 2). Although serum testosterone levels remained unchanged and as low as 0 nmol/L after pulsatile GnRH treatment, testicular volume increased slightly from 1.9 ± 0.7 mL to 2.9 ± 2.0 mL (P = 0.018). Of these patients, 50% (8 of 16) had LH levels <1.0 IU/L, indicating pituitary resistance to GnRH therapy or gonadotrophic cell deficiency. The remaining 50% had LH levels >1.0 IU/L, with serum testosterone levels that did not increase. This result suggested that gonadotropin axis function may have been partially activated. It is possible that with a longer treatment duration, the testicular volume may have increased further. The mean pulsatile GnRH dose for this group was 12.9±1.4 µg/90 minutes (n = 16).
Figure 2.
Changes to (a, b) gonadotropins, (c) testosterone, and (d) testicular volume during pulsatile GnRH therapy in the poor-response group (n = 16). In general, gonadotropin levels and testicular volumes tended to increase gradually during the 3 months of treatment. In (c), the number of patients sharing the same testosterone values is indicated above the line. T, testosterone.
Predictive factors
Patients were divided into four subgroups according to their serum LH levels at 1 month. Group 1 was defined as LH ≥ 3.0 IU/L; group 2 as 2.0 ≤ LH < 3.0 IU/L; group 3 as 1.0 ≤ LH < 2.0 IU/L; and group 4 as LH < 1.0 IU/L (Table 3; Supplemental Fig. 2 (107.4KB, tif) ). At 3 months, 100% (18 of 18) of patients in group 1, 50% (4 of 8) of patients in group 2, 16.7% (1 of 6) of patients in group 3, and 12.5% (1 of 8) of patients in group 4 had normal LH and FSH levels and a low-normal to normal range of serum testosterone. Others had a slightly elevated or a below-normal range for LH and FSH levels along with undetectable serum testosterone levels or levels that showed no increase after treatment. Consequently, serum LH levels >3 IU/L at 1 month seemed to suggest a good response to continued pulsatile GnRH therapy, LH levels <2 IU/L at 1 month seemed to suggest a poor response, but those with LH levels between 2 IU/L and 3 IU/L will require longer follow-up times before any definitive conclusions can be made.
Table 3.
Subgroup Analysis According to Serum LH Levels at 1 Month of Treatment
| Variable | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| LH (IU/L) | ≥3.0 | 2.0−3.0 | 1.0−2.0 | <1.0 |
| Cases | 18 | 8 | 6 | 8 |
| Patients with response | 18 | 4 | 1 | 1 |
| LH range (IU/L) | 2.44−10.7 | 4.00−8.11 | 2.13 | 9.25 |
| FSH range (IU/L) | 3.76−27.18 | 6.00−12.49 | 3.38 | 5.79 |
| T range (nmol/L) | 1.11−18.04 | 4.65−12.8 | 7.98 | 12.49 |
| TV range (mL) | 2.0−15.0 | 4.0−8.0 | 6 | 3 |
| Patients with poor response | 0 | 4 | 5 | 7 |
| LH range (IU/L) | 1.60−2.62 | 0.27−1.60 | 0.00−0.95 | |
| FSH range (IU/L) | 3.62−5.41 | 2.53−5.40 | 0.40−4.90 | |
| T range (nmol/L) | 0.0−0.0 | 0.0−0.0 | 0.0−0.8 | |
| TV range (mL) | 2.5−6.0 | 1.0−8.0 | 1.0−4.0 |
In the table, all ranges are the results of 3-month pulsatile GnRH treatment.
Abbreviations: T, testosterone; TV, testicular volume.
Finally, we used binary logistic analysis and determined that pituitary height (P = 0.308), MRI pituitary stalk visibility (P = 0.619), presence of other pituitary hormone deficiencies (P = 0.196), baseline LH levels (P = 0.779), and baseline testicular volume (P = 0.202) were not predictive for response to pulsatile GnRH therapy (Table 4).
Table 4.
Predictors for Responses to Pulsatile GnRH Therapy (Binary Logistic Analysis)
| Factor | β | P Value | 95% CI Lower Bound | 95% CI Upper Bound |
|---|---|---|---|---|
| Pituitary height | −0.457 | 0.308 | 0.263 | 1.524 |
| Pituitary stalk visibility | 0.268 | 0.619 | 0.455 | 3.760 |
| Pituitary hormone deficiencies | −1.076 | 0.196 | 0.067 | 1.742 |
| Baseline serum LH level | 0.356 | 0.779 | 0.119 | 17.186 |
| Baseline testicular volume | 0.526 | 0.202 | 0.754 | 3.792 |
P < 0.05 indicates statistically significant differences.
Genetic screening
Genomic DNA was extracted from peripheral-blood leukocytes of 25 of 40 participants (Supplemental Table 1 (33.1KB, docx) ) using the QIAGEN Mini Blood kit according to the manufacturer’s instructions. All exonic and proximal intronic (minimum 10 bp from the splice site) DNA sequences of 85 candidate genes involved in pituitary development were screened by targeted next-generation sequencing. Gene mutations in PROP1, HESX1, POU1F1, LHX3, LHX4, OTX2, PITX2, SOX2, SOX3, TBX19 and GPR161 (Supplemental Table 2 (14KB, docx) ), which were previously reported in patients with CCPHD, were not detected in any of the 25 patients studied. Ongoing work in our research group is addressing the contribution of the other possible genes to CCPHD. Therefore, the relationship between gene mutations and the response to GnRH therapy could not be clarified.
Discussion
This study focused on male patients with CCPHD and found that 60% of them responded to GnRH therapy. This surprising finding suggests the existence of a gonadotrophic cell reservoir in the pituitary gland of these patients, despite their hypogonadotropic hypogonadism. This large study that has revealed the effectiveness of pulsatile GnRH therapy in restoring pituitary–gonadal axis function in patients with CCPHD.
Previous studies have shown that pulsatile GnRH therapy is effective in inducing virilization and spermatogenesis in patients with isolated congenital hypogonadotropic hypogonadism (16, 17, 25). Moreover, some case reports have demonstrated effectiveness of pulsatile GnRH therapy in patients with acquired hypopituitarism caused by pituitary tumor resection or cranial radiotherapy (26). An earlier study included six patients with CCPHD and used 36 hours of pulsatile GnRH stimulation. These results showed a rise in both LH and FSH levels (median LH increased from 1.6 IU/L to 2.55 IU/L, and median FSH increased from 1.4 IU/L to 2.95 IU/L) but no change in serum testosterone levels (27). These limited studies led to the following hypothesis: If some gonadotrophic cells survived in the damaged pituitary, they may be responsive to pulsatile GnRH therapy. Our study added support for this hypothesis through use of a larger sample of patients with CCPHD.
In the response group, LH and FSH serum levels increased to within the normal range. Furthermore, testosterone increased to the low-normal or normal range after 3 months of treatment. Five cases showed increased LH and FSH levels to within the normal range after 1 month of therapy. However, their testosterone levels did not increase significantly even after 3 months of treatment. These findings indicated that testosterone increases tend to lag behind the increase seen in gonadotropin levels. This phenomenon has been observed during the progression of normal pubertal development. During this process, LH increases at the very beginning of puberty onset, with testosterone levels showing a delayed, more gradual increase (28, 29). This phenomenon suggests that pulsatile GnRH therapy may mimic a pubertal developmental course, thus making it a more physiologic therapy. Similar phenomena have also been observed in patients with congenital hypogonadotropic hypogonadism (17) and with hypopituitarism (26) treated with pulsatile GnRH.
How long should we treat patients with pulsatile GnRH before we declare its effectiveness (or failure)? For patients with isolated congenital hypogonadotropic hypogonadism, gonadotropins increase within 1 to 7 days after pulsatile GnRH therapy (18) and testosterone increases in 1 to 3 months. For patients with CCPHD, it seems that a longer time is required for reactivation of the gonadal axis. Our data have shown that, between 1 and 3 months of treatment, five additional patients became responsive. This suggests that 1 month of treatment may not be a sufficient period to identify all patients who will respond well. Furthermore, in the poor-response group, both LH and FSH levels and testicular size tended to continue to increase during the 3 months of treatment (Fig. 2). It can be speculated that with a longer treatment duration, some patients in the poor-response group might achieve the ability to produce sufficient testosterone and achieve spermatogenesis. Therefore, clinicians and researchers should be prudent in their evaluation of the efficacy of pulsatile GnRH treatment in patients with CCPHD, particularly if their LH levels, FSH levels, and/or testicular volumes are progressively increasing.
In our study, 8 of 24 patients (in the response group) achieved spermatogenesis after 3 months of treatment. This result revealed that pulsatile GnRH was effective at inducing spermatogenesis in at least some patients with CCPHD. Our previous study showed that spermatogenesis could be successfully induced in 100% of patients with CCPHD after 24 months of combined gonadotrophin therapy; the mean time until first sperm appearance in seminal fluid was 10.4 ± 3.8 months (15). Although head-to-head comparison studies are lacking, it appears that pulsatile GnRH therapy may be equal or superior to combined gonadotropin therapy, at least in some patients, because the former can produce more physiologic levels of gonadotropins. Moreover, we did not find that higher serum testosterone levels during treatment were associated with better spermatogenesis. In this series, the 8 patients who had the appearance of sperm in their semen after 3 months of treatment had serum testosterone levels of 4.09 to 18.04 nmol/L. Interestingly, this mimics normal pubertal development, in which adolescents with testicular size greater than 4 mL may have the appearance of sperm in the ejaculate or urine, regardless of their testosterone levels (30).
In our patient population, 40% responded poorly to GnRH therapy after 3 months of treatment. In this poor-response group, 50% (8 of 16) had LH levels <1 IU/L, indicating pituitary resistance to pulsatile GnRH or gonadotrophic cell deficiency. The remaining 50% had LH levels >1 IU/L, with low testosterone levels, indicating insufficient gonadotropin secretion, testicular impairment, or short treatment duration. It is possible that with a longer treatment time, serum gonadotropin and testosterone levels in addition to testicular volume may increase further. The varied response to GnRH therapy may relate to the severity of pituitary gonadotrope impairment, or gonadal responses (16), depending on the nature of the etiology/pathophysiology.
Caution should still be taken with these results in that a hypothesized pituitary gonadotroph reservoir is difficult to evaluate by using short periods of pulsatile GnRH therapy. Because the remaining GnRH receptors on gonadotrophic cells may be downregulated because of a lack of trophic stimulation, the initial administration of pulsatile GnRH would produce a priming effect on gonadotrophic cells before actual, functional activation of the hypothalamic–pituitary–testis axis. As expected, our study showed that LH and FSH levels slowly and progressively increased during the first month of treatment, after which they were maintained at a relatively normal level. Similar phenomena have also been observed in patients with isolated congenital hypogonadotropic hypogonadism (17). It seems that longer time periods are required for gonadotrophic cells to synchronize in order to produce gonadotropins in patients with CCPHD.
The mechanism of hormonal response to pulsatile GnRH therapy may involve two components. First, there is a gonadotrophic cell reserve in the pituitary. As other work has shown, anterior pituitary gland function can remain “normal” even after most of the gland has been destroyed, as in Sheehan syndrome or empty sella syndrome. For patients with CCPHD, although the pituitary gland looks dysplastic on MRI examination, gonadotrophic cells may still remain that can respond to stimulation by pulsatile GnRH. Second, gonadotroph cell regeneration from the pluripotent stem cells may play an important role. Stem/progenitor cells have been identified in the pituitary gland (31). Recent studies have shown that SOX2- and SOX9-expressing progenitors can differentiate into the appropriate endocrine cell types in response to physiologic stress (32). Although the cell turnover rate in the pituitary is as low as 1.58% per day, the number of hormone-producing cells could change according to physiologic demands. For example, the number of prolactin-producing cells may increase during pregnancy and lactation (33). Therefore, it is expected that gonadotroph regeneration may partly contribute to the increased gonadotropin production after 3 months of treatment. Regardless, our results have shown that a gonadotroph cell reservoir is sufficiently activated by pulsatile GnRH therapy—despite pituitary hypoplasia.
We attempted to identify markers that could predict treatment outcomes with pulsatile GnRH therapy. All patients with LH levels greater than 3.0 IU/L (n = 18) after 1 month of treatment were in the response group and 85.7% of patients with LH levels <2.0 IU/L (n = 14) after 1 month of treatment were in the poor-response group. These results indicate that a higher level of LH at the 1-month treatment time point was a favorable predictor for later outcomes. We found that neither pituitary height nor stalk visibility was correlated with treatment outcome. In addition, no pathogenic gene mutations were identified in our patients. This therefore makes it difficult to predict outcomes by genotype.
In this study, the prevalence of perinatal complications (e.g., breech delivery) was 100%. These data are much higher than in previous reports (1, 2, 13, 34, 35), suggesting a close relationship between breech delivery and CCPDH. However, a causal role of breech delivery in CCPDH has not been well established. Mutations in some genes, such as PROP1, HESX1, POU1F1, LHX3, and LHX4, have been identified in <5% patients with CCPHD, suggesting a genetic cause. We did not identify any pathogenic mutations in the aforementioned genes in our cohort. Although it remains possible that unidentified genetic defects contributed to CCPHD in some (or all) of the patients in this study, we did find a clear association with perinatal complications in all studied patients. Given this, environmental factors probably play key roles in the development of CCPHD. However, additional study is needed to further evaluate the underlying, causal mechanisms.
The main advantage of our study was its large sample size, with few confounding factors. Participants all presented with similar clinical and MRI manifestations, including hypopituitarism, pituitary dysplasia, and stalk interruption. This large study investigated the effect of pulsatile GnRH in patients with CCPHD. Some limitations should be briefly mentioned. First, if a longer treatment period were used, results might show more patients who are responsive to pulsatile GnRH therapy, ultimately resulting in successful sperm production. Second, the GnRH dose in this study was based on work done in patients with congenital hypogonadotropic hypogonadism (22) in which the maximum dose was approximately 200 ng/kg. It has been reported that for some female patients with congenital hypogonadotropic hypogonadism, further increases of GnRH doses to 250 ng/kg may stimulate gonadotropin secretion (36). Thus, it is possible that further increases in the GnRH dose may overcome pituitary resistance and result in more patients being responsive to pulsatile GnRH therapy.
In conclusion, pulsatile GnRH therapy restored pituitary–testis axis function in approximately 60% of patients with CCPHD. This study provides insight into CCPDH, in that gonadotroph cell reservoirs may remain in many patients, despite pituitary aplasia or dysplasia.
Acknowledgments
This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences Grant 2016-12M-1-002, National Key Research and Development Program of China Grant 2016YFC0905102, and National Institutes of Health Grants R01 HD019938 and HD082314.
Clinical trial registry: ClinicalTrials.gov no. NCT02705014 (registered 22 February 2016).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- CCPHD
- congenital combined pituitary hormone deficiency
- FSH
- follicle-stimulating hormone
- GH
- growth hormone
- GnRH
- gonadotropin-releasing hormone
- LH
- luteinizing hormone
- MRI
- magnetic resonance imaging
- PSIS
- pituitary stalk interruption syndrome
- TSH
- thyroid-stimulating hormone.
References
- 1.Fernandez-Rodriguez E, Quinteiro C, Barreiro J, Marazuela M, Pereiro I, Peinó R, Cabezas-Agrícola JM, Dominguez F, Casanueva FF, Bernabeu I. Pituitary stalk dysgenesis-induced hypopituitarism in adult patients: prevalence, evolution of hormone dysfunction and genetic analysis. Neuroendocrinology. 2011;93(3):181–188. [DOI] [PubMed] [Google Scholar]
- 2.Reynaud R, Albarel F, Saveanu A, Kaffel N, Castinetti F, Lecomte P, Brauner R, Simonin G, Gaudart J, Carmona E, Enjalbert A, Barlier A, Brue T. Pituitary stalk interruption syndrome in 83 patients: novel HESX1 mutation and severe hormonal prognosis in malformative forms. Eur J Endocrinol. 2011;164(4):457–465. [DOI] [PubMed] [Google Scholar]
- 3.Tsai SL, Laffan E, Lawrence S. A retrospective review of pituitary MRI findings in children on growth hormone therapy. Pediatr Radiol. 2012;42(7):799–804. [DOI] [PubMed] [Google Scholar]
- 4.Triulzi F, Scotti G, di Natale B, Pellini C, Lukezic M, Scognamiglio M, Chiumello G. Evidence of a congenital midline brain anomaly in pituitary dwarfs: a magnetic resonance imaging study in 101 patients. Pediatrics. 1994;93(3):409–416. [PubMed] [Google Scholar]
- 5.Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, Minami S, Sagoh T, Hiraoka T, Momoi T. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165(2):487–489. [DOI] [PubMed] [Google Scholar]
- 6.Bar C, Zadro C, Diene G, Oliver I, Pienkowski C, Jouret B, Cartault A, Ajaltouni Z, Salles JP, Sevely A, Tauber M, Edouard T. Pituitary stalk interruption syndrome from infancy to adulthood: clinical, hormonal, and radiological assessment according to the initial presentation. PLoS One. 2015;10(11):e0142354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karaca E, Buyukkaya R, Pehlivan D, Charng WL, Yaykasli KO, Bayram Y, Gambin T, Withers M, Atik MM, Arslanoglu I, Bolu S, Erdin S, Buyukkaya A, Yaykasli E, Jhangiani SN, Muzny DM, Gibbs RA, Lupski JR. Whole-exome sequencing identifies homozygous GPR161 mutation in a family with pituitary stalk interruption syndrome. J Clin Endocrinol Metab. 2015;100(1):E140–E147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macchiaroli A, Kelberman D, Auriemma RS, Drury S, Islam L, Giangiobbe S, Ironi G, Lench N, Sowden JC, Colao A, Pivonello R, Cavallo L, Gasperi M, Faienza MF. A novel heterozygous SOX2 mutation causing congenital bilateral anophthalmia, hypogonadotropic hypogonadism and growth hormone deficiency. Gene. 2014;534(2):282–285. [DOI] [PubMed] [Google Scholar]
- 9.Reynaud R, Jayakody SA, Monnier C, Saveanu A, Bouligand J, Guedj AM, Simonin G, Lecomte P, Barlier A, Rondard P, Martinez-Barbera JP, Guiochon-Mantel A, Brue T. PROKR2 variants in multiple hypopituitarism with pituitary stalk interruption. J Clin Endocrinol Metab. 2012;97(6):E1068–E1073. [DOI] [PubMed] [Google Scholar]
- 10.Ziemnicka K, Budny B, Drobnik K, Baszko-Błaszyk D, Stajgis M, Katulska K, Waśko R, Wrotkowska E, Słomski R, Ruchała M. Two coexisting heterozygous frameshift mutations in PROP1 are responsible for a different phenotype of combined pituitary hormone deficiency. J Appl Genet. 2016;57(3):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rienzo F, Mellone S, Bellone S, Babu D, Fusco I, Prodam F, Petri A, Muniswamy R, De Luca F, Salerno M, Momigliano-Richardi P, Bona G, Giordano M; Italian Study Group on Genetics of CPHD . Frequency of genetic defects in combined pituitary hormone deficiency: a systematic review and analysis of a multicentre Italian cohort. Clin Endocrinol (Oxf). 2015;83(6):849–860. [DOI] [PubMed] [Google Scholar]
- 12.Kim SS, Kim Y, Shin YL, Kim GH, Kim TU, Yoo HW. Clinical characteristics and molecular analysis of PIT1, PROP1,LHX3, and HESX1 in combined pituitary hormone deficiency patients with abnormal pituitary MR imaging. Horm Res. 2003;60(6):277–283. [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Yang Y, Mu Y, Lu J, Pan C, Dou J, Lv Z, Ba J, Wang B, Zou X, Yang L, Ouyang J, Yang G, Wang X, Du J, Gu W, Jin N, Chen K, Zang L, Erickson BJ. Pituitary stalk interruption syndrome in Chinese people: clinical characteristic analysis of 55 cases. PLoS One. 2013;8(1):e53579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottembourg D, Linglart A, Adamsbaum C, Lahlou N, Teinturier C, Bougnères P, Carel JC. Gonadotrophic status in adolescents with pituitary stalk interruption syndrome. Clin Endocrinol (Oxf). 2008;69(1):105–111. [DOI] [PubMed] [Google Scholar]
- 15.Mao J, Xu H, Wang X, Huang B, Liu Z, Zhen J, Nie M, Min L, Wu X. Congenital combined pituitary hormone deficiency patients have better responses to gonadotrophin-induced spermatogenesis than idiopathic hypogonadotropic hypogonadism patients. Hum Reprod. 2015;30(9):2031–2037. [DOI] [PubMed] [Google Scholar]
- 16.Sykiotis GP, Hoang XH, Avbelj M, Hayes FJ, Thambundit A, Dwyer A, Au M, Plummer L, Crowley WF, Jr, Pitteloud N. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab. 2010;95(6):3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF, Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87(9):4128–4136. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman AR, Crowley WF, Jr. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307(20):1237–1241. [DOI] [PubMed] [Google Scholar]
- 19.Growth Hormone Research Society; GH Research Society . Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990–3993. [DOI] [PubMed] [Google Scholar]
- 20.Kazlauskaite R, Evans AT, Villabona CV, Abdu TAM, Ambrosi B, Atkinson AB, Choi CH, Clayton RN, Courtney CH, Gonc EN, Maghnie M, Rose SR, Soule SG, Tordjman K; Consortium for Evaluation of Corticotropin Test in Hypothalamic-Pituitary Adrenal Insufficiency . Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245–4253. [DOI] [PubMed] [Google Scholar]
- 21.Moses AM, Miller M. Osmotic threshold for vasopressin release as determined by saline infusion and by dehydration. Neuroendocrinology. 1971;7(4):219–226. [DOI] [PubMed] [Google Scholar]
- 22.Mao JF, Liu ZX, Nie M, Wang X, Xu HL, Huang BK, Zheng JJ, Min L, Kaiser UB, Wu XY. Pulsatile gonadotropin-releasing hormone therapy is associated with earlier spermatogenesis compared to combined gonadotropin therapy in patients with congenital hypogonadotropic hypogonadism [published online ahead of print December 27, 2016]. Asian J Androl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 25.Christiansen P, Skakkebaek NE. Pulsatile gonadotropin-releasing hormone treatment of men with idiopathic hypogonadotropic hypogonadism. Horm Res. 2002;57(1-2):32–36. [DOI] [PubMed] [Google Scholar]
- 26.Skarin G, Nillius SJ, Ahlsten G, Tuvemo T, Wide L. Induction of male puberty by long-term pulsatile subcutaneous LH-RH therapy. Ups J Med Sci. 1984;89(1):73–80. [DOI] [PubMed] [Google Scholar]
- 27.Partsch CJ, Sippell WG. Short-term pulsatile administration of luteinizing hormone releasing hormone in male adolescents with multiple idiopathic pituitary hormone deficiencies. Horm Res. 1987;25(2):88–96. [DOI] [PubMed] [Google Scholar]
- 28.Albertsson-Wikland K, Rosberg S, Lannering B, Dunkel L, Selstam G, Norjavaara E. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. J Clin Endocrinol Metab. 1997;82(2):541–549. [DOI] [PubMed] [Google Scholar]
- 29.Goji K, Tanikaze S. Spontaneous gonadotropin and testosterone concentration profiles in prepubertal and pubertal boys: temporal relationship between luteinizing hormone and testosterone. Pediatr Res. 1993;34(2):229–236. [DOI] [PubMed] [Google Scholar]
- 30.Zachmann M, Prader A, Kind HP, Häfliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29(1):61–72. [PubMed] [Google Scholar]
- 31.Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, Sasai Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7:10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13(4):419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goluboff LG, Ezrin C. Effect of pregnancy on the somatotroph and the prolactin cell of the human adenohypophysis. J Clin Endocrinol Metab. 1969;29(12):1533–1538. [DOI] [PubMed] [Google Scholar]
- 34.Pham LL, Lemaire P, Harroche A, Souberbielle JC, Brauner R. Pituitary stalk interruption syndrome in 53 postpubertal patients: factors influencing the heterogeneity of its presentation. PLoS One. 2013;8(1):e53189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Guo QH, Wang BA, Dou JT, Lv ZH, Ba JM, Lu JM, Pan CY, Mu YM. Pituitary stalk interruption syndrome in 58 Chinese patients: clinical features and genetic analysis. Clin Endocrinol (Oxf). 2013;79(1):86–92. [DOI] [PubMed] [Google Scholar]
- 36.Seminara SB, Beranova M, Oliveira LM, Martin KA, Crowley WF, Jr, Hall JE. Successful use of pulsatile gonadotropin-releasing hormone (GnRH) for ovulation induction and pregnancy in a patient with GnRH receptor mutations. J Clin Endocrinol Metab. 2000;85(2):556–562. [DOI] [PubMed] [Google Scholar]