Abstract
Pituitary tumors are the second most common adult primary brain tumor, with a variable clinical course. Recent work has identified a number of genetic determinants of pituitary tumor subtypes, which may augment traditional histopathologic classification schemes. We sought to determine whether pituitary tumors could be stratified based on objective molecular characteristics using a clinical genomics assay. We performed a retrospective analysis of patients operated on at the Brigham and Women's Hospital from 2012 to 2016 whose pituitary tumors were profiled using multiplexed next-generation sequencing. We analyzed 127 pituitary tumors, including 114 adenomas, 5 craniopharyngiomas, and 8 tumors of other histologies. We observed recurrent BRAFV600E mutations in papillary craniopharyngiomas, CTNNB1 mutations in adamantinomatous craniopharyngiomas, and activating GNAS mutations in growth hormone–secreting adenomas. Furthermore, we validated the presence of two distinct genomic subclasses in adenomas (i.e., those with disrupted or quiet copy-number profiles) and the significant association of disruption with functional hormone status (P < 0.05). We report the clinical implementation of next-generation sequencing of pituitary tumors. We confirmed previously identified molecular subclasses for these tumors and show that routine screening as part of clinical practice is both feasible and informative. This large-scale proof-of-principle study may help to guide future institutional efforts for pituitary tumor classification as well as the incorporation of such techniques into prospective analysis as part of clinical trials.
We profiled 127 pituitary tumors with a clinical assay, confirming known mutations, uncovering undescribed alterations, and validating stratification of pituitary adenomas by copy-number alterations.
Pituitary tumors are the second most common primary brain tumors in adults and include a spectrum of pathologies, including adenomas, craniopharyngiomas, and granular cell tumors (1). The clinical outcome of patients with pituitary adenomas, in particular, can be difficult to predict. A subset of pituitary adenomas with frequent mitoses, elevated P53 protein expression, and elevated MIB-1 proliferative indices (>3%) have been considered “atypical” due to an association with a potentially more aggressive natural history. Longitudinal follow-up suggests, however, that the presence of atypical features is not consistently predictive of recurrence and poorer outcome (2, 3). New methods of stratifying the behavior of pituitary tumors are therefore needed.
The genomic profile of neoplasms is increasingly recognized as a valuable molecular adjunct in facilitating diagnosis, management, and prognostication (4–6). Without atypical status as a molecular marker to help distinguish adenomas, alternative classification schemes are needed to help guide clinical decision making and accurately stratify patients. Recently, several studies have begun to decode the genomic profile of pituitary tumors and other tumors of the sellar region (7–16). Notably, these efforts have identified targetable mutations such as BRAFV600E in papillary craniopharyngiomas as well as potential molecular classification strategies based on genomic disruption in pituitary adenomas (7, 10, 17).
We sought to examine the genomic profile of a large cohort of pituitary and sellar region tumors from a contemporary consecutive clinical series to determine the ability of a clinically based sequencing assay to differentiate tumor subtypes and to provide the framework for a molecular genetic classification scheme.
Materials and Methods
Sample cohort
All patients with pituitary tumors resected at Brigham and Women’s Hospital who consented to have their tumors profiled were eligible for the study. Informed consent was obtained from all patients included in this study. Patients without sufficient available tissue for extraction of high-quality DNA were excluded. A total of 127 cases of pituitary tumors resected from 2012 to 2016 in the Department of Neurosurgery, Brigham and Women’s Hospital, underwent genotyping within the Brigham and Women’s Hospital Center for Advanced Molecular Diagnostics, a Clinical Laboratory Improvement Amendments–certified laboratory environment. All samples underwent central neuropathology review using World Health Organization criteria (18). Null cell and clinically silent gonadotroph (follicle-stimulating hormone/luteinizing hormone–expressing) tumors were considered nonfunctional adenomas; somatotroph [growth hormone (GH)-expressing], corticotroph [adrenocorticotropic hormone (ACTH)-expressing], and galactroph (prolactin-expressing) adenomas were considered clinically functional. Clinical and imaging data were retrospectively gathered. The designation of typical or atypical status was derived from the pathology reports. The study was conducted after approval from the Dana-Farber/Brigham and Women’s Cancer Center Institutional Review Board.
Genomic analysis
A total of 127 patients with pituitary tumors underwent somatic mutation profiling of 300 canonical cancer-associated genes and 30 intronic regions as part of the Dana-Farber/Brigham and Women’s Cancer Center PROFILE clinical research study (OncoPanel v1 and v2) (19). DNA was isolated from 5-μm formalin-fixed, paraffin-embedded sections (n = 5 to 10) containing at least 50% tumor nuclei. Copy-number analysis was performed using one of two internally developed algorithms (19). Both algorithms, VisCapCancer and RobustCNV, calculate copy number using a normalized read depth of coverage against a panel of normal (noncancer) samples. VisCapCancer compares the tumor sample coverage to a median value from the panel of normal samples. Circular binary segmentation is used to segment the data, and segments are determined via strict thresholding. The algorithm is configurable for different tumor fractions. RobustCNV performs a robust linear regression against the panel of normal samples to calculate copy ratios. Circular binary segmentation is again used to segment the data, and event identification is performed based on the observed variance of the data points. Estimated copy number was calculated using the following published formula: CR = (TC/2) × P + (2/2)(1 − P), where CR is the median copy ratio for all intervals in a gene, TC is the number of copies in the tumor sample, and P is purity (20).
Coverage plots of sequence reads were individually inspected for all samples by two independent reviewers to confirm chromosome-level copy-number variations. Chromosomal gain or loss was defined by a 0.5 or greater log-fold change. Samples with more than two chromosome arm–level gains or losses were scored as disrupted. Rearrangements were called using BreakMer (21). Gene clusters were identified using GeNets (https://apps.broadinstitute.org/genets).
Immunohistochemistry for β-catenin
We performed immunohistochemical studies on 5-μm–thick sections of formalin-fixed, paraffin-embedded tissues using an antibody that recognizes β-catenin (catalog no. 610154, mouse-monoclonal, clone: 14, 1:1000 dilution; BD Pharmigen). We performed antigen retrieval using a pressure cooker with citrate buffer (pH 6.0). Primary antibody was applied for 45 minutes followed by Dako anti-mouse horseradish peroxidase for 30 minutes at room temperature. We generated semiquantitative estimates of the percentage of pituitary tumor cells with nuclear and membranous for each case.
Statistical analysis
All statistical analyses were performed using the R software package (http://www.r-project.org/). Differences in incidence between populations were assessed using Fisher exact test. Differences in means were assessed using a t test or Wilcoxon rank-sum test. A P value of <0.05 was considered significant.
Results
Study cohort
We used next-generation sequencing of a panel of 300 cancer-related genes (Oncopanel) to characterize tumor specimens from the pituitary region from 127 patients (62 men, 65 women). The cohort included 114 pituitary adenomas, 5 craniopharyngiomas, and 8 tumors of other pathologies (spindle cell oncocytoma, pituicytoma, glioneuronal tumor, metastasis, plasma cell neoplasma, pituitary carcinoma, Rathke’s cleft cyst) (Table 1). Tumor samples were composed of 71% neoplastic cells on average (range, 25% to 95%). A median of 12,240,000 reads (range, 315,200 to 28,020,000) were sequenced per tumor.
Table 1.
Pituitary Tumor Pathologies
| Pathology | Case No. | Primary/Recurrent | Typical/Atypical |
|---|---|---|---|
| Adenoma | 114 (90%) | 91/23 | 105/9 |
| Null cell | 59 | 44/15 | 57/2 |
| GH | 21 | 20/1 | 19/2 |
| ACTH | 14 | 9/5 | 13/1 |
| Prolactin | 14 | 12/2 | 10/4 |
| Follicle-stimulating hormone/luteinizing hormone | 6 | 6/0 | 6/0 |
| Craniopharyngioma | 5 (4%) | 4/1 | n.a. |
| Papillary | 2 | 1/1 | n.a. |
| Adamantinomatous | 3 | 3/0 | n.a. |
| Other | 8 (6%) | 4/4 | n.a. |
Tumor pathologies in the cohort, as stratified by tumor subtype, primary or recurrent status, and typical or atypical designation.
Abbreviation: n.a., not applicable.
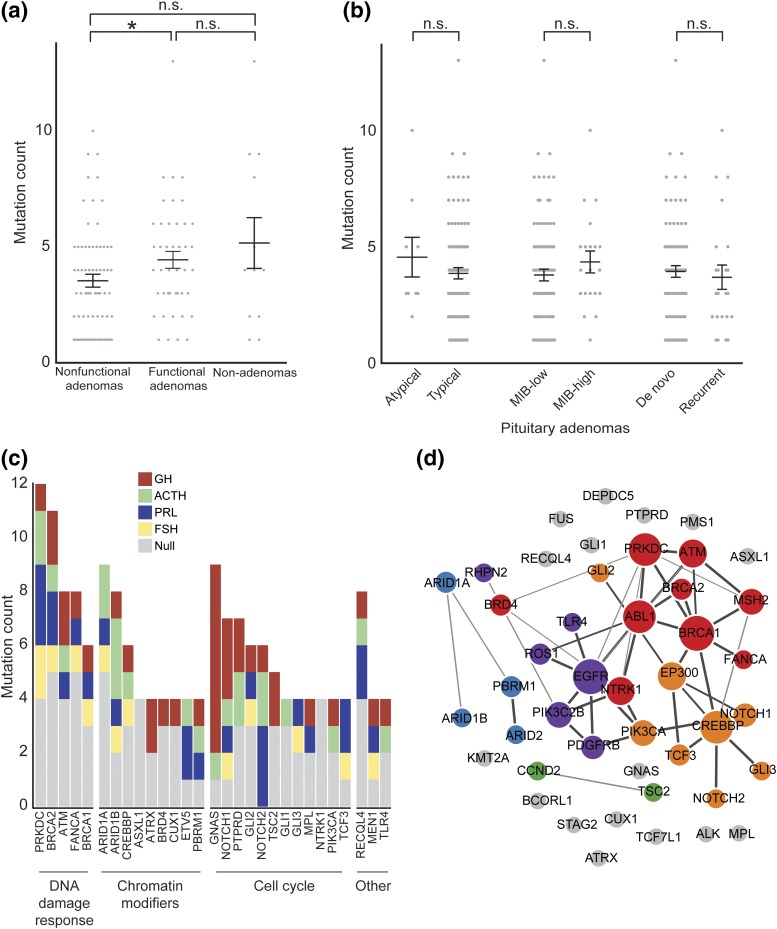
Of the 127 samples, 121 (95%) were interpreted to harbor DNA variants in at least one of the assayed genes. Chromosomal copy-number alterations were reported in 47 (37%) tumors. Nonfunctional pituitary adenomas had a significantly reduced mutational load compared with functional pituitary adenomas (P = 0.02) but not compared with other nonadenomatous tumors (P = 0.12) [Fig. 1(a)]. The mutational load of adenomas did not vary on the basis of atypical status, MIB-1 index, or recurrence [Fig. 1(b)]. Rearrangements were observed in nine tumors (7%); however, this likely understates the true prevalence of such events because only a select panel of introns was covered by the assay.
Figure 1.
Mutation burden in pituitary adenomas as stratified by tumor subtype. (a) Mutation count across pituitary tumor subtypes. (b) Mutation count in pituitary adenomas stratified by histopathologic criteria. MIB-high, >3%. (c) Mutation count among pituitary adenomas stratified by hormonal subtype of genes mutated in at least four patients grouped by gene families. (d) Cluster diagram of related pathways for genes mutated in at least three patients, as predicted by GeNets (each color represents a distinct cluster). *P < 0.05. FSH, follicle-stimulating hormone; n.s., not significant; PRL, prolactin.
Recurrent mutations in pituitary adenomas
We observed mutations in 196 of the 300 (65%) genes analyzed by OncoPanel among the 114 pituitary adenomas in our cohort (Supplemental Table 1 (11.7KB, xlsx) ). There was an average of 4 mutations (range, 0 to 13) detected per patient. We observed 29 genes that were mutated in at least four patients [Fig. 1(c)]. These recurrently mutated genes were members of three main pathways: cell cycle regulation, growth, or differentiation; chromatin modification and transcriptional regulation; or DNA damage response. We observed an additional 22 genes that were mutated three times each across the cohort; these genes were largely members of the same pathways [Fig. 1(d)].
Of the top eight most frequently altered genes in our cohort, four were members of the DNA damage response pathway. Germline and somatic DNA damage response pathway mutations have been implicated across a wide range of tumors and, in our cohort, included PRKDC, BRCA1, BRCA2, ATM, and FANCA mutations. The second most commonly altered class of genes was cell-cycle pathway members, including PIK3CA, NOTCH1/2, and GLI1/2/3. We also identified frequent mutations in chromatin modifiers, including ARID1A, ARID1B, ASXL1, BRD4, and CREBBP, and recurrent alterations in TLR4, affecting T-cell signaling and immune response, in four adenomas.
Nine genes harbored recurrent mutations affecting the same amino acid, or “hotspots,” among adenomas in our cohort (Supplemental Table 2 (9.9KB, xlsx) ). In particular, we observed previously validated oncogenic alterations in in GNAS (GNASR201C and GNASQ227L) in 6 of 21 (29%) GH-expressing adenomas and in none of the adenomas of other pathologies (P = 2 × 10−5), consistent with previous literature demonstrating enrichment in tumors from patients with acromegaly (12). Three other GNAS mutations were observed across the cohort; however, these specific mutations have not been previously observed in cancer or pituitary adenomas. Other genes mutated in a subtype-specific manner included ASXL1 and NTRK1, which were exclusively altered in null cell adenomas (n = 4 each), and NOTCH2, which was mutated only in functional adenomas (n = 6), but these were not significantly enriched (q = 0.45).
A number of genes have been identified that play a role in familial or inherited adenoma syndromes. We observed four mutations in MEN1 and two mutations each in MLL and MLL2. Germline alterations in MEN1 on chromosome 11, leading to loss of Menin, have been implicated in multiple endocrine neoplasia 1 (MEN1), as well as a number of sporadic pituitary adenomas (22, 23). Interestingly, one of the patients with an MEN1 mutation also had focal deletion of the wild-type copy of MEN1, suggesting that biallelic activation may be driving this tumor. In addition, the familial endocrine syndromes Carney complex and MEN4 are attributed to mutations in PRKAR1A, which we did not observe, and CDKN1B, of which we observed one alteration. Other genes responsible for familial adenoma syndromes, including AIP, GPR101, and USP8, were not assessed by the assay in this study.
Recent analysis of pituitary adenomas using whole-exome sequencing indicated that there was unlikely to be any single gene mutated in >15% of all adenomas (10, 13). The finding here of sporadic mutations at relatively low prevalence (<10% of the cohort for any specific gene) is in line with these observations and suggests that pituitary tumorigenesis may occur via different mechanisms across different histologic and hormonal subtypes.
Genomic alterations in nonadenomatous sellar tumors
Among the five craniopharyngiomas in the cohort, both papillary craniopharyngiomas harbored the known oncogenic BRAFV600E mutation, and two out of three adamantinomatous craniopharyngiomas harbored mutations in the β-catenin gene CTNNB1, consistent with prior reports (7, 24). One adamantinomatous craniopharyngioma also contained a BRAF rearrangement involving a breakpoint at intron 12 of BRAF on chromosome 8 to a ribosomal RNA region on chromosome 7.
One case of a metastasis from a renal primary had classic mismatch repair mutations in both MSH2 and MSH6. Mutations in this pathway often lead to tumors with elevated mutational burdens; this sample had the highest number of mutations of all samples in our cohort. We also detected a TP53 mutation in a plasma cell neoplasm, which had been previously reported to be oncogenic (COSMIC ID COSM43952). Furthermore, a case of a spindle cell oncocytoma harbored a mutation in HRAS in both the primary tumor and its recurrence, with associated immunohistochemical alterations in mitogen-activated protein kinase pathway signaling (11).
Pituitary tumors demonstrate variability in copy-number profiles
We recently reported the results of whole exome sequencing of 42 pituitary adenomas (10). We identified losses of chromosomes 1 and 11 as the most common genomic alteration and found that overall levels of chromosomal disruption correlated with functional subtype.
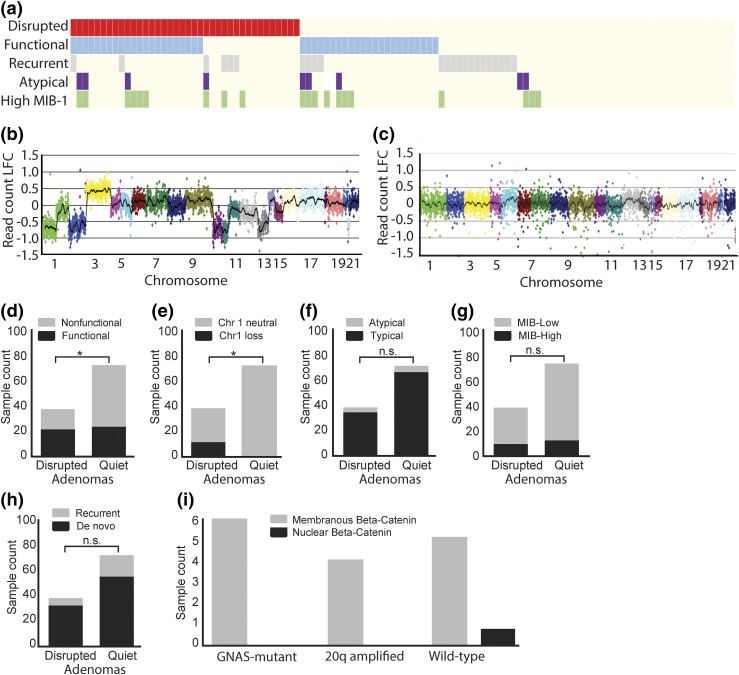
We expanded the analysis of genomic disruption and pituitary tumor subtypes in the current study [Fig. 2(a)]. We classified each sample as either genomically disrupted [Fig. 2(b)] or quiet [Fig. 2(c)] based on the level of chromosomal copy-number alterations. Among 114 adenomas in our cohort, we detected a significant association between disrupted subtype and clinical hormone status (P = 0.01) [Fig. 2(d)]. Chromosome 1 loss was the most common genomic alteration in our cohort (10%) and was likewise associated with disrupted status (P = 0.02) [Fig. 2(e)]. We did not detect an association between disrupted subtype and atypical histopathology, MIB-1 proliferative index, or recurrent status [Fig. 2(f–h)].
Figure 2.
Chromosomal copy-number alterations in pituitary adenomas. (a) Comut plot of pituitary adenomas demonstrating the association between disrupted subtype and histopathologic criteria of interest. (b) Log-fold change (LFC) in read count across all chromosomes for a sample with a disrupted copy-number profile. (c) Log-fold change in read count across all chromosomes for a sample with a quiet copy-number profile. (d) Number of samples that were classified as disrupted or quiet stratified by functional status. (e) Number of samples that were classified as disrupted or quiet stratified by chromosome 1 loss. (f) Number of samples that were classified as disrupted or quiet stratified by atypical status. (g) Number of samples that were classified as disrupted or quiet stratified by MIB-1 classification. (h) Number of samples that were classified as disrupted or quiet stratified by recurrence status. (i) Number of samples that demonstrated either nuclear or membranous β-catenin staining. *P < 0.05. n.s., not significant.
Among nonadenomatous pituitary tumors, two pituicytomas had relatively quiet genomes, whereas a pituitary carcinoma and a sellar metastasis were markedly disrupted. A number of studies have demonstrated a correlation between chromosomal instability and tumor aggressiveness (25–28). Our data suggest that a similar relationship may exist for pituitary tumors; although a relatively sparse number of samples was available within each of these pathologies, their genomic profiles are consistent with their overall clinical course. Further work will be needed to determine whether pituitary tumors with increased chromosomal instability are associated with poor clinical outcomes.
GNAS mutations do not activate canonical Wnt signaling in GH-secreting adenomas
Although GNAS mutations have been reported in approximately 30% of GH-secreting adenomas, the oncogenic drivers in those tumors without GNAS mutations have yet to be elucidated. We observed frequent gain of chromosome 20q, the arm containing GNAS, in GH-secreting adenomas. This gain nearly always occurred in tumors lacking a GNAS mutation. We hypothesized that these copy-number alterations could be serving as an alternative mechanism of activation in GNAS wild-type GH adenomas.
To determine the functional effects of this amplification, we examined 16 GH adenomas in our cohort (1) without 20q alterations but with GNAS mutations (n = 6), (2) with 20q gain and without GNAS mutations (n = 4), or (3) without either 20q gain or GNAS mutation (n = 6). We performed immunohistochemical staining of β-catenin, a canonical downstream marker of Wnt pathway activation, which has been shown to be triggered by mutant GNAS. Interestingly, neither GNAS mutated nor 20q amplified adenomas expressed nuclear β-catenin [Fig. 2(i)].
This finding suggests that GNAS alterations in GH adenomas are not activating canonical Wnt pathway signaling. Rather, GNAS mutations may be exerting a mitogenic influence through alternative mechanisms, such as cAMP activation or noncanonical Wnt pathways. Further work will be required to determine the precise relationship between GNAS mutation, downstream pathway activation, and proliferative advantage.
Discussion
Recent insights into the genomic characterization of pituitary adenomas and craniopharyngiomas highlight the potential for a formal molecular stratification of these tumors (7, 10). Previous studies investigating the genomic profile of pituitary tumors were influenced by sample selection and hence may not reflect the clinical spectrum of adenomas. We present a large chronological clinical series of pituitary tumors with genomic profiling and demonstrate the feasibility of routine clinical genomic profiling in these patients.
Molecular profiling has shown a striking ability to predict both patient response to therapy and treatment-naive survival across a number of cancers (29–31). The identification of subtype-specific mutations illustrates the potential to molecularly categorize pituitary tumors, with GNAS mutations exclusively appearing in GH adenomas, USP8 mutations defining a subset of ACTH adenomas, BRAFV600E mutations characterizing papillary craniopharyngiomas, and CTNNB1 mutations defining adamantinomatous craniopharyngiomas (9, 12). Moreover, we recently demonstrated that two classes of pituitary adenomas emerge based on their copy-number profile. One class had widespread genomic disruption with high rates of chromosome arm–level copy-number alterations, whereas the other class exhibited somatic copy–number alterations involving <6% of the genome. Levels of genomic disruption correlated with tumor pathology: 75% of the disrupted subclass were functional adenomas or atypical null-cell adenomas, compared with only 13% of the less-disrupted groups (10).
The routine incorporation of tumor sequencing efforts into clinical practice will be necessary to refine the relationship between genomic signature, traditional histopathology, and clinical outcomes. We demonstrate the implementation of such an approach. We confirmed the exclusivity of GNASR201C mutations in GH-secreting adenomas and identified a number of additional mutations. We found that genes in the DNA-damage response pathway, cell cycle, and chromatin modifier families were frequently altered. These alterations may represent somatic drivers, germline susceptibility alterations, or passenger events during tumorigenesis. We also showed the ability of this clinical sequencing assay to provide copy-number alteration data and validated the enrichment of disrupted copy-number profiles in functional tumors.
We further validated the association between chromosomal disruption and hormone-secreting status (functional vs nonfunctional) of adenomas in our clinical cohort. Interestingly, this relationship demonstrated incomplete penetrance, as only half of all functional adenomas displayed a disrupted genome, whereas 80% (4/5) of silent ACTH adenomas exhibited significant genomic disruption. Copy-number alterations did not associate with the atypical or recurrence status of the adenomas in our cohort. Longer follow-up periods may reveal whether such a genomic signature provides an objective measure of clinical outcome to better aid current histopathologic classification schemes.
To our knowledge, we performed the first large-scale clinical sequencing effort of pituitary region tumors and successfully identified previously published genomic determinants. Prompt real-time analysis of genetic alterations in pathologic specimens can lend insight into clinically actionable molecular targets for pituitary tumors, such as the BRAF mutation in craniopharyngiomas. Moreover, the ability to accurately identify critical molecular signatures associated with tumor type and clinical outcome may provide sharper insight into predicted tumor behavior and expand therapeutic options in recurrent or treatment refractory cases. Future studies in larger cohorts of patients will be needed to determine the potential role of the novel mutations observed in this study. Furthermore, because the clinical assay that was used to characterize samples was designed for the assessment of a broad range of solid tumors, a number of genes that are relevant to pituitary biology are not covered. As such, further work with a more tailored assay, along with matched normal samples, will likely be of benefit to more systematically investigate possible driver alterations.
Acknowledgments
We thank Robert Wiemann and Sherry Iuliano for assistance with case identification from the Brigham and Women’s Hospital Department of Neurosurgery and Pituitary Center clinical databases.
Acknowledgments
This work was supported in part by National Institutes of Health Grant R01 HD019938 (to U.B.K.).
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| β-catenin | Mouse anti-catenin, β-monoclonal antibody, unconjugated, clone 14 | BD Biosciences, 610154 | Mouse; monoclonal | 1:1000 | AB_397555 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- ACTH
- adrenocorticotropic hormone
- GH
- growth hormone
- MEN
- multiple endocrine neoplasia.
References
- 1.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Aguiar PH, Aires R, Laws ER, Isolan GR, Logullo A, Patil C, Katznelson L. Labeling index in pituitary adenomas evaluated by means of MIB-1: is there a prognostic role? A critical review. Neurol Res. 2010;32(10):1060–1071. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi HA, Cote DJ, Dunn IF, Laws ER Jr. Predictors of aggressive clinical phenotype among immunohistochemically confirmed atypical adenomas. J Clin Neurosci. 2016;34:246–251. [DOI] [PubMed] [Google Scholar]
- 4.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8(6):340–351. [DOI] [PubMed] [Google Scholar]
- 5.Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, Benner A, Hielscher T, Milde T, Remke M, Jones DT, Northcott PA, Garzia L, Bertrand KC, Wittmann A, Yao Y, Roberts SS, Massimi L, Van Meter T, Weiss WA, Gupta N, Grajkowska W, Lach B, Cho YJ, von Deimling A, Kulozik AE, Witt O, Bader GD, Hawkins CE, Tabori U, Guha A, Rutka JT, Lichter P, Korshunov A, Taylor MD, Pfister SM. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20(2):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR; TCGA Research Network . The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, Dias-Santagata D, Thorner AR, Lawrence MS, Rodriguez FJ, Bernardo LA, Schubert L, Sunkavalli A, Shillingford N, Calicchio ML, Lidov HG, Taha H, Martinez-Lage M, Santi M, Storm PB, Lee JY, Palmer JN, Adappa ND, Scott RM, Dunn IF, Laws ER Jr, Stewart C, Ligon KL, Hoang MP, Van Hummelen P, Hahn WC, Louis DN, Resnick AC, Kieran MW, Getz G, Santagata S. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46(2):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy PA, Ferrante E, Strebkova N, Mazerkina N, Zatelli MC, Lodish M, Horvath A, de Alexandre RB, Manning AD, Levy I, Keil MF, Sierra MdeL, Palmeira L, Coppieters W, Georges M, Naves LA, Jamar M, Bours V, Wu TJ, Choong CS, Bertherat J, Chanson P, Kamenický P, Farrell WE, Barlier A, Quezado M, Bjelobaba I, Stojilkovic SS, Wess J, Costanzi S, Liu P, Lupski JR, Beckers A, Stratakis CA. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371(25):2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CL, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31–38. [DOI] [PubMed] [Google Scholar]
- 10.Bi WL, Horowtiz P, Greenwald N, Abedalthagafi M, Agarwalla PK, Gibson WJ, Mei Y, Schumacher SE, Ben-David U, Chevalier A, Carter SL, Tiao G, Brastianos PK, Ligon AH, Ducar M, MacConaill LE, Laws ER, Santagata S, Beroukhim R, Dunn IF. Landscape of genomic alterations in pituitary adenomas. Clin Cancer Res. 2017;23(7):1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller MB, Bi WL, Ramkissoon LA, Kang YJ, Abedalthagafi M, Knoff DS, Agarwalla PK, Wen PY, Reardon DA, Alexander BM, Laws ER Jr, Dunn IF, Beroukhim R, Ligon KL, Ramkissoon SH. MAPK activation and HRAS mutation identified in pituitary spindle cell oncocytoma. Oncotarget. 2016;7(24):37054–37063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronchi CL, Peverelli E, Herterich S, Weigand I, Mantovani G, Schwarzmayr T, Sbiera S, Allolio B, Honegger J, Appenzeller S, Lania AG, Reincke M, Calebiro D, Spada A, Buchfelder M, Flitsch J, Strom TM, Fassnacht M. Landscape of somatic mutations in sporadic GH-secreting pituitary adenomas. Eur J Endocrinol. 2016;174(3):363–372. [DOI] [PubMed] [Google Scholar]
- 13.Song ZJ, Reitman ZJ, Ma ZY, Chen JH, Zhang QL, Shou XF, Huang CX, Wang YF, Li SQ, Mao Y, Zhou LF, Lian BF, Yan H, Shi YY, Zhao Y. The genome-wide mutational landscape of pituitary adenomas. Cell Res. 2016;26(11):1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228–1230. [DOI] [PubMed] [Google Scholar]
- 15.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke R, Gimm O, Koch CA, Gündogdu S, Lucassen A, Tischkowitz M, Izatt L, Aylwin S, Bano G, Hodgson S, De Menis E, Launonen V, Vahteristo P, Aaltonen LA. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92(8):3321–3325. [DOI] [PubMed] [Google Scholar]
- 16.Välimäki N, Demir H, Pitkänen E, Kaasinen E, Karppinen A, Kivipelto L, Schalin-Jäntti C, Aaltonen LA, Karhu A. Whole-genome sequencing of growth hormone (GH)-secreting pituitary adenomas. J Clin Endocrinol Metab. 2015;100(10):3918–3927. [DOI] [PubMed] [Google Scholar]
- 17.Brastianos PK, Shankar GM, Gill CM, Taylor-Weiner A, Nayyar N, Panka DJ, Sullivan RJ, Frederick DT, Abedalthagafi M, Jones PS, Dunn IF, Nahed BV, Romero JM, Louis DN, Getz G, Cahill DP, Santagata S, Curry WT Jr, Barker FG II. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst. 2015;108(2):djv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis D, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 19.Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, Ducar M, Van Hummelen P, Macconaill LE, Hahn WC, Meyerson M, Gabriel SB, Garraway LA. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA, Beroukhim R, Pellman D, Levine DA, Lander ES, Meyerson M, Getz G. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30(5):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abo RP, Ducar M, Garcia EP, Thorner AR, Rojas-Rudilla V, Lin L, Sholl LM, Hahn WC, Meyerson M, Lindeman NI, Van Hummelen P, MacConaill LE. BreaKmer: detection of structural variation in targeted massively parallel sequencing data using kmers. Nucleic Acids Res. 2015;43(3):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29(1):22–32. [DOI] [PubMed] [Google Scholar]
- 23.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1). Best Pract Res Clin Endocrinol Metab. 2010;24(3):355–370. [DOI] [PubMed] [Google Scholar]
- 24.Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrübl F, Hahnen E, Kreutzer J, Fahlbusch R. Common mutations of β-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 2005;109(6):589–597. [DOI] [PubMed] [Google Scholar]
- 25.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38(9):1043–1048. [DOI] [PubMed] [Google Scholar]
- 26.How C, Bruce J, So J, Pintilie M, Haibe-Kains B, Hui A, Clarke BA, Hedley DW, Hill RP, Milosevic M, Fyles A, Liu FF. Chromosomal instability as a prognostic marker in cervical cancer. BMC Cancer. 2015;15:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cope L, Wu RC, Shih IeM, Wang TL. High level of chromosomal aberration in ovarian cancer genome correlates with poor clinical outcome. Gynecol Oncol. 2013;128(3):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco G, Puiggros A, Baliakas P, Athanasiadou A, García-Malo M, Collado R, Xochelli A, Rodríguez-Rivera M, Ortega M, Calasanz MJ, Luño E, Vargas M, Grau J, Martínez-Laperche C, Valiente A, Cervera J, Anagnostopoulos A, Gimeno E, Abella E, Stalika E, Hernández-Rivas JM, Ortuño FJ, Robles D, Ferrer A, Ivars D, González M, Bosch F, Abrisqueta P, Stamatopoulos K, Espinet B. Karyotypic complexity rather than chromosome 8 abnormalities aggravates the outcome of chronic lymphocytic leukemia patients with TP53 aberrations. Oncotarget. 2016;7(49):80916–80924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. [DOI] [PubMed] [Google Scholar]
- 30.Attiyeh EF, London WB, Mossé YP, Wang Q, Winter C, Khazi D, McGrady PW, Seeger RC, Look AT, Shimada H, Brodeur GM, Cohn SL, Matthay KK, Maris JM; Children’s Oncology Group . Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353(21):2243–2253. [DOI] [PubMed] [Google Scholar]
- 31.Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, Socci ND, Heguy A, Cherry A, Vance G, Higgins RR, Ketterling RP, Gallagher RE, Litzow M, van den Brink MR, Lazarus HM, Rowe JM, Luger S, Ferrando A, Paietta E, Tallman MS, Melnick A, Abdel-Wahab O, Levine RL. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]