Abstract
Glucocorticoids are potent modulators of metabolic and behavioral function. Their role as mediators in the “stress response” is well known, but arguably their primary physiological function is in the regulation of cellular and organismal metabolism. Disruption of normal glucocorticoid function is linked to metabolic disease, such as Cushing syndrome. Glucocorticoids are also elevated in many forms of obesity, suggesting that there are bidirectional effects of these potent hormones on metabolism and metabolic function. Adolescence is a time of rapid physical growth, and disruptions during this critical time likely have important implications for adult function. The hypothalamic-pituitary-adrenal axis continues to mature during this period, as do tissues that respond to glucocorticoids. In this work, we investigate how chronic noninvasive exposure to corticosterone affects metabolic outcomes (body weight, body composition, insulin, and glucose homeostasis), as well as changes in bone density in both adult and adolescent male mice. Specifically, we report a different pattern of metabolic effects in adolescent mice compared with adults, as well as an altered trajectory of recovery in adolescents and adults. Together, these data indicate the profound influence that adolescent development has on the metabolic outcomes of chronic corticosterone exposure, and describe a tractable model for understanding the short- and long-term impacts of hypercortisolemic states on physiological and neurobehavioral functions.
Chronic exposure to corticosterone drives differential metabolic effects in adult vs adolescent mice, with the trajectory of recovery also being different between these life stages.
Adolescence in mammals represents a period of broad changes in somatic growth, physiology, and behavior (1–3). Both epidemiological and empirical data demonstrate that insults or perturbations during adolescence strongly predict long-term health trajectories into and throughout adult life (4, 5). The specific mechanisms of these effects are still not completely understood, but neuroendocrine signaling seems to be particularly important for mediating these developmental processes, because reproductive maturation, brain development, and body growth are all mediated by neuroendocrine signals. As such, disruptions of neuroendocrine systems during adolescence often have large impacts on health. The hypothalamic-pituitary-adrenal (HPA) axis is a neuroendocrine system required for coordination of the stress response in vertebrates (6, 7), and regulates many metabolic processes (8–10). The juvenile HPA axis shows substantial maturational changes compared with the adult HPA, suggesting that adolescence is a time when the HPA axis begins to take the adult form (11). Our work explores how altering HPA function during this critical period may affect adult function. Specifically, we focus on the metabolic consequences of disrupted HPA activity.
Cushing syndrome, a health disorder characterized by disrupted HPA function, highlights how neuroendocrine dysfunction can lead to many negative health outcomes, including obesity and a broad breakdown of metabolic homeostasis (12–14). Cushing syndrome has different impacts on health based on age of onset. Early onset Cushing syndrome is marked by distinct differences from late onset Cushing syndrome, including growth retardation, delayed bone age, and abnormal reproductive development (12). Furthermore, these health issues have been shown to persist and lead to long-term consequences into adulthood, even when treated successfully (15–17).
We have characterized an animal model of Cushing syndrome in rodents that involves chronic oral treatment with corticosterone (CORT), the primary endocrine effector of the HPA axis. This treatment leads to many of the physiological and metabolic outcomes observed in clinical Cushing syndrome, and allows us to probe the mechanisms by which neuroendocrine disruption alters physiological and metabolic homeostasis (18–20). Here, we attempt to model early-onset Cushing syndrome in mice and characterize the distinct physiological and metabolic impacts that this disorder has, both during treatment and following “recovery” from treatment. This allows us to monitor the age-specific impacts of neuroendocrine dysfunction and also report on any lasting changes that may occur.
In the current study, adult C57BL6/N mice treated chronically with CORT displayed increased weight gain and adiposity, as well as impaired glucose tolerance, consistent with our previous findings and with clinical symptoms of adult-onset Cushing syndrome (18–20). In contrast, adolescent mice treated with CORT displayed blunted growth rates, increased adiposity, enhanced glucose clearance, and decreased bone density. These findings are consistent with clinical early-onset Cushing, and thus may provide a useful model for studying the nuances of this disorder as it pertains to children and adolescents. Although there are caveats to our model, a benefit of our model is the ability to tightly control age of treatment onset, and its noninvasive nature also permits investigation of the trajectory of recovery following treatment removal. Thus, we are able to track changes that occur following removal of CORT, and demonstrate substantial differences between the effects of a Cushingoid phenotype in adolescence, and the effects of the phenotype in adulthood.
Materials and Methods
Animals and CORT treatment
Adult male mice (C57/B6N, 62 days old) and adolescent male mice (22 days old) were ordered from Charles River Laboratories (Wilmington, MA). Animals were group-housed (n = 88 total, four per cage) upon arrival in standard cages on a 12-hour light-dark cycle (lights off at 1800 hours). Animals were acclimated to the facility for 7 days following arrival, during which standard rodent chow and drinking water were available ad libitum. After the acclimation phase, chow remained available, but drinking water was replaced with a solution containing 25 µg/mL (low) or 100 µg/mL (high) free-CORT (Sigma Inc, St. Louis, MO) dissolved in 100% ethanol (EtOH), and then diluted in regular drinking water to a final EtOH concentration of 1% [vehicle (VEH)]. We have conducted pilot studies to show that 1% EtOH had no significant effects on these end points in either adults or adolescents (unpublished observation). Mice were weighed weekly during cage change at which time solutions were replaced with freshly made solution. Body composition analysis was performed immediately prior to cage change each week. All procedures with animals were approved by the Institutional Animal Care and Use Committees of Washington State University and Columbia University.
Two cohorts of mice were used. The first cohort (n = 40; 20 adolescents, 20 adults) for week 4 plasma measures was placed on VEH (n = 8/age), low CORT (n = 6/age), or high CORT (n = 6/age) for 4 weeks, and then euthanized via rapid decapitation between (0800 hours and 1200 hours, the nadir of the endogenous CORT rhythm) at which time trunk blood was collected. Water bottles were turned around 2 hours before each terminal collection. The second (longitudinal) cohort of mice (n = 48; 24 adolescents, 24 adults) were treated for 4 weeks (VEH n = 8/age; low CORT n = 8/age; high CORT n = 8/age). These mice had weekly body composition measurements, and underwent glucose tolerance testing at the end of week 4. Mice were then switched to VEH for an additional 4 weeks to assess recovery from CORT treatment, similarly undergoing weekly body composition analysis. Following the recovery period, mice underwent glucose tolerance testing and after 2 days of further recovery were euthanized, with blood collected as described previously.
In the first cohort, one adult low CORT, and one adolescent high CORT mouse were excluded due to health problems during treatment. In the second (longitudinal) cohort of mice, one adolescent VEH treated mouse died unexpectedly, and one cage of adolescent high CORT mice (n = 4) were excluded from the entire experiment as during recovery there was substantial fighting and several mice were injured.
Nuclear magnetic resonance body composition analysis
Measurements of lean, fat, and fluid mass were acquired weekly in the longitudinal cohort (adult, n = 8/treatment; adolescent n = VEH 7, low CORT 8, high CORT 4) using the Minispec LF90 system (Bruker Optics, Billerica, MA). This system allows for noninvasive nuclear magnetic resonance (NMR) analysis (90-second duration) of whole-body composition in live, unanesthetized animals. The “other” components of body composition besides body fat, lean mass, and fluid were not reported.
Radioimmunoassay and enzyme-linked immunosorbent assay
After collection (see previous description), blood was centrifuged (1500 rcf for 15 minutes at 4°C) to separate plasma from the whole blood, and stored at −20°C until analysis. Plasma CORT concentrations were measured using a radioimmunoassay and performed as indicated by the supplier (MP Biomedicals; Solon, OH). All samples were run in duplicate and values were averaged. The intra-assay coefficient of variation and lower limit of detectability were 6.2% and 22.6 ng/mL (week 4) and 6.2% and 25.13 ng/mL (week 8). Plasma insulin was measured via enzyme-linked immunosorbent assay (EMD Millipore, Inc, Billerica, MA) using manufacturer specifications and protocols. Samples were run in duplicate. The intra-assay coefficient of variation and lower limit of detectability were 7.6% and 0.2 ng/mL (week 4) and 5.7% and 0.49 ng/mL (week 8). For the insulin, samples from the 100 µg/mL CORT condition in both the adolescent- and adult-exposed groups were diluted 1:10 in assay buffer to insure the samples were within the detectable range of the standard curve. Thus, values obtained from the assay were multiplied by 10. Due to space constraints in the assay, one adult VEH from the week 4 cohort was not run. Similarly, two adult VEH and low CORT, and two adolescent VEH and low CORT from the longitudinal cohort were also not run.
Glucose tolerance test
For glucose tolerance testing, in the longitudinal cohort, mice were tested at both week 4 and week 8 (i.e., 4 weeks recovery) of the experiment (in both cases adult n = 8/treatment; adolescent n = 7 VEH, 8 low CORT, 4 high CORT). At lights-on (0600 hours) on testing days, food was removed from the cages. Six hours later (1200 hours), mice were weighed, and a tail blood sample was obtained and analyzed using a commercially available glucose monitoring kit (One-Touch; Lifescan Inc, Wayne, PA). Filtered, sterilized d-glucose was then injected intraperitoneally (IP; 200 ng/mL), at a dose of 2 g/kg in normal saline (kept at 37°C prior to injection). Blood was collected at baseline, 15, 30, 60, and 120 minutes after injection and placed onto a glucometer strip for measurement of blood glucose. Following testing, mice were returned to their home cage and food hoppers were refilled.
Spectrum In-Vivo Imaging System computed tomography scan analysis
In the same longitudinal cohort as described previously, at the end of the 4-week treatment period and again following 4 weeks of recovery from treatment, computed tomography (CT) scans of each mouse were obtained using the Spectrum In-Vivo Imaging System (IVIS; PerkinElmer, Inc, Waltham, MA). Mice were anesthetized with isoflurane (2%) during imaging (∼2-minute duration). CT scan images were reconstructed into three-dimensional images using Living Image software (PerkinElmer, Inc). X-ray absorption is closely associated with bone density (21–23). As such, relative bone density in the diaphysis (shaft) of the femur was determined via measurement of total X-ray absorption counts in three-dimensional region-of-interest frames measuring approximately 55,000 voxels (5.5 mm3) in size.
Statistics
One-way analyses of variance were used to analyze plasma CORT and insulin concentrations, as well as relative bone density. Mixed two-way analyses of variance (repeated measures by time) were used to analyze body weight, body composition, and glucose tolerance data. Post hoc analyses were undertaken using Tukey- or Dunnett-corrected t tests where appropriate. Results were considered significant at the P < 0.05. All statistical analyses were undertaken using GraphPad Prism (version 7).
Results
Body weight and composition
Our previous data demonstrate a clear effect of CORT on body weight gain. To determine if developmental stage alters this effect, adolescent and adult mice were exposed to low (25 µg/mL) or high (100 µg/mL) CORT, or VEH (1% EtOH), and various aspects of physiology were measured. These measurements were made during treatment and throughout a 4-week “recovery” period in which CORT solutions were replaced with VEH.
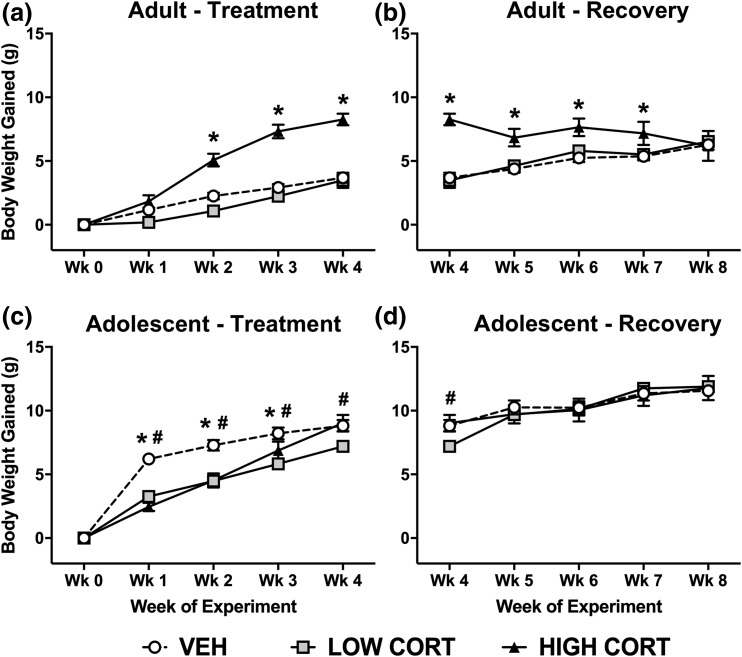
Consistent with previous reports, we found high CORT causes significant weight gain in adult mice, with no significant effect of low CORT compared with VEH. Post hoc analysis revealed that high CORT mice gained significantly more weight than both VEH and low CORT mice from weeks 2 to 4, whereas no difference was observed between low CORT and VEH mice [Fig. 1(a)]. During the recovery period, high CORT mice gradually lost weight and by the end, all groups had gained the equivalent amount of body weight [Fig. 1(b)].
Figure 1.
CORT exposure has divergent, age-dependent effects on body weight gain. (a) In adult mice, treatment with high CORT, but not low CORT, increased weight gain, with a main effect of treatment time (F4, 84 = 240.4, P < 0.0001), dose of CORT (F2, 21 = 31.06, P < 0.0001), and a significant interaction (F8, 84 = 27.99, P < 0.0001). (b) During recovery, body weight normalized in adult CORT-treated mice, with a main effect of treatment time (F4, 84 = 8.307, P < 0.0001), dose of CORT (F2, 21 = 6.579, P = 0.0060), and a significant interaction (F8, 84 = 9.391, P < 0.0001). (c) In adolescent mice, body weight gain is reduced in both high and low CORT groups, with a main effect of treatment time (F4, 64 = 561.1, P < 0.0001), dose of CORT (F2, 16 = 15.72, P = 0.0002), and a significant interaction (F8, 64 = 16.37, P < 0.0001). (d) During recovery, body weight normalized in adolescent CORT-treated mice, with a main effect of treatment time (F4, 64 = 206.8, P < 0.0001) and a significant interaction (F8, 64 = 11.49, P < 0.0001). Post hoc Tukey tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups and # indicating statistical significance between low CORT and VEH groups (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
In contrast to the effect in adult mice, we found that both doses of CORT slowed normal body weight gain in adolescent mice. Post hoc analysis revealed that adolescent mice treated with high CORT gained less weight than VEH-treated mice at week 1 to week 3, low CORT mice gained less weight than VEH mice at week 1 to week 4, and low CORT mice gained less than high CORT mice at week 4 [Fig. 1(c)]. Similar to adult mice, we found that body weight normalized after the recovery period. Body weight gain for all treatment groups became normalized by week 5, and remained so for the duration of the experiment [Fig. 1(d)]. Thus, CORT exposure has divergent effects on body weight depending on developmental stage; however, weight gain normalizes after a recovery period in both adult and adolescent mice.
Body fat
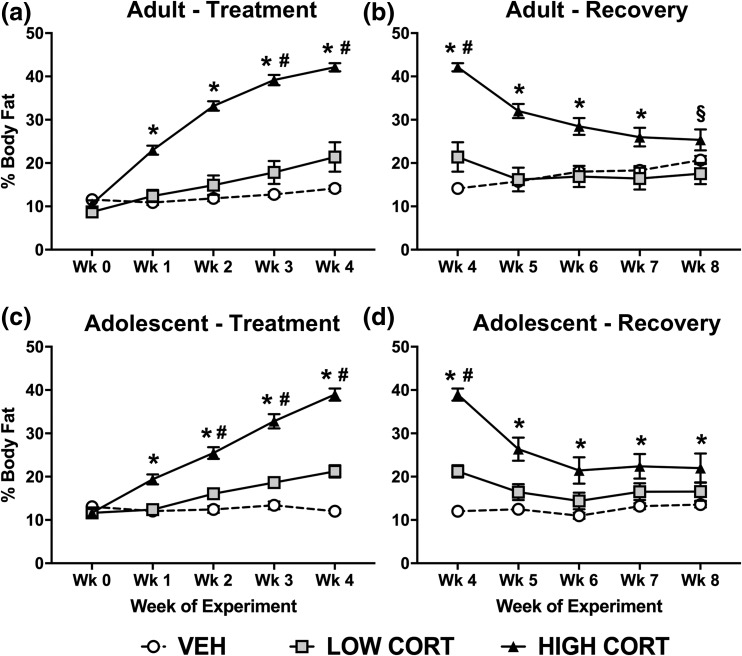
Given the change in weight and the known effects of our model on white adipose accumulation, we used NMR body composition analysis to investigate if developmental stage affected accumulation of body fat, lean mass, and body fluid following CORT exposure. Consistent with previous results, CORT exposure increased body fat in adult mice during treatment. Post hoc analysis revealed that mice treated with the high dose of CORT displayed greater body fat than both VEH and low CORT mice at week 1 to week 4, whereas those treated with the low dose of CORT displayed greater body fat than VEH mice at week 3 to week 4 [Fig. 2(a)]. Similar to body weight, body fat normalized during the recovery period in adult mice. Specifically, body fat in high CORT mice normalized to levels of VEH mice by week 8, whereas body fat in low CORT mice normalized to VEH mice levels by week 5 [Fig. 2(b)]. Body fat in high CORT mice remained greater than low CORT even at week 8.
Figure 2.
CORT exposure leads to lasting increases in body fat in adolescent, but not adult mice. (a) In adult mice, treatment with both high and low doses of CORT increased body fat, with a main effect of treatment time (F4, 84 = 239.8, P < 0.0001), dose of CORT (F2, 21 = 46.71, P < 0.0001), and a significant interaction (F8, 84 = 77.93, P < 0.0001). (b) During recovery, body fat normalized in adult CORT-treated mice, with a main effect of treatment time (F4, 84 = 24.6, P < 0.0001), dose of CORT (F2, 21 = 17.29, P < 0.0001), and a significant interaction (F8, 84 = 35.09, P < 0.0001). (c) In adolescent mice, treatment with both doses of CORT increased body fat, with a main effect of treatment time (F4, 64 = 253.5, P < 0.0001), dose of CORT (F2, 16 = 50.13, P < 0.0001), and a significant interaction (F8, 64 = 92.51, P < 0.0001). (d) During recovery, body fat did not normalize in adolescent mice, with a main effect of treatment time (F4, 64 = 63.64, P < 0.0001), dose of CORT (F2, 16 = 14.73, P = 0.0002), and a significant interaction (F8, 64 = 26.12, P < 0.0001). Post hoc Tukey tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups, # indicating statistical significance between low CORT and VEH groups, and § indicating statistical significance between low CORT and high CORT, but not VEH (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
Similar to adult mice, CORT exposure increased body fat in adolescent mice during treatment. Post hoc analysis revealed that mice treated with high CORT displayed greater body fat than both VEH and low CORT mice at week 1 to week 4, and low CORT mice displayed greater body fat than VEH mice at week 2 to week 4 [Fig. 2(c)]. In contrast to adult mice, body fat did not normalize after the recovery period in adolescent mice. Specifically, mice treated with high CORT had greater body fat than VEH mice at week 4 to week 8 and low CORT mice at week 4 to week 6, whereas body fat of low CORT mice normalized to that of VEH mice by week 5 [Fig. 2(d)]. Thus, CORT exposure increases body fat accumulation independent of developmental stage, and this increase is maintained for a period of time after exposure ends in adolescent mice only. This suggests that adolescent mice are more susceptible to lasting changes in body composition caused by elevated CORT compared with adults. It is important to note that, even though CORT reduced overall body weight gain in adolescents, it led to increased adiposity.
Lean mass
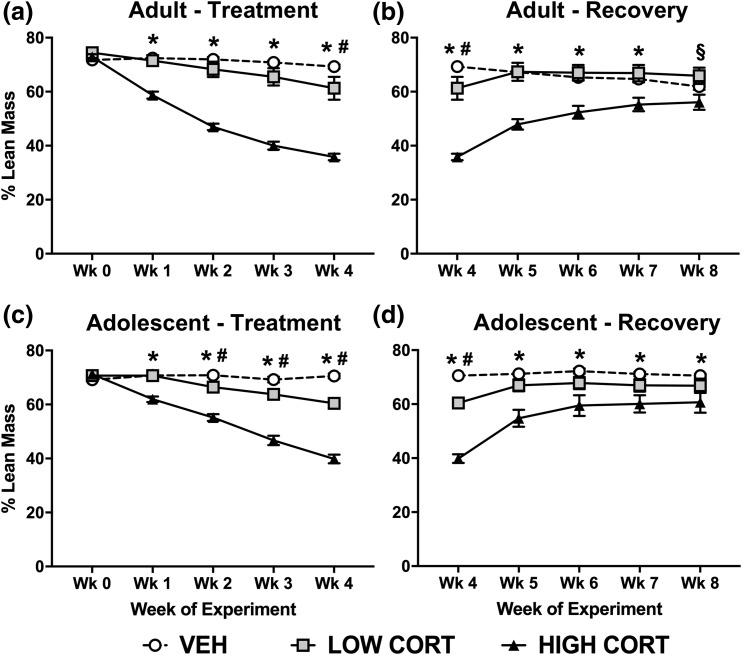
Although CORT exposure increased adiposity, it concomitantly decreased lean mass in adult mice. Post hoc analysis revealed that mice treated with high CORT had reduced lean mass compared with both VEH and low CORT mice at week 1 to week 4, and low CORT mice had lower lean mass than VEH mice at week 4 [Fig. 3(a)]. Similar to body weight and body fat, lean mass in adult mice normalized during the recovery period. Specifically, lean mass in high CORT mice normalized to levels of VEH mice at week 8, and lean mass in low CORT mice normalized to VEH levels by week 5 [Fig. 3(b)]. Lean mass in adult, high CORT mice remained lower than low CORT mice even at week 8.
Figure 3.
CORT exposure leads to lasting decreases in lean mass in adolescent, but not adult mice. (a) In adult mice, treatment with both high and low doses of CORT decreased lean mass, with a main effect of treatment time (F4, 84 = 188.2, P < 0.0001), dose of CORT (F2, 21 = 42.54, P < 0.0001), and a significant interaction (F8, 84 = 69.07, P < 0.0001). (b) During recovery, lean mass normalized in adult CORT-treated mice, with a main effect of treatment time (F4, 84 = 22.97, P < 0.0001), dose of CORT (F2, 21 = 17.71, P < 0.0001), and a significant interaction (F8, 84 = 30.41, P < 0.0001). (c) In adolescent mice, treatment with both doses of CORT decreased lean mass, with a main effect of treatment time (F4, 64 = 179.5, P < 0.0001), dose of CORT (F2, 16 = 53.93, P < 0.0001), and a significant interaction (F8, 64 = 69.41, P < 0.0001). (d) During recovery, lean mass did not normalize in adolescent mice, with a main effect of treatment time (F4, 64 = 66.96, P < 0.0001), dose of CORT (F2, 16 = 14.01, P = 0.0003), and a significant interaction (F8, 64 = 22.27, P < 0.0001). Post hoc Tukey tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups, # indicating statistical significance between low CORT and VEH groups, and § indicating statistical significance between low CORT and high CORT, but not VEH (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
As with adult mice, we observed decreased lean mass in adolescent mice after CORT exposure. Post hoc analysis revealed that high CORT mice displayed lower lean mass than both VEH and low CORT mice from week 1 to week 4, and low CORT mice displayed less lean mass than VEH mice from week 2 to week 4 [Fig. 3(c)]. In contrast to adult mice, lean mass did not normalize following the recovery period in adolescents. Specifically, adolescent mice treated with the high dose of CORT displayed reduced lean mass compared with VEH mice at week 4 to week 8 and low CORT mice at weeks 4 to 6, whereas lean mass in low CORT mice normalized to that of VEH mice by week 5 [Fig. 3(d)].
Thus, CORT exposure decreases lean mass in both adult and adolescent mice, and this decrease is sustained after a recovery period only in adolescent mice.
Body fluid
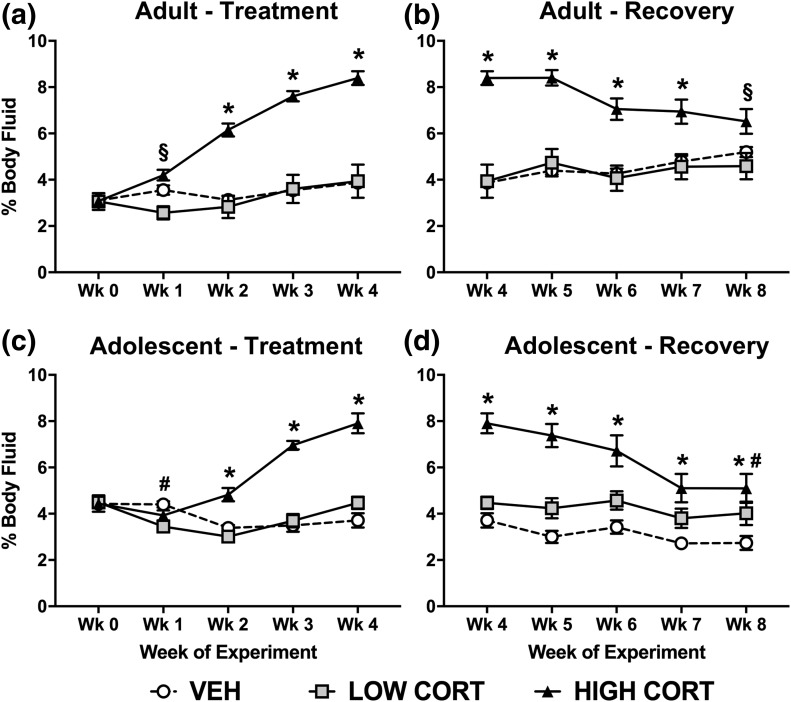
In addition to changes in body fat and lean mass, we found that high CORT increased body fluid accumulation in adult mice, with no effect of low CORT compared with VEH. Post hoc analysis revealed that mice treated with high CORT displayed greater body fluid compared with VEH mice at week 2 to week 4 and to low CORT mice at week 1 to week 4, whereas no difference was observed between low CORT and VEH mice [Fig. 4(a)]. Similar to previous measures, body fluid measures normalized after recovery in adult mice by week 8 [Fig. 4(b)]. Body fluid remained higher in high CORT mice compared with low CORT mice even at week 8.
Figure 4.
CORT exposure leads to lasting increases in body fluid in adolescent, but not adult mice. (a) In adult mice, treatment with both high and low doses of CORT increased body fluid, with a main effect of treatment time (F4, 84 = 74.59, P < 0.0001), dose of CORT (F2, 21 = 24.61, P < 0.0001), and a significant interaction (F8, 84 = 33.54, P < 0.0001). (b) During recovery, body fluid normalized in adult CORT-treated mice, with a main effect of treatment time (F4, 84 = 5.275, P = 0.0008), dose of CORT (F2, 21 = 18.39, P < 0.0001), and a significant interaction (F8, 84 = 12.76, P < 0.0001). (c) In adolescent mice, high CORT increased, whereas low CORT decreased body fluid (only at week 1), with a main effect of treatment time (F4, 64 = 34.09, P < 0.0001), dose of CORT (F2, 16 = 22.84, P < 0.0001), and a significant interaction (F8, 64 = 27.27, P < 0.0001). (d) During recovery, body fluid did not normalize in adolescent mice, with a main effect of treatment time (F4, 64 = 38.99, P < 0.0001), dose of CORT (F2, 16 = 15.96, P = 0.0002), and a significant interaction (F8, 64 = 8.927, P < 0.0001). Post hoc Tukey tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups, # indicating statistical significance between low CORT and VEH groups, and § indicating statistical significance between low CORT and high CORT, but not VEH (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
In adolescent mice, exposure to CORT also increased body fluid. Specifically, high CORT mice displayed greater body fluid measures compared with both VEH and low CORT mice at week 2 to week 4, whereas low CORT mice displayed decreased body fluid compared with VEH mice at week 1 only [Fig. 4(c)]. In contrast to adult mice, body fluid levels did not normalize after the recovery period in adolescent mice. Body fluid remained elevated in high CORT mice compared with VEH mice even at week 8, and compared with low CORT until week 7 [Fig. 4(d)]. Body fluid in low CORT mice increased compared with VEH mice at week 8.
Together, our body composition analysis reveals consistent effects of CORT exposure in both adult and adolescent mice in measures of body fat, lean mass, and body fluid levels. These effects normalize after recovery in adult mice, but are sustained in adolescent mice. This indicates that adolescents are more susceptible to the prolonged effects of high levels of CORT exposure compared with adults.
Hormone and plasma measures
Corticosterone
Given previous findings that CORT treatment alters circulating CORT and insulin levels, we next asked how developmental stage of treatment affects these measures. Mice were sampled at the time of the endogenous CORT nadir (approximately midway through the light phase; ZT6). At this time point, we found that the high CORT increased plasma CORT concentrations in both adult and adolescent mice, with no effect of low CORT (Table 1). In adult mice, high CORT mice had higher plasma CORT than VEH mice, whereas no difference was observed between low CORT mice and high CORT mice, or low CORT mice and VEH mice. In adolescent mice, high CORT mice had higher plasma CORT than both VEH and low CORT mice, whereas no difference was observed between low CORT and VEH mice. After the recovery period, we observe a decrease in levels of plasma CORT in adult low and high CORT mice to those even lower that VEH mice, whereas plasma CORT levels normalize in adolescent mice (Table 1).
Table 1.
CORT Exposure Increases Plasma CORT Levels in Both Adult and Adolescent Mice
| CORT Dose |
Treatment Plasma CORT (ng/mL) |
Postrecovery Plasma CORT |
||||
|---|---|---|---|---|---|---|
| VEH | Low CORT | High CORT | VEH | Low CORT | High CORT | |
| Adult | 26.39 ± 2.57 | 96.36 ± 28.52 | 258.51 ± 115.04a | 48.06 ± 6.99 | 27.31 ± 1.11b | 30.29 ± 3.25a |
| Adolescent | 42.36 ± 9.28 | 40.40 ± 15.62 | 421.58 ± 142.60a | 26.31 ± 1.18 | 26.76 ± 1.63 | 25.13 ± 0.00 |
High, but not low, CORT exposure increased plasma CORT concentrations in both adult (F2, 16 = 3.968, P = 0.0399) and adolescent (F2, 16 = 10.16, P = 0.0014) mice. After recovery, plasma CORT decreased in high and low CORT adult mice (F2, 21 = 6.233, P = 0.0075). In adolescent mice, plasma CORT normalized to VEH levels after recovery. Post hoc Tukey tests were undertaken to probe interactions. Values are considered statistically significant at the P < 0.05 level.
Statistical significance between high CORT and VEH groups.
Statistical significance between low CORT and VEH groups (n = 4 to 8/age/treatment).
Thus, CORT exposure results in increased plasma CORT levels regardless of developmental stage. Importantly, this effect does not persist following recovery in adult and adolescent mice, as levels decrease to concentrations within normal basal nadir values. However, in adult mice, basal plasma CORT levels are lower than VEH mice after recovery, suggesting that adrenal function may not have fully recovered, whereas mice treated during adolescence show no difference between treatments after recovery.
Insulin
We found that high CORT exposure increased plasma insulin in both adult and adolescent mice, with no effect of low CORT (Table 2). In both age groups, high CORT mice had higher plasma insulin than both VEH and low CORT mice, whereas no difference was observed between low CORT and VEH mice. It is interesting to note that insulin levels in high CORT treated adolescent mice were more than double those observed in high CORT treated adults. After 4-week recovery, in both age groups, high CORT mice still had greater plasma insulin than both low CORT and VEH mice after the recovery period (Table 2). Thus, high CORT exposure results in sustained changes in plasma insulin levels in both adolescent and adult mice, though the absolute levels of plasma insulin were twice as high in adolescent mice as in adult mice after treatment.
Table 2.
CORT Exposure Leads to Sustained Increases Plasma Insulin Levels in Both Adult and Adolescent Mice
| CORT Dose |
Treatment Plasma Insulin (ng/mL) |
Postrecovery Plasma Insulin |
||||
|---|---|---|---|---|---|---|
| VEH | Low CORT | High CORT | VEH | Low CORT | High CORT | |
| Adult | 3.50 ± 1.12 | 5.47 ± 1.28 | 40.53 ± 9.30a | 1.91 ± 0.29 | 1.72 ± 0.31 | 6.02 ± 0.28a |
| Adolescent | 1.78 ± 0.14 | 5.63 ± 2.02 | 83.69 ± 16.76a | 1.41 ± 0.24 | 2.08 ± 0.44 | 6.23 ± 1.05a |
High, but not low, CORT exposure increased plasma insulin concentrations in both adult (F2, 15 = 14.74, P = 0.0003) and adolescent (F2, 16 = 33.12, P < 0.0001) mice. After recovery, plasma insulin remained elevated in high CORT adult (F2, 17 = 73.75, P < 0.0001) and adolescent (F2, 12 = 17.75, P = 0.0003) mice. Post hoc Tukey tests were undertaken to probe interactions. Values are considered statistically significant at the P < 0.05 level.
Statistical significance between high CORT and VEH groups.
Statistical significance between low CORT and VEH groups (n = 4 to 8/age/treatment).
Glucose tolerance
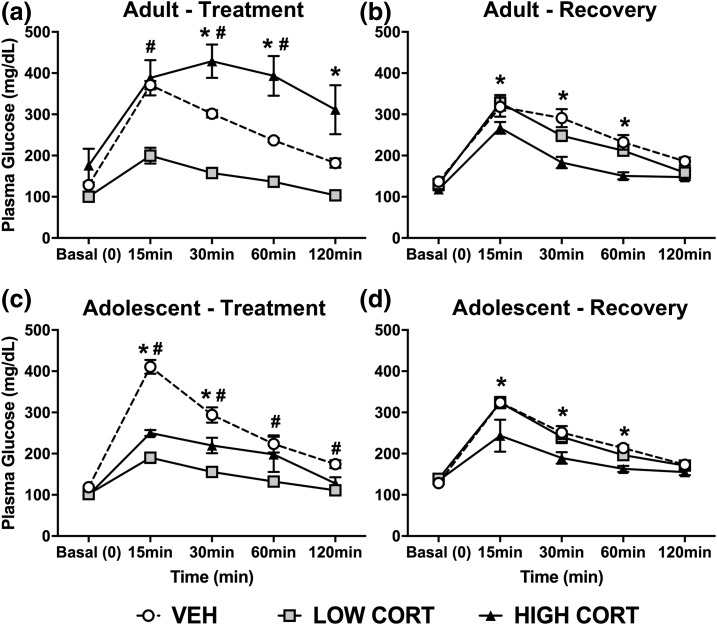
Given our previous findings that CORT treatment impairs glucose regulation, and the observed effects on insulin, we next assessed how glucose tolerance could be differentially affected by CORT treatment during adolescence. In accordance with previous findings, adult mice treated with high CORT have impaired glucose clearance after 4 weeks following glucose challenge. We also show that low CORT mice display enhanced glucose clearance. Post hoc analysis revealed that high CORT mice had greater plasma glucose levels compared with VEH mice at the 30-to 120-minute time points, and to low CORT mice at the 15- to 120-minute time points. In contrast, low CORT mice had lower plasma glucose levels compared with VEH mice at the 15- to 60-minute time points [Fig. 5(a)]. After the recovery period, adult high CORT mice no longer show impaired glucose clearance, and in contrast display enhanced glucose clearance. No difference was observed between low CORT and VEH mice after the recovery period. At the 15- to 60-minute time points, high CORT mice had lower plasma glucose levels than both VEH and low CORT mice [Fig. 5(b)].
Figure 5.
CORT exposure has sustained, age-dependent effects on glucose tolerance. (a) In adult mice, following a glucose challenge, high CORT exposure resulted in decreased glucose clearance (higher concentration in plasma over time), whereas low CORT exposure resulted in enhanced glucose clearance (lower glucose concentration over time). We observe a main effect of sample time (F4, 84 = 185.3, P < 0.0001), dose of CORT (F2, 21 = 13.81, P = 0.0001), and a significant interaction (F8, 84 = 23.2, P < 0.0001). (b) After recovery, adult high CORT mice exhibited enhanced glucose clearance, with a main effect of sample time (F4, 84 = 134.3, P < 0.0001), dose of CORT (F2, 21 = 9.142, P = 0.0014), and a significant interaction (F8, 84 = 3.862, P = 0.0006). (c) In adolescent mice, both high and low CORT groups displayed enhanced glucose clearance, with a main effect of sample time (F4, 64 = 124.8, P < 0.0001), dose of CORT (F2, 16 = 28.57, P < 0.0001), and a significant interaction (F8, 64 = 17.95, P < 0.0001). (d) After recovery, adolescent high CORT mice still had enhanced glucose clearance, with a main effect of sample time (F4, 64 = 114.6, P < 0.0001), dose of CORT (F2, 16 = 4.906, P = 0.0218), and a significant interaction (F8, 64 = 3.06, P = 0.0056). Post hoc Dunnett tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups and # indicating statistical significance between low CORT and VEH groups (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
In adolescent mice, both high and low CORT mice display enhanced glucose clearance following glucose challenge. Specifically, high CORT mice had lower plasma glucose levels compared with VEH mice at the 15- to 30-minute time points, and higher plasma glucose levels compared with low CORT mice at the 15- to 60-minute time points. However, low CORT mice had lower plasma glucose compared with VEH mice at the 15- to 120-minute time points [Fig. 5(c)]. Following the recovery period, high CORT adolescent mice still displayed enhanced glucose clearance compared with VEH mice, whereas no difference between low CORT and VEH mice was observed. High CORT mice had lower plasma glucose than VEH mice at the 15- to 60-minute time points, and lower levels than low CORT mice at the 15- to 30-minute time points [Fig. 5(d)].
Therefore, we show an age-dependent effect of CORT exposure on glucose metabolism, by which adult mice display impaired glucose clearance, and adolescent mice display enhanced glucose clearance. In addition, we observe that normal glucose regulation is not restored following the recovery period, indicating lasting effects of CORT treatment on glucose metabolism.
IVIS CT bone density analysis
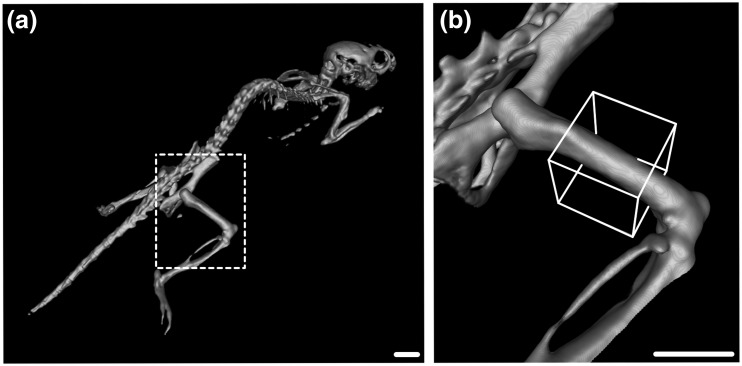
To explore the discrepant results between body weight loss and adipose gain in adolescent mice, we performed CT scans to determine if changes in bone density could underlie these effects. In these experiments, X-ray absorption counts in the diaphysis of the right femur were used to determine relative differences in bone density between treatment groups (Fig. 6).
Figure 6.
Reconstructed CT imaging used to determine relative bone density. (a) Mice were imaged in an IVIS CT Imager, and total X-ray absorption was measured in the diaphysis of the right femur (b; white box). Scale bar = 5 mm.
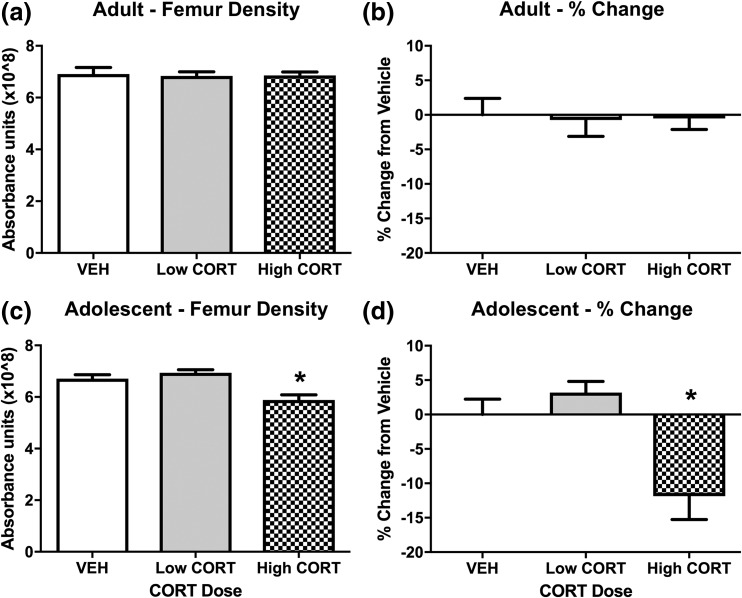
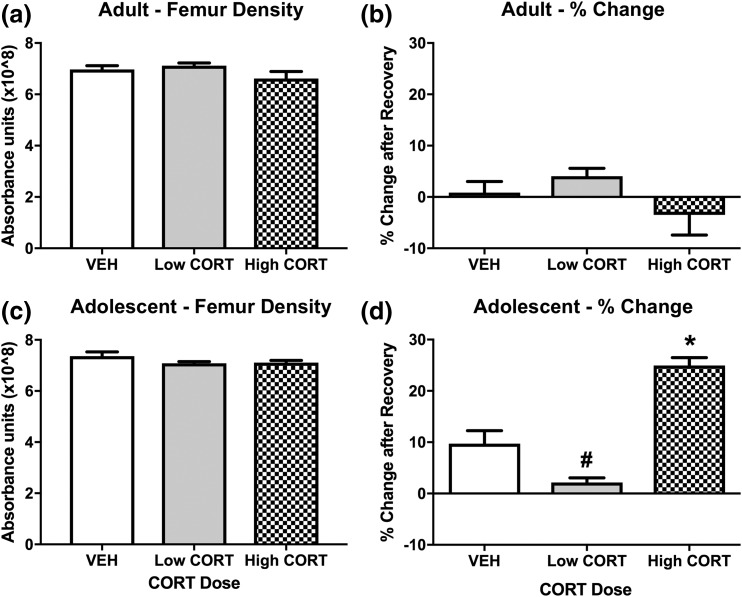
We observed no difference in femur density in adult CORT mice after treatment [Figs. 7(a) and 7(b)], or following recovery [Figs. 8(a) and 8(b)]. In contrast, we found a decrease in femur density in adolescent high CORT mice, showing reduced femur density compared with mice in both the VEH and low CORT groups. No difference was observed in femur density between VEH and low CORT mice [Figs. 7(c) and 7(d)]. Following the recovery period, bone density normalized in adolescent CORT mice, with no difference observed between treatment groups [Fig. 8(c)]. This normalization was accompanied by a very large percent change in femur density within treatment groups from week 4 to week 8, with high CORT mice displaying drastically increased percent change in femur density compared with VEH and low CORT mice, and a small but significant reduction in percent change in femur density in low CORT mice compared with VEH mice [Fig. 8(d)]. Along with this substantial increase in bone density (nearly 25% to 30%) over just 4 weeks, we also found various bone abnormalities at week 8 in adolescent exposed high CORT mice, including bone masses in the tail and fused bones in the extremities (not shown). This suggests that the rapid increase in bone mass observed in adolescent CORT mice during recovery from treatment may lead to deleterious effects. Together, these findings suggest that adolescence represents a sensitive period to the effects of CORT exposure on bone development.
Figure 7.
CORT exposure during adolescence decreases bone density. (a, b) In adult mice, we observe no effect of CORT exposure on X-ray absorption in the right femur (P > 0.05). (c, d) In adolescent mice, high CORT exposure resulted in decreased X-ray absorption in the right femur, with a main effect of CORT dose (F2, 16 = 10.47, P = 0.0012). Post hoc Tukey tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
Figure 8.
Bone density normalizes following recovery from CORT exposure in adolescent mice. (a) In adult mice, we observed no effect of CORT exposure on X-ray absorption in the right femur after the recovery period (P > 0.05), and (b) no difference in right femur X-ray absorption when comparing within treatment groups from week 4 to week 8 (before and after recovery) (P > 0.05). (c) In adolescent mice, X-ray absorption in the right femur normalized to that of VEH mice after the recovery period (P > 0.05). (d) When comparing within treatment groups from week 4 to week 8, we observed a robust increase in femur X-ray absorption in high CORT mice, and a decrease in low CORT mice, with a main effect of CORT dose (F2, 16 = 32.31, P < 0.0001). Post hoc Tukey tests were undertaken to probe interactions, with * indicating statistical significance between high CORT and VEH groups and # indicating statistical significance between low CORT and VEH groups (n = 4 to 8/age/treatment). Values are considered statistically significant at the P < 0.05 level.
Discussion
The current study explored two aspects of the physiological effects of chronic high exposure to glucocorticoids. First, we demonstrate that several aspects of metabolic dysregulation observed during CORT exposure can be reversed following cessation of treatment, whereas others have a lasting effect. Second, we have shown that developmental stage affects somatic and endocrine features of this mouse model of hypercortisolemia, as well as the trajectory of recovery following end of treatment. Using noninvasive treatment with CORT in the drinking water, we model key features of Cushing syndrome observed in humans, including increased weight and adiposity, decreased glucose tolerance, and dysregulated insulin levels (12–14). The present results explore the effects of this model during adolescence—a common stage of early onset Cushing syndrome (12, 24). In addition to replicating Cushing-like physiology in adult mice, we show that applying our model during adolescence leads to marked changes in body weight, body composition, and bone density, while also causing changes in glucose metabolism and circulating plasma levels of insulin and CORT. We also show that after a recovery period where CORT-treatment was removed, body weight and bone density are normalized, but body composition, glucose metabolism, and plasma insulin remained abnormal. These results closely mimic the phenotype observed in early onset Cushing syndrome, and provide a potentially powerful animal model to explore factors that lead to pathophysiological outcomes, both in the short term during disease onset and progression, and the long term during disease treatment and remission.
Glucocorticoids, obesity, and metabolic dysregulation
In our model, CORT in the drinking water leads to a sustained increase in plasma levels of CORT in high CORT mice, but not in low CORT mice, during the CORT nadir (middle of the light phase) regardless of age (Table 1). This replicates our previous work in adults (19, 20), which we posit is due to the pattern of drinking in nocturnal rodents, which will consume much of their water earlier in the active (dark) phase. Our published data (19) further demonstrate that it is only after the first 2 weeks of treatment that high CORT mice begin to drink significantly more, an effect that we have observed in other studies in our laboratories in both adults and adolescents (unpublished observation), which may lead to a more constant dosing in high CORT mice driven by polydipsia (more discussion of this below). Additionally, high CORT treated adolescents show elevated (though not statistically significant) plasma CORT compared with similarly treated adults (Table 1). It is unclear if this difference is physiologically relevant, but undoubtedly a caveat is that our model may result in different levels of plasma CORT depending on age. Basal levels of plasma CORT are somewhat suppressed in high CORT mice following recovery, perhaps suggesting that the adrenal has not yet fully recovered from the effects of chronic treatment. Our previous work supports this conclusion, with gross assessment of adrenals after 2 weeks recovery still showing some decrease in weight, as well as increased vacuolization (albeit less than that observed during treatment) (19). That mice treated with CORT during adolescence do not show a difference in plasma CORT (at least at this time point) would suggest that the adolescent adrenal gland may have greater recuperative capacity than the adult. Detailed morphological analyses, coupled with ACTH stimulation experiments could potentially test this hypothesis.
Glucocorticoids are clearly linked to metabolic function, and dysfunction, with chronic exposure to stress and the resulting increase in glucocorticoids likely contributing to the rise of obesity in the modern world (25–29). In addition, exogenous treatment with glucocorticoids, as observed in individuals prior to certain surgeries, or for treatment of inflammatory disorders, can lead to signs of the metabolic syndrome (30). Organically dysregulated glucocorticoid signaling, as observed in Cushing disease, leads to substantial increases in adiposity, and eventually negative effects on lean tissues (31). Previous work in mice by our laboratory and others has demonstrated that chronic noninvasive treatment with high levels of CORT leads to metabolic dysregulation that is similar to Cushing syndrome (18–20). Our present work has focused on the trajectory of recovery following cessation of treatment, and whether age of onset of treatment can alter this trajectory.
In the present results, we replicated our previous findings, with adult mice showing significantly increased weight gain (18–20). We have extended these original findings by tracking the specific changes in body composition throughout treatment, clearly demonstrating that the increased weight gain is due to a gradual increase in adiposity at the cost of decreased lean mass. We have previously demonstrated that chronic high CORT leads to hepatopathy that is likely a result of increased glycogen accumulation caused by dyslipidemia and hyperglycemia (19), which has also been demonstrated in both humans and nonhuman animals (32–35). Disruption of normal hepatic function and subsequent dyslipidemia occurs concomitantly with an effect of CORT directly on adipocytes, driving accumulation of lipids in this organ (36). The decrease in lean mass should be considered in the context of the hyperinsulinemia and decreased glucose tolerance observed in high CORT mice (discussed below). With a loss of insulin sensitivity and chronically elevated circulating glucocorticoids, a shift for catabolic actions of CORT on muscle tissue is likely occurring.
Following recovery in adults, body weight between groups is indistinguishable (Fig. 1), and body composition reveals neither high nor low CORT mice show any statistical difference compared with VEH mice in adiposity, lean mass, or fluids (Figs. 2–4). Adolescent mice show different changes in growth and body composition in response to CORT treatment. Specifically, we observe reduced body weight gain and lean mass following CORT treatment, whereas adiposity and body fluid accumulation increase (Figs. 1–4). Following recovery, body weight in adolescent-exposed mice normalizes, but high CORT mice continue to show a persistent elevation in adiposity compared with controls, suggesting a potential for long-lasting changes in body composition. It is intriguing to consider if this change in adiposity is maintained for even longer periods of time, or if the exposure of adolescent mice to high CORT during development render them more vulnerable to weight gain later in life. This pattern of results is interesting for several reasons. First, previous studies in rats exposed to chronic stress demonstrate that ponderal growth is protected at the cost of reproductive maturation (37), suggesting normal body weight gain is being tightly regulated at this stage of development in which there are rapid changes in somatic development. However, the loss of lean mass and increased adiposity might indicate that although overall weight is protected, body composition is less tightly regulated. The long-term consequences of these changes are not clear, though our data suggest they persist for at least 1 month following cessation of CORT treatment.
In addition to changes in adiposity and lean mass, CORT treatment caused a substantial increase in fluid retention in both age groups. This effect is likely driven by the well-known effects of glucocorticoids on electrolyte balance. Our previous work demonstrated substantial changes in electrolytes following high CORT, particularly hyponatremia and hypokalemia (19), which would contribute to polyuria and polydipsia. Additionally, the effects of CORT on the mineralocorticoid receptor can be similar to the effects of aldosterone (38), which would also contribute to increased fluid retention by the kidneys. Our previous work showed that high CORT mice increase water consumption after week 2 of treatment, an effect which is sustained through the end of week 4, compared with VEH and low CORT groups (19), and unpublished data from our work further indicate that this increase in water consumption occurs independent of age. This increased water consumption and fluid retention could also explain our observation of consistently elevated circulating CORT concentrations in high, but not low, CORT mice during treatment. Our data on recovery illustrate this effect persists at least for 4 weeks, which may be due to continued electrolyte imbalance, or potentially a consequence of development of other conditions during CORT treatment, such as diabetes insipidus, in which changes in sensitivity to antidiuretic hormone may have occurred. This outcome is plausible given the observed polyuria and polydipsia in high CORT mice (19), as hypercortisolemia can lead to suppression of antidiuretic hormone (39, 40).
We show a clear effect of chronic treatment of CORT on growth and metabolism, as evidenced by abnormal changes in body weight and body composition, with divergent effects dependent on age. In adult mice, we observe increased body weight, adiposity, and fluid accumulation, and a concomitant decrease in lean mass. After a recovery period where CORT was no longer administered, body weight normalized, whereas body composition remained somewhat dysregulated. This is in line with clinical Cushing syndrome, as well as previous findings using this model in mice.
Glucocorticoids, insulin, and glucose homeostasis
Under normal conditions, glucocorticoids and insulin can be considered to operate in opposition to each other. Although glucocorticoids are largely responsible for increasing plasma glucose levels, insulin is primarily responsible for reducing blood glucose. Another way to consider this relationship is that glucocorticoids promote energy utilization, whereas insulin promotes energy storage (41, 42). As such, disrupting the balance between glucocorticoids and insulin can lead to substantial metabolic dysregulation. However, it has been demonstrated that there is a cooperative effect of glucocorticoids in some body compartments, particularly in adipocytes, where they promote development of mature adipocytes (43, 44). This pattern of results is similar to what we observe in our model: hypercortisolemia coupled with hyperinsulinemia and dysregulated glucose tolerance. Moreover, this occurs with increased adiposity, all of which are analogous to the symptoms of Cushing syndrome.
A primary physiological role for glucocorticoids is to increase plasma glucose, driven by increased catabolism of glycogen stores, and increased gluconeogenesis (42, 45). Thus, multiple mechanisms in high CORT treated mice can contribute to the increased plasma insulin concentrations observed in both age groups. Following a recovery period, although CORT concentrations returned to the normal range, insulin remained elevated in both adult and adolescent mice. It is unclear if this change is a result of a permanent shift in islet cell activity, or a shift in insulin receptor sensitivity (which would thus require higher levels of insulin). However, these changes in plasma insulin, although parallel at both ages, seems to result in a very different pattern of glucose handling.
As in our previous work, we demonstrate altered glucose handling following CORT treatment. In adult mice, we observe that treatment with high CORT results in impaired glucose tolerance, by which plasma glucose concentrations remained elevated for longer after a glucose challenge compared with VEH mice. Curiously, adult mice treated with low CORT show enhanced glucose clearance, which may be due to an increase in insulin release without a decrease in insulin receptor sensitivity, though this remains to be tested. Following the recovery period, high CORT mice display enhanced glucose clearance, whereas low CORT mice are not different from VEH mice.
In adolescent mice, treatment with both high and low CORT results in enhanced glucose clearance following a glucose challenge. This enhanced glucose clearance is maintained through the recovery period in high CORT mice, but glucose tolerance normalized in low CORT mice. These results are noteworthy because plasma insulin levels in high CORT treated adolescents are twice those observed in high CORT treated adults, but in adults a marked decrease in glucose tolerance suggests impaired insulin sensitivity. If these responses are in fact due to changes in insulin sensitivity, it would imply that adolescent mice are more resilient to defects in glucose handling because glucose tolerance is maintained during CORT treatment, even with substantially higher levels of plasma insulin. Future studies clamping insulin levels could address this outcome more directly, as well as changes in insulin receptor signaling pathways in both liver and skeletal muscle. If it is demonstrated that this maintenance of glucose tolerance is indeed mediated by sustained insulin sensitivity even in the face of hyperinsulinemia (which in adults causes profound defects in glucose handling), it would provide a potential model to uncover mechanisms by which insulin sensitivity can be maintained (e.g., type 2 diabetes).
Bone
Glucocorticoids can significantly modulate bone structure, with exogenous glucocorticoid therapy being one of the most commons causes of secondary iatrogenic osteoporosis, with a major component being decreased bone formation (46–48). Similarly, Cushing syndrome is associated with osteoporosis (48–50). Specifically, CORT can increase osteoblast apoptosis (51, 52), whereas several findings suggest that CORT can lead to increased activity of osteoclasts (51, 53, 54), thus CORT can alter the balance of bone formation by reducing the production of new bone and increasing the breakdown of existing bone tissue. Given that adolescence is an important time for bone growth, we hypothesized that there may be differences in the effects of chronic CORT on bone density at these two ages.
In adults, we did not find any major effect of chronic CORT on femur bone density. This would be in line with findings that suggest glucocorticoids affect bone growth more than bone loss (55). However, in adolescent mice, high CORT causes a striking reduction in bone density of nearly 15% (Fig. 7). After cessation of CORT, and a 4-week recovery period, bone density normalized. However, this normalization was caused by a rapid increase in bone density in high CORT mice, at a rate nearly double that observed in VEH mice (Fig. 8). Moreover, this rapid building of bone was accompanied by several abnormalities, including fused joints and aberrant bone formations in the tail (data not shown). The mechanisms by which this occurs is unclear, but our previous work demonstrated that high CORT in adults leads to a significant (8%) increase in plasma calcium (19), which may be a result both of altered kidney function and increases in osteoclast activity. Thus, if CORT inhibits osteoblast function, the removal of CORT treatment may unmask a compensatory increase in osteoblast function. Similarly, if high CORT has been driving increased osteoclast activity, withdrawal of CORT could then lead to reduced overall osteoclast function. When coupled with increased availability of calcium in the blood, these changes could lead to aberrant bone formation.
Cushing syndrome: age of onset and long-term consequences
Cushing syndrome can have both iatrogenic and organic etiologies. Our model, although closer to iatrogenic Cushing, also mimics the hallmark symptoms of organic Cushing. Iatrogenic Cushing can be caused by any number of treatments with corticosteroids, including topical ointments (56, 57), ophthalmic dexamethasone (58, 59), and combination of glucocorticoid therapies with some retroviral medications (60–62). In children, and adolescents, the increased use of topical steroids and inhaled corticosteroids for the treatment of asthma and related disorders (63–66), may also increase the risk of developing iatrogenic Cushing. Thus, it is important to understand if the mechanisms that drive the metabolic and hormonal dysfunction associated with Cushing in adults are similar to those in adolescents, and if the trajectory of recovery following cessation of treatment may be different between these two developmental stages.
Pediatric Cushing is accompanied by growth failure (12, 15, 67, 68) and abnormal pubertal development (69). In many cases, growth is reduced for several years, and is likely due to reduced growth hormone secretion even after surgical treatment or radiotherapy (70–72), while body mass index remains elevated (73). Our results mirror many of these effects, with adolescent treated mice showing reduced growth and lean mass, with increased adiposity. Following recovery, our findings show that body weight and bone density normalize in adolescent treated mice, whereas body composition and glucose handling remain dysregulated. Rapid changes in bone density also seem to be accompanied with bone growth abnormalities, which could result in problems in adulthood. These findings align with clinical literature on child/adolescent-onset Cushing syndrome, in which patients often present with decreased height, delayed bone age, and increased body mass index (12). As indicated by Storr et al. (74), there are few comparisons between adult and pediatric Cushing, and our model should provide a useful tool for preclinical research to explore mechanisms of disease course, and recovery, in multiple tissues.
Conclusions
These findings are from a mouse model of early onset Cushing-like syndrome, which recapitulate many of the somatic and endocrine outcomes of the human disorder. We also contrast these effects to the adult onset Cushing, and demonstrate significant differences in the response of adolescent and adult mice. Finally, we show that there are lasting effects even after removal of the treatment, particularly in body composition and bone structure. The establishment of this mouse model could prove a useful approach to understand both the long-term changes caused by early onset Cushing, while also enabling the study of the underlying molecular mechanisms, given the powerful genetic tools that can be leveraged in mouse models. Overall, our findings lend further support to developmental approaches when investigating metabolic and homeostatic dysregulation, because instances where juvenile animals respond differently than adults could provide important insights into mechanisms of disease etiology, progression, and potential targets for intervention.
Acknowledgments
The authors thank Dr. David Dewitt for assistance with the IVIS CT imaging and the skilled animal technicians that supported this work.
Acknowledgments
The work was supported in part by National Science Foundation Faculty Early Career Development (CAREER) Award 1553067 and National Institutes of Health Grant R21 AG050054 (to I.N.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CORT
- corticosterone
- CT
- computed tomography
- EtOH
- ethanol
- HPA
- hypothalamic-pituitary-adrenal
- IVIS
- Spectrum In-Vivo Imaging System
- NMR
- nuclear magnetic resonance
- VEH
- vehicle.
References
- 1.McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–233. [DOI] [PubMed] [Google Scholar]
- 2.Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. [DOI] [PubMed] [Google Scholar]
- 3.Varlinskaya EI, Vetter-O’Hagen CS, Spear LP. Puberty and gonadal hormones: role in adolescent-typical behavioral alterations. Horm Behav. 2013;64(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. [DOI] [PubMed] [Google Scholar]
- 5.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–1139. [DOI] [PubMed] [Google Scholar]
- 6.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr Comp Biol. 2002;42(3):541–551. [DOI] [PubMed] [Google Scholar]
- 8.Björntorp P, Rosmond R. The metabolic syndrome—a neuroendocrine disorder? Br J Nutr. 2000;83(Suppl 1):S49–S57. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann N Y Acad Sci. 1995;771:730–742. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94(2):169–177. [DOI] [PubMed] [Google Scholar]
- 11.Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev. 2016;70:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan LF, Storr HL, Grossman AB, Savage MO. Pediatric Cushing’s syndrome: clinical features, diagnosis, and treatment. Arq Bras Endocrinol Metabol. 2007;51(8):1261–1271. [DOI] [PubMed] [Google Scholar]
- 13.Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593–5602. [DOI] [PubMed] [Google Scholar]
- 14.Ferraú F, Korbonits M.. Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol. 2015;173(4):M133–M157. [DOI] [PubMed] [Google Scholar]
- 15.Leong GM, Abad V, Charmandari E, Reynolds JC, Hill S, Chrousos GP, Nieman LK. Effects of child- and adolescent-onset endogenous Cushing syndrome on bone mass, body composition, and growth: a 7-year prospective study into young adulthood. J Bone Miner Res. 2007;22(1):110–118. [DOI] [PubMed] [Google Scholar]
- 16.Barahona MJ, Sucunza N, Resmini E, Fernández-Real JM, Ricart W, Moreno-Navarrete JM, Puig T, Farrerons J, Webb SM. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(9):3365–3371. [DOI] [PubMed] [Google Scholar]
- 17.Resmini E. Persistent comorbidities in Cushing’s syndrome after endocrine care. Adv Endocrinol. 2014;2014. doi:10.1155/2014/231432. [Google Scholar]
- 18.Bowles NP, Karatsoreos IN, Li X, Vemuri VK, Wood JA, Li Z, Tamashiro KL, Schwartz GJ, Makriyannis AM, Kunos G, Hillard CJ, McEwen BS, Hill MN. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc Natl Acad Sci USA. 2015;112(1):285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassano AE, White JR, Penraat KA, Wilson CD, Rasmussen S, Karatsoreos IN. Anatomic, hematologic, and biochemical features of C57BL/6NCrl mice maintained on chronic oral corticosterone. Comp Med. 2012;62(5):348–360. [PMC free article] [PubMed] [Google Scholar]
- 20.Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151(5):2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouznetsov A, Tambasco M. A hybrid approach for rapid, accurate, and direct kilovoltage radiation dose calculations in CT voxel space. Med Phys. 2011;38(3):1378–1388. [DOI] [PubMed] [Google Scholar]
- 22.Papavero L, Zwönitzer R, Burkard I, Klose K, Herrmann HD. A composite bone graft substitute for anterior cervical fusion: assessment of osseointegration by quantitative computed tomography. Spine. 2002;27(10):1037–1043. [DOI] [PubMed] [Google Scholar]
- 23.Sheets CG, Hui DD, Bajaj V, Earthman JC. Quantitative percussion diagnostics and bone density analysis of the implant-bone interface in a pre- and postmortem human subject. Int J Oral Maxillofac Implants. 2013;28(6):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storr HL, Isidori AM, Monson JP, Besser GM, Grossman AB, Savage MO. Prepubertal Cushing’s disease is more common in males, but there is no increase in severity at diagnosis. J Clin Endocrinol Metab. 2004;89(8):3818–3820. [DOI] [PubMed] [Google Scholar]
- 25.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2-3):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: a meta-analysis of longitudinal studies. Obesity (Silver Spring). 2011;19(4):771–778. [DOI] [PubMed] [Google Scholar]
- 28.Patterson ZR, Abizaid A. Stress induced obesity: lessons from rodent models of stress. Front Neurosci. 2013;7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson SE, Kirschbaum C, Steptoe A. Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity (Silver Spring). 2017;25(3):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trikudanathan S, McMahon GT. Optimum management of glucocorticoid-treated patients. Nat Clin Pract Endocrinol Metab. 2008;4(5):262–271. [DOI] [PubMed] [Google Scholar]
- 31.Schuff KG. Issues in the diagnosis of Cushing’s syndrome for the primary care physician. Prim Care. 2003;30(4):791–799. [DOI] [PubMed] [Google Scholar]
- 32.Fittschen C, Bellamy JE. Prednisone-induced morphologic and chemical changes in the liver of dogs. Vet Pathol. 1984;21(4):399–406. [DOI] [PubMed] [Google Scholar]
- 33.Iancu TC, Shiloh H, Dembo L. Hepatomegaly following short-term high-dose steroid therapy. J Pediatr Gastroenterol Nutr. 1986;5(1):41–46. [DOI] [PubMed] [Google Scholar]
- 34.Rogers WA, Ruebner BH. A retrospective study of probable glucocorticoid-induced hepatopathy in dogs. J Am Vet Med Assoc. 1977;170(6):603–606. [PubMed] [Google Scholar]
- 35.Schaer M, Ginn PE. Iatrogenic Cushing’s syndrome and steroid hepatopathy in a cat. J Am Anim Hosp Assoc. 1999;35(1):48–51. [DOI] [PubMed] [Google Scholar]
- 36.Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94–103. [DOI] [PubMed] [Google Scholar]
- 37.Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143(8):2852–2862. [DOI] [PubMed] [Google Scholar]
- 38.de Kloet ER, Joëls M. Brain mineralocorticoid receptor function in control of salt balance and stress-adaptation. Physiol Behav. 2017;S0031-9384(16)30565-0. [DOI] [PubMed] [Google Scholar]
- 39.Bahr V, Franzen N, Oelkers W, Pfeiffer AF, Diederich S. Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man. Eur J Endocrinol. 2006;155(6):845–848. [DOI] [PubMed] [Google Scholar]
- 40.Diederich S, Franzen NF, Bahr V, Oelkers W. Severe hyponatremia due to hypopituitarism with adrenal insufficiency: report on 28 cases. Eur J Endocrinol. 2003;148(6):609–617. [DOI] [PubMed] [Google Scholar]
- 41.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145(5):2174–2185. [DOI] [PubMed] [Google Scholar]
- 42.Warne JP, Akana SF, Ginsberg AB, Horneman HF, Pecoraro NC, Dallman MF. Disengaging insulin from corticosterone: roles of each on energy intake and disposition. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1366–R1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zennaro MC, Caprio M, Fève B. Mineralocorticoid receptors in the metabolic syndrome. Trends Endocrinol Metab. 2009;20(9):444–451. [DOI] [PubMed] [Google Scholar]
- 44.Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987;64(4):832–835. [DOI] [PubMed] [Google Scholar]
- 45.Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr Alzheimer Res. 2007;4(2):199–204. [DOI] [PubMed] [Google Scholar]
- 46.Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015;1(1):e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehman Q, Lane NE. Effect of glucocorticoids on bone density. Med Pediatr Oncol. 2003;41(3):212–216. [DOI] [PubMed] [Google Scholar]
- 48.Warriner AH, Saag KG. Glucocorticoid-related bone changes from endogenous or exogenous glucocorticoids. Curr Opin Endocrinol Diabetes Obes. 2013;20(6):510–516. [DOI] [PubMed] [Google Scholar]
- 49.Ceccato F, Barbot M, Albiger N, Zilio M, De Toni P, Luisetto G, Zaninotto M, Greggio NA, Boscaro M, Scaroni C, Camozzi V. Long-term glucocorticoid effect on bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2016;175(2):101–106. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann K, Koenen M, Schauer S, Wittig-Blaich S, Ahmad M, Baschant U, Tuckermann JP. Molecular actions of glucocorticoids in cartilage and bone during health, disease, and steroid therapy. Physiol Rev. 2016;96(2):409–447. [DOI] [PubMed] [Google Scholar]
- 51.Komori T. Glucocorticoid signaling and bone biology. Horm Metab Res. 2016;48(11):755–763. [DOI] [PubMed] [Google Scholar]
- 52.Yun SI, Yoon HY, Jeong SY, Chung YS. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3beta. J Bone Miner Metab. 2009;27(2):140–148. [DOI] [PubMed] [Google Scholar]
- 53.Whittier X, Saag KG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 2016;42(1):177–189, x (x). [DOI] [PubMed] [Google Scholar]
- 54.Jia D, O’Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147(12):5592–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. [DOI] [PubMed] [Google Scholar]
- 56.Ermis B, Ors R, Tastekin A, Ozkan B. Cushing’s syndrome secondary to topical corticosteroids abuse. Clin Endocrinol (Oxf). 2003;58(6):795–796. [DOI] [PubMed] [Google Scholar]
- 57.Fairris GM, White JE, Tymms DJ, Leatherdale BA. Cushing’s syndrome, topical steroids, and cling film. Lancet. 1986;2(8500):228. [DOI] [PubMed] [Google Scholar]
- 58.Messina MF, Valenzise M, Aversa S, Arrigo T, De Luca F. Iatrogenic Cushing syndrome caused by ocular glucocorticoids in a child. BMJ Case Rep. 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orton S, Censani M. Iatrogenic Cushing’s syndrome due to intranasal usage of ophthalmic dexamethasone: a case report. Pediatrics. 2016;137(5):137. [DOI] [PubMed] [Google Scholar]
- 60.Albert NE, Kazi S, Santoro J, Dougherty R. Ritonavir and epidural triamcinolone as a cause of iatrogenic Cushing’s syndrome. Am J Med Sci. 2012;344(1):72–74. [DOI] [PubMed] [Google Scholar]
- 61.Gillett MJ, Cameron PU, Nguyen HV, Hurley DM, Mallal SA. Iatrogenic Cushing’s syndrome in an HIV-infected patient treated with ritonavir and inhaled fluticasone. AIDS. 2005;19(7):740–741. [DOI] [PubMed] [Google Scholar]
- 62.Mahlab-Guri K, Asher I, Gradstein S, Zung A, Radian-Sade S, Elbirt D, Sthoeger Z. Inhaled fluticasone causes iatrogenic Cushing’s syndrome in patients treated with Ritonavir. J Asthma. 2011;48(8):860–863. [DOI] [PubMed] [Google Scholar]
- 63.Paton J, Jardine E, McNeill E, Beaton S, Galloway P, Young D, Donaldson M. Adrenal responses to low dose synthetic ACTH (Synacthen) in children receiving high dose inhaled fluticasone. Arch Dis Child. 2006;91(10):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sattar H, Manzoor J, Mirza L, Sheikh AM, Butt TA. Iatrogenic Cushing’s syndrome in children presenting at Children’s Hospital Lahore using nappy rash ointments. J Pak Med Assoc. 2015;65(5):463–466. [PubMed] [Google Scholar]
- 65.Sim D, Griffiths A, Armstrong D, Clarke C, Rodda C, Freezer N. Adrenal suppression from high-dose inhaled fluticasone propionate in children with asthma. Eur Respir J. 2003;21(4):633–636. [DOI] [PubMed] [Google Scholar]
- 66.Wilson AM, Blumsohn A, Jung RT, Lipworth BJ. Asthma and Cushing’s syndrome. Chest. 2000;117(2):593–594. [DOI] [PubMed] [Google Scholar]
- 67.Peters CJ, Ahmed ML, Storr HL, Davies KM, Martin LJ, Allgrove J, Grossman AB, Savage MO. Factors influencing skeletal maturation at diagnosis of paediatric Cushing’s disease. Horm Res. 2007;68(5):231–235. [DOI] [PubMed] [Google Scholar]
- 68.Scommegna S, Greening JP, Storr HL, Davies KM, Shaw NJ, Monson JP, Grossman AB, Savage MO. Bone mineral density at diagnosis and following successful treatment of pediatric Cushing’s disease. J Endocrinol Invest. 2005;28(3):231–235. [DOI] [PubMed] [Google Scholar]
- 69.Dupuis CC, Storr HL, Perry LA, Ho JT, Ahmed L, Ong KK, Dunger DB, Monson JP, Grossman AB, Besser GM, Savage MO. Abnormal puberty in paediatric Cushing’s disease: relationship with adrenal androgen, sex hormone binding globulin and gonadotrophin concentrations. Clin Endocrinol (Oxf). 2007;66(6):838–843. [DOI] [PubMed] [Google Scholar]
- 70.Lebrethon MC, Grossman AB, Afshar F, Plowman PN, Besser GM, Savage MO. Linear growth and final height after treatment for Cushing’s disease in childhood. J Clin Endocrinol Metab. 2000;85(9):3262–3265. [DOI] [PubMed] [Google Scholar]
- 71.Magiakou MA, Mastorakos G, Chrousos GP. Final stature in patients with endogenous Cushing’s syndrome. J Clin Endocrinol Metab. 1994;79(4):1082–1085. [DOI] [PubMed] [Google Scholar]
- 72.Davies JH, Storr HL, Davies K, Monson JP, Besser GM, Afshar F, Plowman PN, Grossman AB, Savage MO. Final adult height and body mass index after cure of paediatric Cushing’s disease. Clin Endocrinol (Oxf). 2005;62(4):466–472. [DOI] [PubMed] [Google Scholar]
- 73.Storr HL, Savage MO. Management of endocrine disease: paediatric Cushing’s disease. Eur J Endocrinol. 2015;173:R35–R45. [DOI] [PubMed] [Google Scholar]
- 74.Storr HL, Alexandraki KI, Martin L, Isidori AM, Kaltsas GA, Monson JP, Besser GM, Matson M, Evanson J, Afshar F, Sabin I, Savage MO, Grossman AB. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing’s disease. Eur J Endocrinol. 2011;164:667–674. [DOI] [PubMed] [Google Scholar]