Abstract
The tachykinins substance P (SP) and neurokinin A (Tac1) have emerged as novel regulators of kisspeptin/GnRH release. Recently, we documented that SP modulates reproductive function in the female mouse. Here, we extended this characterization to the male mouse. Tac1−/− male mice showed delayed puberty onset. They also presented significantly decreased expression levels of Pdyn (dynorphin) and Nos1 (nitric oxide synthase) in the mediobasal hypothalamus and elevated Gnrh1 levels. Unexpectedly, the response of Tac1−/− mice to central kisspeptin or senktide (neurokinin B receptor–agonist) administration was significantly decreased compared with controls, despite the preserved ability of GnRH neurons to stimulate luteinizing hormone release as demonstrated by central N-methyl-D-aspartate receptor administration, suggesting a deficit at the GnRH neuron level. Importantly, we demonstrated that kisspeptin receptor and SP receptor (NK1R) heterodimerize, indicating that changes in the SP tone could alter the responsiveness of GnRH neurons to kisspeptin. Finally, electrophysiological recordings from arcuate Kiss1 neurons showed that, although virtually all Kiss1 neurons responded to NKB and senktide, only half responded to an NK1R agonist and none to the neurokinin A receptor agonist at a 1-μM dose. In summary, we provide compelling evidence for a role of Tac1 in the control of reproductive function in the male mouse, suggesting a predominant central action that may involve a change in the balance of neural factors that control GnRH expression.
This work focuses on the role of Tac1 products (SP and NKA) on reproduction in male mice. Tac1 products are involved in puberty onset and facilitate full responsiveness of GnRH neurons to kisspeptin.
The members of the tachykinin family of peptides, comprising substance P (SP), neurokinin A (NKA), and neurokinin B (NKB) (1), have been shown to modulate GnRH release in a number of species in a process that is mediated, at least in part, through the stimulation of kisspeptin release at the hypothalamic level (2–4). NKB action in the control of the gonadotropic axis has been thoroughly characterized in the past two decades (5) and its critical role on reproduction demonstrated by (1) its ability to modulate GnRH release and (2) the identification of inactivating mutations in TAC3/TACR3 genes, encoding NKB and its receptor (NK3R) in humans, as the cause of hypogonadotropic hypogonadism (6–8). However, the roles of SP and NKA, encoded by Tac1, have received less attention. SP is expressed mainly in the central nervous system, where it has been suggested to be involved in the development of some psychiatric disorders and nociceptive processes (9). SP is widely distributed in the brain and is highly expressed in the hypothalamus in close contact with Kiss1 and GnRH neurons, suggesting a likely direct interaction on these neurons (3, 10–16). In this line, we have recently demonstrated that approximately half of Kiss1 neurons in the arcuate nucleus and a subset of GnRH neurons of the mouse express Tacr1 [encoding SP receptor (NK1R)], whereas neither of these populations of neurons express Tacr2 [encoding NKA receptor (NK2R)] (3). All tachykinins are able to significantly induce gonadotropin release in male and female mice under the proper sex steroid milieu in a process that, despite the presence of NK1R and NK3R in some GnRH neurons, appears to be kisspeptin dependent (3, 17, 18). We have also shown that SP plays a role in the control of puberty onset and estrous cyclicity in the female mouse (19), similar to what had been described for NKB (20); however, the contribution of the products of the Tac1 gene to the maturation of reproductive function and control of fertility in the male mouse remains unexplored. Puberty onset in female mammals typically occurs earlier than in males (21, 22), suggesting the existence of different mechanisms governing puberty onset in each sex and therefore justifying the need to assess whether the mechanisms involved in female maturation also take place in the male. In this study, we hypothesized that SP can directly activate Kiss1/NKB/dynorphin neurons in the male, and the lack of this activation will lead to impairments in the timing of sexual maturation and/or fertility in male mice.
Materials and Methods
Animal care
The animal studies were approved by the Harvard Medical Area Standing Committee on the Use of Animals in Research and Teaching in the Harvard Medical School Center for Animal Resources and Comparative Medicine. The mice were maintained under a 12:12-hour light/dark cycle and provided with standard rodent chow and water ad libitum. Tac1−/− mice were purchased from The Jackson Laboratories, stock No. 004103; see Cao et al. (23) for the knockout strategy. Kiss1-creGFP mice were obtained from The Jackson Laboratory, stock No. 017701 [see Gottsch et al. (24) for knockout strategy] and housed at Yale University. Electrophysiological studies in Kiss1-creGFP mice were performed following the Yale University Standing Committee on the Use of Animals in Research and Teaching.
Puberty assessment
Prepubertal littermate Tac1+/+ [wild-type (WT)] and Tac1−/− (knockout) males (n = 8 to 13 in each group) were monitored daily from postnatal day (PND) 21 for preputial separation as an indirect marker of puberty onset, and body weight was measured at the average age of puberty onset (PND28).
Fertility assessment
Tac1−/− and WT littermate mice (>PND75) were placed with proven fertile WT females. Time to deliver pups was monitored.
Characterization of the postgonadectomy response of luteinizing hormone
Bilateral removal of testes from 6- to 8-week-old males was performed with light isoflurane anesthesia. Briefly, the ventral skin was shaved and cleaned to perform one small incision in the skin and abdominal musculature of the abdomen. Once the gonads were identified and excised, the muscle incision was sutured and the skin was closed with surgical clips. Luteinizing hormone (LH) levels were measured in intact WT and Tac1−/− mice and 1 week after bilateral gonadectomy (GDX) (n = 5/group). Blood samples were collected by retro-orbital bleeding and serum stored at −20°C until hormonal determination.
Effect of central administration of N-methyl-D-aspartate receptor, senktide, or kisspeptin on gonadotropin release in adult male WT and Tac1−/− mice
Adult male mice (>PND75, n = 4 to 5/group) were anesthetized with isoflurane anesthesia and received 5 μL N-methyl-D-aspartate receptor (NMDA) (1 nmol; Tocris Bioscience), senktide (600 pmol; Tocris Biosience), kisspeptin 10 (1 nmol; Phoenix Pharmaceuticals), NK1R agonist (Ag) (GR 73632, 600 pmol; Tocris Biosience), or vehicle (0.9% NaCl) through an intracerebroventricular (ICV) injection, as previously described (25). Briefly, mice were anesthetized with isoflurane delivered by a vaporizer. On achieving a surgical plane of anesthesia, a small hole was bored in the skull 1 mm lateral and 0.5 mm posterior to bregma with a Hamilton syringe attached to a 27-gauge needle fitted with polyethylene tubing, leaving 3.5 mm of the needle tip exposed. Once the initial hole was made, all subsequent injections were made at the same site. Mice were allowed to recover for at least 2 days before treatment. For ICV injections, mice were anesthetized with isoflurane for a total of 2 to 3 minutes, during which time 5 μL of solution was slowly and continuously injected into the lateral ventricle. The needle remained inserted for approximately 60 seconds after the injection to minimize backflow up the needle track. Mice typically recovered from the anesthesia within 3 minutes after the injection. Blood samples (200 μL) were collected by retro-orbital bleeding (26) 25 minutes postinjection. The dose and time of collection were selected based on our previous studies (3).
Hormone assays
LH and follicle-stimulating hormone levels were measured using a Milliplex MAP immunoassay (Mouse Pituitary Panel; Millipore) in the Luminex 200 (27). Testosterone was measured through radioimmunoassay at the University of Virginia Ligand Core.
Quantitative real-time reverse transcription-polymerase chain reaction
We aimed to confirm the lack of Tac1 expression in Tac1−/− mice and determine if there are changes in the expression of Tacr1, Tacr2, Tac2, Tacr3, Kiss1, Pdyn, and Nos1 in the mediobasal hypothalamus (MBH), the site that includes the arcuate nucleus (ARC) as the likely hypothalamic area where the mechanisms that initiate puberty take place, and Kiss1r, Gnrh1, Tacr1, and Tacr3 in the preoptic area (POA). Intact >PND75 WT (n = 5) and Tac1−/− (n = 4) male mice were euthanized and brains were collected. The hypothalamic tissue was sectioned using a coronal brain matrix (Braintree Scientific) and immediately frozen in liquid nitrogen and stored at −80°C as described above (n = 3/group). Briefly, the rostral and caudal fragments, including the POA and the ARC, respectively, were isolated from each section under a dissection microscope with fine instruments by two bilateral parasagittal cuts, each 0.5 mm lateral to the midline between the optic chiasm and the posterior commissure, and one horizontal cut 1 mm dorsal of the ventral surface, yielding total tissue sizes of approximately 1 mm3 that were immediately frozen in dry ice and stored at −80°C (28). Total RNA from the MBH was isolated using TRIzol reagent (Invitrogen) followed by chloroform/isopropanol extraction. RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific), and 1 μg RNA was reverse transcribed using Superscript III cDNA synthesis kit (Invitrogen). Quantitative real-time polymerase chain reaction assays were performed in triplicates of each sample on an ABI Prism 7000 sequence detection system and analyzed using ABI Prism 7000 SDS software (Applied Biosystems). The cycling conditions were as follows: 2-minute incubation at 50°C, 10-minute incubation at 95°C (hot start), and 40 amplification cycles (95°C for 15 seconds, 60°C for 1 minute, and 45 seconds at 75°C, with fluorescence detection at the end of cycles 3 to 40), followed by the melting curve of the amplified products obtained by ramped increase of the temperature from 55°C to 95°C to confirm the presence of a single-amplification product per reaction. The primers used are listed in Table 1. The data were normalized using L19 primers as an internal control (19) and expressed as fold-change relative to the WT value.
Table 1.
Primers Used for Polymerase Chain Reaction Assays
| Gene Name | Primer Sequence | Gene Accession Number |
|---|---|---|
| Gnrh1 | F: GGGAAAGAGAAACACTGAACA | NM_008145.2 |
| R: TCTGCCATTTGATCCACCTC | ||
| Kiss1R | F: GGTGCTGGGAGACTTCATG | NM_053244.5| |
| R: ACATACCAGCGGTCCACAC | ||
| Nos1 | F: TCGATGCCAAGGCTATGTCC | NM_008712.2 |
| R: CGGACCTTGTAGCTCTTCCTC | ||
| Tac1 | F: ATGAAAATCCTCGTGGCCGT | NM_009311.2 |
| R: GTTCTGCATCGCGCTTCTTT | ||
| Tacr1 | F: GTCTGCCAAGAGCCAAGAAC | NM_009313 |
| R: CCAGCCACATCTGAGAGACA | ||
| Tacr2 | F: TCAACTTCATCTATGCCAGTCAC | NM_009314 |
| R: ATGACAGCAATAACCGCCTTG | ||
| Tac2 | F: GCTCCACAGCTTTGTCCTTC | NM_001199971.1 |
| R: GCTAGCCTTGCTCAGCACTT | ||
| Tacr3 | F: GCCATTGCAGTGGACAGGTAT | NM_021382.6 |
| R: ACGGCCTGGCATGACTTTTA | ||
| Kiss1 | F: CTCTGTGTCGCCACCTATGC | AF472576.1| |
| R: TTCCCAGGCATTAACGAGTTC | ||
| Pdyn | F: ACAGGGGGAGACTCTCATCT | NM_018863.4 |
| R: GGGGATGAATGACCTGCTTACT |
Abbreviations: F, forward; R, reverse.
Coimmunoprecipitation
HEK-293T cells were maintained in Dulbecco’s modified Eagle medium (Corning) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). For the coimmunoprecipitation studies, cells were seeded into a 6-well plate and transfected at 60% to 70% confluence with 1 μg pcDNA encoding hKISS1R or hTACR1, with a 3X-HA or 3X-FLAG tag at the carboxyterminal end, using Lipofectamine 2000 (Invitrogen) following the manufacturer’s recommended protocol. To ensure transfection efficiency, we monitored fluorescence from cotransfected plasmids encoding GFP or mCherry. Two days later, cells were washed three times with ice-cold phosphate-buffered saline, then lysed with M-PER lysis buffer (Pierce) containing protease inhibitor cocktail (Roche). After incubation on ice for 30 minutes, the lysates were centrifuged and supernatants were harvested. After preclearing with 30 μL protein A agarose beads (Invitrogen) at 4°C for 1 hour, supernatants were incubated overnight at 4°C with anti-HA antibody (Abcam) and protein A agarose beads. The beads were collected via centrifugation and washed three times, and then proteins were eluted for 1 hour at room temperature using sodium dodecyl sulfate–polyacrylamide gel electrophoresis protein sample buffer. Proteins were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and detected by immunoblotting using antibodies as indicated. Plasmids and antibodies are listed in Tables 2 and 3.
Table 2.
Plasmids Used for In Vitro Coimmunoprecipitation Assays
| Plasmid | Species |
|---|---|
| pcDNA3.1 | |
| pcDNA-hKISS1R-3XFLAG | Human |
| pcDNA-hKISS1R-3XHA | Human |
| pcDNA-hTACR1-3XFLAG | Human |
| pcDNA-hTACR1-3XHA | Human |
| pcDNA-hGABBR1-3XFLAG | Human |
Abbreviation: GABBR1, γ-aminobutyric acid type B receptor subunit.
Table 3.
Antibodies Used
| Antibodies (Source) | Catalog No. | Company | Usage | RRID |
|---|---|---|---|---|
| Anti-HA (rabbit) | ab9110 | Abcam | IP (0.5–1 μg) | AB_307019 |
| Anti-HA (mouse) | ab18181 | Abcam | WB (1:5000) | AB_444303 |
| Anti-FLAG-HRP (mouse) | A8592 | Sigma-Aldrich | WB (1:3000) | AB_439702 |
| Anti-mouse (donkey) | sc-2314 | Santa Cruz Biotech | WB (1:5000) | AB_641170 |
Abbreviations: IP, immunoprecipitation; RRID, research resource identifier; WB, Western blot.
Slice preparation and recordings from Kiss1 neurons
Animals were anesthetized with chloral hydrate (400 mg/kg intraperitoneally) and euthanized by decapitation between 1200 and 1900 hours. After decapitation, brains were removed, placed in a Petri dish containing artificial cerebrospinal fluid (aCSF), and trimmed to yield a small block containing the region of interest. Coronal slices of 300 μm thickness were obtained with a Vibratome 1500 (Vibratome Co.) and transferred to a Plexiglass recording chamber (1.5 mL) on the fixed stage of an Olympus BX50WI scope for visualized whole-cell recording. The chamber was perfused continuously with normal aCSF at a rate of 2 to 3 mL/min, with its temperature maintained at 33 ± 0.5°C. One to 2 hours later, the slice was used for recording.
Whole-cell current and voltage-clamp recordings were performed through the use of previously described methods (29). The low-resistance (2.5 to 3.5 mΩ) patch pipettes were filled with a solution containing 125 mm K gluconate, 10 mm HEPES, 5 mm [1,2-bis(o-aminophenoxy)ethane-N,N,N′N-tetraacetic acid] K4, 2.38 mm CaCl2, 4 mm Mg-ATP, 10 mm Na phosphocreatine, and 0.3 mm Na2-GTP (pH 7.32 to 7.35). Cell-attached recordings were performed by using patch pipettes filled with aCSF. Data were acquired using an Axoclamp-2B and pClamp 9 (Axon Instruments). No correction was made for the calculated liquid junction potential of approximately 11 mV for the internal solution.
A medium of aCSF containing 128 mm NaCl, 3 mm KC, 1.25 mm NaH2PO4, 10 mm d-glucose, 26 mm NaHCO3, 2 mm CaCl2, and 2 mm MgCl2 (pH 7.35 to 7.38) was prepared and equilibrated with 95% O2–5% CO2. NKB, GR73632 (the NK1R-A), GR64349 (the NK2R-A), and senktide (purchased from Tocris) were diluted in aCSF from previously prepared stock solutions that were stored at −20°C. Agonists were applied through a Y-tube (30).
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM) for each group. A two-tailed unpaired Student t-test or a one- or two-way analysis of variance test followed by a Tukey or Newman Kleus post hoc test was used to assess variation among experimental groups. Significance level was set at P < 0.05. All analyses were performed with GraphPad Prism software.
Results
Electrophysiological responses of Kiss1 neurons to specific agonists of tachykinin receptors
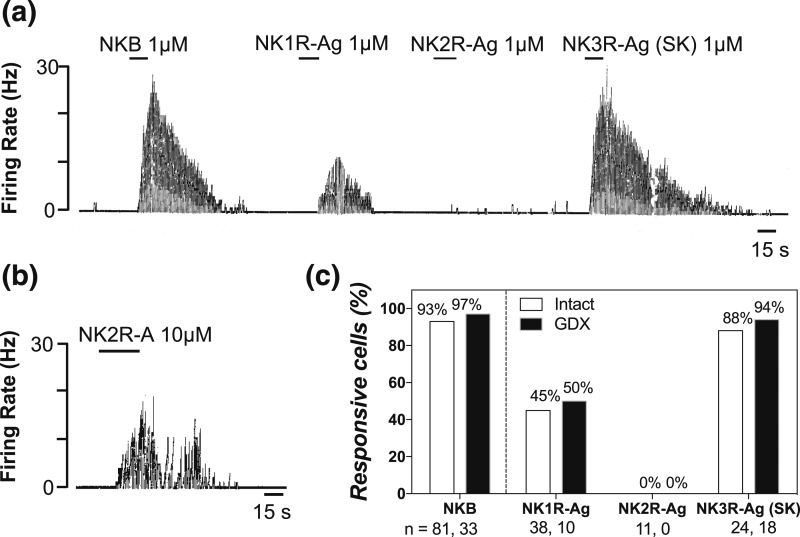
All three tachykinins induce gonadotropin release in male mice (3). To address whether Kiss1 neurons in the male mouse can respond to all tachykinins directly, we assessed the response of GFP-labeled ARC Kiss1 neurons to a sequential 15-second application of specific agonists of each tachykinin receptor. Brain slices were prepared from intact (n = 59) and GDX (n = 23) male mice. Cell-attached recordings showed that NKB (0.1–1 μM, 15 seconds), used as a positive control, increased the firing rate of 93% and 97% of Kiss1 neurons tested in the intact (n = 81) and GDX (n = 33) groups, respectively [Fig. 1(a) and 1(c)]. Similarly, virtually all Kiss1 neurons responded to senktide (0.1 to 1 μM, 15 seconds) regardless of the gonadal state (88% intact, n = 24; 94% GDX, n = 18) [Fig. 1(a) and 1(c)]. However, only about half of Kiss1 neurons responded to the NK1R-Ag (0.1 to 1 μM, 15 seconds) (45% intact, n = 18; 50% GDX, n = 10) [Fig. 1(a) and 1(c)] and none of them to the NK2R-Ag (0.1 to 1 μM, 15 seconds) [Fig. 1(c)]. Interestingly, even when we further assessed the response to NK2R-Ag at a higher dose (10 μM, 15 seconds), we observed that only three of 10 Kiss1 neurons (from six intact males) showed activation after the application of the agonist [Fig. 1(b)].
Figure 1.
(a) A continuous trace depicting an electrophysiological cell-attached recording from ARC Kiss1-creGFP neurons exposed to 15 seconds of 1 μM NKB, GR73632 (NK1R-Ag), GR64349 (NK2R-Ag), and senktide (NK3R-Ag) in a sequential manner. (b) A cell that responded to a higher concentration of NK2R agonist. (c) Bar graph depicting the percentage of Kiss1 responsive cells.
Assessment of pubertal development and fertility of Tac1−/− male mice
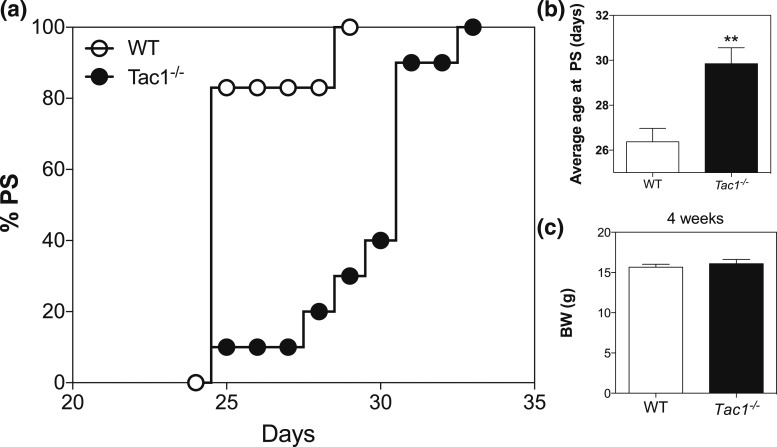
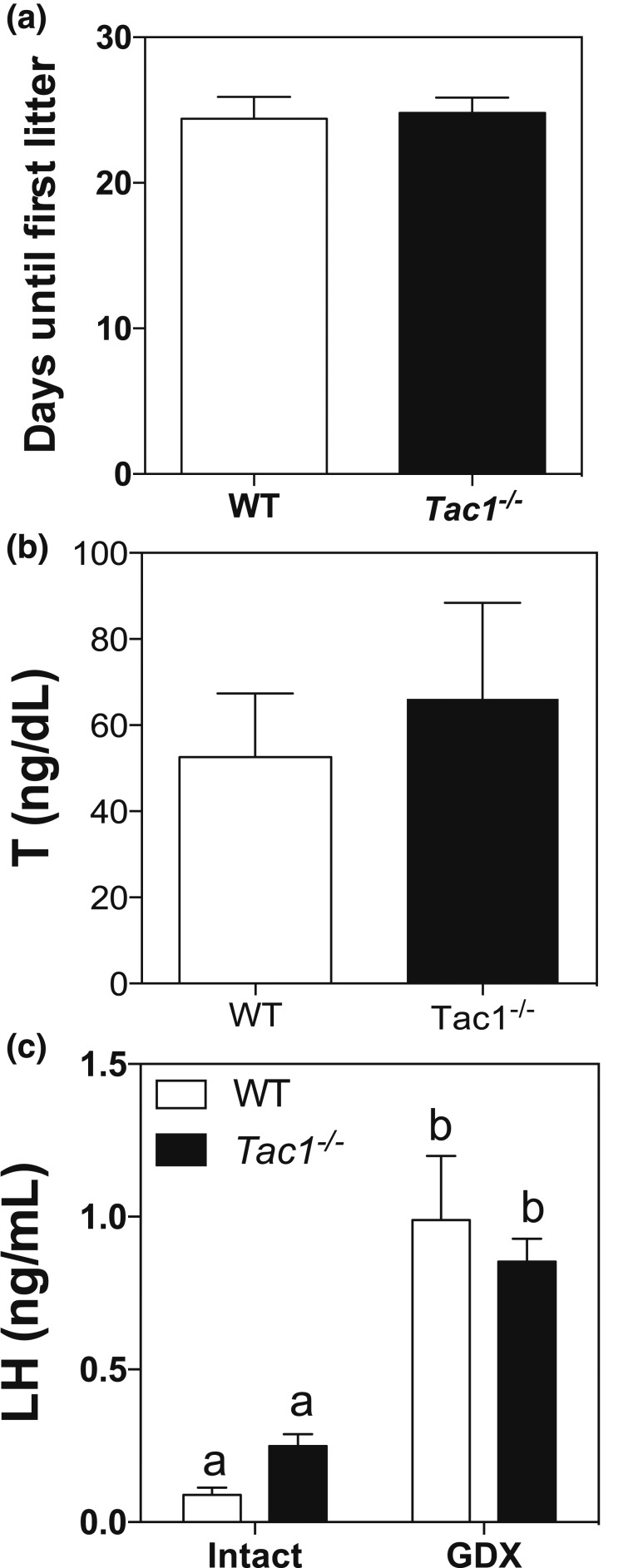
To determine whether the absence of a functional Tac1 gene leads to a delay in puberty onset and a reproductive defect as in females (19), male Tac1−/− mice were monitored daily after weaning (PND21). Indeed, knockout mice showed a significant delay in preputial separation compared with controls (controls: 26.38 ± 0.59, n = 8 vs Tac1−/−: 29.85 ± 0.71, n = 13; P = 0.0032, t = 3.374, df = 19) [Fig. 2(a) and 2(b)] despite similar body weight [Fig. 2(c)]. In addition, to test whether these animals are fertile, we mated them with WT females and observed that the time until first litter was similar in WT and Tac1−/− mice [Fig. 3(a)]. Serum testosterone levels were also similar to control mice [Fig. 3(b)].
Figure 2.
Pubertal progression of WT and Tac1−/− male mice. (a) Percentage of mice with preputial separation. (b) Age of preputial separation. (c) Body weight at 4 weeks. Student t test (**P < 0.01). BW, body weight; PS, preputial separation.
Figure 3.
(a) Fertility assessment of Tac1−/− male mice (Student t test). (b) Testosterone levels in intact adult mice. (c) Basal and GDX LH levels of WT and Tac1−/− male mice. Different letters indicate statistically significant differences. Statistical analysis was performed using two-way analysis of variance followed by Newman-Keuls post hoc test.
Characterization of the postgonadectomy response of LH
The previous experiment evidenced a delay in puberty onset in Tac1−/− mice. Therefore, we proceeded to assess whether these mice exhibit any central deficit in GnRH release. To this aim, mice were gonadectomized as a model of maximal expression and release of kisspeptin (and therefore GnRH) when the negative feedback of sex steroids is removed (31, 32). LH levels in intact WT (n = 8) and Tac1−/− (n = 8) mice were measured and compared with 1 week after GDX. Interestingly, both groups of animals displayed a similar compensatory rise of LH levels with no significant difference between the two genotypes [Fig. 3(c)], suggesting normal increases in kisspeptin and GnRH release following testosterone withdrawal. Interestingly, Tac1−/− mice showed a trend to higher basal levels of LH, which became significant if analyzed by a Student t test (P = 0.0034, t = 3.271, df = 23).
Effect of central administration of NMDA, senktide, or kisspeptin on gonadotropin release in intact adult male WT and Tac1−/− mice
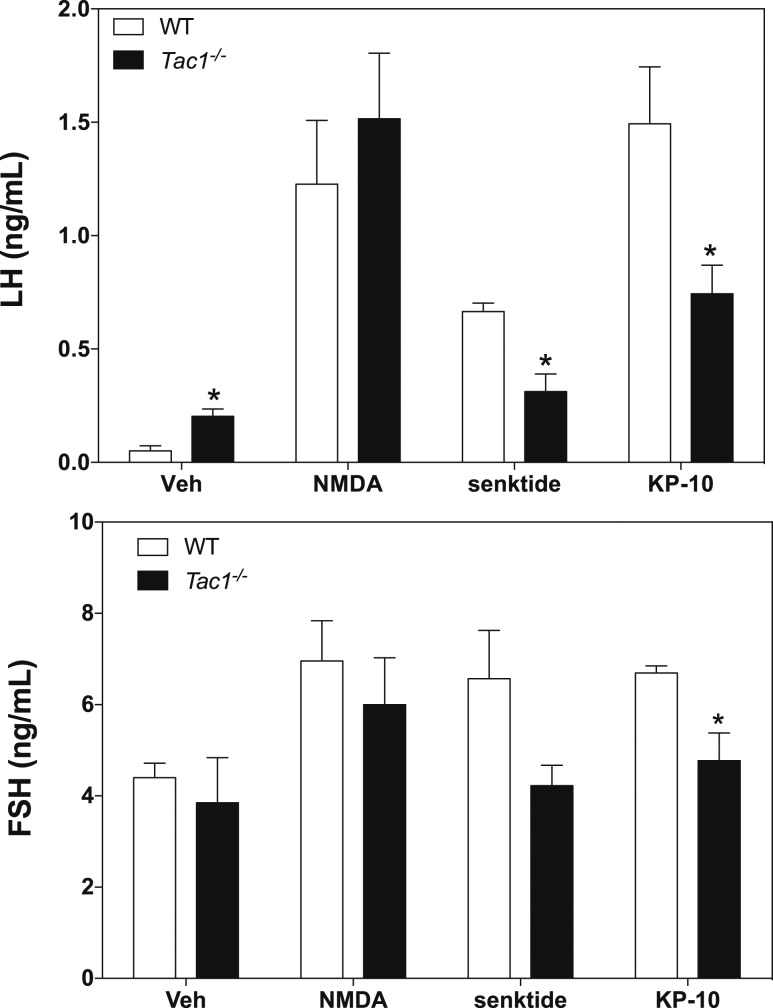
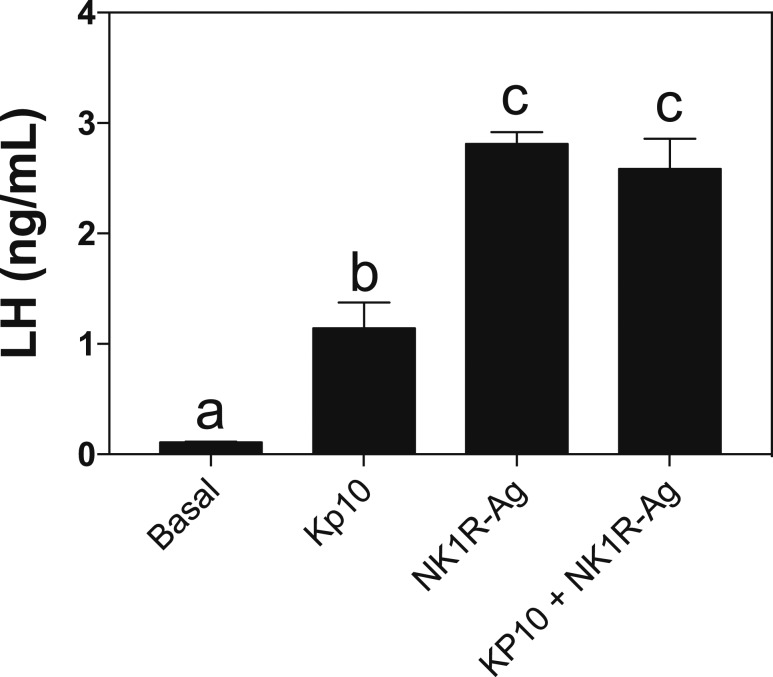
Puberty onset is mostly driven by the combination of increased stimulatory and reduced inhibitory hypothalamic factors that ultimately induce the increase in GnRH release. Thus, the delay in puberty onset observed in Tac1−/− mice suggests the existence of a central defect in knockout animals. To assess this further, we tested the ability of GnRH neurons to respond to known direct (NMDA and kisspeptin) and indirect (senktide) stimulators in WT (n = 5) and Tac1−/− (n = 4) mice. Interestingly, although both groups responded similarly to NMDA with a robust increase in LH release (Fig. 4) (P = 0.52), Tac1−/− mice showed half of the response of controls to senktide (P = 0.006, t = 4.08, df = 6) and kp-10 (P = 0.037, t = 2.66, df = 6) (Fig. 4, upper panel). In terms of follicle-stimulating hormone release, knockout mice tended to respond less than controls to senktide. This difference was significant after kp-10 treatment (P = 0.045, t = 2.64, df = 5) (Fig. 4, lower panel). To determine whether Tac1−/− mice require the activation of NK1R to increase their responsiveness to kp-10, we tested the action of the NK1R-Ag and kp-10 independently and in combination in Tac1−/− mice (n = 4). We observed that the response of knockout animals to kp-10 is approximately half of that evoked by NK1R-Ag, replicating the effect seen in Fig. 4, and the coadministration of both did not induce any further increase in GnRH release (Fig. 5).
Figure 4.
Serum LH (upper) and FSH (lower) values of adult mice 25 minutes after central ICV injection of 1 nmol NMDA, 600 pmol senktide, and 1 nmol kp-10. Statistical analysis was performed using two-way analysis of variance followed by Newman-Keuls post hoc test to compare all groups with their respective controls (*P < 0.05). FSH, follicle-stimulating hormone.
Figure 5.
Serum LH values of adult Tac1−/− male mice 25 minutes after central ICV injection of 1 nmol Kp-10, 600 pmol NK1R-Ag, or the coadministration of both. Different letters indicate statistically significant differences. Statistical analysis was performed using one-way analysis of variance followed by Tukey post hoc test.
Analysis of the expression of genes involved in the central control of GnRH release
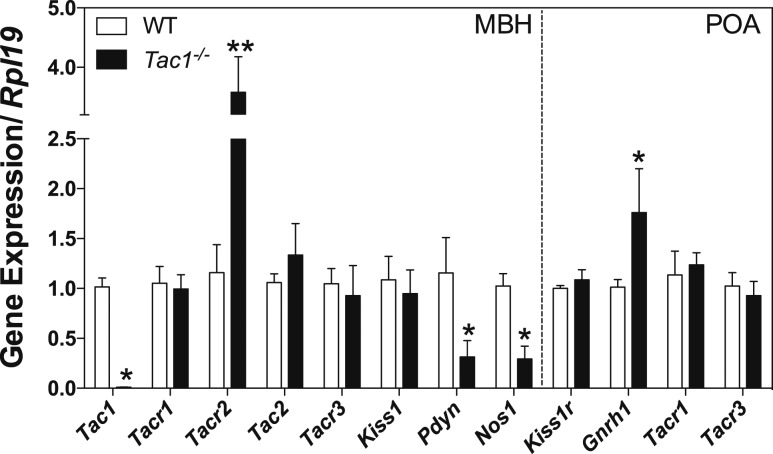
To investigate the mechanism for the decreased response of Tac1−/− mice to senktide and kp-10, we examined the expression in adult males of genes in the MBH encoding the ligands and receptors of the tachykinin system, kisspeptin and its cotransmitter dynorphin (Pdyn), nitric oxide synthase (Nos1) as nitric oxide has been implicated in the amplification of SP effects (33–35), and the expression in the POA of genes encoding for Kiss1r, GnRH, and NK1R and NK3R [also identified in GnRH neurons (3)] in male WT (n = 5) and Tac1−/− (n = 4) mice (Fig. 6). Tac1−/− mice, as expected, did not show any expression of Tac1. Interestingly, although the expression of the SP receptor (Tacr1) and NKB receptor (Tacr3) did not change between controls and knockouts, NKA receptor (Tacr2) displayed a significant increase in Tac1−/− mice (P < 0.001, t = 7.55, df = 76). Pdyn and Nos1 expression were significantly reduced in Tac1−/− mice (Pdyn: P = 0.015, t = 2.488, df = 76; Nos1: P = 0.03, t = 2.16, df = 76). Within the POA, neither Kiss1r nor Tacr1 and Tacr3, described in GnRH neurons (3), showed any significant change. However, Gnrh1 was increased in Tac1−/− mice (P = 0.022, t = 2.33, df = 76).
Figure 6.
Expression profile of hypothalamic genes involved in the control of GnRH secretion in the MBH and POA of WT and Tac1−/− male mice. Two-way analysis of variance followed by Newman-Keuls post hoc test was performed to compare all groups with their respective controls (*P < 0.05; **P < 0.001).
Analysis of the interaction Between KISS1R and NK1R
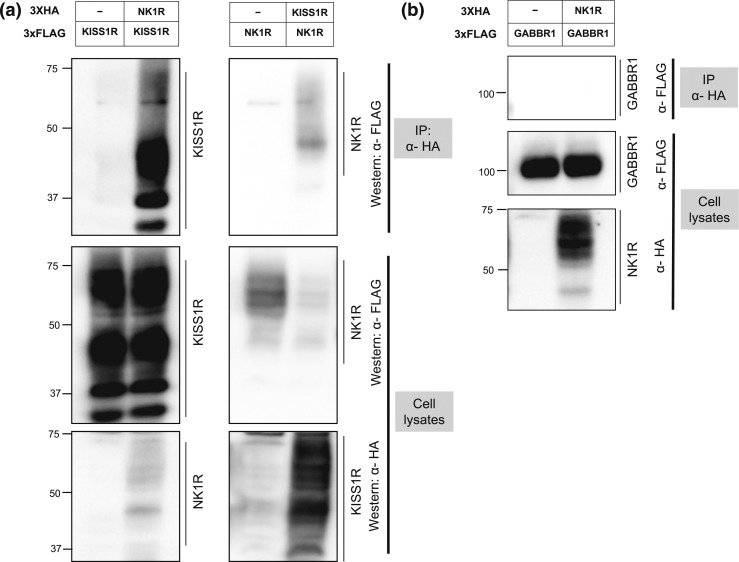
SP exerts a priming effect on NK1R (36). This receptor has been described to form heterodimers with other G protein-coupled receptors, such as the mu opioid receptor, whose ability to respond to opioids is highly regulated by its dimerization with NK1R (37, 38). Moreover, NK1R displays a slow recycling process after internalization and prolonged retention of β-arrestins in endosomes (39). We hypothesized that NK1R and KISS1R might form heterodimers in GnRH neurons that could, at least in part, contribute to the diminished response of GnRH neurons to kisspeptin observed in Tac1−/− mice. G protein-coupled receptor dimeric complexes exhibit specific functional properties that are distinct from monomeric receptors, involving differences in agonist recognition, intracellular signaling, or receptor trafficking. We tested the ability of NK1R and KISS1R to heterodimerize in a heterologous transient transfection system. Indeed, coimmunoprecipitation studies using vectors expressing HA- or FLAG-tagged human NK1R and human KISS1R provide evidence that these receptors can heterodimerize in vitro [Fig. 7(a)]. Furthermore, this interaction is specific, because NK1R did not heterodimerize with gamma-aminobutyric acid type B receptor subunit 1, a G protein-coupled receptor used as negative control [Fig. 7(b)].
Figure 7.
Coimmunoprecipitation of KISS1R and NK1R. (a) Left panels: an expression vector encoding KISS1R-3XFLAG was cotransfected with or without an expression vector encoding NK1R-3XHA. Right panels: NK1R-3XFLAG was cotransfected with or without KISS1R-3XHA. Both studies were done by transient transfection in HEK-293T cells. Cells were lysed and HA-immunoprecipitates (upper panels) or total proteins (lower panels) were analyzed by Western blot using anti-FLAG or anti-HA antibodies as indicated. Both KISS1R and NK1R form multiple bands, which likely represent variable posttranslational modifications such as glycosylation (40). (b) An expression vector encoding hGABBR1-3XFLAG was cotransfected with or without NK1R-3XHA, followed by HA-immunoprecipitation and Western blot analysis using anti-FLAG or anti-HA antibodies as indicated, as a negative control. GABBR1, γ-aminobutyric acid type B receptor subunit 1; IP, immunoprecipitation.
Discussion
We recently demonstrated that all three tachykinins are able to modulate gonadotropic response in the mouse (3, 19). Importantly, our studies in female mice showed that not only are Kiss1 neurons a direct target for NKB and SP, but a subset of GnRH neurons also expresses these receptors (3), despite the apparently kisspeptin-dependent action reported (3, 17, 18). Kiss1 neurons in the ARC are a nodal regulatory center of GnRH release (41); however, whether these neurons in the male brain, subjected to a different sexual differentiation process, are direct targets to SP and NKA remained unexplored. Here, we show that the percentage of neurons that show neuronal firing to each receptor agonist in males and the percentage of neurons that express each tachykinin receptor in female (3) mice are highly correlative (i.e., virtually all ARC Kiss1 neurons express NK3R and respond to NKB/senktide, around half express NK1R and respond to NK1R-Ag, and none express NK2R or respond to the NK2R-Ag), suggesting that there is no sexual differentiation in the ratio of tachykinin receptors in ARC Kiss1 neurons. There is some discrepancy between these results and a previous study by de Croft et al. (42), who showed that all endogenous agonists of the tachykinin receptors (SP, NKA, NKB) can activate Kiss1 neurons. Given the evidence for cross-reactivity between the tachykinin receptor systems (1), our findings suggest that in the previous study (42), NKA may have activated Kiss1 neurons through NK1R or NK3R.
The similarity of the Tac1 system between both sexes is further substantiated by our puberty onset studies. As previously reported in female mice (19), the constitutional absence of the Tac1 gene leads to delayed sexual maturation in the male. Puberty onset is considered a centrally driven process (22); therefore, although Tac1 is expressed in a variety of tissues, the absence of SP (and NKA) must interfere with the proper timing of GnRH release, although the exact mechanism of action underlying this process remains unknown. Interestingly, once puberty is achieved, Tac1 null mice display normal reproductive function, as they can father litters and present normal testosterone levels, but whether this is the consequence of compensatory mechanisms that develop in adulthood is unknown.
We next focused on the characterization of the central processes that govern GnRH release and that may be altered in Tac1−/− mice. Tac1−/− mice showed similar LH responses to the central administration of the glutamate ionotropic receptor agonist NMDA, suggesting that GnRH neurons (and pituitary gonadotropes) are functional and able to respond normally. Surprisingly, the response of LH to kisspeptin was reduced to approximately half of that in controls. This was a striking finding because kisspeptin action on GnRH neurons is direct (43). Moreover, senktide evoked a similarly decreased LH response in the Tac1−/− mice, likely due to the kisspeptin-mediated action of the NKB pathway (3, 17, 18). This reduction was also significant for the follicle-stimulating hormone response after kisspeptin treatment. Interestingly, gonadectomy in these mice did not lead to the same decrease in the ability of the animal to respond to high kisspeptin levels, as expected after GDX. It is possible that the continued exposure to high kisspeptin levels over 1 week in our GDX model achieves sufficient stimulation, or priming effect, to normalize the gonadotropin response in both models, which is otherwise absent after acute central stimulation. We hypothesized that this blunted response to kisspeptin acutely could be a consequence of the decreased expression of the kisspeptin receptor in GnRH neurons, but both groups of animals showed similar expression levels of Kiss1r in the POA, where GnRH neurons are located. Admittedly, RNA expression levels may not reflect protein content, which cannot be determined for Kiss1r due to the absence of a reliable antibody. Nonetheless, our expression studies showed a significant decrease in expression levels for the inhibitory signal Pdyn and nitric oxide synthase Nos1, which could account for the modest—but significant—increase in Gnrh1 expression observed in knockouts. This increase in Gnrh1 expression, despite the fact that expression and protein levels may differ, as mentioned previously, is in keeping with the higher basal LH levels in Tac1−/− mice than in controls, consistent with the slight but significant removal of direct inhibitory signals on GnRH neurons in the basal (nonstimulated) state. In addition, Tac1−/− mice showed a specific increase in NKA receptor expression (Tacr2) in the MBH area compared with controls, whereas the expression of Tacr1 and Tacr3 remained unchanged. These changes may reflect an attempt to compensate for the lack of NKA, as described for other neuronal ligand-receptor systems (44) or the absence of some downstream response of NK2R activation, and the resultant loss of negative feedback from that downstream response. This phenomenon provides further evidence of differences between NK1R/NK3R and NK2R in terms of location and regulation.
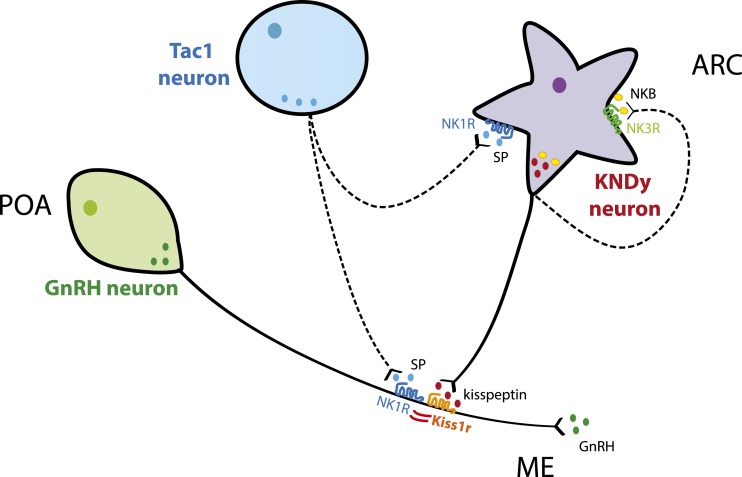
These experiments uncovered a paradoxical scenario in which GnRH neurons, which release GnRH normally after glutamate stimulation, show a significant decrease in the response to a direct activator (i.e., kisspeptin) when a second ligand (i.e., SP) is not present, suggesting that an interaction between these two receptors may occur at the cellular level. Although the expression of these receptors in GnRH neurons is different, with Kiss1r present in virtually all neurons and NK1R in only a subset of neurons (3), it has been described that a minimal fraction of GnRH neurons is required for reproduction (45), which allows the possibility of a significant biological effect to the subpopulation of GnRH neurons coexpressing Kiss1r and NK1R. Here, we have confirmed that these two receptors heterodimerize in vitro. To our knowledge, this finding offers the first insight into a novel level of regulation of kisspeptin action, and therefore reproductive function, by linking SP signaling from Tac1 neurons with kisspeptin signaling at the level of GnRH neurons (Fig. 8). Interestingly, Kiss1r and NK1R have already been described to form heterodimers with other GPCRs [e.g., GnRHR (46) and mu opioid receptor (37), respectively]. The present results suggest that the lack of SP priming of NK1R (36) may extend the recycling time of Kiss1r/NK1R heterodimers, as described for mu opioid receptor/NK1R (39), resulting in lower cell surface Kiss1r levels available to respond to an acute kisspeptin challenge. However, the confirmation of this hypothesis in vivo is limited by the absence of reliable Kiss1r antibodies.
Figure 8.
Schematic representation of the hypothetical interaction between Tac1, Kiss1/NKB/Dynorphin (KNDy), and GnRH neurons in the caudal part of the hypothalamus. SP may modulate GnRH by (1) stimulating kisspeptin release at the KNDy neurons level and (2) acting directly on GnRH neurons through the activation of NK1R and the modulation of Kiss1r via heterodimerization of both receptors.
In sum, we offer compelling evidence for a role of the products of the Tac1 gene in the control of puberty onset and fertility in male mice. This action is at least in part direct on Kiss1 neurons for SP but indirect on unknown intermediate neurons for NKA. Furthermore, we have uncovered a novel regulatory level of kisspeptin responses, previously unknown to our knowledge, that entails the intracellular regulation of a Kiss1r second-messenger pathway by the formation of heterodimers with NK1R. This heterodimerization remains to be further investigated in vivo to fully understand the contribution of this regulatory pathway to the overall response of GnRH neurons to kisspeptin.
Acknowledgments
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research grants from the National Institutes of Health (NIH); NIH Grants R01 HD019938 and HD082314 to U.B.K. and R00 HD071970 to V.M.N.; Charles H. Hood Foundation for Child Health Research Program to V.M.N.; and the Microgrant Program from The Biomedical Research Institute and the Center for Faculty Development and Diversity’s Office for Research Careers at the Brigham and Women’s Hospital to V.M.N.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- Ag
- agonist
- ARC
- arcuate nucleus
- GDX
- gonadectomy
- ICV
- intracerebroventricular
- LH
- luteinizing hormone
- MBH
- mediobasal hypothalamus
- NK1R
- substance P receptor
- NK2R
- neurokinin A receptor
- NK3R
- neurokinin B receptor
- NKA
- neurokinin A
- NKB
- neurokinin B
- NMDA
- N-methyl-D-aspartate receptor
- PND
- postnatal day
- POA
- preoptic area
- SP
- substance P
- WT
- wild-type.
References
- 1.Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32(9):1972–1978. [DOI] [PubMed] [Google Scholar]
- 2.Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, Carroll RS, Seminara SB, Tena-Sempere M, Rønnekleiv OK, Kaiser UB. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99(1):18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2008;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295. [DOI] [PubMed] [Google Scholar]
- 8.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–272. [DOI] [PubMed] [Google Scholar]
- 10.Harlan RE, Garcia MM, Krause JE. Cellular localization of substance P- and neurokinin A-encoding preprotachykinin mRNA in the female rat brain. J Comp Neurol. 1989;287(2):179–212. [DOI] [PubMed] [Google Scholar]
- 11.Hökfelt T, Pernow B, Nilsson G, Wetterberg L, Goldstein M, Jeffcoate SL. Dense plexus of substance P immunoreactive nerve terminals in eminentia medialis of the primate hypothalamus. Proc Natl Acad Sci USA. 1978;75(2):1013–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makara GB, Kakucska I, Lenoir V, Kerdelhue B, Palkovits M. A substance P-containing hypothalamic neuronal system projects to the median eminence. Brain Res. 1986;374(2):399–401. [DOI] [PubMed] [Google Scholar]
- 13.Rance NE, Young WS III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 14.Rønnekleiv OK, Kelly MJ, Eskay RL. Distribution of immunoreactive substance P neurons in the hypothalamus and pituitary of the rhesus monkey. J Comp Neurol. 2004;224(1):51–59. [DOI] [PubMed] [Google Scholar]
- 15.Tsuruo Y, Hisano S, Okamura Y, Tsukamoto N, Daikoku S. Hypothalamic substance P-containing neurons: sex-dependent topographical differences and ultrastructural transformations associated with stages of the estrous cycle. Brain Res. 1984;305(2):331–341. [DOI] [PubMed] [Google Scholar]
- 16.Tsuruo Y, Kawano H, Hisano S, Kagotani Y, Daikoku S, Zhang T, Yanaihara N. Substance P-containing neurons innervating LHRH-containing neurons in the septo-preoptic area of rats. Neuroendocrinology. 1991;53(3):236–245. [DOI] [PubMed] [Google Scholar]
- 17.García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. [DOI] [PubMed] [Google Scholar]
- 18.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, Millar RP, Lightman SL, O’Byrne KT. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7(9):e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, Kaiser UB, Navarro VM. Substance P regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212–E1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojeda SR, Lomniczi A, Sandau U, Matagne V. New concepts on the control of the onset of puberty. Endocr Dev. 2009;17:44–51. [DOI] [PubMed] [Google Scholar]
- 23.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–394. [DOI] [PubMed] [Google Scholar]
- 24.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 26.van Herck H, Baumans V, Brandt CJ, Hesp AP, Sturkenboom JH, van Lith HA, van Tintelen G, Beynen AC. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim. 1998;32(4):377–386. [DOI] [PubMed] [Google Scholar]
- 27.Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, Wondisford FE, Radovick S. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol Reprod. 2009;81(3):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5(7):e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M, Hajszan T, Xu C, Leranth C, Alreja M. Group I metabotropic glutamate receptor activation produces a direct excitation of identified septohippocampal cholinergic neurons. J Neurophysiol. 2004;92(2):1216–1225. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003;18(5):1155–1168. [DOI] [PubMed] [Google Scholar]
- 31.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. [DOI] [PubMed] [Google Scholar]
- 33.Andoh T, Kuraishi Y. Nitric oxide enhances substance P–induced itch-associated responses in mice. Br J Pharmacol. 2003;138(1):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joad JP, Kott KS, Bonham AC. Nitric oxide contributes to substance P–induced increases in lung rapidly adapting receptor activity in guinea-pigs. J Physiol. 2004;503(Pt 3):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Shaughnessy MC, Vetsika EK, Inglis JJ, Carleson J, Haigh R, Kidd BL, Winyard PG. The effect of substance P on nitric oxide release in a rheumatoid arthritis model. Inflamm Res. 2006;55(6):236–240. [DOI] [PubMed] [Google Scholar]
- 36.Lai JP, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM, Douglas SD. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci USA. 2006;103(20):7771–7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schröder H, Höllt V, Schulz S. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278(51):51630–51637. [DOI] [PubMed] [Google Scholar]
- 38.Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405(6783):180–183. [DOI] [PubMed] [Google Scholar]
- 39.Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J Neurosci. 2009;29(1):222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abreu AP, Noel SD, Xu S, Carroll RS, Latronico AC, Kaiser UB. Evidence of the importance of the first intracellular loop of prokineticin receptor 2 in receptor function. Mol Endocrinol. 2012;26(8):1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1316. [DOI] [PubMed] [Google Scholar]
- 42.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 43.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2005;80(4):264–272. [DOI] [PubMed] [Google Scholar]
- 44.Condic ML, Letourneau PC. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature. 1997;389(6653):852–856. [DOI] [PubMed] [Google Scholar]
- 45.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quaynor S, Hu L, Leung PK, Feng H, Mores N, Krsmanovic LZ, Catt KJ. Expression of a functional g protein-coupled receptor 54-kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol. 2007;21(12):3062–3070. [DOI] [PubMed] [Google Scholar]