Abstract
Endurance exercise has been shown to improve lipid oxidation and increase mitochondrial content in skeletal muscle, two features that have shown dependence on increased expression of the peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α). It is also hypothesized that exercise-related alterations in PGC1α expression occur through epigenetic regulation of nucleosome positioning in association with differential DNA methylation status within the PGC1α promoter. In this study, we show that when primary human myotubes from obese patients with type 2 diabetes are exposed to lipolytic stimulus (palmitate, forskolin, inomycin) in vitro, nucleosome occupancy surrounding the −260 nucleotide (nt) region, a known regulatory DNA methylation site, is reduced. This finding is reproduced in vivo in the vastus lateralis from 11 healthy males after a single, long endurance exercise bout in which participants expended 650 kcal. Additionally, we show a significant positive correlation between fold change of PGC1α messenger RNA expression and −1 nucleosome repositioning away from the −260 nt methylation site in skeletal muscle tissue following exercise. Finally, we found that when exercise participants are divided into high and low responders based on the −260 nt methylation status, the −1 nucleosome is repositioned away from the regulatory −260 nt methylation site in high responders, those exhibiting a significant decrease in −260 nt methylation, but not in low responders. Additionally, high but not low responders showed a significant decrease in intramyocellular lipid content after exercise. These findings suggest a potential target for epigenetic modification of the PGC1α promoter to stimulate the therapeutic effects of endurance exercise in skeletal muscle.
Acute exercise epigenetically regulates skeletal muscle PGC1α in conjunction with beneficial metabolic alterations. Greater responses occur in high vs low exercise responders.
Exercise training is known to increase skeletal muscle mitochondrial content and function as well as improve insulin sensitivity, contributing to amelioration of the adverse effects of obesity and type 2 diabetes (T2D) on skeletal muscle physiology (1, 2). Recent reports indicate that the beneficial effects of exercise associated with improvements in obesity and insulin resistance (IR) may occur through epigenetic regulation of skeletal muscle peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) (3–8). Hypermethylation specifically at the −260 nucleotide (nt) has been observed in T2D skeletal muscle and during diet-induced IR (9, 10). PGC1α is a transcriptional coactivator whose expres-sion coordinates gene expression from the nuclear and mitochondrial genomes to determine metabolic adaptations, such as changes in mitochondrial number and function, in response to environmental input (2, 11, 12). Concordantly, acute exercise epigenetically regulates skeletal muscle PGC1α expression through alterations in promoter methylation, leading to hypomethylation of the −260 nt in the PGC1α promoter (9), and hypomethylation leads to increased gene expression in association with exercise-induced increases in skeletal muscle mitochondrial number (13). PGC1α epigenetic regulation at the −260 nt may occur through a mechanism involving alterations in nucleosome positioning within the PGC1α promoter, as DNA methyltransferase 3b, the methyltransferase required for −260 nt methylation (9), recruitment is dependent on nucleosome occupancy and positioning (14–17) and the −1 nucleosome (N) has been shown to position over this the regulatory −260 nt methylation site in PGC1α in association with decreased gene expression and increased cardiovascular disease risk in overweight and obese individuals (18) and in response to diet-induced obesity and IR in C57BL/6J mice (10).
Exercise responsiveness, such as differences in PGC1α expression, involves interindividual variations that contribute to the metabolic response and beneficial effects of exercise (1, 19). Differences in responsiveness provide important insights into mechanisms that determine preventative and therapeutic exercise prescription. Genetic factors account for some interindividual differences (20–24); however, genetic influences are often expressed before exercise but not after exercise (19), suggesting that other factors may contribute to interindividual variation in response to exercise. The epigenetic contributions to exercise-related interindividual responses, leading to “high” and “low” exercise responders, are unknown. We hypothesize that the plasticity of epigenetic regulation of PGC1α contributes to interindividual variation in the metabolic responses to exercise within skeletal muscle. Specifically, we hypothesize that acute exercise leads to hypomethylation of skeletal muscle PGC1α and repositioning of the PGC1α −1 N away from the regulatory −260 nt methylation site in association with exercise-induced metabolic benefits. The degree to which exercise induces beneficial metabolic responses may be attributed to the responsiveness of the epigenetic modifications within the PGC1α promoter. We tested this hypothesis by measuring exercise-induced variation in PGC1α DNA methylation, −1 N position, gene expression, and whole body and skeletal muscle metabolic responses in muscle biopsies from sedentary, lean men [N = 11; age, 24 ± 1 years; body mass index (BMI), 23.2 ± 0.55 kg/m2) before and after an acute bout of exercise (50% maximal oxygen consumption, ∼650 kcal).
Materials and Methods
Participants and exercise
The methods of the exercise bout were published previously by Galgani et al. (1). Healthy, normoglycemic males (N = 11) 24 ± 1 years of age with a BMI of 23.2 ± 0.5 kg/m2 reported to the laboratory after a 10-hour overnight fast, and a vastus lateralis biopsy (before) was obtained. After performing a 2-minute cycling warmup at 0 W, participants exercised on a cycle ergometer at 50% maximal oxygen consumption until reaching an energy expenditure of 650 kcal. Steady-state oxygen consumption was measured using a TruOne 2400 metabolic cart between 5 and 8 minutes after initiating the exercise and at intervals equivalent to 20%, 40%, 60%, and 100% of the entire exercise period to ensure fulfillment of the target exercise intensity. When necessary, workload was adjusted accordingly to ensure that participants were at 50% maximal oxygen consumption. Immediately after the exercise bout, a second muscle biopsy was obtained (after) from the vastus lateralis. Anthropometric measurements for study participants can be found in Table 1. All experiments were reviewed and approved by the Pennington Biomedical Research Center Institutional Review Board.
Table 1.
Anthropometric Measurements
| Responder |
|||
|---|---|---|---|
| All Participants (n = 11) | Low (n = 5) | High (n = 6) | |
| Age, y | 24 ± 1 | 25 ± 2 | 23 ± 2 |
| BMI, kg/m2 | 23.2 ± 0.5136 | 23.23 ± 0.9214 | 23.18 ± 0.6331 |
| Weight, kg | 76.35 ± 1.907 | 78.52 ± 3.225 | 74.55 ± 2.228 |
| Height, cm | 181.4 ± 1.19 | 183.9 ± 1.836 | 179.3 ± 1.032 |
| Fat mass, kg | 11.96 ± 0.8805 | 12.94 ± 1.623 | 11.14 ± 0.9053 |
| Fat-free mass, kg | 64.4 ± 1.212 | 65.58 ± 1.753 | 63.41 ± 1.705 |
| Percentage fat mass | 15.52 ± 0.8153 | 16.29 ± 1.37 | 14.88 ± 1.003 |
| Percentage fat-free mass | 84.48 ± 0.8153 | 83.71 ± 1.37 | 85.12 ± 1.003 |
Measurements of serum insulin and free fatty acids and intramyocellular lipid in skeletal muscle before and after acute exercise bout
Serum insulin and free fatty acid (FFA) levels were measured as previously described (25). Measures of intramyocellular lipid (IMCL) were as previously described using immunofluorescence techniques with Bodipy 494 dye (Molecular Probes, Eugene, OR) to stain for IMCL (1). IMCL was determined using the Sigma Scan Pro 5 software (SPSS, Chicago, IL) by delineating Bodipy staining within myofibers.
In vitro myotube experiments
A complete description of the in vitro myotube methods can be found in Covington et al. (25, 26). Briefly, pooled primary human myotubes from five healthy (lean) male donors (BMI, 24.2 ± 0.6 kg/m2) and four obese, T2D male donors (BMI, 39.7 ± 7.0 kg/m2) were differentiated and analyzed under either basal conditions or following 3 days of treatment with a cocktail of 30 µM palmitate, 2 µM forskolin (a drug that increases cyclic adenosine monophosphate levels), and 1.6 µM ionomycin [palmitate, forskolin, ionomycin (PFI) cocktail], all purchased from Sigma-Aldrich (St. Louis, MO). We previously showed that PFI treatment mimics exercise response in myotubes by increasing palmitate oxidation and mitochondrial oxidative phosphorylation complex expression and by improving glucose uptake (27).
Nucleosome positioning in the PGC1α promoter
Mononucleosomal and genomic DNA were extracted by dounce homogenization in a 0.25 M sucrose buffer, as previously described (10). Following release of the chromatin from nuclei, mononucleosomal DNA samples were treated with micrococcal nuclease for 15 minutes at 37°C whereas genomic DNA was left untreated. All samples were lysed and treated with proteinase K to digest away histone proteins, and EtOH was precipitated before use in scanning polymerase chain reaction (PCR) assays.
For scanning PCR, overlapping primer pairs were designed to target the promoter region of the PGC1α gene from approximately the −800 nt to the −100 nt upstream of the transcriptional start site (TSS), specifically encompassing the known regulatory −260 nt (Fig. 1; Table 2), as previously described (18). Scanning PCR products for each primer pair were run on a 1.2% agarose gel and quantified by densitometry with ImageJ software. Mononucleosomal DNA band intensity was normalized by genomic DNA input band intensity for each sample and reaction. Quantified and normalized results were analyzed by a Student t test or repeated measures analysis of variance and post hoc Tukey test as necessary.
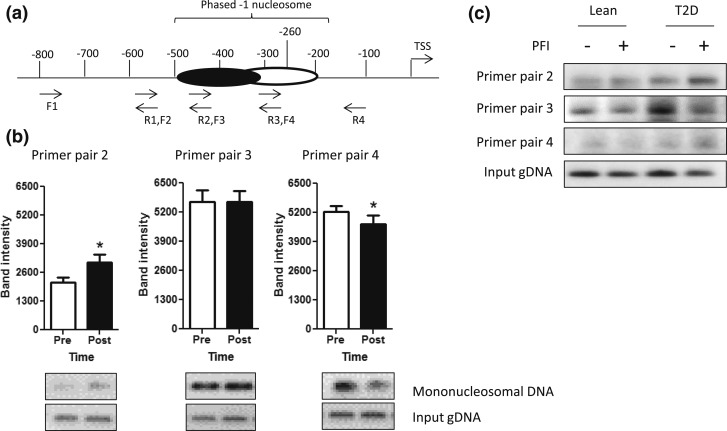
Figure 1.
PGC1α −1 N position in lean and diabetic primary myotubes and in skeletal muscle before and after acute endurance exercise. (a) Nucleosome position was determined via scanning PCR, using four overlapping primer pairs that spanned the PGC1α promoter from approximately the −100 nt to approximately the −800 nt. A phased −1 N was found to be present from approximately the −200 to −500 nt and is depicted. (b) Densitometry of scanning PCR results are shown as the mean ± standard error of the mean for primer pairs in which the phased −1 N was present in the PGC1α promoter (primer pairs 2, 3, and 4) in skeletal muscle samples before (Pre; open bars) and immediately after (Post; filled bars) an acute exercise bout. Representative gel images are shown below each graph. *P < 0.05. (c) Scanning PCR results from primer pairs 2, 3, and 4 are shown in myotubes isolated from lean or T2D individuals with or without the exercise mimetic PFI. F, forward; gDNA, genomic DNA; R, reverse.
Table 2.
Primer Sequences for Bisulfite Sequencing and Scanning PCR
| Primer Name | Primer | Sequence |
|---|---|---|
| PGC1α bisulfite amplification 1 | Forward | 5′-TAT AGT TAT TTT GTT ATG AAA TAG GGA GTT TT G-3′ |
| Reverse | 5′-CCA ATC ACA TAA CAA AAC TAT TAA AAA ATA A-3′ | |
| PGC1α bisulfite nested amplification 2 | Forward | 5′-TTA TTT GTT TGT TTT GGA AGG AAA AT-3′ |
| Reverse | /5Biosg/AAC AAC CCT CTA CTT AAT AAA ATA ACG C-3′ | |
| PGC1α pyrosequencing primer | 5′-AAG ATT GTA GGG GAT TTT GG-3′ | |
| Scanning PCR primer pair 1 | Forward | 5′-AGA GCA GCA GCG ACT GTA T-3′ |
| Reverse | 5′-TAC CAG CTC CCG AAG AGT TG-3′ | |
| Scanning PCR primer pair 2 | Forward | 5′-CAA CTC TTC GGG AGC TGG TA-3′ |
| Reverse | 5′-TGA GGG AGT GTT TGA AAG CG-3′ | |
| Scanning PCR primer pair 3 | Forward | 5′-CGC TTT CAA ACA CTC CCT CA-3′ |
| Reverse | 5′-GCA AAG CTC CCT GTT TCA TGA C-3′ | |
| Scanning PCR primer pair 4 | Forward | 5′-GTC ATG AAA CAG GGA GCT TTG C-3′ |
| Reverse | 5′-GAG GCT TCA AGC ATC ATG CT-3′ |
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed to determine gene expression as previously reported (10). Briefly, total RNA was isolated using TRIzol per the manufacturer’s protocol, followed by column purification using a Qiagen RNeasy kit. Messenger RNA (mRNA) was converted to complementary DNA using Promega Moloney murine leukemia virus reverse transcription and used in subsequent qRT-PCR. qRT-PCR was performed on the ABI 7900HT platform using commercially available ABI TaqMan assays to measure PGC1α (Hs01016719_m1), PPARα (Hs00947538_m1), and pyruvate dehydrogenase kinase isozyme 4 (PDK4; Hs01037712_m1) expression as previously described (25). qRT-PCR data were analyzed using the delta delta cycle threshold (ΔΔCt) method.
Pyrosequencing
DNA methylation of the regulatory −260 nt in PGC1α was determined in duplicate via pyrosequencing, as previously described (10). Briefly, genomic DNA was isolated using a Qiagen DNeasy kit, amplified with nested primers, bisulfite converted with the Qiagen EpiTect bisulfite kit, and the −260 nt was pyrosequenced on the Qiagen PyroMark platform using primers previously published (9) and shown in Table 2. Based on pyrosequencing results, subjects were divided into high (change in DNA methylation percentage ≤ 0; n = 6) or low (change in DNA methylation percentage > 0; n = 5) responders to exercise training.
Statistical analysis
Data were analyzed via a Pearson r correlation, a Student t test or repeated measures analysis of variance, and a post hoc Tukey test to determine significant differences as appropriate, using GraphPad Prism software, version 6.0 (GraphPad Software, La Jolla, CA). A P value of ≤0.05 was considered statistically significant. All data are presented as mean ± standard error of the mean.
Results
Nucleosome positioning in the PGC1α promoter
Scanning PCR experiments in skeletal muscle from before and after samples used overlapping primer pairs that spanned the PGC1α promoter region from −800 nt to the −100 nt, as depicted in Fig. 1(a). Scanning PCR results showed that the first nucleosome located upstream of the TSS or −1 N exhibited phasing within approximately the −200 to −500 nt region [Fig. 1(a)]. In response to an acute exercise bout, a significant increase the PCR product from mononucleosomal DNA primer pair 2 and reduced expression in primer pair 4 with no change in primer pair 3 were observed [Fig. 1(b)]. In complementary in vitro experiments, cultured primary human myotubes from T2D individuals showed an increase in nucleosome occupancy of the −1 N under basal conditions in comparison with lean individuals [Fig. 1(c)]. Following treatment with the exercise mimetic cocktail (PFI), −1 N occupancy near the −260 nt decreased in T2D cultures to similar levels as those seen in lean individuals whereas occupancy in PFI-treated T2D cultures increased further upstream of the TSS [Fig. 1(c)]. These data indicate the presence of a phased −1 N within the PGC1α promoter whose position is altered acutely by exercise, with the −1 N being positioned closer to the TSS before exercise, spanning from approximately nt −350 to −190, and being repositioned farther upstream from the TSS after exercise, approximately spanning the region from nt −500 to −300.
Fold change of PGC1α gene expression significantly increased in response to exercise-induced −1 N repositioning
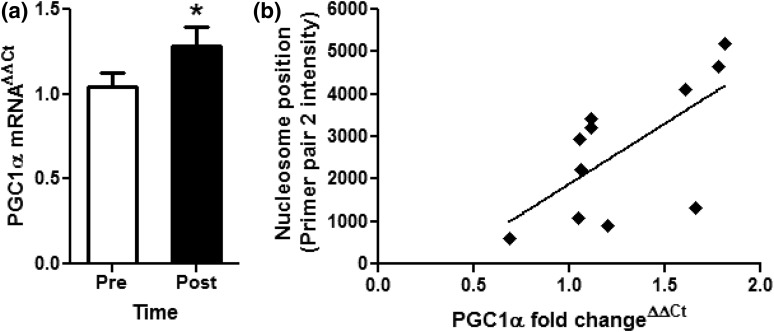
The −1 N plays an important regulatory role in determining gene expression and may be epigenetically regulated, and epigenetic upregulation of PGC1α expression through DNA hypomethylation at the −260 nt has been shown to correlate with increased skeletal muscle mitochondrial number and function (9, 13). To determine whether exercise-induced repositioning of the −1 N in the present study may alter gene expression, PGC1α mRNA was quantified in before and after muscle biopsy samples by qRT-PCR. The acute bout of exercise significantly increased fold change of PGC1α gene expression in after compared with before samples [1.05 ± 0.08 to 1.29 ± 0.11 fold change; Fig. 2(a)]. Additionally, we found a significant positive correlation (r2 = 0.43, P = 0.03) between PGC1α gene expression and the −1 N position upstream of the TSS, away from the −260 nt [Fig. 2(b)]. These data suggest that, in skeletal muscle, exercise-induced repositioning of the −1 N away from the −260 nt site may lead to increases in PGC1α expression.
Figure 2.
Fold change of PGC1α gene expression and DNA methylation. (a) qRT-CPR was performed to measure fold change of PGC1α expression in skeletal muscle sample before (Pre; open bar) and immediately after (Post; filled bar) exercise. Results are as the mean ± standard error of the mean fold change using the ΔΔCt method. *P < 0.05. (b) Correlation analysis was performed between mRNA expression of PGC1α and after primer pair 2 intensity measured via scanning PCR. PGC1α expression was positively correlated with nucleosome occupancy in the primer pair 2 region of the PGC1α promoter region, indicating repositioning of the −1 N farther upstream of the TSS in samples with higher PGC1α expression.
Fold change of PGC1α gene expression significantly increased in response to exercise-induced hypomethylation at the −260 nt in high but not low responders
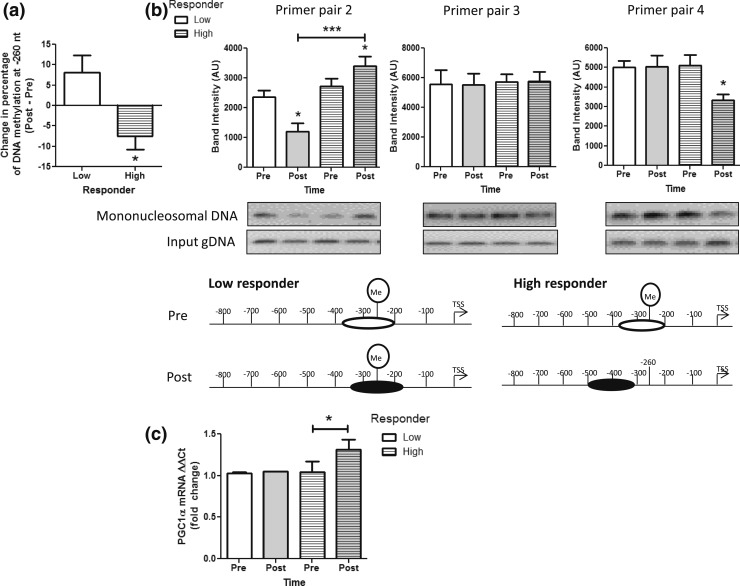
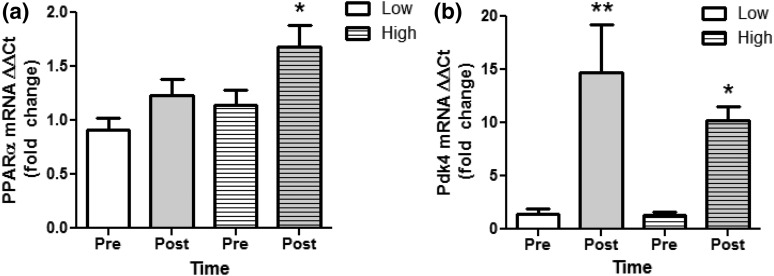
The −260 nt DNA methylation is known to epigenetically regulate PGC1α and downstream mitochondrial adaptations in skeletal muscle (9, 10), and the response to exercise training shows interindividual heterogeneity that cannot be explained wholly by genetic variance (20–23). Individual physiological responses to exercise training, such as mitochondrial adaptations and PGC1α levels, may result from differential epigenetic regulation of gene expression. To determine the relationship between −260 nt methylation and −1 N positioning and the contribution of interindividual epigenetic variation in determining acute exercise-induced epigenetic regulation of PGC1α in association with muscle lipid metabolism and mitochondrial content, we divided our participants into high and low responders based on exercise-induced DNA methylation changes at the −260 nt [Fig. 3(a)]. There were no significant differences in anthropometric measurements between high and low responders (Table 1). High responders to exercise showed a significant decrease in PGC1α −260 nt methylation compared with low responders [Fig. 3(a)]. Additionally, scanning PCR results showed a significant increase in primer pair 2 in high responders and a decrease in low responders after vs before exercise with no change in primer pair 3 and a decrease in primer pair 4 in high responders only [Fig. 3(b)]. In conjunction with epigenetic modifications, high but not low responders showed a significant increase in PGC1α gene expression [Fig. 3(c)]. Furthermore, we have previously reported that acute exercise in the present individuals significantly upregulates expression of skeletal muscle PPARα and PDK4, transcription factors known to be coactivated and upregulated, respectively, by PGC1α (26). In this study, we found that in those individuals showing exercise-induced upregulation of PGC1α, both PPARα and PDK4 were also upregulated (Fig. 4). Additionally, PDK4 upregulation occurred in response to exercise irrespective of changes in PGC1α [Fig. 4(b)]. Although PDK4 is a known PGC1α target gene, it has been shown previously that PDK4 upregulation in skeletal muscle occurs through both PGC1α-dependent and independent pathways, as PDK4 is upregulated by adenosine 5′-monophosphate–activated protein kinase activation in PGC1α knockout muscle (28). Collectively, these data indicate that epigenetic modifications within the PGC1α promoter may be necessary for determining PGC1α gene expression and that of related transcription factors and may play a role in determining interindividual variation in exercise responsiveness.
Figure 3.
High vs low DNA methylation responders. (a) The −260 nt DNA methylation levels in the PGC1α promoter were determined by pyrosequencing and the change in methylation levels from pre-exercise to post-exercise were determined. Participants in the acute exercise bout showing no change or an increase in methylation were considered low responders (clear). Participants with a decrease in PGC1α −260 nt DNA methylation were considered high responders (striped). (b) Scanning PCR was performed in the promoter region of PGC1α in low (clear) and high responders (striped bars) before (Pre; white bars) and immediately after exercise (Post; gray bars), and densitometry results are shown for primer pairs 2, 3, and 4 [see Fig. 1(a)]. Representative gel images and a schematic of approximate positions of the −1 N within the PGC1α promoter are depicted below the scanning PCR result graphs for each condition. (c) PGC1α mRNA expression was determined by qRT-PCR in skeletal muscle samples from low (clear) and high responders (striped) before (Pre; white) and immediately after exercise (Post; gray). All data are presented as mean ± standard error of the mean. *P < 0.05; ***P < 0.001. gDNA, genomic DNA.
Figure 4.
PGC1α-related gene expression in high vs low DNA methylation responders. (a) PPARα and (b) PDK4 mRNA expression was determined by qRT-PCR in skeletal muscle samples from low (clear bars) and high responders (striped bars) both before (Pre; white bars) and immediately after exercise (Post; gray bars). All data are presented as mean ± standard error of the mean. *P < 0.05; **P < 0.01.
Whole-body and IMCL responses to acute endurance exercise
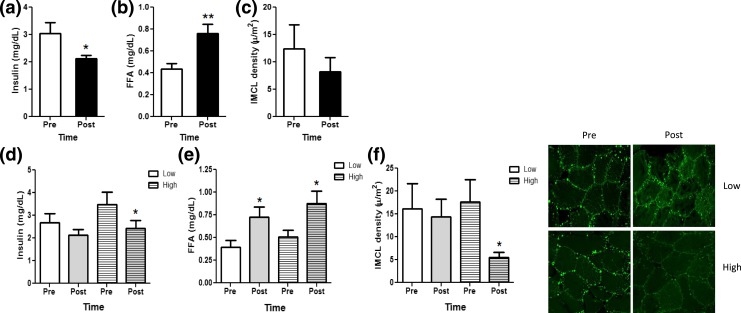
Skeletal muscle is one of the first sites to exhibit IR during the onset of T2D, partially due to decreased mitochondrial β-oxidation of fatty acids and ectopic lipid deposition in the form of IMCL (29, 30). Exercise ameliorates IR by acutely decreasing circulating insulin levels while increasing insulin-independent glucose uptake and reversing fatty acid–induced impairment in insulin sensitivity by decreasing ectopic lipid storage (31, 32) and promoting lipolysis and fatty acid oxidation in muscle (33). To determine whether exercise-induced DNA hypomethylation and repositioning of the −1 N in the PGC1α promoter in high responders is also associated with altered glucose and lipid metabolism, representative systemic and muscle-specific markers were assessed. As expected, the acute bout of endurance exercise significantly decreased serum insulin levels while increasing FFA levels [Fig. 5(a) and 5(b)], suggesting that endurance exercise acutely contributes to insulin sensitization and increases reliance on fatty acids vs glucose for substrate utilization. Interestingly, we observed no change in IMCL following the acute bout of exercise [Fig. 5(c)]. Given that exercise-induced metabolic effects on lipid metabolism show interindividual variation (23), we examined the effects of the acute bout of endurance exercise in high vs low responders on serum FFA and insulin levels and IMCL. Interestingly, whereas exercise similarly increased FFA levels in high and low responders, exercise acutely decreased insulin only in high but not in low responders [Fig. 5(d) and 5(e)]. Additionally, in high but not in low responders, we observed a significant decrease in skeletal muscle IMCL [Fig. 5(f)]. These data indicate increased reliance on fatty acids vs glucose during exercise in individuals demonstrating exercise-induced hypomethylation of the −260 nt and −1 N repositioning away from the −260 nt within the PGC1α promoter. Increased lipolysis resulting in circulating levels of FFA must be matched by oxidation rates in skeletal muscle during exercise to prevent ectopic lipid deposition and to promote decreases in IMCL (33). Thus, our data suggest that only those individuals exhibiting epigenetic upregulation of PGC1α expression may benefit by increased fatty acid utilization and decreased lipid accumulation in muscle, which may result in insulin sensitization (31).
Figure 5.
Whole-body and IMCL responses to acute endurance exercise. (a) Serum insulin, (b) FFAs, and (c) skeletal muscle IMCL content were measured before (Pre; open bars) and immediately after (Post; filled bars) an acute exercise bout. (d) Serum insulin, (e) FFA, and (f) skeletal muscle IMCL content were determined also in low (clear bars) vs high (striped bars) responders before (Pre; white bars) and after (Post; gray bars) an acute exercise bout, and representative immunohistochemical images are shown. All data are shown as mean ± standard error of the mean. *P < 0.05; **P < 0.01.
Discussion and Conclusion
Exercise is known to increase PGC1α expression and insulin sensitivity while improving skeletal muscle mitochondrial function and decreasing ectopic lipid deposition in the form of IMCL. Recently, exercise has also been shown to epigenetically regulate PGC1α expression via DNA and histone methylation in skeletal muscle (13, 34), with exercise-induced decreases in PGC1α promoter methylation resulting in increased gene expression and mitochondrial function and number (13). In this study, we demonstrate that an acute bout of endurance exercise repositions the −1 N in the PGC1α promoter upstream of the regulatory −260 nt methylation site. We also show that exercise-induced PGC1α expression −1 N repositioning is correlated with increased gene expression and associated with decreased −260 nt methylation levels and IMCL content in high but not low responders. Decreased PGC1α expression and skeletal muscle mitochondrial dysfunction, such as incomplete oxidation of fatty acids, and increased lipid deposition have been noted during obesity and IR and may be causative in disease onset and progression (10, 30, 35–37). Consistent with these reports, others have shown that the PGC1α promoter is hypermethylated in T2D muscle (9). We have previously shown that the −1 N position within the PGC1α promoter may be critical in determining cardiovascular disease risk in overweight and obese individuals and that in the obese, insulin-resistant state the −1 N is positioned over the regulatory −260 nt methylation site, and PGC1α is hypermethylated here (10, 18). Additionally, repositioning of the −1 N in conjunction with decreased −260 nt methylation may contribute to amelioration of diet-induced obesity and IR via subsequent increases in mitochondrial function and complete β -oxidation of fatty acids in skeletal muscle (10, 18). Thus, the ability to epigenetically upregulate PGC1α, a major regulator of mitochondrial adaptations that determine lipid metabolism and subsequently deposition, through exercise training may be sufficient to ameliorate chronic disease states.
Unfortunately, exercise prescription for prevention and treatment may need to be adapted to the individual, as exercise training leads to differential individual responses, with some individuals exhibiting a greater response to stimuli than others (19). In some cases, individuals have shown no response to exercise training or even adverse responses (38, 39). Importantly, interindividual responses in PGC1α expression have been noted following exercise training in high vs low responders (40). Although interindividual variation to exercise training may be partially explained by genetic variation, most interindividual variation cannot be explained by genetic factors alone (20–23). As PGC1α is a major regulator of skeletal muscle mitochondrial adaptations that is epigenetically regulated by DNA methylation and epigenetic regulation is dependent on environmental inputs such as exercise, it is possible that interindividual responses to exercise training may also be epigenetically regulated. Indeed, we found phasic responses to an acute bout of cycling exercise resulting in dichotomy in DNA methylation status at the −260 nt of PGC1α in skeletal muscle. Subsequent division of participants into high and low responder–based changes in DNA methylation status alone revealed that exercise-induced repositioning of the −1 N in the PGC1α promoter, upstream of the regulatory −260 nt methylation site, occurred only in high but not in low responders. Additionally, high responders but not low responders showed upregulation of PGC1α expression, decreases in serum insulin in conjunction with increased serum FFA levels, and decreased levels of IMCL. Collectively, these data reinforce the conclusion that exercise acutely epigenetically regulates PGC1α expression through alterations in DNA methylation at the −260 nt and through −1 N repositioning. Furthermore, beneficial metabolic adaptations suggesting an increase in fatty acid utilization and decrease in storage resulting in ectopic lipid deposition as IMCL were observed only in those individuals in which PGC1α was epigenetically upregulated. Muscle adaptations consistent with exercise-induced upregulation of PGC1α are known to occur in conjunction with upregulation in expression of several transcription factors that are coactivated by PGC1α and work synergistically to bring about the effects of PGC1α. A similar mechanism of epigenetic regulation may occur within these genes as part of a coordinated response to exercise. Indeed, it has been recently reported that transcription factor a, mitochondrial, myocyte enhancing factor 1A, and PDK4 promoter methylation levels are decreased by an acute bout of exercise, and hypomethylation in transcription factor a, mitochondrial and PDK4 translated to increased gene expression following exercise (13). Although we did not measure promoter methylation in these associated genes in the present study, we did observe significant increases in PDK4 and PPARα expression following the acute bout of exercise. It is possible that this increase is due to hypomethylation (13), and that PDK4 and PPARα act synergistically with PGC1α to induce exercise responses in skeletal muscle of high responders, and epigenetic regulation of PGC1α may be necessary to convey the beneficial metabolic adaptations of exercise training.
Importantly, note that only lean, sedentary male subjects were included in the present study, and a small sample size was available for hypothesis testing. However, as exercise training results in similar outcomes in PGC1α expression in overweight/obese and in exercise-trained individuals and because the exercise mimetic PFI resulted in decreased −1 N occupancy in the PGC1α promoter in the present study, we would expect similar results in other populations of individuals in which exercise results in metabolic benefits. Another limitation of the present study is the lack of measurement of PGC1α protein levels and posttranslational modifications that may affect its activity (41) and lack of measurement of DNA methylation of other metabolic genes that may act synergistically with PGC1α in determining exercise-induced skeletal muscle responses. Posttranslational modifications to the PGC1α protein have been extensively studied (41), and it is possible that although we see an increase in PGC1α transcript levels, the activity of the protein could be unaffected by acute exercise. However, others have shown that upregulation of PGC1α transcript levels coincides with PGC1α activity and PGC1α-dependent skeletal muscle adaptations in response to an acute exercise bout (13, 42). Furthermore, it is possible that whereas exercise-induced PGC1α hypomethylation and gene upregulation is needed for skeletal muscle mitochondrial adaptations, it is dispensable for determining chronic exercise-induced muscle adaptive changes or obesity and IR (43–46). However, our data in this study and those of others suggest that the acute metabolic effects of exercise are mediated through epigenetic regulation of PGC1α in muscle (13).
In summary, the results presented in this study identify the effects of an acute bout of exercise on −1 N positioning and its role in the epigenetic regulation of PGC1α and downstream metabolic adaptations. They furthermore show that epigenetic regulation of PGC1α through DNA methylation and −1 N positioning may explain interindividual variation in response to exercise training. These findings emphasize the importance of epigenetics in IR and in determining exercise responsiveness.
Acknowledgments
The authors thank Dr. Conrad Ernest and Melissa Lupo for exercise testing supervision, Dr. Neil Johannsen for whole-body exercise calorimetry measures, and Cesar Meza, Cynthia Montenegro, and Catalina De La Pena for assistance with immunohistochemistry. Finally, the authors thank all of the research participants.
Acknowledgments
This work was funded in part by Purdue University Ralph W. and Grace M. Showalter Trust Fund (to T.M.H.) and an early investigator grant from the Obesity Society (to J.E.G.). Core facilities at Pennington Biomedical Research Center, which were used in this study, are supported in part by Nutrition Obesity Research Center (National Institutes of Health Grant 1P30-DK072476) and Centers for Biomedical Research Excellence center grants (National Institutes of Health Grant P20 GM103528).
Author contributions: S.B. performed experiments, contributed to discussion, and reviewed/edited the manuscript. J.D.C. performed experiments, analyzed data, and drafted the manuscript. E.MT. performed experiments and reviewed/edited the manuscript. L.K.S. contributed to discussion and reviewed/edited the manuscript. J.E.G. contributed to discussion, performed experiments, and reviewed/edited the manuscript. T.M.H. conceived and performed experiments, analyzed data, and drafted and edited the manuscript. All authors gave final approval of the manuscript prior to submission. T.M.H. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- FFA
- free fatty acid
- IMCL
- intramyocellular lipid
- IR
- insulin resistance
- mRNA
- messenger RNA
- N
- nucleosome
- nt
- nucleotide
- PCR
- polymerase chain reaction
- PDK4
- pyruvate dehydrogenase kinase isozyme 4
- PFI
- palmitate, forskolin, ionomycin
- PGC1α
- peroxisome proliferator-activated receptor-γ coactivator 1α
- qRT-PCR
- quantitative real-time polymerase chain reaction
- T2D
- type 2 diabetes
- TSS
- transcriptional start site.
References
- 1.Galgani JE, Johannsen NM, Bajpeyi S, Costford SR, Zhang Z, Gupta AK, Ravussin E. Role of skeletal muscle mitochondrial density on exercise-stimulated lipid oxidation. Obesity (Silver Spring). 2012;20(7):1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. [DOI] [PubMed] [Google Scholar]
- 3.Dudley KJ, Sloboda DM, Connor KL, Beltrand J, Vickers MH. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS One. 2011;6(7):e21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallou-Kabani C, Junien C. Nutritional epigenomics of metabolic syndrome: new perspective against the epidemic. Diabetes. 2005;54(7):1899–1906. [DOI] [PubMed] [Google Scholar]
- 5.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. [DOI] [PubMed] [Google Scholar]
- 6.Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB, Hawke TJ. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS One. 2009;4(10):e7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151(10):4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13(2):97–109. [DOI] [PubMed] [Google Scholar]
- 9.Barrès R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, Krook A, Zierath JR. Non-CpG methylation of the PGC-1α promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10(3):189–198. [DOI] [PubMed] [Google Scholar]
- 10.Henagan TM, Stefanska B, Fang Z, Navard AM, Ye J, Lenard NR, Devarshi PP. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br J Pharmacol. 2015;172(11):2782–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12(8):360–365. [DOI] [PubMed]
- 12.Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30(4):145–151. [DOI] [PubMed] [Google Scholar]
- 13.Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–411. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, De Carvalho DD, Jeong S, Jones PA, Liang G. Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance. PLoS Genet. 2011;7(2):e1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeltsch A, Jurkowska RZ. Allosteric control of mammalian DNA methyltransferases—a new regulatory paradigm. Nucleic Acids Res. 2016;44(18):8556–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong S, Liang G, Sharma S, Lin JC, Choi SH, Han H, Yoo CB, Egger G, Yang AS, Jones PA. Selective anchoring of DNA methyltransferases 3A and 3B to nucleosomes containing methylated DNA. Mol Cell Biol. 2009;29(19):5366–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeshima H, Suetake I, Shimahara H, Ura K, Tate S, Tajima S. Distinct DNA methylation activity of Dnmt3a and Dnmt3b towards naked and nucleosomal DNA. J Biochem. 2006;139(3):503–515. [DOI] [PubMed] [Google Scholar]
- 18. doi: 10.1155/2014/895734. Henagan TM, Stewart LK, Forney LA, Sparks LM, Johannsen N, Church TS. PGC1α−1 nucleosome position and splice variant expression and cardiovascular disease risk in overweight and obese individuals. PPAR Res. 2014;2014:895734. [DOI] [PMC free article] [PubMed]
- 19.Mann TN, Lamberts RP, Lambert MI. High responders and low responders: factors associated with individual variation in response to standardized training. Sports Med. 2014;44(8):1113–1124. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol (1985). 2011;110(5):1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankinen T, Sung YJ, Sarzynski MA, Rice TK, Rao DC, Bouchard C. Heritability of submaximal exercise heart rate response to exercise training is accounted for by nine SNPs. J Appl Physiol (1985). 2012;112(5):892–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An P, Borecki IB, Rankinen T, Pérusse L, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Evidence of major genes for exercise heart rate and blood pressure at baseline and in response to 20 weeks of endurance training: the HERITAGE Family Study. Int J Sports Med. 2003;24(7):492–498. [DOI] [PubMed] [Google Scholar]
- 23.An P, Borecki IB, Rankinen T, Després JP, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Evidence of major genes for plasma HDL, LDL cholesterol and triglyceride levels at baseline and in response to 20 weeks of endurance training: the HERITAGE Family Study. Int J Sports Med. 2005;26(6):414–419. [DOI] [PubMed] [Google Scholar]
- 24.Rice T, Després J-P, Pérusse L, Hong Y, Province MA, Bergeron J, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Familial aggregation of blood lipid response to exercise training in the health, risk factors, exercise training, and genetics (HERITAGE) Family Study. Circulation. 2002;105(16):1904–1908. [DOI] [PubMed] [Google Scholar]
- 25.Covington JD, Galgani JE, Moro C, LaGrange JM, Zhang Z, Rustan AC, Ravussin E, Bajpeyi S. Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PLoS One. 2014;9(3):e91675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covington JD, Noland RC, Hebert RC, Masinter BS, Smith SR, Rustan AC, Ravussin E, Bajpeyi S. Perilipin 3 differentially regulates skeletal muscle lipid oxidation in active, sedentary, and type 2 diabetic males. J Clin Endocrinol Metab. 2015;100(10):3683–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW, Thoresen GH, Rustan AC, Smith SR. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One. 2011;6(7):e21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA. 2007;104(29):12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Xu S, Zhang X, Yi Z, Cichello S. Skeletal intramyocellular lipid metabolism and insulin resistance. Biophys Rev. 2015;1:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]
- 31.Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(1):E66–E72. [DOI] [PubMed] [Google Scholar]
- 32.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117(6):1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lochmann TL, Thomas RR, Bennett JP Jr, Taylor SM. Epigenetic modifications of the PGC-1α promoter during exercise induced expression in mice. PLoS One. 2015;10(6):e0129647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116. [DOI] [PubMed] [Google Scholar]
- 36.Consitt LA, Bell JA, Houmard JA. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 2009;61(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–346. [DOI] [PubMed] [Google Scholar]
- 38.Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Häkkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, Rao DC, Sarzynski MA, Skinner JS, Slentz CA, Rankinen T. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5):e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vollaard NBJ, Constantin-Teodosiu D, Fredriksson K, Rooyackers O, Jansson E, Greenhaff PL, Timmons JA, Sundberg CJ. Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J Appl Physiol (1985). 2009;106(5):1479–1486. [DOI] [PubMed] [Google Scholar]
- 40.Marton O, Koltai E, Takeda M, Koch LG, Britton SL, Davies KJ, Boldogh I, Radak Z. Mitochondrial biogenesis-associated factors underlie the magnitude of response to aerobic endurance training in rats. Pflugers Arch. 2015;467(4):779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546(Pt 3):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong KE, Mikus CR, Slentz DH, Seiler SE, DeBalsi KL, Ilkayeva OR, Crain KI, Kinter MT, Kien CL, Stevens RD, Muoio DM. Muscle-specific overexpression of PGC-1α does not augment metabolic improvements in response to exercise and caloric restriction. Diabetes. 2015;64(5):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leick L, Wojtaszewski JFP, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294(2):E463–E474. [DOI] [PubMed] [Google Scholar]
- 45.Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One. 2012;7(7):e41817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, Kelly DP. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12(6):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]