Abstract
Estrogens are essential hormones for the regulation of fertility. Cellular responses to estrogens are mediated by estrogen receptor α (ESR1) and estrogen receptor β (ESR2). In mouse and rat models, disruption of Esr1 causes infertility in both males and females. However, the role of ESR2 in reproductive function remains undecided because of a wide variation in phenotypic observations among Esr2-mutant mouse strains. Regulatory pathways independent of ESR2 binding to its cognate DNA response element have also been implicated in ESR2 signaling. To clarify the regulatory roles of ESR2, we generated two mutant rat models: one with a null mutation (exon 3 deletion, Esr2ΔE3) and the other with an inframe deletion selectively disrupting the DNA binding domain (exon 4 deletion, Esr2ΔE4). In both models, we observed that ESR2-mutant males were fertile. ESR2-mutant females exhibited regular estrous cycles and could be inseminated by wild-type (WT) males but did not become pregnant or pseudopregnant. Esr2-mutant ovaries were small and differed from WT ovaries by their absence of corpora lutea, despite the presence of follicles at various stages of development. Esr2ΔE3- and Esr2ΔE4-mutant females exhibited attenuated preovulatory gonadotropin surges and did not ovulate in response to a gonadotropin regimen effective in WT rats. Similarities of reproductive deficits in Esr2ΔE3 and Esr2ΔE4 mutants suggest that DNA binding-dependent transcriptional function of ESR2 is critical for preovulatory follicle maturation and ovulation. Overall, the findings indicate that neuroendocrine and ovarian deficits are linked to infertility observed in Esr2-mutant rats.
We studied the role of ESR2 in reproductive function, using two mutant rat models. Our findings suggest that DNA binding domain-dependent transcriptional function of ESR2 is critical for ovulation.
Estrogen signaling plays an important regulatory role in the development and function of the reproductive system (1). Two nuclear receptors, estrogen receptor α [also called estrogen receptor 1 (ESR1)] and estrogen receptor β [also called estrogen receptor 2 (ESR2)] transduce cellular responses to estrogens (2–5). Studies with estrogen-receptor–mutant models have significantly advanced our understanding of the physiology of estrogen action. In the mouse and rat, disruption of Esr1 causes infertility in both males and females (6–8). However, the effects of Esr2 gene disruption in the mouse range from subfertility to unexplained infertility in both males and females (7, 9–12). Such variability can be related to differences in the sites targeted for mutagenesis and potential inframe alternative splicing of Esr2 transcripts (11).
The first Esr2 knockout mouse model was created by interrupting the DNA binding domain (DBD) with a neomycin resistance cassette (9). In this model, mutant females were subfertile and mice exhibited abnormalities in the brain, prostate, lung, colon, and immune system (12–17). A separate research group used a similar targeting approach to make an Esr2 knockout mouse exhibiting reproductive phenotypes, ranging from female subfertility to infertility (7). Shughrue et al. (10, 18) generated another Esr2-mutant mouse model by insertion of stop codons at the 19th codon and placement of a neomycin resistance gene in the reverse orientation, and used the model to investigate the involvement of ESR2 in estrogen action within the brain. Unlike Esr1 mutants, males were fertile in each of these three Esr2-mutant models. Subsequent mutant Esr2 mouse models were generated through Cre/LoxP-mediated targeted deletion of exon 3 (11, 12). Antal et al. (11) observed female and male infertility, whereas Maneix et al. (12) reported only female infertility due to a failure of ovulation. Because of these variations in findings among independently generated mutant Esr2 mouse models, the physiological role of ESR2 remains controversial (11, 12, 19).
ESR1 and ESR2 can regulate biological functions through direct DNA binding to a motif referred to as the estrogen response element (ERE), where they act to regulate gene transcription (20). Alternatively, ESR1 and ESR2 can also influence gene transcription through tethering to transcription factors such as activator protein 1, specificity protein 1, or nuclear factor kappa B, and, thus, act independently of an ERE (21–23). Exon 3 of Esr2, which encodes part of the DBD, was the site for mutagenesis in each of the mouse models generated to interfere with ESR2 function (7, 9–12). Maneix et al. (12) have proposed that the wide range of phenotypes associated with mutant Esr2 mouse models was attributed to variable impacts of the mutations on non–ERE-dependent functions.
To date, the mouse has dominated genetic approaches to reproductive biology research (24), including the study of estrogen action (1, 25). The advent of genome editing strategies has expanded the range of animal models available for genetic research (26, 27). The rat has a rich history as a model for mammalian reproduction and is especially amenable to investigations on the endocrine regulation of female fertility (28–30). Recently, mutant rat models have been established for investigating both estrogen and progesterone action in female reproductive function (8, 31).
In this report, we generated two new mutant rat models to investigate the physiological role of ESR2 and to distinguish ERE-dependent function of ESR2 from non–ERE-dependent actions. Using zinc finger nuclease (ZFN)-mediated genome editing, we deleted exon 3 (ΔE3) in one model, and exon 4 (ΔE4) in a second. Deletion of exon 3 resulted in a frameshift and a null mutation, whereas deletion of exon 4 generated an allele that expressed an ESR2 protein lacking a functional DBD. In both mutant models, males were fertile, whereas females were infertile due to a failure in ovulation.
Materials and Methods
Generation of Esr2-mutant rat models
Holtzman Sprague-Dawley rats obtained from Envigo (Indianapolis, IN) were used for the generation of targeted mutations in the Esr2 gene. The University of Kansas Medical Center Animal Care and Use Committee approved all protocols for generation of Esr2-mutant rats and their phenotypic characterization. Exon 3 was targeted to generate a frameshift and null mutation in the rat ESR2 coding sequence, and exon 4 was targeted to generate an inframe mutation resulting in an ESR2 protein lacking part of the DBD. Targeted mutations were generated by ZFN-mediated genome editing. ZFN constructs specific for exon 3 or exon 4 of rat Esr2 were designed, assembled, and validated by Sigma-Aldrich (St. Louis, MO). Selected ZFNs were targeted to exon 3 (TGCTCACTTCTGCCCCGTCtgcagcGATTATGCATCTGGG; nucleotides: 1309-1348, XM_006240221.3) or to exon 4 (CAGGCCTGCCGACTTCGCAagtgtTATGAAGTAGGAATGGTC; nucleotides 1478-1519, XM_006240221.3). In vitro-transcribed ZFN mRNAs were microinjected into single-cell–stage rat embryos. Injected embryos were transferred to the oviduct of day 0.5 pseudopregnant rats and offspring were screened for targeted Esr2 mutations.
Identification of founders and establishment of Esr2-mutant rat strains
Genotyping was performed by polymerase chain reaction (PCR) on genomic DNA samples extracted from tail-tip biopsy specimens, using the RED Extract-N-Amp tissue PCR kit (Sigma-Aldrich) and primers targeting flanking intron sequences. Stepwise increases in the target region flanking the ZFN site (500 bp to 5 kbp) were PCR amplified to assess possible deletions. PCR products were identified by agarose gel electrophoresis and ethidium bromide staining, and the specific sites of mutations were determined by DNA sequencing. Among the multiple founders possessing mutations in exon 3 or exon 4 (Supplemental Tables 1 and 2 (274.6KB, pdf) ), a mutant strain (No. 3389) with complete deletion of exon 3 (Esr2ΔE3) and a mutant strain (No. 1500) with complete deletion of exon 4 (Esr2ΔE4) were selected for phenotypic characterization. Heterozygous mutants were intercrossed, and phenotypic characterization was performed on homozygous mutants and wild-type (WT) littermates. Routine genotyping of Esr2ΔE3 and Esr2ΔE4 mutations were performed with the primers shown in Supplemental Tables 3 and 4 (274.6KB, pdf) . Both Esr2-mutant rat models can be obtained from Rat Resources & Research Center (University of Missouri, Columbia, MO).
Esr2-mutant RNA analyses and expression constructs
Mutant Esr2 mRNAs were detected by reverse transcription-PCR (RT-PCR) using primers within exons flanking the targeted exons 3 and 4 (Supplemental Table 5 (274.6KB, pdf) ). Changes in open reading frames of transcripts were determined by DNA sequencing. Full-length Esr2 cDNA was cloned into pCMV-SC expression vectors (StrataClone Mammalian Expression System, Agilent Technologies, Santa Clara, CA), and ΔE3 and ΔE4 mutations were generated by PCR-based site-directed mutagenesis. Additionally, WT, Esr2ΔE3-, and Esr2ΔE4-mutant cDNAs were PCR amplified using primers shown in Supplemental Table 6 (274.6KB, pdf) and cloned into expression vectors with or without C-terminal FLAG tags (Agilent Technologies). Expression vectors were transfected into 293FT cells (Thermo Fisher Scientific, Carlsbad, CA), using Lipofectamine 2000 (Thermo Fisher Scientific) and recombinant proteins analyzed. A rabbit monoclonal antibody directed to amino acids 63 through 82 of rat ESR2 (clone 68-4; catalog no. 05-824; Research Resource Identifier: AB_11212759; EMD Millipore, Billerica, MA) was used for immunodetection. Flag-tagged proteins were detected using a mouse monoclonal anti-FLAG antibody (A00187; Research Resource Identifier: AB_1720813; GenScript, Piscataway, NJ).
ESR2 transactivation
To assess the ERE-binding and transcriptional activation function of the ΔE4 mutant, ERE-dependent luciferase activity was assessed by cotransfection of WT or ΔE4 ESR2 expression vectors with an ERE-reporter vector (3XERE-TATA-Luc construct, No. 11354; Addgene, Cambridge, MA) into HeLa cells (American Type Culture Collection, Manassas, VA) or an AP1-reporter vector (5XTRE-TATA-luc) into MCF7 cells (American Type Culture Collection) using Lipofectamine 2000 (Thermo Fisher Scientific). A Renilla vector (pGL4.74[hRluc/TK]) was used as an internal control. Twenty-four hours after transfection, cells were stimulated with 10 nM 17β estradiol (E2) or 5 μM tamoxifen (Sigma-Aldrich) for an additional 24 hours. Standard dual-luciferase assays were performed on the cell lysates, using dual-luciferase reporter assay reagents (Promega, Madison, WI).
Phenotypic characterization
The reproductive phenotypes of WT and mutant rats were examined. The onset of puberty was monitored in males by preputial separation (32) and in females by vaginal opening (33). After vaginal opening, vaginal lavages were performed daily and cytology examined microscopically to determine reproductive cyclicity. At 8 to 12 weeks of age, female rats were weighed and killed at key phases of the estrous cycle (34, 35). Blood samples were obtained by cardiac puncture. At 12 to 16 weeks of age, male rats were weighed and killed. Ovaries, uteri, and mammary glands were collected from females, whereas testes, epididymides, and seminal vesicles were recovered from males. Each organ was weighed and fixed in 4% paraformaldehyde or snap-frozen in liquid nitrogen and stored at −80°C until processed for RNA or protein analyses. Histological examinations for all tissues were performed on paraffin-embedded, hematoxylin-and-eosin–stained sections.
Fertility tests
Fertility was assessed by cohabiting 12- to 16-week-old male rats with 8- to 12-week-old female rats for 12 weeks and recording pregnancies and litter sizes. The breeding combinations included WT males with WT or homozygous Esr2-mutant females and homozygous Esr2-mutant males with WT females. Vaginal cytology was monitored daily to record estrous cyclicity, mating (presence of sperm), signs of pregnancy (continuous diestrus), and litter size.
Uterine responses to E2
Four-week-old WT and age-matched Esr2-mutant female rats were tested for uterine responsiveness to E2. E2 (40 μg/kg) or vehicle (sesame oil) was injected subcutaneously for three consecutive days and uteri collected for measurement of gravimetric responses.
Ovulatory responses to exogenous gonadotropins
At 4 weeks of age, female rats were tested for responsiveness to exogenous gonadotropins (31, 33). Females were treated intraperitoneally with 30 IU of equine chorionic gonadotropin (eCG) at 1500 hours; 48 hours later, 30 IU of human chorionic gonadotropin (hCG) was injected.
At 24 hours after the hCG injection, animals were killed, oocytes were recovered from the oviduct, cumulus cells removed using hyaluronidase (Sigma-Aldrich), and oocytes counted. In additional experiments, rats were killed at 4, 10, and 24 hours after the hCG injection, and ovaries and oviducts were collected, weighed, processed for histology, or snap frozen for subsequent RNA or protein analyses. Total RNA was reverse-transcribed using the high-capacity reverse transcription kit (Thermo Fisher Scientific) and expression of target genes (Supplemental Table 7 (274.6KB, pdf) ) was evaluated by quantitative PCR. Primer sequences used in the analyses are shown in Supplemental Table 7 (274.6KB, pdf) .
Hormone measurements
Blood samples were collected at 0800 hours during day 1 of diestrus, proestrus, and estrus, and also at 2000 hours on proestrus, which coincides with the preovulatory surge of luteinizing hormone (LH) (34, 35). Serum LH and follicle-stimulating hormone concentrations were determined by Milliplex MAP kits (EMD Millipore). Serum E2 concentrations were measured by radioimmunoassay, as previously described (36).
Statistical analysis
Statistical analyses were performed using the SPSS Statistical Package (IBM, Armonk, NY). Comparisons of two means were analyzed by Student t test, whereas multiple comparisons were analyzed by analysis of variance followed by Tukey test, using GraphPad prism software (GraphPad Software, La Jolla, CA).
Results
In vitro evaluation of ESR2 transcripts and proteins
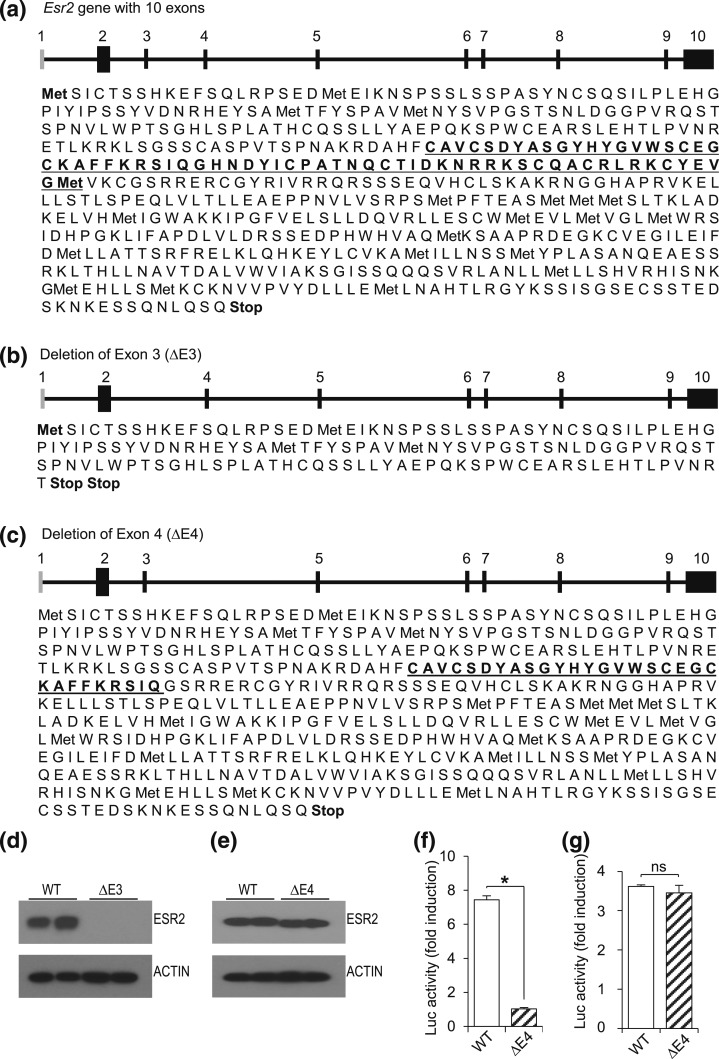
Targeted exon deletion in the rat Esr2 gene was simulated and analyzed for predicted translation products (Fig. 1). Deletion of exon 3 caused loss of ESR2 expression due to an out-of-frame translation product generating a premature stop codon [Fig. 1(b)]. In contrast, deletion of exon 4 resulted in an inframe mutation generating a mutant ESR2 protein that lacks an intact DBD [Fig. 1(c)]. cDNAs with targeted deletion of exons 3 or 4 were subsequently cloned into expression vectors and transfected into 293FT cells to evaluate expression of these mutant proteins. This in vitro expression experiment verified the predicted translation products [Fig. 1(d) and 1(e)]. ESR2 protein was not detected from the ΔE3 expression construct [Fig. 1(d)]; however, a mutant protein with slightly lower molecular weight was detected from the ΔE4 expression construct [Fig. 1(e)]. The absence of ERE binding-dependent transcriptional activity of the exon 4 deletion construct was also confirmed [Fig. 1(f)]. Both WT and ΔE4 ESR2 were capable of transactivation of an AP1 responsive reporter to a similar level [Fig. 1(g)].
Figure 1.
Translation of WT and mutant ESR2 proteins. (a) Schematic presentation of the rat Esr2 gene (NC_005105.4) followed by predicted translation products of Esr2 mRNA (XM_006240221.3) indicate that the full-length rat ESR2 protein possesses 567 amino acids (DBD is underlined). (b) Deletion of exon 3 leads to a frame shift and two premature stop codons after amino acid 140, whereas (c) deletion of exon 4 causes a loss of 39 amino acids from the DBD (truncated DBD is underlined), resulting in a truncated protein of 528 amino acids. Full-length rat Esr2 cDNA was amplified by RT-PCR, cloned into pCMV-SC vector, and exon 3 or exon 4 deletion was performed by site-directed mutagenesis. (d) Transfection of ΔE3 constructs into 293FT cells failed to express detectable ESR2 protein; (e) however, ΔE4 constructs expressed ESR2 proteins of lower molecular weight. (f) ERE-dependent luciferase (Luc) activity was assessed by cotransfection of WT or ΔE4 ESR2 expression vectors and ERE-reporter vector into HeLa cells and stimulated with E2. Partial loss of the DBD caused failure of ΔE4 ESR2 to induce the ERE-reporter activity. (g) AP1-reporter activity was assessed by cotransfection of WT or ΔE4 ESR2 expression vector and a TRE-reporter construct into HeLa cells and stimulated with tamoxifen. No significant differences in transactivation activity were observed between WT and ΔE4 ESR2 expression vectors. Results are expressed as mean ± standard error of the mean and the induction of Luc activity was expressed as fold induction with E2 stimulation. Asterisk indicates significant differences between Esr2-mutant and WT means (P < 0.05). ns, not significant.
Generation of Esr2-mutant rats
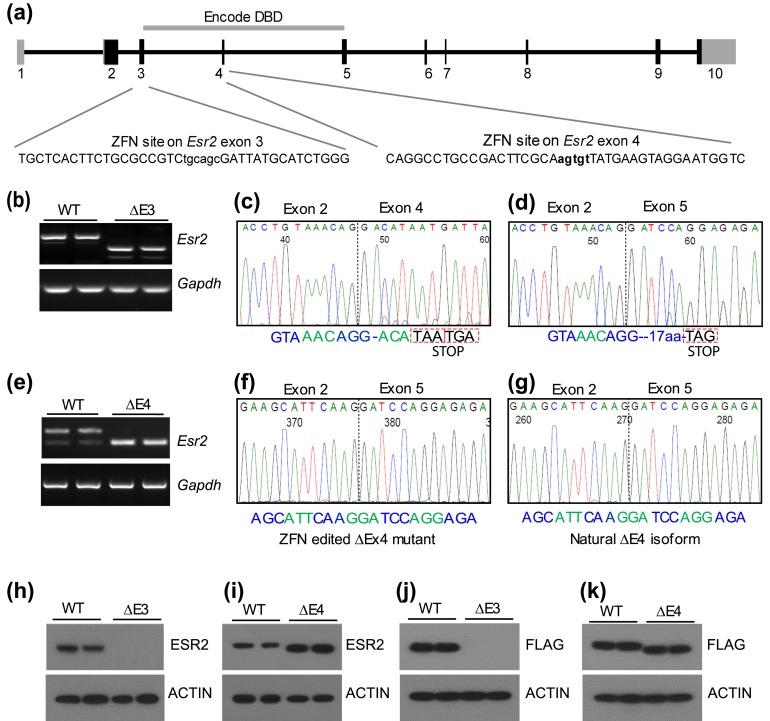
On the basis of the in vitro evaluation of the ESR2 transcripts and proteins, mRNAs encoding ZFNs targeting exon 3 or exon 4 [Fig. 2(a)] were generated and microinjected into single-cell embryos to produce mutant rats. Multiple founders with targeted exon 3 or exon 4 deletions were identified (Supplemental Tables 1 and 2 (274.6KB, pdf) ). After preliminary characterization of ESR2 expression in two independent lines from each mutant group, strain No. 3389 (Esr2ΔE3) and strain No. 1500 (Esr2ΔE4) were selected for phenotyping. The Esr2ΔE3 mutation consists of a 590-bp deletion, encompassing all of exon 3 and portions of its flanking introns, whereas the Esr2ΔE4 mutation consists of a 1501-bp deletion, encompassing all of exon 4 and portions of its flanking introns.
Figure 2.
Targeted disruption of the rat Esr2 gene. (a) Schematic presentation of the rat Esr2 gene (NC_005105.4) and ZFN target sites within exon 3 and exon 4. (b) RT-PCR was performed on RNA samples from WT and Esr2ΔE3 mutant ovaries. PCR primers were designed for exon 2 and exon 5. Sequencing of the RT-PCR products showed the presence of two different ΔE3 mutant transcripts arising from alternative splicing between (c) exons 2 and 4 or (d) exons 2 and 5. (c) Splicing between exons 2 and 4 was predominant generating a frameshift and premature stop codons after amino acid 140. (d) Exon 2 to 5 splicing also generated a frameshift, 17 aberrant codons after amino acid 140 followed by a premature stop codon. (e) RT-PCR was performed on RNA samples from WT and Esr2ΔE4-mutant ovaries to detect ΔE4-mutant transcripts. Sequencing of the RT-PCR products indicated that (f) ZFN-edited ΔE4-mutant transcript is indistinguishable from (g) the naturally occurring endogenous ΔE4 isoform. Western blot analyses of granulosa cell proteins demonstrated undetectable ESR2 protein in (h) ΔE3-mutant ovaries and (i) a truncated ESR2 protein in ΔE4-mutant ovaries. (j, k) Full-length WT and mutant ESR2 coding sequences were amplified by RT-PCR, cloned into a mammalian expression vector with a C-terminal FLAG-tag, and expressed in 293FT cells. Western blot analysis with anti-FLAG antibody did not detect expression of (j) recombinant ΔE3 ESR2 protein but detected (k) a truncated ΔE4 ESR2 protein.
Expression of ESR2 in Esr2ΔE3- and Esr2ΔE4-mutant rat models
The ΔE3 and ΔE4 deletion mutations were analyzed at both the mRNA and protein levels using ovarian tissue samples (Fig. 2). Sequencing of RT-PCR products indicated that two different ΔE3-mutant transcripts were generated from alternative splicing between exons 2 and 4 or exons 2 and 5 [Fig. 2(b–d)]. Splicing between exons 2 and 4 was predominant and generated a frameshift with two premature stop codons after amino acid 140 [Fig. 2(c)]. Splicing between exons 2 to 5 also generated a frameshift 17 aberrant codons after amino acid 140 followed by a premature stop codon [Fig. 2(d)].
Deletion of exon 4 resulted in exclusive splicing between exons 3 and 5 of Esr2 [Fig. 2(e–g)], which is a known endogenous alternative splice variant. Sequencing of the ΔE4 Esr2 RT-PCR products indicated that the ZFN-edited Esr2ΔE4-mutant transcript [Fig. 2(f)] was indistinguishable from a naturally occurring splice variant (37) [Fig. 2(g)].
Western blot analyses confirmed the absence of ESR2 protein in Esr2ΔE3-mutant ovaries [Fig. 2(h)], whereas a truncated ESR2 protein was detected in Esr2ΔE4 ovaries [Fig. 2(i)]. To examine whether the ΔE3 or ΔE4 mutations caused any alternative or aberrant transcription or translation, we also cloned full-length mutant and WT Esr2 mRNAs into expression vectors, and sequenced and verified their expression using carboxy-terminal FLAG tags in 293FT cells. These in vitro expression studies confirmed the predicted translational processing of Esr2ΔE3- and Esr2ΔE4-mutant mRNAs [Fig. 2(j) and 2(k)].
Postnatal development and fertility of Esr2ΔE3- and Esr2ΔE4-mutant rat models
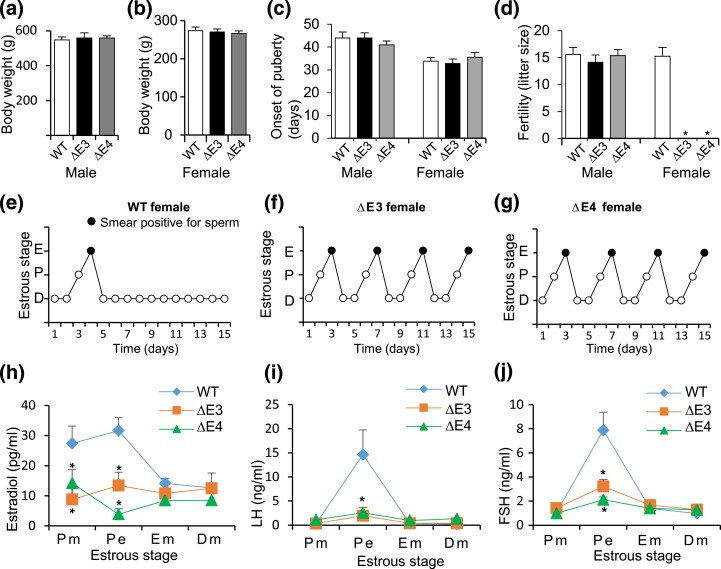
Rats possessing heterozygous mutations for either Esr2ΔE3 or Esr2ΔE4 were fertile. Genotypes of offspring from the Esr2ΔE3 heterozygote × Esr2ΔE3 heterozygote breeding indicated some modest but significant viability issues for Esr2ΔE3 homozygous mutants, whereas the Esr2ΔE4 heterozygote × Esr2ΔE4 heterozygote breeding generated offspring with the expected Mendelian ratio (Supplemental Figs. 1 and 2 (274.6KB, pdf) ). The disruption in the generation of Esr2ΔE3 homozygous mutants was small (expected vs observed, 25% and 22.34%, respectively), suggesting a potential phenotype of low penetrance, which will be difficult to mechanistically dissect. Homozygous Esr2ΔE3 or Esr2ΔE4 mutants exhibited postnatal growth patterns similar to that of WT rats [Fig. 3(a) and 3(b)], and normal onset of puberty [Fig. 3(c)]. Esr2-mutant males were fertile, whereas Esr2-mutant females were infertile [Fig. 3(d)]. Esr2-mutant females exhibited regular estrous cycles [Fig. 3(e–g)] and could be inseminated by WT males but did not become pregnant or pseudopregnant. Furthermore, serum levels of E2 [Fig. 3(h)], LH [Fig. 3(i)], and follicle-stimulating hormone [Fig. 3(j)] were significantly lower in Esr2-mutant females vs WT control rats during the evening of proestrus. Thus, Esr2 mutations were compatible with male reproductive function but interfered with female fertility.
Figure 3.
Assessment of postnatal development and fertility of Esr2 mutant rats. (a, b) Body weight of ΔE3- and ΔE4-mutant male and female rats were compared with WT rats and no significant difference was observed. (c) Onset of puberty in Esr2-mutant males (preputial separation) and mutant females (vaginal opening) was similar to that of their WT littermates. (d) Both Esr2ΔE3- and Esr2ΔE4-mutant males were fertile and their fertility was comparable to that of WT males. However, Esr2ΔE3- and Esr2ΔE4-mutant females did not become pregnant (n ≥ 12 in each group). (e–g) Vaginal cytology of adult (8–12 weeks old) Esr2ΔE3- and Esr2ΔE4-mutant females showed cyclic changes similar to WT female rats. When mutant females cohabitated with WT males, they mated but did not become pregnant or pseudopregnant. (h–j) Changes in serum hormone levels during specific stages of the estrous cycle were examined in WT vs Esr2ΔE3- and Esr2ΔE4-mutant females. Samples were collected at 0800 hours on the morning of proestrus (Pm), 2000 hours on the evening of proestrus (Pe), and 0800 hours on the morning of estrus (Em), and the first day of diestrus (Dm; n = 6 on collection day). Results are presented as mean ± standard error of the mean. Asterisks indicate significant differences between Esr2 mutant and WT means (P < 0.05). Serum E2, LH, and follicle-stimulating hormone (FSH) concentrations were measured. Serum E2 concentrations were significantly lower during proestrus in Esr2-mutant rats vs WT control rats. In addition, LH and FSH levels during the evening of proestrus were significantly lower in Esr2ΔE3- and Esr2ΔE4-mutant female rats vs WT control rats. D, diestrus; E, estrus; P, proestrus.
Reproductive tract in Esr2-mutant rat models
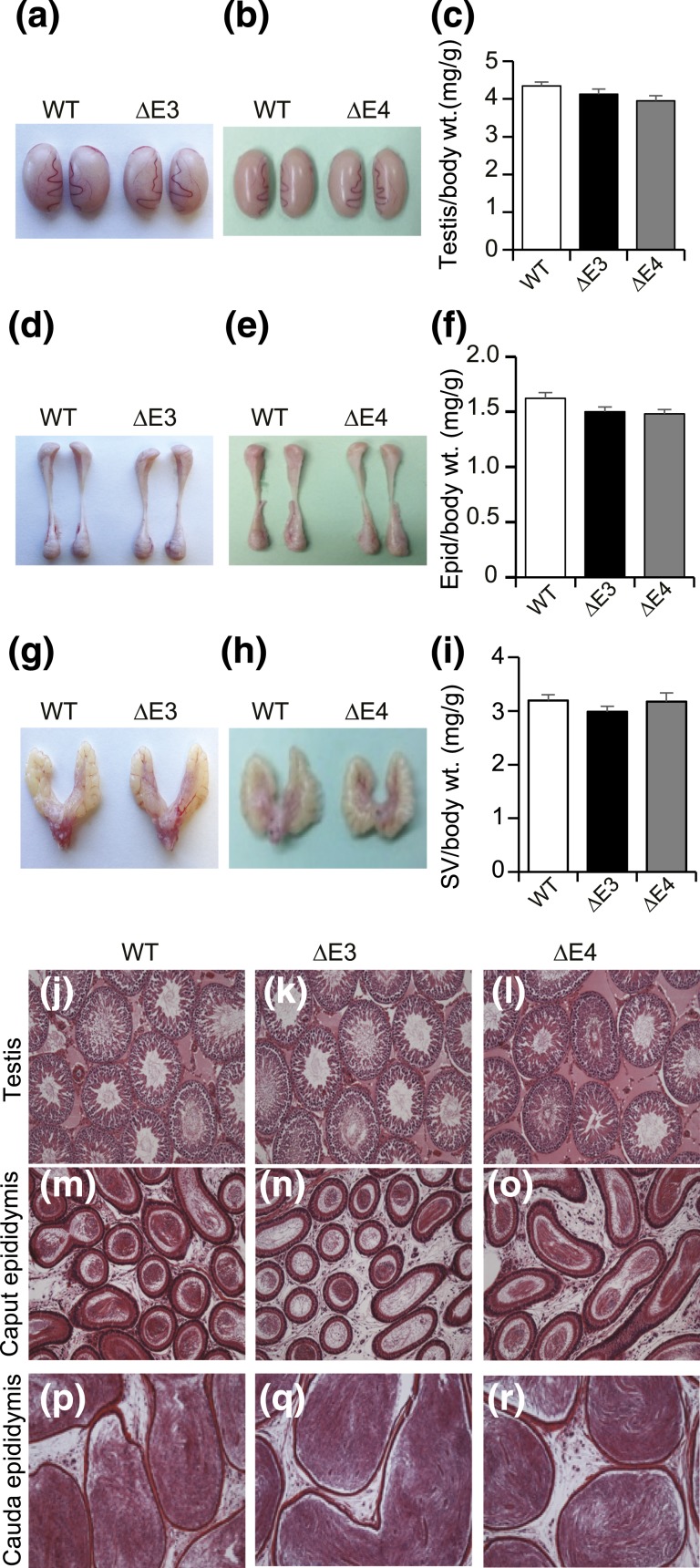
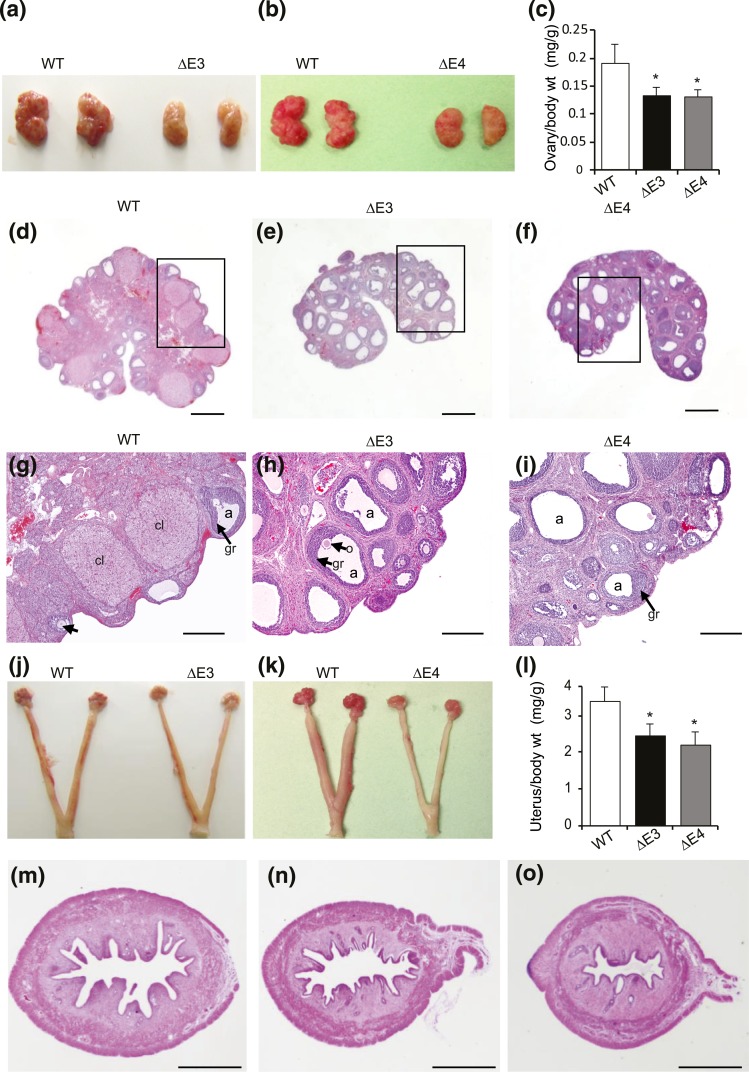
The size and histological organization of testes, epididymides, and seminal vesicles in Esr2ΔE3- and Esr2ΔE4-mutant males were similar to those of WT males (Fig. 4). Ovaries in Esr2ΔE3- and Esr2ΔE4-mutant rats were smaller than those from WT controls [Fig. 5(a–c)]. Ovaries from both of the mutant groups, as well as WT controls, contained follicles in various stages of development. In contrast, there was no evidence of corpora lutea in ovaries from Esr2ΔE3 or Esr2ΔE4 females [Fig. 5(d–i)]. Uteri from Esr2-mutant females were significantly smaller than uteri from WT females [Fig. 5(j–l)]. Uterine histological architecture and responsiveness to E2 did not differ between WT and Esr2-mutant rats [Fig. 5(m–o); Supplemental Fig. 3 (274.6KB, pdf) ).
Figure 4.
Effects of ESR2 disruption on the male reproductive tract. The reproductive tracts of adult WT and Esr2ΔE3- and Esr2ΔE4-mutant males (12 to 14 weeks of age) were examined, including gross appearance and weights for (a–c) testes, (d–f) epididymides, and (g–i) seminal vesicles. Sample sizes for the organ weight measurements were ≥10 per genotype. Representative hematoxylin and eosin–stained tissue sections of (j–l) testis, (m–o) caput epididymis, and (p–r) cauda epididymis from WT and Esr2ΔE3- and Esr2ΔE4-mutant male rats are presented. No significant differences in reproductive tracts were observed between the WT and Esr2-mutant male rats. Scale bars, 500 μM. Epid, epididymis; SV, seminal vesicle; wt., weight.
Figure 5.
Female reproductive tract in Esr2-mutant rats. Ovaries of (a, c) Esr2ΔE3- and (b, c) Esr2ΔE4-mutant rats were smaller than those of WT rats (n = 6). Histological examination of hematoxylin and eosin (H&E)–stained ovary sections shows the presence of (d, g) multiple corpora lutea in WT ovaries, whereas (e, h) Esr2ΔE3- or (f, i) Esr2ΔE4-mutant ovaries were characterized by the absence of corpora lutea despite presence of numerous follicles in various stages of development. [Boxed areas of (d–f) are magnified in (g–i).] Uteri of (j, l) Esr2ΔE3- and (k, l) Esr2ΔE4-mutant rats were smaller than those of WT rats (n = 6). H&E-stained uterine sections show that (n) Esr2ΔE3- and (o) Esr2ΔE4-mutant uteri possessed all three definitive uterine compartments: myometrium, endometrial stroma, and epithelium; however, all were relatively smaller than that found in (m) the WT uterine sections. Scale bars, 1000 μM (d–f and m–o) and 100 μM (g–i). Results are presented as mean ± standard error of the mean. Asterisks indicate significant differences between Esr2 mutant and WT means (P < 0.05). a, antrum; cl, corpus luteum; gr, granulosa layer; o, oocyte; wt, weight.
Ovulatory responses to gonadotropin treatment in Esr2-mutant rat models
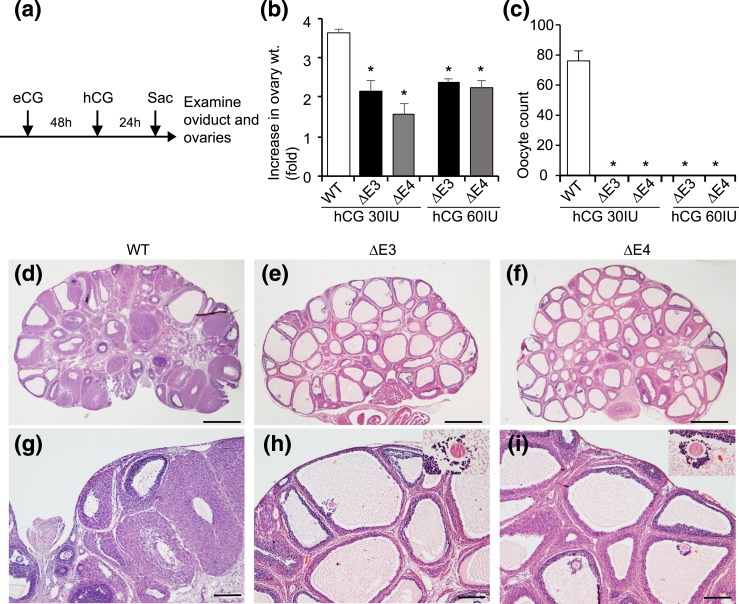
The absence of corpora lutea in Esr2ΔE3- and Esr2ΔE4-mutant ovaries prompted an assessment of the regulation of ovulation in the mutant females. Four-week-old Esr2-mutant females and their WT littermates were treated with gonadotropins, and ovarian weights and oocyte yield were evaluated [Fig. 6(a)]. Gonadotropin treatment stimulated ovarian weight in WT females and, to a lesser extent, in Esr2-mutant females [Fig. 6(b)]. Gonadotropins triggered ovulation and the presence of oocytes in oviducts of WT females but not in Esr2ΔE3- and Esr2ΔE4-mutant females [Fig. 6(c)]. Upon histological examination, Esr2-mutant ovaries exhibited many antral follicles containing trapped oocytes and an absence of corpora lutea, indicating a failure in ovulatory responses to gonadotropins compared with WT ovaries [Fig. 6(d–i)].
Figure 6.
Ovarian responses to gonadotropin stimulation in Esr2-mutant rats. (a) Four-week-old WT and Esr2-mutant female rats were treated with equine chorionic gonadotropin (eCG) and hCG, as indicated. (b) Gonadotropin treatment resulted in an increase in ovarian weight (wt.) in all three genotypes but was less effective with the ΔE3- and ΔE4-mutant ovaries compared with WT. (c) Esr2ΔE3- and Esr2ΔE4-mutant rats did not ovulate after eCG and hCG treatment (n = 10 per group). Histological examination of hematoxylin and eosin–stained ovary sections revealed that (d, g) WT ovaries contained follicles at various stages of maturation and corpora lutea, whereas (e, h) Esr2ΔE3- or (f, i) Esr2ΔE4- mutant ovaries exhibited numerous follicles and the absence of corpora lutea. *P < 0.05. Scale bars, 1000 μM (d–f) and 100 μM (g–i). Sac, sacrifice.
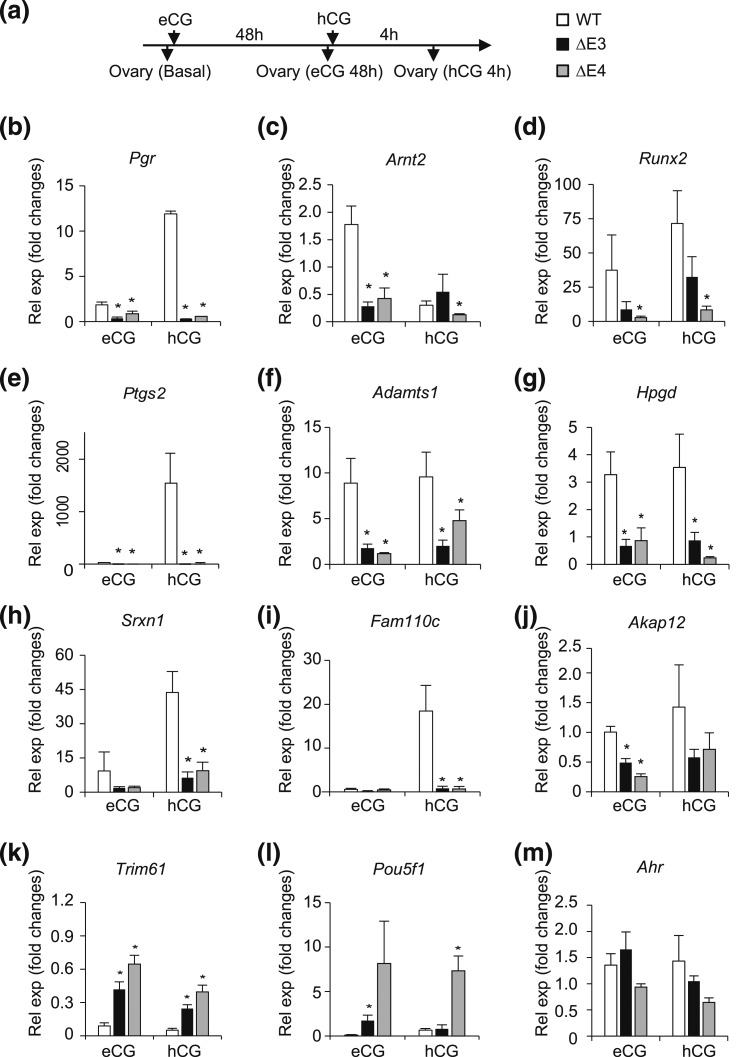
Next, we investigated the expression of genes known to be involved in the control of follicle rupture after gonadotropin stimulation (38–40) (Fig. 7). Unlike WT females, exogenous gonadotropin treatment failed to upregulate the expression of transcriptional regulators Pgr, Arnt2, and Runx2 [Fig. 7(b–d)], enzymes involved in the biosynthesis of prostaglandins, proteolysis, and protection from oxidative stress [Ptgs2, Adamts1, Hpgd, and Srxn1; Fig. 7(e–h)], and protein kinase stabilizers such as Fam110c and Akap12 [Fig. 7(i) and 7(j)]. In contrast, the transcriptional regulators Trim61 and Pou5f1 were uniquely upregulated in ovaries from gonadotropin-treated Esr2 mutant rats [Fig. 7(k–m)]. Other transcripts exhibited distinct profiles in ovaries of WT and Esr2-mutant rats treated with gonadotropins, including Fshr, Lhcgr, Klf4, Cyp11a1, Abcb1b, and Scube1 (Supplemental Fig. 4 (274.6KB, pdf) ). The significance of these latter changes in gonadotropin-dependent transcript responses remains to be determined.
Figure 7.
Gonadotropin-induced ovarian transcript responses in Esr2-mutant rats. RNA was extracted from gonadotropin-stimulated ovaries and expression of potential ESR2 target genes was examined by quantitative RT-PCR. (a) Four-week old WT and Esr2-mutant female rats were treated with eCG and hCG as indicated. Animals were euthanized and ovaries harvested from untreated animals (basal) 48 hours after eCG administration (eCG 48h) and 4 hours after hCG administration (hCG 4h). (b–j) WT ovaries responded with an upregulation of (b) Pgr, (c) Arnt2, (d) Runx2, (e) Ptgs2, (f) Adamts1, (g) Hpgd, (h) Srxn1, (i) Fam110c, and (j) Akap12. (k, l) However, ΔE3- and ΔE4-mutant ovaries did not respond to the gonadotropin treatment. In contrast, expression of (k) Trim61 and (l) Pou5f1 was uniquely upregulated in gonadotropin-treated Esr2-mutant ovaries. (m) Ahr expression remained unchanged after gonadotropin treatment. Results are expressed as mean ± standard error of the mean of gonadotropin-induced fold changes relative to basal levels (n = 4 per group). Asterisks indicate significant differences between Esr2-mutant and WT means (P < 0.05). eCG, equine chorionic gonadotropin; Rel exp, relative expression.
Our findings that both Esr2ΔE3- and Esr2ΔE4-mutant females exhibited failed ovulation, and of the absence of corpora lutea and aberrant ovarian gene expression indicated that the DBD/ERE-dependent function of ESR2 is essential for gonadotropin-mediated ovarian responses, including ovulation.
Discussion
The physiological role of ESR2 in reproductive function remains controversial, mainly due to a wide variation in phenotypic observations among an assortment of different mutant mouse models (7, 9–12). The variability in mutant phenotypes is associated with selection of distinct gene-targeting sites and methods for mutagenesis (11). The mechanism of ESR2 action may also be a confounding factor for such phenotypic variability (12). The estrogen-ESR2 complex can bind directly to an ERE or can tether to other transcription factors (41–43), facilitating interactions with appropriate coregulators to activate or repress its target genes (44–47). In this study, we explored the role of ESR2 in reproductive function and the possible mode of ESR2 action, using two different mutant rat models. One mutant model possessed a null mutation (Esr2ΔE3) and the other contained an inframe disruption of the DBD (Esr2ΔE4). It is important to point out that deletion of exon 4 in the Esr2ΔE4 model also corresponds to a naturally occurring splice variant identified in both rodent (37) and human tissues (48, 49). In contrast to the collective analysis of Esr2-mutant mouse models (7, 9–12), our findings with these two mutant ESR2 rat models were unequivocal. Disruption of ESR2 was compatible with fertility in male rats, whereas both ESR2 null and DBD mutations interfered with female fertility because of deficits in ovarian function.
Infertility in ESR2-mutant rats and subfertility in ESR2-mutant mice are associated with disrupted ovarian production of estrogen (39, 50, 51). Ovarian granulosa cells express ESR2 (52, 53). Loss of ESR2 leads to diminished granulosa cell responsiveness to gonadotropins and impairments in follicle maturation (39, 40, 50, 51). In addition, disruption of ESR2 appears to negatively impact gonadotropin-driven intracellular signaling pathways within granulosa cells (54, 55). The Esr2ΔE3 and Esr2ΔE4 rat models represent new tools for dissecting the mechanism of ESR2 action within granulosa cells.
Unlike ESR2-mutant mice, ESR2-mutant rats did not ovulate when two different doses of gonadotropins were administered. This suggests that ESR2 plays an essential role in regulating preovulatory follicular maturation and ovulation in the rat. This was further exemplified by the aberrant expression of several key genes involved in ovarian/granulosa cell function in ESR2-mutant rats. Changes in expression pattern were similar in both the ESR2-mutant groups; therefore, DNA-binding dependent transcriptional function appears critical for regulation of these genes. Whether this is strictly due to disruption of the estrogen-signaling cascade within the ovary or a developmental anomaly associated with loss of ESR2 is currently unknown.
An attenuated preovulatory gonadotropin surge was identified in ESR2-mutant rats, similar to previous observations in ESR2-mutant mice (56). ESR2 has been implicated in neuroendocrine regulation. The ESR2 protein is present in the rat anterior pituitary and throughout the brain, including areas responsible for regulating gonadotropin-releasing hormone synthesis and secretion (57–61). Furthermore, neuronal disruption of ESR2 has been reported to delay puberty through alterations in kisspeptin expression (62). However, a hypothalamo-hypophyseal site of ESR2 action is not critical for regulation of preovulatory gonadotropin secretion. Korach et al. (56), through a series of ovary transplantation experiments, have implicated the ovary and diminished preovulatory estrogen secretion for dysregulated gonadotropin secretion in the ESR2-mutant female mouse. Whether ovarian ESR2 serves a similar role in regulating the preovulatory gonadotropin surge in the rat remains to be determined.
Naturally occurring isoforms of ESR2 arising from alternative splicing are present in rodents and humans (37, 48, 63–66). The ΔE4 ESR2 protein variant is present in numerous tissues (37). This ESR2 variant can bind estradiol (37) and function through a non-ERE pathway (12). Our findings indicate that the ΔE4 ESR2 protein variant cannot compensate for the absence of the WT ESR2 protein in the regulation of fertility. The ΔE4 ESR2 protein variant may be involved in fine-tuning reproductive function or contributing to the regulation of non–ERE-mediated pathways in other physiological processes.
In conclusion, phenotypic characterization of the mutant Esr2ΔE3 and Esr2ΔE4 rat models has resolved uncertainties regarding ESR2 action and led to the following insights: (1) ESR2 is not critical for the regulation of male fertility; (2) ESR2 is an essential regulator of female fertility; (3) ESR2 contributes to the regulation of the preovulatory gonadotropin surge; (4) ESR2 is essential for gonadotropin-regulated ovulatory events; and (5) the role of ESR2 in the regulation of female fertility depends on ESR2 acting in trans through binding to ERE motifs.
Acknowledgments
We thank Dr. Donald McDonnell (Duke University, Durham, North Carolina) for the 3XERE-TATA-Luc construct, Dr. Melissa Larson in the University of Kansas Medical Center Transgenic and Gene Targeting Institutional Facility (supported by National Institutes of Health Grant P20GM104936), and Stacy McClure for administrative assistance.
Current affiliations: X. Zhao’s current affiliation is the Department of Molecular and Cellular Medicine, Texas A&M University, College Station, Texas 77843-1114. W. Cui’s current affiliation is the Department of Veterinary & Animal Sciences, University of Massachusetts, Amherst, Massachusetts 01003.
Acknowledgments
The research was supported by National Institutes of Health Grants HD066406 and OD01478 and individual postdoctoral fellowships from the American Heart Association and Japan Society for the Promotion of Science (to K.K.) and Lalor Foundation (to P.D.).
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| ESR2 | KLH-conjugated, synthetic peptide corresponding to amino acids 63-82 of rat and mouse estrogen receptor β and amino acids 55-74 of human estrogen receptor β | Anti-estrogen receptor β antibody, clone 68-4, rabbit; monoclonal | EMD Millipore, 05-824 Produced in collaboration with Epitomics | Rabbit; monoclonal | 5000 | AB_11212759 |
| FLAG | A synthetic peptide (DYKDDDDK) coupled to KLH | Mouse anti-DYKDDDDK-tag mAb; mouse anti-flag-tag mAb; | GenScript, A00187-200 | Mouse; monoclonal | 5000 | AB_1720813 |
| Actin | Synthetic b-cytoplasmic actin N-terminal peptide Ac-AspAsp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-GlyLys, conjugated to KLH. | Anti-β-actin antibody, mouse; monoclonal | Sigma-Aldrich, A 1978-100 UL | Mouse; monoclonal | 25,000 | AB_476692 |
Abbreviations: mAB, monoclonal antibody; MS, manuscript; RRID, Research Resource Identifier.
Footnotes
- DBD
- DNA binding domain
- E2
- estradiol
- ERE
- estrogen response element
- ESR1
- estrogen receptor α
- ESR2
- estrogen receptor β
- hCG
- human chorionic gonadotropin
- LH
- luteinizing hormone
- PCR
- polymerase chain reaction
- RT-PCR
- reverse transcription polymerase chain reaction
- WT
- wild-type
- ZFN
- zinc finger nuclease
- ΔE3
- deleted exon 3
- ΔE4
- deleted exon 4.
References
- 1.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green S, Kumar V, Krust A, Walter P, Chambon P. Structural and functional domains of the estrogen receptor. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):751–758. [DOI] [PubMed] [Google Scholar]
- 3.Greene GL, Press MF. Structure and dynamics of the estrogen receptor. J Steroid Biochem. 1986;24(1):1–7. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. [DOI] [PubMed] [Google Scholar]
- 6.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. [DOI] [PubMed] [Google Scholar]
- 8.Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155(5):1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95(26):15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143(5):1643–1650. [DOI] [PubMed] [Google Scholar]
- 11.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA. 2008;105(7):2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maneix L, Antonson P, Humire P, Rochel-Maia S, Castañeda J, Omoto Y, Kim HJ, Warner M, Gustafsson JÅ. Estrogen receptor β exon 3-deleted mouse: the importance of non-ERE pathways in ERβ signaling. Proc Natl Acad Sci USA. 2015;112(16):5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Andersson S, Warner M, Gustafsson JA. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc Natl Acad Sci USA. 2001;98(5):2792–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA. 2003;100(2):703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Mäkelä S, Warner M, Gustafsson JA. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA. 2003;100(11):6694–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23(23):8542–8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta-/-) mice. Proc Natl Acad Sci USA. 2006;103(18):7165–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98(4):1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21(1):1–13. [DOI] [PubMed] [Google Scholar]
- 20.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13(10):1672–1685. [DOI] [PubMed] [Google Scholar]
- 22.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275(8):5379–5387. [DOI] [PubMed] [Google Scholar]
- 23.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling [published correction appears in Proc Natl Acad Sci USA. 2006;103(40):14977]. Proc Natl Acad Sci USA. 2006;103(35):13162–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–s49. [DOI] [PubMed] [Google Scholar]
- 25.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 26.Dow LE, Lowe SW. Life in the fast lane: mammalian disease models in the genomics era. Cell. 2012;148(6):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells KD. Genetic engineering of mammals. Cell Tissue Res. 2016;363(1):289–294. [DOI] [PubMed] [Google Scholar]
- 28.Everett JW. Progesterone and estrogen in the experimental control of ovulation time and other features of the estrous cycle in the rat. Endocrinology. 1948;43(6):389–405. [DOI] [PubMed] [Google Scholar]
- 29.Richards JS, Midgley AR Jr. Protein hormone action: a key to understanding ovarian follicular and luteal cell development. Biol Reprod. 1976;14(1):82–94. [DOI] [PubMed] [Google Scholar]
- 30.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56(2):293–302. [DOI] [PubMed] [Google Scholar]
- 31.Kubota K, Cui W, Dhakal P, Wolfe MW, Rumi MA, Vivian JL, Roby KF, Soares MJ. Rethinking progesterone regulation of female reproductive cyclicity. Proc Natl Acad Sci USA. 2016;113(15):4212–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. [DOI] [PubMed] [Google Scholar]
- 33.Dhakal P, Rumi MA, Kubota K, Chakraborty D, Chien J, Roby KF, Soares MJ. Neonatal progesterone programs adult uterine responses to progesterone and susceptibility to uterine dysfunction. Endocrinology. 2015;156(10):3791–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–1708. [DOI] [PubMed] [Google Scholar]
- 35.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–226. [DOI] [PubMed] [Google Scholar]
- 36.Terranova PF, Garza F. Relationship between the preovulatory luteinizing hormone (LH) surge and androstenedione synthesis of preantral follicles in the cyclic hamster: detection by in vitro responses to LH. Biol Reprod. 1983;29(3):630–636. [DOI] [PubMed] [Google Scholar]
- 37.Petersen DN, Tkalcevic GT, Koza-Taylor PH, et al. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998;139:1082–1092. [DOI] [PubMed] [Google Scholar]
- 38.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146(8):3247–3262. [DOI] [PubMed] [Google Scholar]
- 40.Binder AK, Rodriguez KF, Hamilton KJ, Stockton PS, Reed CE, Korach KS. The absence of ER-β results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology. 2013;154(6):2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res. 2000;55:163–193, discussion 194–195. [PubMed] [Google Scholar]
- 42.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via estrogen receptor beta. J Biol Chem. 2010;285(51):39575–39579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. [DOI] [PubMed] [Google Scholar]
- 45.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25(1):45–71. [DOI] [PubMed] [Google Scholar]
- 46.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. [DOI] [PubMed] [Google Scholar]
- 47.Lonard DM, Lanz RB, O’Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28(5):575–587. [DOI] [PubMed] [Google Scholar]
- 48.Poola I, Abraham J, Baldwin K. Identification of ten exon deleted ERbeta mRNAs in human ovary, breast, uterus and bone tissues: alternate splicing pattern of estrogen receptor beta mRNA is distinct from that of estrogen receptor alpha. FEBS Lett. 2002;516(1-3):133–138. [DOI] [PubMed] [Google Scholar]
- 49.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25(6):869–898. [DOI] [PubMed] [Google Scholar]
- 50.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)alpha and ERbeta null mice indicate a role for ERbeta in follicular maturation. Endocrinology. 2005;146(6):2817–2826. [DOI] [PubMed] [Google Scholar]
- 51.Drummond AE, Fuller PJ. Ovarian actions of estrogen receptor-β: an update. Semin Reprod Med. 2012;30(1):32–38. [DOI] [PubMed] [Google Scholar]
- 52.Couse JF, Lindzey J, Grandien K, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. [DOI] [PubMed] [Google Scholar]
- 53.Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod. 2000;62(2):310–317. [DOI] [PubMed] [Google Scholar]
- 54.Deroo BJ, Rodriguez KF, Couse JF, Hamilton KJ, Collins JB, Grissom SF, Korach KS. Estrogen receptor beta is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol. 2009;23(7):955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez KF, Couse JF, Jayes FL, Hamilton KJ, Burns KA, Taniguchi F, Korach KS. Insufficient luteinizing hormone-induced intracellular signaling disrupts ovulation in preovulatory follicles lacking estrogen receptor-beta. Endocrinology. 2010;151(6):2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayes FL, Burns KA, Rodriguez KF, Kissling GE, Korach KS. The naturally occurring luteinizing hormone surge is diminished in mice lacking estrogen receptor beta in the ovary. Biol Reprod. 2014;90(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchner NA, Garlick C, Ben-Jonathan N. Cellular distribution and gene regulation of estrogen receptors alpha and beta in the rat pituitary gland. Endocrinology. 1998;139(9):3976–3983. [DOI] [PubMed] [Google Scholar]
- 58.Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63(10):498–504. [DOI] [PubMed] [Google Scholar]
- 59.Schreihofer DA, Stoler MH, Shupnik MA. Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERalpha protein and estrogen responsiveness. Endocrinology. 2000;141:2174–2184. [DOI] [PubMed] [Google Scholar]
- 60.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- 61.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067. [DOI] [PubMed] [Google Scholar]
- 62.Naulé L, Robert V, Parmentier C, Martini M, Keller M, Cohen-Solal M, Hardin-Pouzet H, Grange-Messent V, Franceschini I, Mhaouty-Kodja S. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum Mol Genet. 2015;24(25):7326–7338. [DOI] [PubMed] [Google Scholar]
- 63.Chu S, Fuller PJ. Identification of a splice variant of the rat estrogen receptor beta gene. Mol Cell Endocrinol. 1997;132(1-2):195–199. [DOI] [PubMed] [Google Scholar]
- 64.Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws ER Jr. Selective expression of estrogen receptor alpha and beta isoforms in human pituitary tumors. J Clin Endocrinol Metab. 1998;83(11):3965–3972. [DOI] [PubMed] [Google Scholar]
- 65.Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PT. Human oestrogen receptors: differential expression of ER alpha and beta and the identification of ER beta variants. Steroids. 2002;67(12):985–992. [DOI] [PubMed] [Google Scholar]
- 66.Fujimoto J, Sun WS, Misao R, Sakaguchi H, Aoki I, Toyoki H, Tamaya T. Expression of estrogen receptor beta exon-deleted variant mRNAs in ovary and uterine endometrium. J Steroid Biochem Mol Biol. 2003;84(2-3):133–140. [DOI] [PubMed] [Google Scholar]