Abstract
Protein kinase A (PKA) has recently been shown to mimic the actions of follicle-stimulating hormone (FSH) by activating signaling pathways that promote granulosa cell (GC) differentiation, such as phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK). We sought to elucidate the mechanism by which PKA, a Ser/Thr kinase, intersected the PI3K/AKT and MAPK/ERK pathways that are canonically activated by receptor tyrosine kinases (RTKs). Our results show that for both of these pathways, the RTK is active in the absence of FSH yet signaling down the pathways to commence transcriptional responses requires FSH-stimulated PKA activation. For both pathways, PKA initiates signaling by regulating the activity of a protein phosphatase (PP). For the PI3K/AKT pathway, PKA activates the Ser/Thr PP1 complexed with the insulinlike growth factor 1 receptor (IGF-1R) and insulin receptor substrate 1 (IRS1) to dephosphorylate Ser residues on IRS1, authorizing phosphorylation of IRS1 by the IGF-1R to activate PI3K. Treatment of GCs with FSH and exogenous IGF-1 initiates synergistic IRS1 Tyr phosphorylation and resulting gene activation. The mechanism by which PKA activates PI3K is conserved in preovulatory GCs, MCF7 breast cancer cells, and FRTL thyroid cells. For the MAPK/ERK pathway, PKA promotes inactivation of the MAPK phosphatase (MKP) dual specificity phosphatase (DUSP) MKP3/DUSP6 to permit MEK-phosphorylated ERK to accumulate downstream of the epidermal growth factor receptor. Thus, for the two central signaling pathways that regulate gene expression in GCs, FSH via PKA intersects canonical RTK-regulated signaling by modulating the activity of PPs.
Protein kinase A regulates the activity of protein phosphatases to activate the phosphatidylinositol 3-kinase/AKT and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways.
Follicle-stimulating hormone (FSH) signals via its cell-surface G-protein coupled receptor (GPCR) to regulate the expression of ∼3800 genes that mediate granulosa cell (GC) proliferation and differentiation to drive maturation of ovarian follicles from the preantral to the preovulatory stage (1). Principal upregulated genes that define the preovulatory GC include the luteinizing hormone/choriogonadopin receptor (Lhcgr) that mediates selection of the dominant follicle in primates [reviewed in (2)] and is required for ovulation, luteinization of granulosa and theca cells, and oocyte meiosis in response to the preovulatory surge of LH (3); the rate limiting enzyme for estrogen biosynthesis, Cyp19a1 (4); natriuretic peptide precursor C, Nppc, that yields C-type natriuretic peptide that binds the guanylyl cyclase membrane receptor natriuretic peptide receptor 2 on mural and cumulus GCs to trigger production of cGMP that maintains oocyte arrest until the preovulatory surge of LH (5, 6); Inha, the α subunit of the hormone inhibin that inhibits FSH secretion by the anterior pituitary (7); and cyclin D2, Ccnd2, that is required for the proliferative response of GCs (8).
It has been recognized since the mid-1970s that FSH activates adenylyl cyclase to raise intracellular levels of cAMP (9, 10), resulting in the activation of protein kinase A (PKA) (11). Substrates directly phosphorylated by PKA that enhance the transcriptional responses of FSH through interactions with DNA and other transcriptional factors include cAMP-response element–binding protein (Ser133) (12), histone H3 (Ser10) (13), and β-catenin (Ser552 and Ser675) (14). However, like gonadotropin releasing hormone-induced expression of the LH beta subunit gene in gonadotropes that requires an array of combinatorial transcriptional codes [reviewed in (15)], upregulation of FSH gene targets that have been intensely investigated, such as Lhcgr, Inha, Cyp19a1, and Cyp11a1, also requires a complex cast of transcriptional activators and repressors [(1); reviewed in (16)].
Consistent with evidence that multiple transcriptional regulators converge to regulate FSH-dependent gene expression, FSH activates a number of signaling pathways that modulate both translation and/or transcription, including phosphatidylinositol 3-kinase (PI3K)/AKT, mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and p38 MAPK pathways [reviewed in (16)], as well as the hippo/yes-associated protein pathway [reviewed in (17)]. Most results suggest that FSH signals via PKA to activate these pathways, based on the ability of PKA inhibitors, such as the selective PKA catalytic subunit pseudosubstrate PKI (18) or the ATP competitive antagonist H89 (19), to block FSH-dependent activation of these signaling pathways (20–25) and consequent expression of genes such as Lhcgr, Cyp19a1, Star, Cyp11a1, and Map2d (14, 20, 26–28). However, controversial results using PKI (28) coupled with consistent evidence that H89 either fails to inhibit or activates PI3K/AKT (22, 29, 30) suggest that FSH may also signal independently of PKA. [The molecular basis by which H89 paradoxically fails to inhibit FSH-dependent phosphorylation of IRS1(Tyr989) and AKT(Thr308, Ser473), and dephosphorylation of IRS1(Ser789) and myosin light chain (Ser19), all of which are PKA dependent (21, 31, 32, 33), while blocking FSH-stimulated phosphorylation of GRB2-associated binding protein 2 (Ser159) (32) and other direct PKA targets such as histone H3(Ser10) (11), is not known. It is notable, however, that while the phosphorylation of targets downstream of AKT, tuberin(Thr1462) and p70 ribosomal S6 kinase(Thr389), are also not inhibited by H89, H89 abrogates the phosphorylation of ribosomal S6 (Ser235/236) protein (22) by directly inhibiting the activity of p70 ribosomal S6 kinase (34). Thus, the paradoxical effects of H89 likely reflect its ability to inhibit enzymes other than PKA.] Moreover, neither the adenylyl cyclase activator forskolin, cAMP agonists, nor adenoviral vectors that raise intracellular levels of cAMP, such as constitutively active Gαs and the Lhcgr (26, 28, 35), faithfully mimic the induction of Lhcgr and Cyp19a1 by FSH compared with the induction of Inha and Hsd3b1.
Taken together, these results beg the question: “Is activation of PKA sufficient to account for the complex pattern of intracellular signaling that accompanies [FSH-stimulated] GC differentiation?” (31). Utilizing a constitutively active PKA catalytic subunit mutant that cannot bind the inhibitory PKA regulatory subunits, PKA-CQR, the Zeleznik laboratory demonstrated that PKA-CQR not only qualitatively mimics FSH to regulate the FSH program of gene expression in GCs, including the induction of Lhcgr and Cyp19a1 (31, 36), but also is sufficient to promote the initial phosphorylation of AKT, ERK, and yes-associated protein as well as a number of other FSH-responsive targets (31). These results unequivocally demonstrate that the Ser/Thr kinase PKA in some manner signals to regulate a remarkable number of signaling pathways in GCs.
Two of the central signaling pathways activated by FSH via PKA are PI3K/AKT and MAPK/ERK. The PI3K/AKT pathway regulates the expression of 60% of the ∼3800 genes regulated by FSH via AKT-mediated phosphorylation of the transcriptional activator/repressor forkhead box O family member (1), including Ccnd2, Cyp19a1, Inha, Cyp11a1 (37), Lhcgr, Nppc, and Pappa (1). In light of the numerous additional targets phosphorylated by AKT (38), including those that regulate translation initiation (22) as well as nitric oxide synthase and NF-κB signaling, the PI3K pathway may even contribute to additional FSH-dependent responses of GCs. MAPK/ERK signaling is necessary for the induction minimally of Egfr, Cyp19a1, Inha, Lhcgr, Hsd17b1, and Pappa messenger RNAs (mRNAs) in preantral GCs via ribosomal S6 kinase-2–dependent phosphorylation of the transcriptional activator Y-box-binding protein 1 (39). MAPK/ERK signaling also contributes to the induction of Cyp19a1, Lhcgr, Inha, Inhb, Pappa, and Prkar2b via phosphorylation the transcriptional activator GATA-4 (27) and likely GATA-6 (40), based on dual GATA-4/GATA-6 knockout results (41).
We asked how the Ser/Thr protein kinase PKA activates the PI3K/AKT and MAPK/ERK pathways in GCs, both of which are canonically activated in other cells by receptor tyrosine kinases (RTKs). As summarized in this review, the relevant RTKs for these pathways, the insulinlike growth factor 1 receptor (IGF-1R) and epidermal growth factor receptor (EGFR), are active in GCs in the absence of FSH yet the downstream critical targets, insulin receptor substrate 1 (IRS1) and ERK, respectively, are not phosphorylated in the absence of FSH. PKA intersects these two pathways by regulating the activity of two distinct phosphatases. For the PI3K/AKT pathway, PKA activates the Ser/Thr protein phosphatase (PP)1 complexed with the IGF-1R and IRS1 to dephosphorylate at least four Ser residues on IRS1, promoting phosphorylation of IRS1 on Tyr residues by the IGF-1R to activate PI3K. For the MAPK/ERK pathway, PKA directly or indirectly promotes inactivation of the MAPK phosphatase (MKP) dual specificity phosphatase (DUSP) MKP3/DUSP6 to permit MEK-phosphorylated ERK to accumulate downstream of the EGFR.
Constitutively Active PKA Catalytic Subunit Mutant (PKA-CQR) Promotes Phosphorylation of IRS1(Tyr989) and AKT (Thr308, Ser473) and Activation of PP1 to Activate PI3K/AKT, as Well as Phosphorylation of ERK (Thr202/Tyr204) to Activate MAPK/ERK Signaling
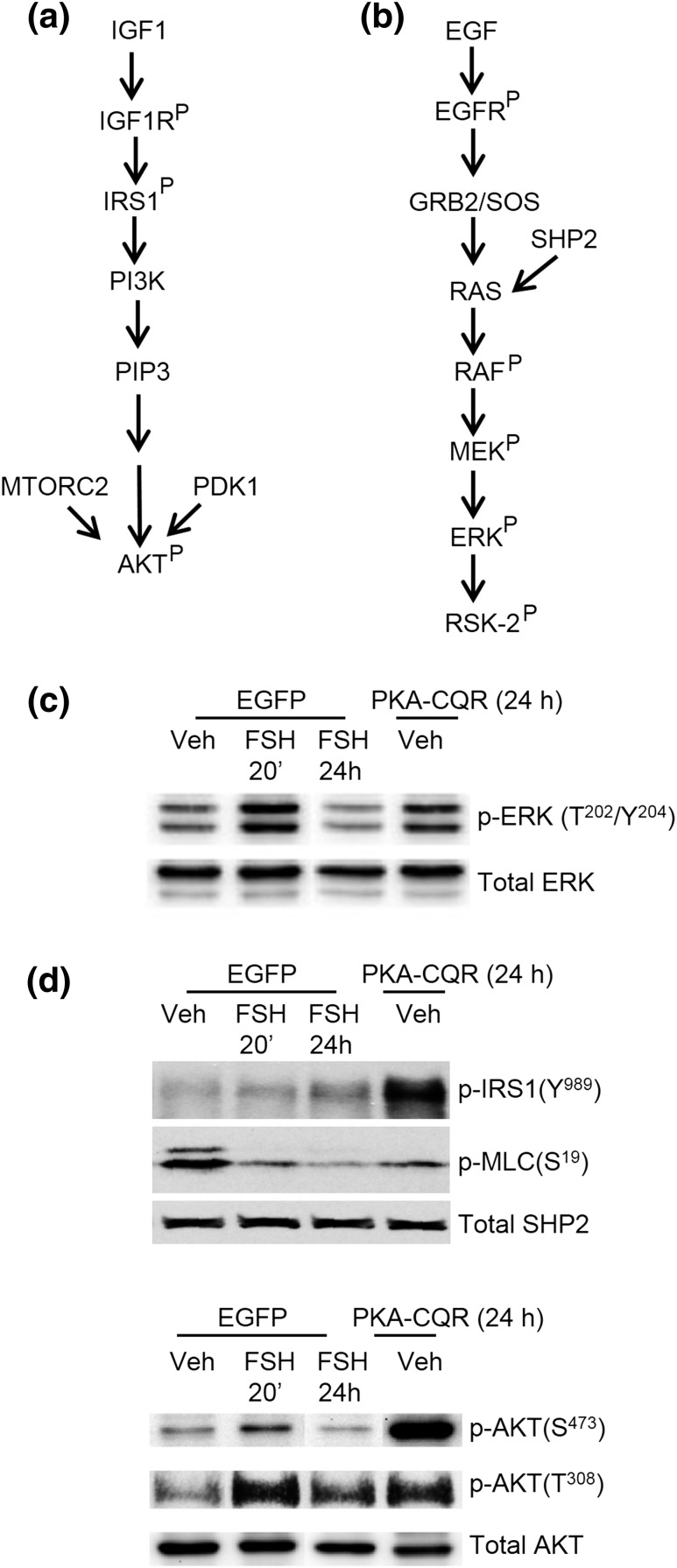
Figure 1(a) and 1(b) show the canonical signaling pathways that promote activation of PI3K/AKT and MAPK/ERK by IGF-1 and EGF, respectively. PKA-CQR or control enhanced green fluorescent protein was transduced into GCs for 24 hours, the earliest time point that showed cAMP-response element binding protein (Ser133) phosphorylation equivalent to that of FSH treatment of 20 minutes (31). Key targets that reveal the activation state of these pathways are the phosphorylation of IRS1 on Tyr989 and AKT on Thr308 and Ser473 and the dual phosphorylation of ERK on Thr202 and Tyr204. PKA-CQR [Fig. 1(c) and 1(d), fourth lane] was sufficient to mimic a 20-minute treatment of GCs with FSH (second lane) compared with vehicle-treated GCs (first lane). PKA-CQR also promoted the dephosphorylation of myosin light chain (Ser19), indicative of the activation of PP1.
Figure 1.
Canonical signaling to ERK and PI3K, and PKA signaling in GCs. (a, b) Canonical signaling pathways by which IGF-1 signals to activate PI3K to promote phosphorylation/activation of AKT and EGF signals to promote the phosphorylation/activation of ERK, respectively. AKT phosphorylation targets regulate translation, transcription, metabolism, cell survival, and angiogenesis [reviewed in (38)]; ERK phosphorylation targets regulate transcription, translation, and metabolism [reviewed in (42, 43)]. (c, d) The ability of constitutively active PKA catalytic subunit mutant (PKA-CQR) to promote phosphorylation of IRS1(Tyr989) [modified from (32)] and AKT (Thr308, Ser473) [modified from (31)] and activation of PP1 to dephosphorylate myosin light chain (Ser19) [modified from (32)] to activate PI3K/AKT, and phosphorylation of ERK (Thr202/Tyr204) to activate MAPK/ERK signaling [modified from (31)]. Preantral GCs were transduced with enhanced green fluorescent protein or PKA-CQR for 24 hours, then treated with vehicle (Veh) or FSH, as indicated. Treatment of GCs with FSH for 20 minutes generally yields a maximal phosphorylation response that declines thereafter; however, expression of the PKA-CQR lentivirus recombinant protein required 24 hours to detect consistent protein phosphorylation (31). Thus, the 24-hour stimulation window reflects the initial responses of GCs to active PKA. PDK1, phosphoinositide-dependent kinase-1; MTORC2, mammalian target of rapamycin 2.
PI3K/AKT Pathway: PKA Activates PP1 to Dephosphorylate Ser Residues on IRS1 That Promote Phosphorylation of Tyr Residues on IRS1 by the IGF-1R to Activate PI3K
Rat GCs in vitro constitutively secrete ∼0.3 ng/ml IGF-1, partially activating the IGF-1R, that is necessary but not sufficient to activate the PI3K/AKT pathway (32, 44). The addition of exogenous IGF-1 (>5 ng/mL) enhances autophosphorylation/activation of the IGF-1R, resulting in IGF-1R-dependent phosphorylation of IRS1 on select Tyr residues (Tyr*XXMet motif) that bind to and promote activation of PI3K, generation of phosphatidylinositol 3,4,5-trisphosphate, recruitment of AKT to the plasma membrane, and phosphorylation/activation of AKT on Thr308 by phosphoinositide-dependent kinase-1 and on Ser473 by mammalian target of rapamycin 2 [Fig. 1(a)] (32). However, AKT activation in response to exogenous IGF-1 alone is not sufficient to activate gene expression in GCs (32, 44).
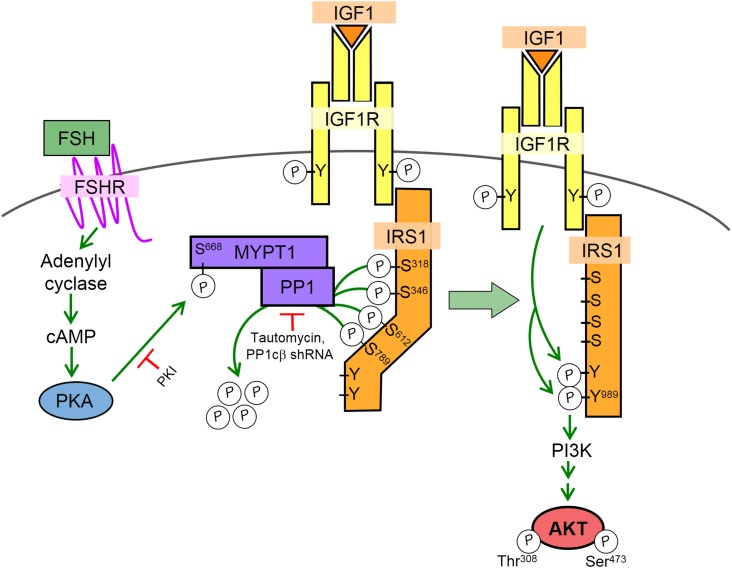
AKT phosphorylation on both Thr308 and Ser473 is detected within 5 minutes of FSH treatment (21). The mechanism by which FSH via PKA activates the PI3K/AKT pathway is diagrammed in Fig. 2. The IGF-1R is phosphorylated (likely only on Tyr1135) (32, 44, 45) and partially active in the absence of FSH but does not phosphorylate IRS1 to activate PI3K. Notably, treatment of GCs with FSH does not enhance the Tyr phosphorylation/activation of the IGF-1R (32, 44) yet FSH promotes the Tyr phosphorylation of IRS1 by the IGF-1R (21, 32, 33). Our results suggest that the ability of the IGF-1R to phosphorylate IRS1 on Tyr residues that activate PI3K is inhibited by the phosphorylation of IRS1 on at least four Ser residues (Ser318, Ser346, Ser612, Ser789) (33). PKA promotes the dephosphorylation of these Ser residues on IRS1 by activating the β-catalytic subunit of PP1 (PP1cβ) that is complexed with its regulatory subunit myosin phosphatase target subunit 1 (MYPT1), IRS1, and the IGF-1R (33). IRS1 also coimmunoprecipitates with the A-kinase anchoring protein GRB2-associated binding protein 2 and PKA (21), suggesting the presence of a multiprotein complex at the plasma membrane. In response to FSH, PKA phosphorylates MYPT1 on Ser668, activating PP1cβ to dephosphorylate the four Ser residues on IRS1. Dephosphorylation of these Ser residues on IRS1 facilitates the phosphorylation of IRS1 on Tyr989, a canonical Tyr*XXMet motif, leading to activation of PI3K and consequent phosphorylation/activation of AKT (33).
Figure 2.
Modeled mechanism by which FSH via PKA promotes the phosphorylation of IRS1 on Tyr989 by the IGF-1R to activate PI3K and downstream AKT in preantral GCs. Schematic model, based on recent publications (21, 32, 33), reflects the presence of endogenous IGF-1 secreted by GCs that promotes Tyr autophosphorylation of the IGF-1R but is not sufficient to promote phosphorylation of IRS1 to activate PI3K. Activation of PI3K/AKT requires FSH-stimulated, PKA-dependent phosphorylation of the PP1 regulatory subunit MYPT1 on Ser668 that catalyzes dephosphorylation of IRS1 on at least four Ser residues (Ser318, Ser346, Ser612, and Ser789), resulting in IGF-1R-dependent phosphorylation of IRS1 on Tyr residues such as Tyr989 that activate PI3K. Sites of action for inhibitors that block PKA (PKI) and PP1 (tautomycin and PP1cβ shRNA) and hence phosphorylation of IRS1(Tyr989) and activation/phosphorylation of AKT(Thr308/Ser473) are indicated.
In support of these conclusions, FSH-stimulated phosphorylations of IRS1(Tyr989), MYPT1(Ser668), AKT(Thr308), and AKT(Ser473) are selectively inhibited by the PP1 inhibitor tautomycin (32, 33) and/or shRNA-mediated downregulation of PP1cβ (32). Tautomycin and PKI also abrogate FSH-stimulated dephosphorylation of IRS1(Ser318) (33) and IRS1(Ser789) (32). Recombinant PKA catalytic subunit phosphorylates immunoprecipitated MYPT1 on Ser668 under cell-free conditions, enhancing the catalytic activity of PP1 that coimmunoprecipitates with IRS1 (33). Finally, a constitutively active MYPT1 transiently transfected into GCs is sufficient to promote the phosphorylations of IRS1(Tyr989), AKT(Thr308), AKT(Ser473), and the dephosphorylation of IRS1(Ser318) in the absence of FSH (33). Phosphorylations of IRS1(Tyr989), AKT(Thr308), MYPT1(Ser668) and dephosphorylation of IRS1(Ser318) in hCG-treated preovulatory GCs and forskolin-treated MCF7 breast cancer and thyroid FRTL cells require the active IGF-1R and are abrogated by PKI. These results demonstrate that the mechanism by which FSH activates PI3K/AKT in preantral GCs in vitro is conserved in preovulatory GCs as well as in MCF7 breast cancer and thyroid FRTL cells. Insulin resistance has been attributed, at least in part, to the presence of specific Ser/Thr phosphorylations on IRS1 that diminish insulin-stimulated PI3K activation [reviewed in (46)]. Our results reveal a general mechanism by which GPCRs can harness IGF-1R and possibly insulin receptors to phosphorylate IRS1 to promote PI3K signaling independent of the cell or tissue type.
Treatment of GCs with increasing concentrations of exogenous IGF-1 and FSH initiates the synergistic phosphorylation of IRS1(Tyr989) that is translated downstream to phosphorylations of AKT(Thr308 and Ser473), forkhead box O family member (Ser256), and S6(Ser235/236), resulting in synergistic expression of Inha, Lhcgr, and Cyp11a1 mRNAs (32). Synergistic phosphorylation of AKT(Ser473) and downstream induction of Cyp19a1, Hsd3b1, and Star mRNAs in response to FSH and exogenous IGF-1 has also been reported (44). These results indicate that the FSH-stimulated dephosphorylation of Ser residues on IRS1 even in the presence of exogenous IGF-1 facilitates IGF-1R-catalyzed phosphorylation of IRS1 on Tyr residues that activate PI3K.
Is the mechanism we have identified by which GPCRs like the FSHR activate the PI3K/AKT pathway via PKA in GCs to promote PP1-dependent dephosphorylation of Ser residues on IRS1 restricted to cultured cells? Many additional studies are required to answer this question, including conditional mutational studies of IRS1 to elucidate the integrated regulatory roles of multisite IRS1 phosphorylations and of MYPT1. However, results using ovarian extracts from vehicle-treated immature rats show that the PI3K/AKT is inactive, and that pregnant mares serum gonadotropin (PMSG)-treatment of immature rats that activates the FSHR is necessary to promote the phosphorylation/activation of AKT and its downstream targets tuberin, p70 ribosomal S6 kinase, S6 (22), and mouse double minute 2 homolog (47). There is also evidence that inhibition of the IGF-1R with the intrabursal injection of an IGF-1R antagonist to intact immature rats blunted PMSG-stimulated follicle growth as well as the expression of Cyp19a1, Cyp11a1, Star, and Lhcgr mRNAs (44). This result suggests that FSH signaling to promote gene expression requires the activity of the IGF-1R in vivo and is consistent with evidence that global deletion of IGF-1 prevents follicular maturation to a preovulatory phenotype (48). Similarly in cultured human cumulus cells, that appear to dedifferentiate in culture to the equivalent of preantral GCs, FSH stimulation of AKT(Ser473) phosphorylation and resulting induction of Cyp19a1 mRNA also require the IGF-1R (49). However, in this cell model, GCs secrete IGF-2 rather than IGF-1 (50). Both IGF-1 and IGF-2 activate the IGF-1R, although IGF-1 binds the IGF-1R with higher affinity than IGF-2 [reviewed in (51)].
In contrast to the low levels of IGF-1 or IGF-2 secreted by GCs in vitro (32, 44), follicular fluid and plasma levels of IGFs are magnitudes higher (52). However, the concentrations of the IGF binding proteins greatly exceed concentrations of IGFs (52), suggesting that there is little free IGF in follicular fluid or plasma. Consistent with this concept, neither follicular fluid nor circulating levels of IGFs are believed to play an endocrine role in follicular development (52, 53). Consistent with evidence that the PI3K/AKT pathway in GCs is inactive in the absence of FSHR activation in vivo (22), these results suggest that the levels of IGFs in follicular fluid or plasma do not regulate the PI3K/AKT pathway. Rather, it is the autocrine/paracrine-regulated local concentrations of IGF that allow GCs to respond to FSH.
MAPK/ERK Pathway: PKA Inactivates MKP3/DUSP6 to Allow Phosphorylated ERK to Accumulate
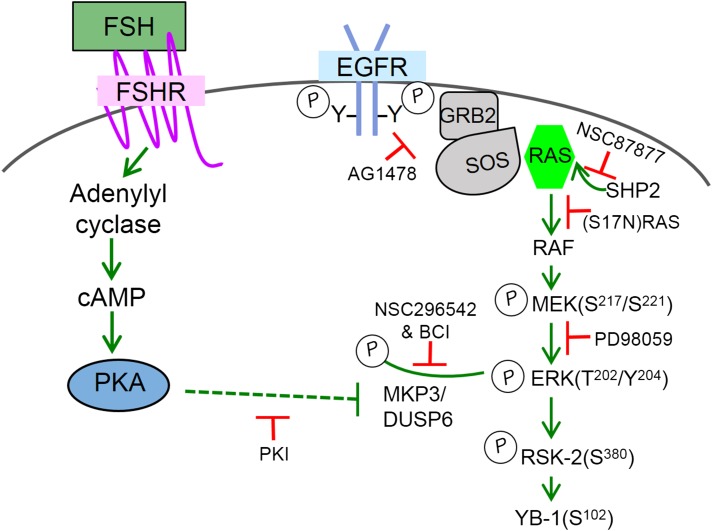
As diagrammed in Fig. 3, signaling from the EGFR to MEK in preantral GCs is constitutively active in the absence of FSH, evidenced by the ability of the EGFR antagonist AG1478, a dominant negative (S17N)RAS, a Src homology 2–domain-containing tyrosine phosphatase-2 inhibitor NSC-87877, and the MEK inhibitor PD98059 to interrupt basal MEK phosphorylation (54). Thus, in the absence of FSH, the EGFR is actively signaling downstream to RAS, RAF, and MEK, albeit at a reduced capacity, the latter evidenced by the enhanced phosphorylation of MEK and ERK detected with the addition of exogenous EGF (50 ng/mL) (20). However, like AKT activation in response to exogenous IGF-1, activation of ERK by exogenous EGF alone is not sufficient to initiate the program of GC differentiation [reviewed in (55)].
Figure 3.
Modeled mechanism by which FSH via PKA inhibits MKP3/DUSP6 to promote the accumulation of phosphorylated ERK in preantral GCs. Schematic model, based on recent publications (39, 54), reflects the observation that the EGFR pathway is constitutively active in the absence of FSH, such that MEK phosphorylation is abrogated by the EGFR antagonist AG1478, the Src homology 2–domain-containing tyrosine phosphatase-2 inhibitor NSC87877, a dominant negative (S17N) RAS, and the MEK inhibitor PD98059. FSH promotes an accumulation of phosphorylated ERK, in a PKA-dependent manner, without affecting MEK phosphorylation. ERK is dephosphorylated in the absence of FSH by MKP3/DUSP6; FSH in a PKA-dependent manner inhibits this phosphatase to allow the accumulation of MEK-phosphorylated ERK that then signals to ribosomal S6 kinase-2 and Y-box-binding protein 1. Dotted line indicates that the mechanism by which PKA inhibits MKP3/DUSP6 is not known.
Within 5 minutes of FSH treatment, phosphorylated ERK is readily detected (39). Pretreatment of GCs with PKI abrogates FSH-dependent ERK but not MEK phosphorylation (54). We concluded that a phosphatase constitutively dephosphorylated ERK on Thr202 and/or Tyr204 in the absence of FSH, and that FSH via PKA inhibited the ERK phosphatase, allowing the accumulation of MEK-phosphorylated ERK. Neither Ser/Thr nor Tyr phosphatase inhibitors inactivated the ERK phosphatase (54). However, the ERK phosphatase was inactivated by inhibitors of MKP3/DUSP6, evidenced by an increase in ERK phosphorylation in vehicle-treated cells equivalent to that of FSH-treated cells, and was inhibited by PKI in a cell-free lysate phosphatase assay (54). Immunoprecipitation results demonstrated that ERK and a peptide tagged-MKP3/DUSP6 exist in a complex. However, the mechanism by which PKA directly or indirectly inactivates MKP3/DUSP6 was not discerned. As the global MKP3/DUSP6 knockout mouse is fertile (56), we hypothesize that its activity is compensated by another ERK-selective DUSP, such as MKPX/PYST2 (DUSP7) (57).
We questioned whether the mechanism by which FSH enhances the accumulation of active/phosphorylated ERK was a more universal event. However, in preovulatory GCs, LHCGR activation by hCG enhances MEK phosphorylation (58). Thus, the EGFR-dependent pathway that promotes MEK activation in the absence of FSH in preantral GCs is not maintained in preovulatory GCs. Indeed, we are aware of only one other report of a constitutively active MEK (59). In ovaries of mice and in a rat luteal cell line expressing only the short form of the prolactin receptor, MEK is constitutively active and promotes the accumulation of phosphorylated ERK (59). Prolactin treatment promotes the activation of DUSP27 that in turn dephosphorylates/inactivates ERK.
Is MKP3/DUSP6 the relevant phosphatase that dephosphorylates ERK in the absence of FSH? An initial step to answer this question would be to determine if MKP3/DUSP6 inhibitors block the expression of identified MAPK/ERK gene targets, such as Egfr, Cyp19a1, Inha, Lhcgr, Hsd17b1, Pappa, and Prkar2b in GCs. Conditional deletion of MKP3/DUSP6 and potentially MKPX/PYST2 (DUSP7) in preantral GCs is also required to confirm our in vitro results.
Summary
A constitutively active PKA catalytic subunit mutant not only promotes the phosphorylation/activation of key targets in the PI3K/AKT pathway [IRS1(Tyr989), AKT(Thr308)(Ser473), and PP1] and the accumulation of phosphorylated ERK(Thr202)(Tyr204) in GCs but also mimics gene expression patterns of FSH (31, 32). These results unambiguously establish that PKA is sufficient to activate the signaling pathways that regulate gene expression that culminates in the formation of a preovulatory follicle. PKA not only directly phosphorylates transcriptional regulators but also intercepts canonical RTK pathways. For both the PI3K/AKT and MAPK/ERK pathways, the respective RTK is active. Yet, FSH is required to fully activate these pathways to regulate gene expression. For PI3K/AKT, phosphorylation of IRS1 on Tyr residues that activate PI3K by the IGF-1R requires the PP1-mediated dephosphorylation of Ser residues on IRS1. Importantly, this pathway is conserved in preovulatory GCs, MCF7 breast cancer cells, and FRTL thyroid cells, suggesting that PKA activates PI3K/AKT at the level of IRS1 in a more universal manner. The accumulation of phosphorylated/active ERK requires the PKA-dependent inhibition of MKP3/DUSP6. Future studies are required to determine how common this pathway is in other cells. Thus, by regulating the activity of a DUSP for ERK, and the Ser/Thr phosphatase PP1 for the PI3K/AKT pathway, PKA activates signaling of select RTK pathways to promote gene expression in preantral GCs.
Acknowledgments
This is the last scientific contribution from the Hunzicker-Dunn Laboratory. We acknowledge the many trainees and colleagues over the last 38 years (too numerous to list here but you know who you are) who have provided critical insights, advice, and mentoring. We leave happy and fulfilled and hope that this review will serve as a useful springboard for future investigations seeking to understand in even greater detail how FSH drives the maturation of preantral GCs to preovulatory GCs.
Current affiliation: E.M. Donaubauer’s current affiliation is the Department of Pharmacology, Case Western Reserve University, Cleveland, Ohio 44106.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1HD065859 and RO1HD062053 (to M.H.-D), RO1HD059901 (to A.J.Z.), and Training Grant T32GM083864 (to N.C.L. and E.M.D.), the Poncin Scholarship Fund (to N.C.L.), and The Fund for Science (to M.H.-D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DUSP
- dual specificity phosphatase
- ERK
- extracellular signal-regulated kinase
- FSH
- follicle-stimulating hormone
- GC
- granulosa cell
- GPCR
- G-protein coupled receptor
- IGF-1
- insulinlike growth factor 1
- IGF-1R
- insulinlike growth factor 1 receptor
- IRS1
- insulin receptor substrate 1
- Lhcgr
- luteinizing hormone/choriogonadopin receptor
- MAPK
- mitogen-activated protein kinase
- MKP
- mitogen-activated protein kinase phosphatase
- mRNA
- messenger RNA
- MYPT1
- myosin phosphatase target subunit 1
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- protein kinase A
- PP
- protein phosphatase
- PP1cβ
- β-catalytic subunit of protein phosphatase 1
- RTK
- receptor tyrosine kinase.
References
- 1.Herndon MK, Law NC, Donaubauer EM, Kyriss B, Hunzicker-Dunn M. Forkhead box O member FOXO1 regulates the majority of follicle-stimulating hormone responsive genes in ovarian granulosa cells. Mol Cell Endocrinol. 2016;434:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15(1):172–183. [DOI] [PubMed] [Google Scholar]
- 4.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95(12):6965–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136(11):1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff TK, Mayo KE. Regulation of inhibin synthesis in the rat ovary. Annu Rev Physiol. 1990;52:807–821. [DOI] [PubMed] [Google Scholar]
- 8.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384(6608):470–474. [DOI] [PubMed] [Google Scholar]
- 9.Hunzicker-Dunn M, Birnbaumer L. Adenylyl cyclase activities in ovarian tissues. III. Regulation of responsiveness to LH, FSH, and PGE1 in the prepubertal, cycling, pregnant, and pseudopregnant rat. Endocrinology. 1976;99(1):198–210. [DOI] [PubMed] [Google Scholar]
- 10.Zeleznik AJ, Keyes PL, Menon KM, Midgley AR Jr, Reichert LE Jr. Development-dependent responses of ovarian follicles to FSH and hCG. Am J Physiol. 1977;233(3):E229–E234. [DOI] [PubMed] [Google Scholar]
- 11.DeManno DA, Cottom JE, Kline MP, Peters CA, Maizels ET, Hunzicker-Dunn M. Follicle-stimulating hormone promotes histone H3 phosphorylation on serine-10. Mol Endocrinol. 1999;13(1):91–105. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993;13(8):4852–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvador LM, Park Y, Cottom J, Maizels ET, Jones JC, Schillace RV, Carr DW, Cheung P, Allis CD, Jameson JL, Hunzicker-Dunn M. Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J Biol Chem. 2001;276(43):40146–40155. [DOI] [PubMed] [Google Scholar]
- 14.Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr expression in granulosa cells: roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol. 2013;27(8):1295–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilson JH. Molecular and cellular biology of gonadotropes: an integrated view with new horizons. Mol Cell Endocrinol. 2014;385(1–2):1. [DOI] [PubMed] [Google Scholar]
- 16.Hunzicker-Dunn M, Mayo KE. Gonadotropin Signaling in the Ovary In: Plant TM, and Zeleznik AJ, eds. Physiology of Reproduction, volume 1 New York, NY: Elsevier Academic Press; 2015:895–992. [Google Scholar]
- 17.Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp BE, Cheng HC, Walsh DA. Peptide inhibitors of cAMP-dependent protein kinase. Methods Enzymol. 1988;159:173–183. [DOI] [PubMed] [Google Scholar]
- 19.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24(3-4):261–274. [DOI] [PubMed] [Google Scholar]
- 20.Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278(9):7167–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunzicker-Dunn ME, Lopez-Biladeau B, Law NC, Fiedler SE, Carr DW, Maizels ET. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc Natl Acad Sci USA. 2012;109(44):E2979–E2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem. 2004;279(19):19431–19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andric N, Ascoli M. A delayed gonadotropin-dependent and growth factor-mediated activation of the extracellular signal-regulated kinase 1/2 cascade negatively regulates aromatase expression in granulosa cells. Mol Endocrinol. 2006;20(12):3308–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M. Follicle stimulating hormone (FSH) activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology. 1998;139(7):3353–3356. [DOI] [PubMed] [Google Scholar]
- 25.Yu FQ, Han CS, Yang W, Jin X, Hu ZY, Liu YX. Activation of the p38 MAPK pathway by follicle-stimulating hormone regulates steroidogenesis in granulosa cells differentially. J Endocrinol. 2005;186(1):85–96. [DOI] [PubMed] [Google Scholar]
- 26.Zeleznik AJ, Saxena D, Little-Ihrig L. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 2003;144(9):3985–3994. [DOI] [PubMed] [Google Scholar]
- 27.Kwintkiewicz J, Cai Z, Stocco C. Follicle-stimulating hormone-induced activation of Gata4 contributes in the up-regulation of Cyp19 expression in rat granulosa cells. Mol Endocrinol. 2007;21(4):933–947. [DOI] [PubMed] [Google Scholar]
- 28.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21(8):1940–1957. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol. 2000;14(8):1283–1300. [DOI] [PubMed] [Google Scholar]
- 30.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002;16(3):580–599. [DOI] [PubMed] [Google Scholar]
- 31.Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein kinase A: a master kinase of granulosa cell differentiation. Sci Rep. 2016;6:28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law NC, Hunzicker-Dunn ME. Insulin receptor substrate 1, the hub linking follicle-stimulating hormone to phosphatidylinositol 3-kinase activation. J Biol Chem. 2016;291(9):4547–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law NC, White MF, Hunzicker-Dunn ME. G protein-coupled receptors (GPCRs) that signal via protein kinase A (PKA) cross-talk at insulin receptor substrate 1 (IRS1) to activate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. J Biol Chem. 2016;291(53):27160–27169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bebia Z, Somers JP, Liu G, Ihrig L, Shenker A, Zeleznik AJ. Adenovirus-directed expression of functional luteinizing hormone (LH) receptors in undifferentiated rat granulosa cells: evidence for differential signaling through follicle-stimulating hormone and LH receptors. Endocrinology. 2001;142(6):2252–2259. [DOI] [PubMed] [Google Scholar]
- 36.Escamilla-Hernandez R, Little-Ihrig L, Orwig KE, Yue J, Chandran U, Zeleznik AJ. Constitutively active protein kinase A qualitatively mimics the effects of follicle-stimulating hormone on granulosa cell differentiation. Mol Endocrinol. 2008;22(8):1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005;280(10):9135–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donaubauer EM, Hunzicker-Dunn ME. Extracellular signal-regulated kinase (ERK)-dependent phosphorylation of Y-box-binding protein 1 (YB-1) enhances gene expression in granulosa cells in response to follicle-stimulating hormone (FSH). J Biol Chem. 2016;291(23):12145–12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adachi Y, Shibai Y, Mitsushita J, Shang WH, Hirose K, Kamata T. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene. 2008;27(36):4921–4932. [DOI] [PubMed] [Google Scholar]
- 41.Bennett J, Baumgarten SC, Stocco C. GATA4 and GATA6 silencing in ovarian granulosa cells affects levels of mRNAs involved in steroidogenesis, extracellular structure organization, IGF-I activity, and apoptosis. Endocrinology. 2013;154(12):4845–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012; 66(2):105–143. [DOI] [PubMed] [Google Scholar]
- 43.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441(2):553–569. [DOI] [PubMed] [Google Scholar]
- 44.Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27(3):511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly GM, Buckley DA, Kiely PA, Adams DR, O’Connor R. Serine phosphorylation of the insulin-like growth factor I (IGF-1) receptor C-terminal tail restrains kinase activity and cell growth. J Biol Chem. 2012;287(33):28180–28194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology. 2009;150(2):915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellvé AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10(7):903–918. [DOI] [PubMed] [Google Scholar]
- 49.Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF-1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99(8):2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgarten SC, Convissar SM, Zamah AM, Fierro MA, Winston NJ, Scoccia B, Stocco C. FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J Clin Endocrinol Metab. 2015;100(8):E1046–E1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76(4):1005–1026. [DOI] [PubMed] [Google Scholar]
- 52.Thierry van Dessel HJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH, Braat DD, Fauser BC, Giudice LC. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81(3):1224–1231. [DOI] [PubMed] [Google Scholar]
- 53.Rabinovici J, Dandekar P, Angle MJ, Rosenthal S, Martin MC. Insulin-like growth factor I (IGF-I) levels in follicular fluid from human preovulatory follicles: correlation with serum IGF-I levels. Fertil Steril. 1990;54(3):428–433. [DOI] [PubMed] [Google Scholar]
- 54.Donaubauer EM, Law NC, Hunzicker-Dunn ME. Follicle-stimulating hormone (FSH)-dependent regulation of extracellular regulated kinase (ERK) phosphorylation by the mitogen-activated protein (MAP) kinase phosphatase MKP3. J Biol Chem. 2016;291(37):19701–19712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsueh AJW, Adashi EY, Jones PBC, Welsh TH Jr. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5(1):76–127. [DOI] [PubMed] [Google Scholar]
- 56.Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283(45):31246–31255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin Cell Dev Biol. 2016;50:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143(8):2986–2994. [DOI] [PubMed] [Google Scholar]
- 59.Devi YS, Seibold AM, Shehu A, Maizels E, Halperin J, Le J, Binart N, Bao L, Gibori G. Inhibition of MAPK by prolactin signaling through the short form of its receptor in the ovary and decidua: involvement of a novel phosphatase. J Biol Chem. 2011;286(9):7609–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]