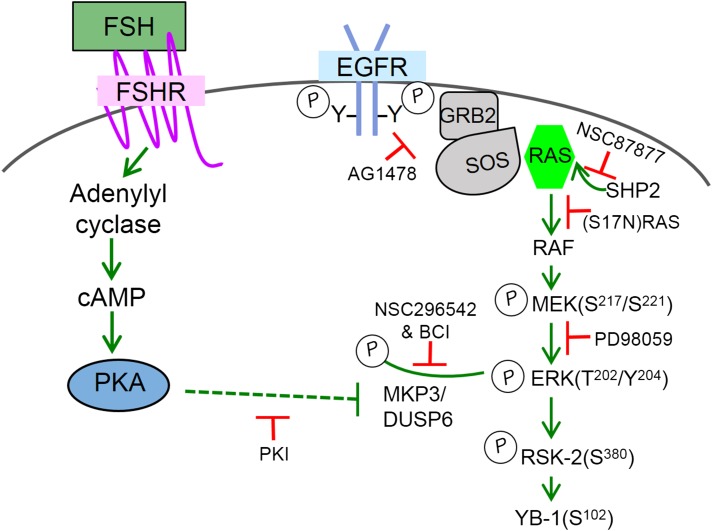
Figure 3.
Modeled mechanism by which FSH via PKA inhibits MKP3/DUSP6 to promote the accumulation of phosphorylated ERK in preantral GCs. Schematic model, based on recent publications (39, 54), reflects the observation that the EGFR pathway is constitutively active in the absence of FSH, such that MEK phosphorylation is abrogated by the EGFR antagonist AG1478, the Src homology 2–domain-containing tyrosine phosphatase-2 inhibitor NSC87877, a dominant negative (S17N) RAS, and the MEK inhibitor PD98059. FSH promotes an accumulation of phosphorylated ERK, in a PKA-dependent manner, without affecting MEK phosphorylation. ERK is dephosphorylated in the absence of FSH by MKP3/DUSP6; FSH in a PKA-dependent manner inhibits this phosphatase to allow the accumulation of MEK-phosphorylated ERK that then signals to ribosomal S6 kinase-2 and Y-box-binding protein 1. Dotted line indicates that the mechanism by which PKA inhibits MKP3/DUSP6 is not known.