Abstract
The glucagon-like peptide 1 (GLP-1) system plays an important role in blood glucose regulation, in great part through coordinate control of insulin and glucagon secretion. These effects are generally attributed to GLP-1 produced in peripheral sites, principally the intestine. GLP-1 is also produced in hindbrain neurons that signal through GLP-1 receptors (GLP-1rs) expressed in brain regions involved in metabolic regulation. GLP-1 in the central nervous system (CNS) induces satiety, visceral illness, and stress responses. However, recent evidence suggests CNS GLP-1 is also involved in glucose regulation. To test the hypothesis that central GLP-1 regulates islet hormone secretion, conscious rats were given intracerebroventricular (ICV) GLP-1, GLP-1r antagonist exendin-[9-39] (Ex-9), or saline during fasting or hyperglycemia from intravenous glucose. Administration of CNS GLP-1 increased fasting glucose, glucagon, corticosterone, and epinephrine and blunted insulin secretion in response to hyperglycemia. Paradoxically, GLP-1r blockade with ICV Ex-9 also reduced glucose-stimulated insulin secretion, and administration of ICV Ex-9 to freely feeding rats caused mild glucose intolerance. Thus, direct administration of CNS GLP-1 affected islet hormone secretion counter to what is seen with peripherally administered GLP-1, an effect likely due to stimulation of sympathetic nervous system activity. In contrast, blockade of brain GLP-1r supports a role for CNS GLP-1 on glucose-stimulated insulin secretion and glucose control after a meal. These findings suggest a model in which activation of CNS GLP-1r by endogenous peptide promotes glucose tolerance, an effect that can be overridden by stress responses stimulated by exogenous GLP-1.
Blockade of GLP-1 receptors in the midbrain of rats supports a role for central nervous system GLP-1 in the regulation of islet hormone secretion and prandial glucose tolerance.
The brain-gut factor glucagon-like peptide 1 (GLP-1) plays a central role in normal glucose homeostasis (1, 2). GLP-1 is released into the circulation from enteroendocrine l-cells scattered throughout the intestinal mucosa, with meal ingestion as the primary physiologic stimulus. The major action by which GLP-1 controls blood glucose is coordinate control of islet hormone secretion, stimulation of insulin, and inhibition of glucagon release (3–5). Beyond these actions, GLP-1 affects glycemia by delaying gastric emptying (6, 7) and reducing hepatic glucose production (8–10).
In addition to synthesis in the intestine, GLP-1 is a neurotransmitter produced by a discrete population of neurons found in the nucleus of the solitary tract (NTS) that, although few in number, have rich axonal projections within the hindbrain and to the hypothalamus and other midbrain regions (11, 12). GLP-1 receptors (GLP-1rs) have widespread distribution in these target areas (13, 14), and there is a high degree of overlap between GLP-1–containing synaptic terminals and GLP-1 binding sites in the brain (12). GLP-1 action in the central nervous system (CNS) has been implicated in the regulation of food intake (15), hypothalamic-pituitary-adrenal (HPA) axis function (16–18), sympathetic nervous system (SNS) activation (16), and visceral illness (19, 20). More recently, studies have shown that CNS GLP-1 may also be involved in the control of peripheral glucose homeostasis. Knauf et al. (21) reported that a GLP-1 agonist, Ex4, administered directly into the brain during experimental hyperinsulinemia and hyperglycemia, increased insulin secretion and hepatic glucose uptake in mice. Sandoval et al. (9) found that intracerebroventricular (ICV) GLP-1 increased glucose stimulated insulin secretion in rats. Other investigators have recently duplicated these effects with acute but not chronic GLP-1r stimulation (22). These results suggest that among the myriad GLP-1r–mediated actions in the brain, central GLP-1 also contributes to insulin secretion and the control of blood glucose.
The primary aim of this study was to investigate whether brain GLP-1 affects insulin and glucagon secretion during fasting and hyperglycemia, as well as to assess the effects of brain GLP-1 on glucose regulation associated with meal ingestion.
Research Design and Methods
Animals
Male Long Evans rats (250 g upon arrival) from Harlan Laboratories (Indianapolis, IN) were individually housed in a temperature-controlled room under a 12-hour/12-hour light-dark cycle (lights on from 0600 to 1800 hours). Standard pelleted chow (Teklad 7012; Harlan Laboratories) and drinking water were available ad libitum. The rats were allowed to acclimate to these conditions for at least 1 week. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Surgical procedures
Rats had catheters placed in the jugular vein (silicone tubing, 0.64 mm inside diameter × 1.19 mm outside diameter; Technical Products, Inc of Georgia, Lawrenceville, GA) and/or carotid artery (polyethylene tubing, 0.58 mm ID × 0.97 mm OD; Instech Solomon, San Antonio, TX) during anesthesia with ketamine (24 mg/kg, intraperitoneal) and xylazine (3 mg/kg, intraperitoneal). In the same surgical session, a 22-gauge stainless steel guide cannula (C313G; Plastics One, Roanoke, VA) was implanted into the brain with the tip aimed at the third cerebral ventricle. The stereotactic coordinates were 2.2 mm posterior to the bregma, a 7.5-mm depth from the dura mater, and in the midline (23). Screws and dental acrylic were used to secure the guide cannula to the skull. After implantation, the guide cannula was blocked with a removable obturator (C313DC; Plastics One). The recovery period was at least 1 week; only rats that had regained more than 90% of presurgery body weight were used in experiments.
Peptides
GLP-1 (7-36NH2) (American Peptide Company, Inc., Sunnyvale, CA), exendin-[9-39] (Ex-9) (21st Century Biochemicals, Marlboro, MA), and angiotensin II (Sigma-Aldrich, St. Louis, MO) were reconstituted in saline, aliquoted, and stored at −20°C.
Experiment 1: Effects of CNS GLP-1 and Ex-9 on islet-cell hormone secretion
This experiment was performed to investigate whether brain GLP-1 affects islet-cell hormone secretion during fasting and hyperglycemia induced by intravenous (IV) glucose. Rats were studied at 1300 hours, after fasting from 1700 hours the day before the experiment; the rats were conscious and unrestrained during the experimental period and studied in their home cage to minimize stress. They were weighed and placed in individual cages, the venous catheter was connected to a precalibrated infusion pump (KDS syringe pump; KD Scientific, Holliston, MA), and the arterial catheter was connected to tubing for blood sampling. Once the lines were connected, the rats were allowed to habituate for 30 minutes. The experiment started with blood samples taken from −90 minutes to −60 minutes for determination of baseline blood glucose and plasma levels of insulin, glucagon, and corticosterone. After the blood sampling at t = −60 minutes, an ICV bolus of GLP-1 (3 µg), the GLP-1r antagonist Ex-9 (50 µg), or saline was administered in a 2-µL volume. Immediately after the bolus, a continuous ICV infusion of GLP-1 (18 µg/h), Ex-9 (100 µg/h), or saline was started; all rats received 8 µL/h of infusate for 125 minutes via a precalibrated infusion pump (Harvard Apparatus, South Natick, MA). At t = 0 minutes, blood glucose was raised to a target of 12 mM with a primed IV infusion of 25% dextrose, and the infusion rate was varied to maintain stable hyperglycemia. Arterial blood was sampled every 5 to 10 minutes during the infusion period for measurements of blood glucose as well as collection of plasma for assay of hormones. Blood glucose concentrations were measured in duplicate using Freestyle glucose meters (Abbott Laboratories, Chicago, IL). To avoid anemia, erythrocytes remaining after an hour of sampling were pooled and returned to the rats at t = −5 minutes and t = 65 minutes. After the last blood sample at t = 65 minutes, the infusions were stopped, and the rats were returned to their cages.
Experiment 2: Effects of CNS GLP-1 on the SNS and the HPA axis
To investigate whether CNS GLP-1, in the dose used in experiment 1, activated the SNS and the HPA axis, markers for activation of central and peripheral stress circuits were measured in animals with minimal handling. Overnight-fasted, conscious, and unrestrained rats were studied in the room where they were housed to minimize stress. Their ICV cannula was connected to a precalibrated infusion pump (Harvard Apparatus), after which they were allowed to habituate for 2 hours. To avoid handling stress, no bolus ICV injection was given. The rats were infused ICV with GLP-1 (19.5 µg/h) or saline; all rats received 8 µL/h of infusate. The dose of GLP-1 was the same as the total amount of GLP-1 administered ICV in experiment 1. One cohort of rats was infused for 2 hours and then deeply anesthetized with intraperitoneal Fatal Plus (Vortech Pharmaceuticals, Inc., Dearborn, MI). Before perfusion, blood was sampled from the tail vein for determination of corticosterone, following which the rats had trans-cardiac perfusion with 0.9% saline followed by 4% paraformaldehyde. The brains and adrenal glands were removed and postfixed in 4% paraformaldehyde overnight. The tissues were then submerged in 30% sucrose for 24 hours before preparation for sectioning. A second cohort of rats had the same infusion protocol with either GLP-1 or saline infused ICV with blood sampled at t = 30, 60, 90, and 120 minutes for measurements of corticosterone and epinephrine. After the final sample at t = 120 minutes, the ICV infusion was stopped, and the rats were returned to their cages.
Experiment 3: Effects of CNS Ex-9 on glucose regulation associated with meal ingestion
This experiment was performed to investigate whether GLP-1 produced in the CNS contributes to glucose regulation before and after meal ingestion. As in the previous experiments, the rats were fasted overnight and studied conscious and unrestrained. On the day of the experiment, they were weighed, and the arterial catheter was connected to tubing for blood sampling. Rats were placed individually in cages and allowed to habituate for 30 minutes. The experiment started with a blood sample at t = −30 minutes for measurement of baseline blood glucose and plasma levels of insulin and glucagon. After the blood sampling, an ICV bolus dose of Ex-9 (50 µg in 2 µL) or saline (2 µL) was administered, and immediately thereafter a continuous ICV infusion of Ex-9 (100 µg/h) or saline was started. All rats received 8 µL/h of infusate for 150 minutes via precalibrated infusion pumps (Harvard Apparatus). At t = 0 minutes, bottles of chocolate-flavored Ensure liquid diet (5.5 mL; Abbott Ross, Columbus, OH) were made available for 10 minutes. Because the rats had been exposed to Ensure several times over the previous week, they voluntarily consumed the meal within 5 minutes; none of the animals in this study failed to consume their meal. d-xylose was added to the Ensure meals, 5% (w/v), to provide an index of gastric emptying. After meal ingestion, arterial blood was sampled every 15 minutes for 120 minutes for measurements of blood glucose and plasma levels of insulin, glucagon, and d-xylose. Blood glucose concentration was measured and anemia treated as described above. After the last blood sample at t = 120 minutes, the ICV infusion was stopped, and the rats were returned to their cages.
Verification of cerebroventricular cannula placement
One week after the infusion studies, cannula placement was verified by performing an angiotensin II test in those rats in which no cerebrospinal fluid was observed when the obturator was removed from the guide cannula. During the light phase, rats were injected ICV with angiotensin II (10 ng in 2 µL) and observed for a positive dipsogenic response. Rats that failed to drink at least 5 mL of water within 60 minutes were removed from the data analyses; three rats assigned to experiment 1 (two treated with GLP-1 and one with saline) and four animals in experiment 3 (three saline and one Ex-9) were excluded.
Plasma analyses
Blood was collected in Eppendorf tubes prefilled with a cocktail of EDTA (0.5 M), heparin (800 U/mL), and aprotinin (0.28 mM); 20 µL of the cocktail was used for 200 µL blood. For measurements of plasma d-xylose and corticosterone, blood was collected in EDTA-coated tubes (Sarstedt, Nürnbrecht, Germany). Blood for catecholamine measurements was collected in Eppendorf tubes prefilled with EGTA/glutathione solution (20 µL per 1 mL blood). Blood samples were immediately placed on ice and centrifuged (1100g, 10 minutes, 4°C) within 60 minutes. Plasma samples for the measurement of catecholamines were stored at −80°C; all other plasma samples were kept at −20°C until assayed. Plasma concentrations of insulin, d-xylose, and corticosterone were measured by assays previously described (24). Plasma glucagon was measured using a modification of a commercial RIA kit (Millipore, St. Charles, MO). Samples, standards, and antibody were added at half the recommended concentrations and incubated for 3 days in a noncompetitive format (25). Tracer was added on the fourth day and the assay completed on day 5. This modification decreased the ED80 of the assay from 45 to 50 pg/mL to 20 to 25 pg/mL with a proportional increase in sensitivity (ED95 = 10 pg/mL). Plasma epinephrine was measured using high-performance liquid chromatography and electrochemical detection by the Assay Core Laboratory at Vanderbilt University of School of Medicine (26).
Single-antigen immunohistochemistry of Fos
Brain and adrenal gland samples were cut (35 µm and 30 µm, respectively) on a freezing microtome (1:4 series). For immunohistochemistry, sections were pretreated with 1% sodium borohydride in phosphate-buffered saline (PBS) for 30 minutes, rinsed in PBS, and incubated in 1% hydrogen peroxide in PBS for 10 minutes. After washing in PBS, sections were incubated in 4% normal goat serum (Protos Immunoresearch, Burlingame, CA) with 0.4% Triton-X-100 in PBS, followed by overnight incubation in Fos rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; dilution 1:2500) in cell signaling antibody diluent (Cell Signaling Technology, Inc., Danvers, MA) at room temperature. After washing in PBS, sections were incubated in biotinylated goat anti-rabbit IgG (Vector Laboratories, Inc., Burlingame, CA; diluted 1:300 in PGT) and then incubated with avidin-biotin complex (Vector Laboratories, Inc.). A mixture of 0.02% diaminobenzidine tetrahydrochloride, 0.02% nickel sulfate, and 0.012% hydrogen peroxide dissolved in PBS was used for the chromogen reaction with three successive rinses in PBS. To avoid heavy background staining, the adrenal sections were not treated with nickel sulfate. All tissue sections were mounted on gelatinized slides, air dried, cleared in xylene, and then coverslipped with DPX (Sigma-Aldrich).
Image analysis
Images (objective: ×10) of brain and adrenal sections were captured with a digital camera mounted directly on the microscope (Zeiss Axio Imager Z1 and Zeiss Axioplan 2 Imaging, respectively; Carl Zeiss, Inc., Thornwood, NY). The counts of Fos-immunoreactive nuclei were done using Scion image (Scion Corporation, Frederick, MD). Anatomical regions of interest were determined based on the brain atlas of Paxinos and Watson (23). Expression of Fos-immunoreactive cells was quantified in the paraventricular nucleus, NTS, and adrenal medulla. One section (bilateral paraventricular nucleus and NTS) was counted for each rat and used in the statistical analysis. All analyses were performed by an observer blinded to the treatment groups.
Statistical analyses
All analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). The data are presented as mean ± standard error of the mean. For experiment 1, fasting plasma hormones and glucose during the ICV infusion studies were compared among the three groups using two-way analysis of variance with time and treatment as the two factors, Tukey’s test was used to distinguish pairwise differences among the groups, and before and after treatment effects within groups were compared using paired t tests. During the hyperglycemic clamp, mean values of insulin and glucagon were compared among the saline, GLP-1, and Ex-9 groups using one-way analysis of variance. Comparisons of mean values and area under the curve in experiments 2 and 3 were made with two-tailed, unpaired t tests. P < 0.05 was considered statistically significant.
Results
Experiment 1: Effects of CNS GLP-1 and Ex-9 on islet-cell hormone secretion
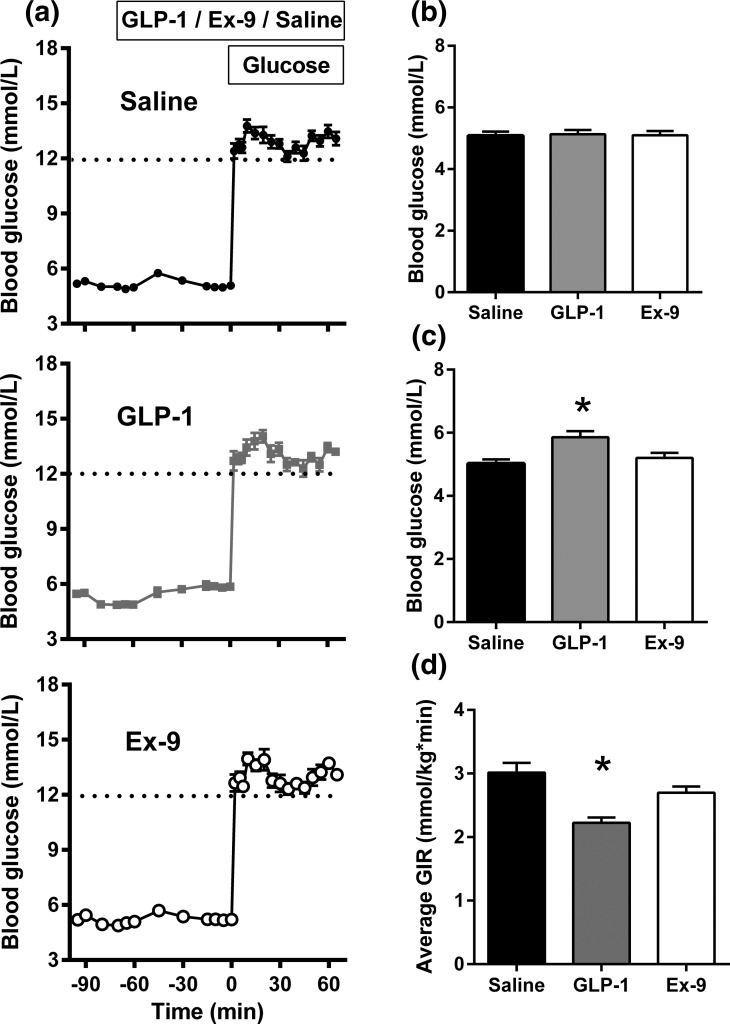
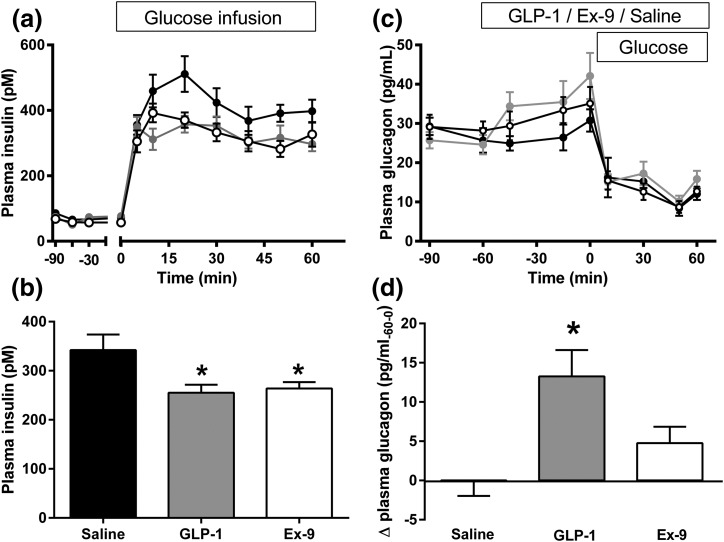
Fasting glucose did not differ among the rats infused with saline, GLP-1, or Ex-9 [Table 1; Fig. 1(b)]. Compared with saline-treated animals, infusion of ICV GLP-1 significantly increased fasting blood glucose and glucagon levels [Table 1; Figs. 1(c) and 2(d)] without a statistically significant change in fasting insulin. Ex-9 infusion also tended to increase fasting glucagon levels [Table 1; Fig. 2(d)] but had no impact on glucose or insulin concentrations before the start of the glucose infusion. Blood glucose concentrations were comparable among the three groups during the hyperglycemic clamp [Fig. 1(a)], although the glucose infusion rate was significantly lower when GLP-1 was infused ICV [Fig. 1(d)]. Animals receiving ICV saline infusions had a prompt increase in plasma insulin that was five- to sixfold basal levels [Fig. 2(a)]. There were also positive insulin responses to hyperglycemia with the infusions of GLP-1 and Ex-9, but the mean increments above basal levels were significantly lower compared with the saline group and did not differ with GLP-1r agonist or antagonist treatment [Table 1; Fig. 2(b)]. Hyperglycemia induced by IV glucose infusion reduced plasma glucagon similarly among the saline, GLP-1, and Ex-9 treated rats [Fig. 2(c)].
Table 1.
Plasma Concentrations of Glucose, Insulin, Glucagon, and Corticosterone Before (−70 to −60 Minutes) and After (−15 to 0 Minutes) ICV Infusion of Saline, GLP-1, or Ex-9 and the GIR Needed to Maintain Stable Hyperglycemia During the ICV Infusions
| Characteristic | Fasting | Postinfusion | P Value |
|---|---|---|---|
| Glucose, mM | |||
| Saline | 89.7 ± 2.3 | 91.5 ± 3.2 | <0.0001 |
| GLP-1 | 87.9 ± 2.4 | 105.5 ± 4.4a | |
| Ex-9 | 91.0 ± 2.1 | 92.8 ± 3.1 | |
| Insulin, pM | |||
| Saline | 66.6 ± 5.3 | 71.7 ± 6.7 | 0.09 |
| GLP-1 | 50.6 ± 9.2 | 76.8 ± 9.9 | |
| Ex-9 | 56.1 ± 3 | 57.3 ± 6.6 | |
| Glucagon, pg/mL | |||
| Saline | 25.7 ± 3.1 | 28.1 ± 2.0 | 0.18 |
| GLP-1 | 24.6 ± 2.5 | 38.4 ± 4.0a | |
| Ex-9 | 28.5 ± 2.4 | 33.4 ± 2.9 | |
| Corticosterone, ng/mL | |||
| Saline | 680 ± 60 | 552 ± 53 | <0.0001 |
| GLP-1 | 662 ± 76 | 819 ± 41a | |
| Ex-9 | 705 ± 38 | 449 ± 62a | |
| GIR, mL/min | |||
| Saline | 54.3 ± 2.8 | 0.002 | |
| GLP-1 | 40.0 ± 1.5b | ||
| Ex-9 | 48.5 ± 1.8 |
Data are presented as the mean ± standard error of the mean. P values are for interactions in a two-way analysis of variance (glucose, insulin, glucagon, corticosterone) or differences in a one-way analysis of variance (GIR).
Abbreviation: GIR, glucose infusion rate.
P < 0.05 for within group comparison before and after fasting.
P < 0.05 vs saline and Ex-9.
Figure 1.
Blood glucose values in rats receiving infusions with (a, top) ICV saline, (a, middle) GLP-1, and (a, bottom) Ex-9 during fasting and intravenously induced hyperglycemia. Comparisons of fasting glucose (b) before (t = −70 to −60 minutes) and (c) after a 60-minute (t = −15 to 0 minutes) infusion of saline, GLP-1, or Ex-9. (d) Glucose infusion rates to maintain hyperglycemia (t = 0 to 60 minutes) in rats receiving ICV infusions. Ten rats per group; data are mean ± standard error of the mean. *P < 0.05.
Figure 2.
(a) Plasma insulin before and after intravenous hyperglycemia (t = 0 to 60 min) in rats given ICV saline (black), GLP-1 (gray), or Ex-9 (white). (b) Mean insulin levels (average increment from baseline) for the same groups over 60 minutes of hyperglycemia. (c) Plasma glucagon before and after the glucose clamp in rats given ICV saline (black), GLP-1 (gray), or Ex-9 (white). (d) Mean change in fasting glucagon levels during 60 minutes (t = −60 to 0 minutes) infusion of saline (black), GLP-1 (gray), or Ex-9 (white). Ten rats per group; data are mean ± standard error of the mean. *P < 0.05.
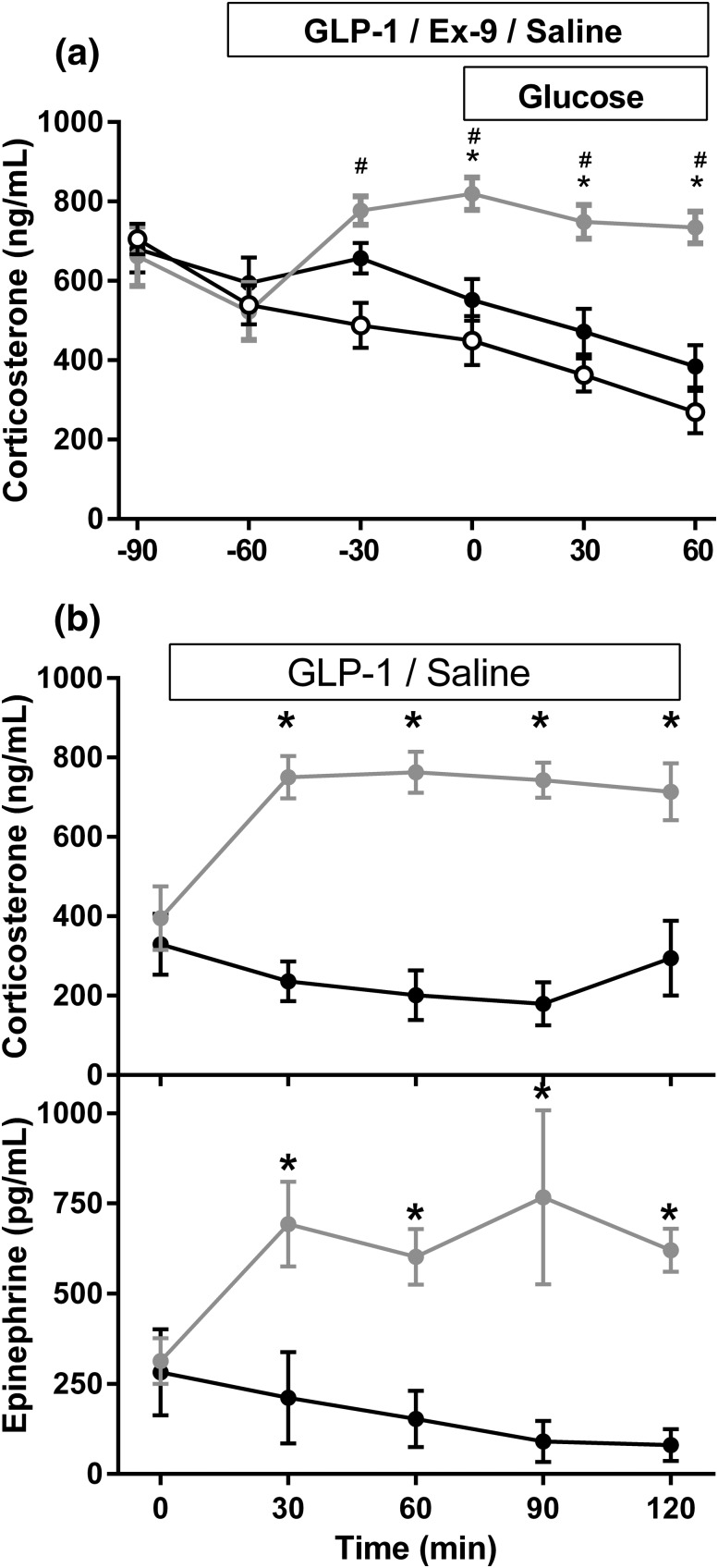
As a measure of stress during these ICV studies, plasma corticosterone was measured. GLP-1 significantly elevated plasma corticosterone compared with saline [Table 1; Fig. 3(a)], whereas there was a tendency for levels to decline during central Ex-9 administration.
Figure 3.
(a) Plasma corticosterone in rats given ICV saline (black), GLP-1 (gray), or Ex-9 (white) before and after a hyperglycemic clamp (experiment 1). (b) Plasma corticosterone and epinephrine in fasted rats receiving ICV GLP-1 (gray) or saline (black) (experiment 2). Eight to 10 rats were included in each group; data are mean ± standard error of the mean. *P < 0.05.
Experiment 2: Effects of CNS GLP-1 on the SNS and the HPA axis
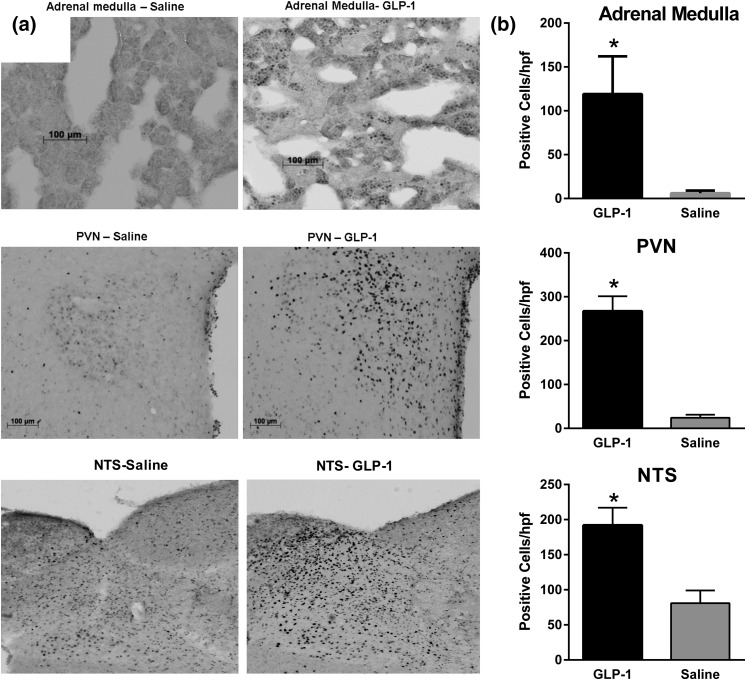
Based on the difference in corticosterone concentrations during experiment 1, a broader assessment of stress activation by the CNS GLP-1 system was performed in rats with minimal handling during ICV infusions. Consistent with experiment 1, plasma corticosterone levels were significantly increased 2 hours after ICV infusion of GLP-1 relative to saline in both the cohort of rats sampled only at the conclusion of the infusion (870.9 ± 98.0 vs 456.4 ± 25.1, P = 0.0046) and in the cohort sampled throughout the infusion [Fig. 3(b)]. GLP-1–treated rats had significantly higher neural activation brain areas associated with stress responses, as marked by Fos-immunoreactivity in the paraventricular nucleus and NTS compared with controls (Fig. 4). There was also increased Fos in adrenal medullary cells of rats given GLP-1 compared with those infused with saline (Fig. 4), suggesting sympathetic activation of the gland. Consistent with this were significantly elevated epinephrine levels in rats given GLP-1 [Fig. 3(c)]. Taken together, these findings support robust activation of the SNS and HPA axis by ICV GLP-1.
Figure 4.
(a) Representative sections of adrenal cortex, paraventricular nucleus (PVN), and nucleus of the solitary tract (NTS) from rats treated with 120 minutes of ICV saline or GLP-1 and stained for Fos-immunoreactivity (experiment 2). (b) Numbers of cells staining for Fos in the adrenal medulla, PVN, or NTS. Ten rats per group; data are mean ± standard error of the mean. *P < 0.05.
Effects of CNS Ex-9 on glucose regulation associated with meal ingestion (experiment 3)
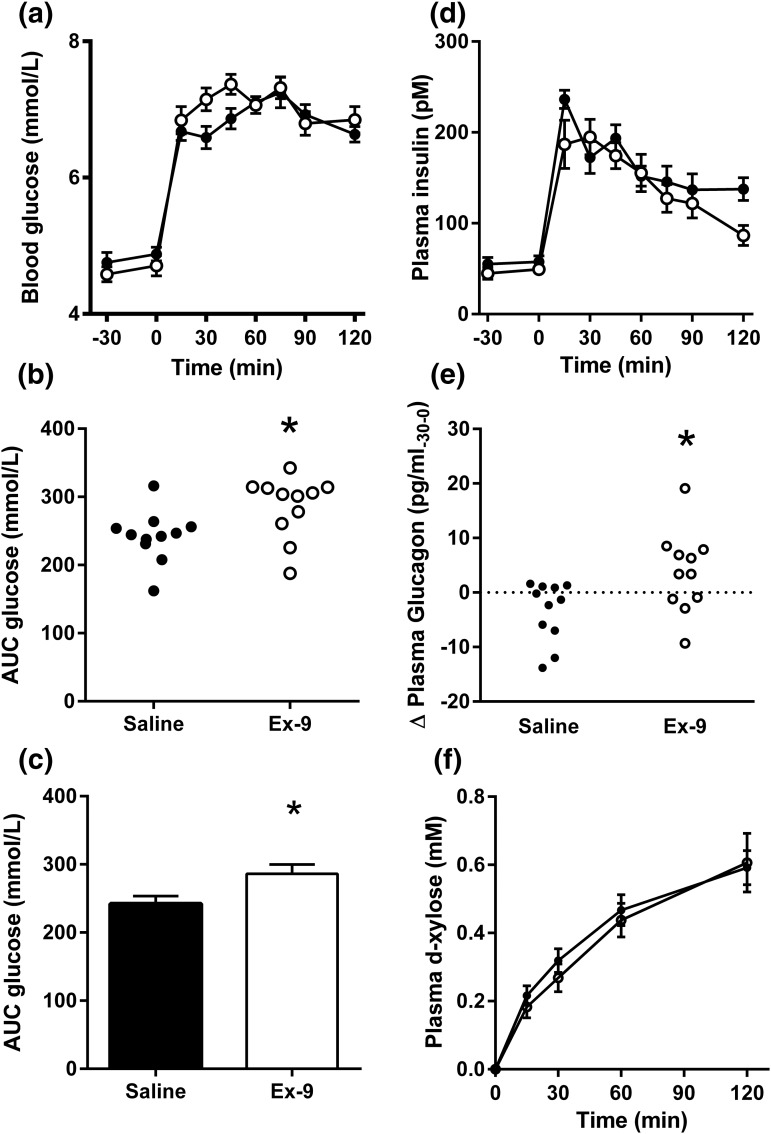
To investigate whether CNS GLP-1 contributes to prandial glucose regulation, rats were infused ICV with Ex-9 or saline during the consumption of a liquid meal. Ex-9–infused ICV increased blood glucose levels after meal ingestion relative to saline [Fig. 5(a)], suggesting that blockade of brain GLP-1 receptors can induce glucose intolerance during normal feeding. The absolute effect on area under the curve was 15% to 20% and consistent within the treatments in this comparison of 11 animals per group [P = 0.015; Fig. 5(b) and 5(c)]. There was no statistically significant difference in plasma insulin levels between the Ex-9–treated and saline-treated rats [Fig. 5(d)]. Compatible with the results in experiment 1, ICV Ex-9 caused increased glucagon secretion during the fasting state [P < 0.05; Fig. 5(e)] but had no effect on plasma levels after the meal. Plasma concentrations of ingested d-xylose did not differ between the Ex-9 and saline groups [Fig. 5(f)], suggesting that meal passage to the intestine occurred at a similar rate and was not a likely explanation for the higher blood glucose levels observed in rats infused with Ex-9. Taken together, these results suggest that CNS GLP-1 has a physiologic role in the control of meal-induced hyperglycemia.
Figure 5.
(a) Blood glucose before and after mixed meal ingestion in rats infused with ICV saline (black) or Ex-9 (white). (b) Individual values for glucose area under the curve (AUC) and (c) mean values for the two groups: saline (black) and Ex-9 (white). (d) Mean postprandial insulin concentrations during the meal study; (e) change in fasting plasma glucagon during ICV infusion of saline or Ex-9; plasma concentrations of d-xylose before and after the meal test. Eleven rats were included in each group; data are mean ± standard error of the mean. *P < 0.05.
Discussion
GLP-1 is essential for normal glucose tolerance (1), an action generally attributed to the actions of circulating, gut-derived peptide (2). However, recent findings suggest that CNS actions of GLP-1 may contribute to insulin secretion and glucose regulation (9, 21, 22). Consistent with these previous reports, we report here that blockade of GLP-1r accessible from the third cerebral ventricle impairs the insulin response to hyperglycemia and causes relative postprandial glucose intolerance. These findings indicate that endogenous GLP-1, produced in the brain, contributes to glucose regulation. Notably, ICV GLP-1 had negative effects on insulin release and also elevated glucagon secretion and fasting glucose. However, this was in the context of robust activation of the HPA and SNS axes, effects likely to override and confound the actions of endogenously produced CNS GLP-1. Taken together, this study adds islet hormone secretion and glucose tolerance to the list of GLP-1–mediated actions in the brain and demonstrates that some of these activities (e.g., stress vs homeostatic responses) can be oppositional.
In this set of experiments, we chose ICV infusions into the third cerebral ventricle of rats as our method of activating or blocking central GLP-1r. This is a model we have used in previous studies to demonstrate effects of central GLP-1 on visceral illness (19), HPA axis activation (17), and insulin secretion (9). Although the specific brain areas that contribute to GLP-1 effects on peripheral metabolism are not known, the ICV approach allowed us several hours of GLP-1r agonism and antagonism during which we could study conscious, freely moving animals. During the peptide infusions, we were able to assess effects of GLP-1 on fasting and postchallenge islet hormone secretion as well as meal tolerance. A 60-minute hyperglycemic clamp was used to examine insulin secretion to control the primary β-cell stimulus and allow time to discriminate among experimental conditions. In addition, we used a test meal ingestion protocol that we have developed for rodent studies (24, 27) that allows prandial responses to be measured in a physiologic setting and with less handling and manipulation than typical gavage protocols. Given the many potential confounders of CNS interventions, minimizing the stress of our animal subjects was central to these experimental designs.
Activation of the HPA and SNS components of the stress response by central and peripheral GLP-1r agonists has been described in several settings in rats and mice (16, 17, 28, 29). It seems plausible that this effect occurs in humans as well, because several studies have reported elevations in circulating cortisol during GLP-1 infusion (30, 31). We have previously observed that rats given the GLP-1r agonist exendin-4 peripherally or in the CNS develop hyperglycemia, despite an insulinotropic effect (28). This response was due to activation of the SNS and release of epinephrine as it was abolished by adrenal medullectomy. In the current study, central GLP-1 increased neural activity in brain regions known to mediate ANS and HPA activation (32), as well as the adrenal medulla. Coincident with these responses, rats had elevations of corticosterone and epinephrine, both of which can cause glucose intolerance and likely contributed to the increase in fasting blood glucose during the ICV infusion. In addition, epinephrine has been described to inhibit insulin secretion and stimulate glucagon release through α2 and β2 adrenoreceptors on islet cells (33, 34); we cannot exclude similar effects from norepinephrine released locally in the islet through sympathetic nerves. Based on these results, we think it is likely that the effects of central GLP-1 to activate the SNS and inhibit insulin secretion masked any stimulatory effect of central activation of CNS GLP-1r, consistent with our previous study (28).
In contrast to ICV GLP-1, central administration of Ex-9 did not have a significant effect to activate stress pathways, at least as reflected by plasma corticosterone. However, blockade of GLP-1r on neurons accessible from the third cerebral ventricle had significant effects on the release of islet hormones. Both in experiments 1 and 3, ICV Ex-9 was associated with an increase in fasting plasma glucagon, although only the second result reached statistical significance. Based on our experimental design, we cannot determine whether this was a direct effect mediated by neural inputs to the α-cell or through an indirect mechanism. Similarly, the decrease in IV glucose-stimulated insulin secretion during Ex-9 treatment indicates that GLP-1 made in CNS neurons potentiates β-cell function. This observation is consistent with previous studies demonstrating an insulinotropic effect of exogenous GLP-1 given in the brain (9, 21, 22). It is notable that the results of experiment 1 suggest a tonic effect of CNS GLP-1 signaling on islet function, because the animals were not subject to the types of stimuli previously reported to activate the hindbrain proglucagon neurons such as gastric distention or satiety-inducing peptides (35).
The results of experiment 3 extend the role of endogenous brain GLP-1r to glucose tolerance. Rats freely ingesting a test meal had relatively tight regulation of postprandial blood glucose that was disrupted by central Ex-9 infusion. Although this effect was not large, it is comparable in relative magnitude with the effect of global deletion of the GLP-1r in mice (36) and occurred in the context of the multiple physiologic processes working to maintain glucose homeostasis after meals. This response could not be attributed to changes of insulin secretion or gastric emptying, which were similar to the control treatment. This raises the possibility that during meals, central GLP-1 action on blood glucose is not insulin dependent, an explanation in keeping with our previous finding that arcuate nucleus injections of GLP-1 increase hepatic insulin sensitivity (9). In the absence of measurements of glucose turnover or insulin sensitivity, it is not possible to rule out insulin action in mediating this effect. Nonetheless, this experiment provides the most direct evidence yet reported for a role of central GLP-1r signaling in physiologic glucose tolerance.
Although our results are discordant with a recent study in mice with CNS-specific deletion of the GLP-1r (37), they are consistent with the observations from several other pharmacologic studies (9, 21, 22). In mice with a floxed Glp-1r, expression of cre recombinase under control of the Nestin promoter created a CNS GLP-1r–null line (37). In contrast to controls with intact CNS GLP-1r, these animals did not suppress food intake in response to the GLP-1r agonist liraglutide but had normal glucose tolerance and treatment responses to liraglutide comparable to controls. Although these results provide evidence for the necessity of CNS GLP-1r in the satiety response to exogenous agonists, they are less informative on the glucoregulatory role of brain GLP-1. Liraglutide has potent effects to lower glucose in mice, an effect mediated in great part at the level of the β-cell (27). It is entirely possible that any CNS effects of GLP-1r on glucose tolerance could be masked by the peripheral actions of this long-acting, pharmacologic agent, especially because central effects contribute <20% to differences in the postprandial glycemic excursion. Previous studies indicate that the effects of CNS GLP-1 are attenuated during fasting (29, 38, 39), suggesting that energy balance must be considered in assessments of glucose regulation by brain GLP-1r. Although it is not surprising that GLP-1 effects in the CNS are modulated by multiple other inputs, the findings reported here demonstrate that interference with CNS GLP-1 signaling has measurable effects on islet function and glucose regulation.
Limitations to this study must be considered in interpreting the results. First, we cannot rule out the possibility that peptides instilled in the third cerebral ventricle did not leak into the peripheral circulation. This is particularly a concern for Ex-9, for which we used a dose that causes glucose intolerance when given systemically (40) and for which the effects we show in the CNS are similar. However, previous experiments in rats have shown that microgram quantities of leptin (41) and insulin (42, 43) given into the cerebrospinal fluid do not spill over into the peripheral bloodstream, even when administered chronically. Second, although ICV administration of peptides is a reliable way to activate CNS receptors specifically, it does not allow identification of the key GLP-1r neural populations mediating the responses observed here. Moreover, general midbrain delivery of peptide as occurs with third ventricular administration can activate groups of neurons with differential or distinct responses to GLP-1, reflected here by the coincident activation of the stress response that likely confounded direct actions on islet hormone release. Future studies designed to identify the specific sites of brain GLP-1r that control the ANS and islet/glucose regulation are an important next step in this field. A third limitation is that we were not able to distinguish direct from indirect mechanisms for central GLP-1r agonism and antagonism on the islet and glucose tolerance. The first of these seems tractable in that models of islet autonomic denervation are now available (44) and could be used to test the responses to central GLP-1. Finally, although we tested the effects of central GLP-1r action during hyperglycemia and meal stimuli, both robust tests of β-cell function, these are not ideal settings for examining α-cell secretion; future studies should be directed at control of glucagon release to more specific challenges such as hypoglycemia or protein meals.
To our knowledge, the results of this study add new information to support a role for endogenous CNS GLP-1 in the regulation of islet function and blood glucose. Our findings are consistent with other reports suggesting that GLP-1r signaling in the brain is insulinotropic and demonstrate that the effects of central GLP-1 signaling to activate the stress axes can mitigate this effect. In addition, extension of CNS GLP-1 effects to glucagon secretion suggests coordinate regulation of islet hormones parallel to that seen with peripherally administered peptide. Finally, the effects of Ex-9 on meal-induced glycemia implicate the brain GLP-1 system in glucose homeostasis and add to the enormous accumulation of data over the past two decades supporting acute and chronic CNS control of nutrient metabolism.
Acknowledgments
We thank Kay Ellis, Brianne Reedy, Dale Merz, and Todd Greer for careful and skilled technical assistance.
Acknowledgments
This work was supported by PHS R01 DK057900.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CNS
- central nervous system
- Ex-9
- exendin-[9-39]
- GLP-1
- glucagon-like peptide 1
- GLP-1r
- glucagon-like peptide 1 receptor
- HPA
- hypothalamic-pituitary-adrenal
- ICV
- intracerebroventricular
- IV
- intravenous
- NTS
- nucleus of the solitary tract
- PBS
- phosphate-buffered saline
- SNS
- sympathetic nervous system.
References
- 1.D’Alessio DA, Vahl TP. Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab. 2004;286(6):E882–E890. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. [DOI] [PubMed] [Google Scholar]
- 3.Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab. 2008;93(12):4909–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59(7):1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woerle HJ, Carneiro L, Derani A, Göke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes. 2012;61(9):2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, Burgstad C, Jones KL, Chapman MJ, Rayner CK, Horowitz M. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95(1):215–221. [DOI] [PubMed] [Google Scholar]
- 7.Schirra J, Houck P, Wank U, Arnold R, Göke B, Katschinski M. Effects of glucagon-like peptide-1(7-36)amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans. Gut. 2000;46(5):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285(4):E701–E707. [DOI] [PubMed] [Google Scholar]
- 9.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57(8):2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E, Ferrannini E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia. 2012;56(1):156–161. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Fekete C, Légrádi G, Lechan RM. Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985(2):163–168. [DOI] [PubMed] [Google Scholar]
- 12.Tauchi M, Zhang R, D’Alessio DA, Stern JE, Herman JP. Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat. 2008;36(3–4):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280. [DOI] [PubMed] [Google Scholar]
- 14.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2013;63(4):1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7(9):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D’Alessio D. The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20(4):1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22(23):10470–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Grémeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115(12):3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tudurí E, Beiroa D, Porteiro B, López M, Diéguez C, Nogueiras R. Acute but not chronic activation of brain glucagon-like peptide-1 receptors enhances glucose-stimulated insulin secretion in mice. Diabetes Obes Metab. 2015;17(8):789–799. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson, C. The Rat Brain in Stereotaxic Coordinates. 3rd ed. Waltham, MA: Academic Press.
- 24.Vahl TP, Aulinger BA, Smith EP, Drazen DL, Ulrich-Lai Y, Seeley RJ, Woods SC, D’Alessio DA. Meal feeding improves oral glucose tolerance in male rats and causes adaptations in postprandial islet hormone secretion that are independent of plasma incretins or glycemia. Am J Physiol Endocrinol Metab. 2014;307(9):E784–E792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277(13):11225–11232. [DOI] [PubMed] [Google Scholar]
- 26.Robertson SD, Matthies HJ, Owens WA, Sathananthan V, Christianson NS, Kennedy JP, Lindsley CW, Daws LC, Galli A. Insulin reveals Akt signaling as a novel regulator of norepinephrine transporter trafficking and norepinephrine homeostasis. J Neurosci. 2010;30(34):11305–11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, Li B, Mahbod P, Sandoval D, Perez-Tilve D, Tamarina N, Philipson LH, Stoffers DA, Seeley RJ, D’Alessio DA. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 2014;19(6):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Tilve D, González-Matías L, Aulinger BA, Alvarez-Crespo M, Gil-Lozano M, Alvarez E, Andrade-Olivie AM, Tschöp MH, D’Alessio DA, Mallo F. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298(5):E1088–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav. 2013;121:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk FW, Holst JJ, Rizza RA. Effect of glucagon-like peptide-1(7-36)-amide on initial splanchnic glucose uptake and insulin action in humans with type 1 diabetes. Diabetes. 2001;50(3):565–572. [DOI] [PubMed] [Google Scholar]
- 31.Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA. Lack of effect of exendin-4 and glucagon-like peptide-1-(7,36)-amide on insulin action in non-diabetic humans. Diabetologia. 2002;45(10):1410–1415. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia. 2000;43(4):393–410. [DOI] [PubMed] [Google Scholar]
- 34.Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab. 2011;13(Suppl 1):82–88. [DOI] [PubMed] [Google Scholar]
- 35.Trapp S, Cork SC. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol. 2015;309(8):R795–R804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2(11):1254–1258. [DOI] [PubMed] [Google Scholar]
- 37.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124(6):2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D’Alessio DD, Seeley RJ. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS One. 2012;7(12):e51870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D’Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965–4973. [DOI] [PubMed] [Google Scholar]
- 41.van Dijk G, Donahey JC, Thiele TE, Scheurink AJ, Steffens AB, Wilkinson CW, Tenenbaum R, Campfield LA, Burn P, Seeley RJ, Woods SC. Central leptin stimulates corticosterone secretion at the onset of the dark phase. Diabetes. 1997;46(11):1911–1914. [DOI] [PubMed] [Google Scholar]
- 42.Ono T, Steffens AB, Sasaki K. Influence of peripheral and intracerebroventricular glucose and insulin infusions on peripheral and cerebrospinal fluid glucose and insulin levels. Physiol Behav. 1983;30(2):301–306. [DOI] [PubMed] [Google Scholar]
- 43.Manin M, Balage M, Larue-Achagiotis C, Grizard J. Chronic intracerebroventricular infusion of insulin failed to alter brain insulin-binding sites, food intake, and body weight. J Neurochem. 1988;51(6):1689–1695. [DOI] [PubMed] [Google Scholar]
- 44.Rossi J, Santamäki P, Airaksinen MS, Herzig KH. Parasympathetic innervation and function of endocrine pancreas requires the glial cell line–derived factor family receptor alpha2 (GFRalpha2). Diabetes. 2005;54(5):1324–1330. [DOI] [PubMed] [Google Scholar]