Abstract
Background
Recently, we demonstrated that transdermal alcohol monitors could be used in a contingency management procedure to reduce problematic drinking; the frequency of self-reported heavy/moderate drinking days decreased and days of no to low drinking increased. These effects persisted for three months after intervention. In the current report, we used the transdermal alcohol concentration (TAC) data collected prior to and during the contingency management procedure to provide a detailed characterization of objectively measured alcohol use.
Methods
Drinkers (n = 80) who frequently engaged in risky drinking behaviors were recruited and participated in three study phases: a 4-week Observation phase where participants drank as usual; a 12-week Contingency Management phase where participants received $50 each week when TAC did not exceed 0.03 g/dl; and a 3-month Follow-up phase where self-reported alcohol consumption was monitored. Transdermal monitors were worn during the first two phases, where each week they recived $105 for visiting the clinic and wearing the monitor. Outcomes focused on using TAC data to objectively characterize drinking and were used to classify drinking levels as either no, low, moderate, or heavy drinking as a function of weeks and day of week.
Results
Compared to the Observation phase, TAC data indicated that episodes of heavy drinking days during the Contingency Management phase were reduced and episodes of no drinking and low to moderate drinking increased.
Conclusions
These results lend further support for linking transdermal alcohol monitoring with contingency management interventions. Collectively, studies to date indicate that interventions like these may be useful for both abstinence and moderation-based programs.
Keywords: Alcohol, Transdermal Alcohol Monitoring, Harm Reduction, Alcohol Detection
1. INTRODUCTION
Recent advances in technology have improved the ability to objectively monitor alcohol use. Transdermal alcohol monitoring devices such as the Secure Continuous Remote Alcohol Monitor [SCRAM-II™, Alcohol Monitoring Systems Inc. (AMS), Highlands Ranch, CO] provide a real-time measure (every 30 minutes, 24 hours a day) of alcohol excreted through the skin (Swift, 2000; 2003). This transdermal alcohol concentration (TAC) data can then be used to estimate both breath alcohol levels and quantity of alcohol consumed as well as identify patterns of alcohol consumption (Dougherty et al., 2012; Hill-Kapturczak et al., 2014).
Recent research has sought to test the feasibility and effectiveness of using transdermal alcohol monitors in contingency management interventions to reduce or eliminate alcohol consumption (Barnett et al., 2011; Dougherty et al., 2014a). Contingency management interventions are based on operant conditioning principles whereby individuals receive reinforcers (e.g., money or vouchers) when treatment goals such as abstinence or moderation of substance use are achieved (Higgins et al., 1994; Stitzer et al., 1980; Stitzer and Petry, 2006). This approach has been shown to be an effective intervention for a variety of substances (reviewed in Prendergast et al., 2006; Roll et al., 2006). Because contingency management requires the use of real and important consequences to reinforce abstinence or reduced drug use, it requires an objective measure of drug use that will be unbiased by self-report and identify levels of drinking. With other drugs of abuse (e.g., cocaine, opaites, marijuana), contingency management interventions have been possible through the use of urine-drug screen testing (Markway and Baker, 2011). In contrast to other drugs of abuse, biological markers for identifying alcohol use are not as straightforward for the use of financial contingencies.
Current alcohol use detection methods are typically a combination of self-report and alcohol use biomarkers. Self-reported alcohol use often underestimates drinking in real-world settings due to memory impairment, poor insight, or an unwillingness to self-report (de Visser and Birch, 2012; Devos-Comby and Lange, 2008; Kerr and Stockwell, 2012; May and Gossage, 2011; Sobell and Sobell, 2003; White et al., 2003). In the context of contingency management, financial motivation would be expected to make self-report especially vulnerable to inaccurate under-reporting of drinking. Clinical interventions often rely on self-report measures, but there is a definite move towards and increased reliance upon other alcohol use biomarkers (e.g., Anton et al., 2002; Kranzler et al., 2004; Mann et al., 2013; Pettinati et al., 2010) to objectively detect alcohol use.
However, biomarkers can be unreliable indicators of alcohol use. For example, non-specific alcohol use markers [e.g., γ-glutamyltransferase (GGT) or carbohydrate deficient transferrin (CDT)] measured in blood have long half-lives (weeks to months), but they are only reliable at detecting heavy drinking and may provide false positive results (reviewed in Javors and Johnson, 2003; Maenhout et al., 2013; Muñiz-Hernández et al., 2014)]. For example, increased serum GGT or CDT levels can occur in conditions not related to alcohol use. In the case of GGT, these conditions include obesity, diabetes, hypertension, hypertriglyceridemia, and non-alcoholic liver disease (reviewed in Muñiz-Hernández et al., 2014). In the case of CDT alternative causes for increases include phosphomannose isomerase deficiency, genetic transferrin variants, untreated galactosemia, hereditary fructose intolerance, and pregnancy (reviewed in Helander et al., 2014). In contrast, direct markers (e.g., ethanol or the ethanol metabolites ethyl-glucuronide and phosphatidylethanol) while specific for alcohol, have shorter half-lives (hours to weeks) which limit the window of detection (reviewed in Javors and Johnson, 2003; Maenhout et al., 2013). However, transdermal alcohol monitoring provides a continuous, objective measure of alcohol use which has the ability to detect different levels of drinking (i.e., no, low, moderate, and heavy; Dougherty et al., 2014a). As such, transdermal alcohol monitoring offers a new level of analysis for interventional studies and may even be used in the intervention itself. With the development of minimally invasive transdermal alcohol monitoring procedures, contingency management has only recently been validated as a possible intervention to moderate or control alcohol consumption.
There have been two preliminary studies that have used transdermal alcohol monitoring to implement contingency management designed to reduce alcohol use (Barnett et al., 2011; Dougherty et al., 2014a). The first study, conducted by Barnett and colleagues (2011), used transdermal alcohol monitors in a 2-week contingency management intervention to achieve abstinence. They demonstrated that financial contingencies did reduce the frequency of heavy drinking among a small sample of heavy drinkers (n = 13) across a 2-week period. However, these reductions were achieved primarily through increased abstinence and there was no evidence for reduced levels of drinking when drinking occurred. In the second study, we (Dougherty et al., 2014a) used transdermal alcohol monitors in an 8-week contingency management intervention designed to moderate alcohol consumption among a group of non-treatment seeking drinkers (n = 26) who frequently engaged in at-risk drinking (as defined by NIAAA, 2010). Instead of using an abstinence criteria, our study used transdermal alcohol monitoring to reinforce lower-level or less harmful patterns of drinking. More specifically, the contingency intervention provided monetary incentives each week when transdermal alcohol concentrations did not exceed 0.03 g/dl (i.e., approximately one or two standard drinks) on any day of the week. Not only did participants decrease their frequency of any drinking, but the amount of alcohol consumed per drinking episode also decreased – most importantly, this included reductions in heavy weekend binge drinking. During the course of this latter study, it became apparent that transdermal alcohol monitors can be used not only to dichotomously define whether or not any drinking occurred, but also to characterize levels of consumption defined as no, low, moderate, or heavy drinking. Together, these two preliminary studies indicate that transdermal alcohol monitors can be used to contingently reduce drinking, but also to moderate the amount of alcohol use.
More recently, we have shown in a 12-week contingency management study that self-reported problematic levels of drinking could be reduced to safer levels among a large group (n = 80) of at-risk heavy drinkers (Dougherty et al., 2014b). That publication focused on the self-reported drinking observed using standard calendar-based methods of Timeline Followback interview (Sobell and Sobell, 1992). However, the contingency was actually implemented using TAC readings to reinforce non-heavy drinking patterns; it is important to understand exactly what the strictly objective observations of TAC data will tell us about drinking behavior. The present report now presents the completed analyses of the TAC outcome data collected during the study. Specifically, we use the TAC data collected during transdermal alcohol monitoring to objectively characterize the patterns of alcohol use (no, low, moderate, and heavy drinking days) during the 4-week observation phase that preceded contingency management and then throughout the 12-week contingency management phase.
2. METHODS
2.1 Participants and criteria
Eighty-two adults were recruited from the community using newspaper, radio, and television advertisements. Potentially eligible participants were identified using a brief phone interview and came into the clinic for a more in-depth screening. This screening procedure included a substance use history, psychiatric assessment (Structured Clinical Interview for DSM-IV-TR Axis I Disorders; First et al., 2001), intelligence testing (Wechsler Abbreviated Scale of Intelligence; Wechsler, 1999), urine-drug and pregnancy tests, and a physical examination. Eligible participants included those reporting patterns of drinking that met or exceed the NIAAA (2010) “at-risk” drinking criteria: > 3 drinks for women or > 4 drinks for men at least 3 times in the prior 28 days (using a Timeline Followback interview, see 2.3.2). Participants were deemed ineligible for the following reasons, including: IQ < 70, current Axis I disorder, history of substance dependence, a positive urinalysis for drugs of abuse (cocaine, THC, opiates, barbiturates, benzodiazepines, and methamphetamine), pregnancy, or medical condition that would be contraindicated for alcohol consumption. All participants gave written consent and the protocol was approved by the Institutional Review Board at The University of Texas Health Science Center at San Antonio. Of the 82 participants who entered the study, 2 participants were not included in the final analyses because one dropped out prior to the Contingency Management phase and one completed only one week of the Contingency Management phase (see Section 2.2 for description of the study phases).
2.2 Procedure
There were three study phases. The first phase was an Observation phase where participants wore a transdermal alcohol ankle monitor (see 2.3.1) for 4 weeks and were told to drink as usual. Participants were instructed that they could not remove the monitor or immerse the device in water (e.g., no swimming or taking a bath). At each weekly visit, any issues with the device (such as comfort of the device tightness and placement) were discussed and resolved. Participants came to the clinic weekly for approximately 30 minutes to download transdermal ankle monitor data (see 2.3.1) and conduct a 7-day Timeline Followback (TLFB) interview. At each weekly clinic visit, participants were paid $105 per visit ($25 for the clinic visit and $80 for wearing the ankle monitor).
After completing the Observation phase, participants entered the Contingency Management phase where they continued to wear the transdermal monitor for an additional 12 weeks. They were paid an additional weekly bonus of $50 for each week that they met TAC contingency criteria of never having 3 readings above a 0.03 g/dl on any day of the week using only drinking events confirmed by AMS (AMS, the manufacturer of SCRAM, reviewed all uploaded TAC data and, using proprietary methods, confirmed whether or not a drinking event occurred). This was exactly the same criterion we employed in our pilot study (Dougherty et al., 2014a). Participants were told that a single drink (e.g., 1 beer), for most people, would not exceed this criterion. Participants came to the clinic once per week for approximately 30 minutes to download transdermal ankle monitor data and conduct a 7-day TLFB interview. As in the Observation phase, all participants were paid $105 per visit. The additional weekly $50 bonuses were paid (or not) solely based on transdermal alcohol monitoring data. At the completion of the Contingency Management phase, the transdermal alcohol monitors were removed.
The final phase was a 3-month Follow-up phase, where participants returned to the clinic monthly and a 28-day TLFB interview was completed. No drinking restrictions were in place during this phase. Participants were paid $85 for each monthly visit.
2.3 Alcohol Use Measurements
2.3.1 Transdermal alcohol monitoring
The transdermal alcohol monitoring devices used in this study were SCRAM-II™ monitors. Each participant was fitted with a device on their ankle and continued to wear it for the duration of their participation in the Observation and Contingency management phases. SCRAM-II measured transdermal alcohol concentration (TAC) continuously every 30 minutes 24 hours/day, 7 days/week until the devices were removed. Infrared signals and body temperature were also recorded at each reading to verify that no tampering or device disruption occurred. Data (TAC, infrared signals, and body temperature) from the monitors were downloaded at each weekly visit using Direct Connect™ (Alcohol Monitoring Systems Inc.).
2.3.2 Timeline Followback interview (TLFB; Sobell and Sobell, 1992)
The TLFB procedure was used to characterize self-reported drinking at study entry and at subsequent study visits. Information was gathered regarding the number, type, size of drinks, and duration of each drinking event. This information was used to calculate the number of standard units of alcohol consumed (NIAAA, 2010)
2.4 Data Analysis
Descriptive statistics were used to summarize participant characteristics and sex differences were examined using t-tests or chi-squared tests for continuous and categorical variables, respectively.
The final analyses included 80 participants who completed two or more weeks of the Contingency phase. Fourteen participants withdrew at various times during the Contingency Management phase for reasons unrelated to the study (for a complete description of why and when participants withdrew, see Figure 1 in Dougherty et al., 2014b). We compared the dropouts (n = 14) and completers (n = 66) on demographics (sex, age, BMI, ethnicity, and race), average drinks per drinking day, at-risk drinking days (men consuming > 4 and women > 3 alcoholic beverages), and total number of binges (the number of occurrences of > 5 drinks for men and > 4 drinks for women in 2 hours) in the 28 days prior to entering the Observation phase and found no significant differences between the two groups on any of these variables (see Table 2 in Dougherty et al., 2014b). In addition, for each outcome of interest, we conducted a sensitivity analysis by imputing missing outcome values using multiple imputation with chained questions (MICE) approach. To impute missing values, we adjusted for time of measurement (week/phase), gender, age, race, ethnicity, marital status and total drinking days, and created 10 imputed data sets. The results after imputation were similar to the results without imputation (i.e., using all available data from the Proc Mixed without imputation). Therefore, the final analyses were conducted using all available data from the 80 participants who completed the 4 weeks of Observation and at least 2 weeks of the Contingency Management phase without imputation. Of these 80 participants, 4 had a single week of missing data occurring randomly throughout the phase due to monitor failure, which is less than 1% of the total possible data.
The percent of participants who met the contingency criterion (i.e., TAC < 0.03 g/dl each day throughout each week) during the 4-week Observation phase (where no contingency was in place) and the 12-week Contingency management phase (where participants were paid the $50 bonus each week if they met the contingency criteria) was determined. A repeated measures logistic regression was used to examine the effects of phase and week nested within phase on the odds of meeting the contingency criteria throughout the whole week; a generalized estimating equations approach was employed using an unstructured correlation matrix. Linear trends across weeks within each phase were examined by treating week as a continuous variable in a repeated measures logistic regression model that included phase, week, and the interaction between phase and week as the explanatory variables.
For each day, four levels of drinking were classified as reported previously (Dougherty et al., 2014a): a) no drinking (TAC = 0 all day long); b) low drinking (pkTAC > 0 and < 0.03g/dl); c) moderate drinking (pkTAC ≥ 0.03 and eBrAC < 0.08%); and d) heavy drinking (estimated BrAC ≥ 0.08%). Because NIAAA definitions of heavy drinking are related specifically to breath alcohol (i.e., drinking 5 drinks for men or 4 for women within 2 hrs would be expected to produce intoxicating levels of 0.08% of breath alcohol), we used TAC data [peak TAC (pkTAC) and time-to-peak TAC] to estimate peak BrAC (eBrAC) using an equation (eBrAC = 0.02158 + 0.3940 *pkTAC + 0.000149 * time-to-peak TAC - 0.00366 * sex – 0.1887 * pkTAC * sex) previously reported and validated (Hill-Kapturczak et al., 2014). For each participant, daily drinking level results were consolidated across each week to compute the percentage of days of drinking at each of the four drinking levels. For each drinking level of interest, a mixed effects model with an unstructured covariance matrix was used to examine the effects of phase and week nested within phase. Linear trends across weeks within each phase were examined by treating week as a continuous variable in a mixed effects model that included phase, week, and the interaction between phase and week as the explanatory variables.
To examine the effect of weekday, each participant’s percentage of days with any drinking (TAC > 0) and percentage of days with heavy drinking (eBrAC ≥ 0.08%) were computed for each weekday. A mixed effects model with an unstructured covariance matrix was used to examine the effects of phase, weekday, and the interaction between phase and weekday as the explanatory variables. All statistical tests were conducted with 2-sided significance levels of 0.05 using SAS (Version 9.3, SAS Institute, Inc., Cary, NC).
3. RESULTS
3.1 Participant characteristics
As previously reported (Dougherty et al., 2014b), men (n = 50) and women (n = 30) did not differ in age (29.96 ± 8.57 and 30.60 ± 8.70, respectively), body mass index (29.96 ± 3.23 and 26.51 ± 4.47, respectively), drinks per drinking day (7.98 ± 2.98 and 6.49 ± 2.76, respectively) or their at-risk drinking days (men consuming > 4 and women > 3 alcoholic drinks; 10.30 ± 4.70 and 10.17 ± 5.63, respectively). Participants were largely Hispanic or Latino (men = 52%, women = 80%).
3.2 Percent of participants maintaining TAC < 0.03 g/dl per week
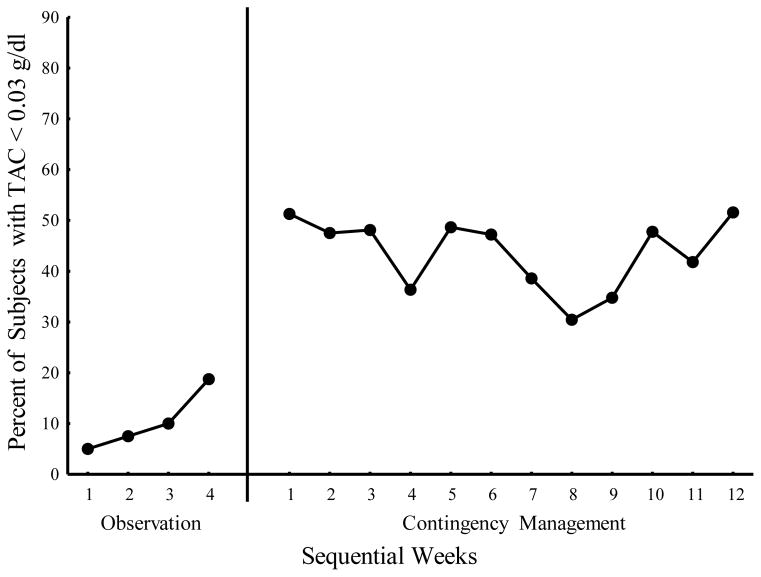
Figure 1 shows the percentage of participants who kept their TAC levels less than the 0.03 g/dl for the entire week. Contingency management significantly (χ2(1) = 40.89, p < 0.001) increased the proportion of subjects (average weekly percentage = 44.2%) meeting the TAC< 0.03 g/dl criteria compared to the observation period (average = 9.6%) before the contingency was implemented. On average, the odds of maintaining TAC below 0.03 g/dl for the entire week was more than 7-fold greater in the Contingency Management phase than the Observation phase (OR = 7.44, 95% CI = 4.39 – 12.60). There was also a significant effect of week within phase (χ2(14) = 29.24, p = 0 .01) and those weekly trends differed by phase (p = 0.014). That is, the odds of keeping TAC levels below 0.03 g/dl did not change over the 12 weeks of contingency management (p = 0.71) but showed an increasing trend over the 4 weeks of observation (p < 0.001) reaching a maximum of 18.8% by week 4.
Fig. 1.
Percent of participants meeting weekly contingency criteria (TAC < .03 g/dl) for both the Observation and Contingency Management phases.
3.3 Characterization of drinking levels (none, low, moderate, and heavy)
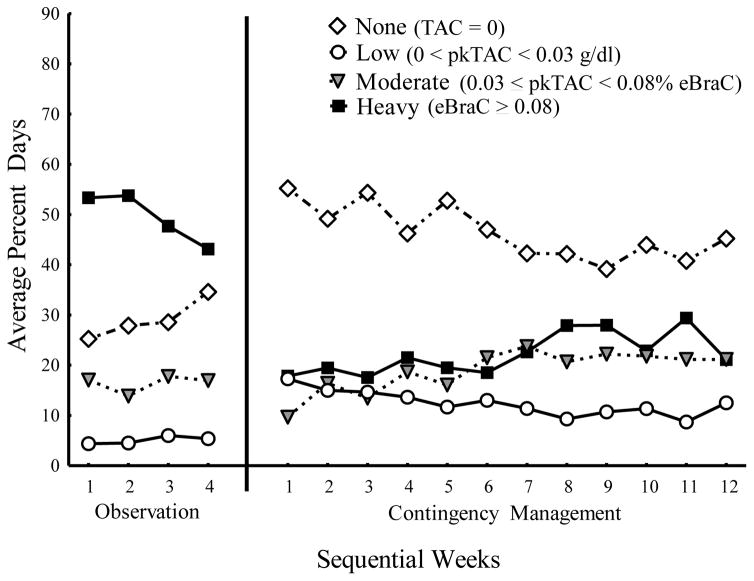
To evaluate our objective aimed at reducing heavy drinking without requiring abstinence, we examined the four possible classifications of daily drinking pattern (no, low, moderate, and heavy drinking) as described in the methods. Figure 2 shows the average percent of days with none, low, moderate, and heavy levels of drinking. The frequency of occurrence for each classification was analyzed separately.
Fig. 2.
Average percent of days per week with No Drinking (white diamonds), Low Drinking (white circles), Moderate Drinking (grey triangles), and Heavy Drinking (black squares) for the Observation and Contingency Management phases.
3.3.1 No drinking
The frequency of no drinking showed significant main effects of phase (F(1, 1089) = 123.86, p < 0.001) and week within phase (F(14, 1089) = 3.39, p < 0.001), and the weekly trends within phase were significantly different between the Observation and Contingency Management phases (F(1, 1102) = 10.93, p = 0.001). During the 4 weeks of the Observation phase, there was a significant increasing trend (slope = 2.82 per week, p = 0.02) of no drinking, and during the 12 weeks of Contingency Management there was a significant decreasing trend (slope = −1.20 per week, p < 0.001). Nonetheless, the average percent of drinking days categorized as none was significantly higher in the Contingency Management phase (M = 46.79%, SD = 31.75%) compared to the Observation phase (M = 29.04%, SD = 24.46%) indicating that participants were less likely overall to drink during the Contingency Management phase compared to the Observation phase.
3.3.2 Low-level drinking
The frequency of low drinking showed significant main effects of phase (F(1, 1089) = 80.56, p < 0.001) and week within phase (F(14, 1089) = 2.35, p = 0.003); however, the weekly trends within phase were not significantly different between the Observation and Contingency Management phases (p = 0.11). During the 4 weeks of the Observation phase, there was an increasing trend (slope = 0.45 per week, p = 0.47) in low-level drinking, and during the 12 weeks of Contingency Management there was a significant decreasing trend (slope = −0.57 per week, p < 0.001). Nonetheless, the average percent of drinking days categorized as low in the Contingency Management phase (M = 12.56%, SD = 14.57%) was greater than in the Observation phase (M = 5.06%, SD = 9.16%). That is, participants were more likely to engage in low-level drinking during the Contingency Management phase than the Observation phase.
3.3.3 Moderate-level drinking
The frequency of moderate drinking showed significant main effects of phase (F(1, 1089) = 5.63, p = 0.02) and week within phase (F(14, 1089) = 3.98, p < 0.0001); however, the weekly trends within phase were not significantly different between the Observation and Contingency Management phases (p = 0.48). During the 4 weeks of the Observation phase, there was an increasing trend (slope = 0.36 per week, p = 0.67) in moderate drinking, and during the 12 weeks of Contingency Management there was a significant increasing trend (slope = 0.96 per week, p < 0.001). Furthermore, the average percent of drinking days categorized as moderate was greater in the Contingency Management phase (M = 18.65%, SD = 19.54%) compared to the Observation phase (M = 16.40%, SD = 17.26%). Overall, participants drank moderately more often in the Contingency Management phase than in the Observation phase.
3.3.4 Heavy-level drinking
The frequency of heavy drinking showed significant main effects of phase (F(1, 1089) = 352.45, p < 0.001) and week within phase (F(14, 1089) = 2.80, p < 0.001), and the weekly trends within phase were significantly different between the Observation and Contingency Management phases (F(1, 1101) = 15.42, p < 0.001). During the 4 weeks of the Observation phase, there was a significant decreasing trend (slope = −3.63 per week, p = 0.001) in heavy drinking, and during the 12 weeks of Contingency Management there was a significant increasing trend (slope = 0.80 per week, p < 0.001). In addition, the average percent of drinking days categorized as heavy was less during the Contingency Management phase (M = 22.01%, SD = 26.72%) compared to the Observation phase (M = 49.49%, SD = 27.21%). That is, participants drank heavily more often in the Observation phase than in the Contingency Management phase.
3.4 Characterization of drinking as a function of day of week
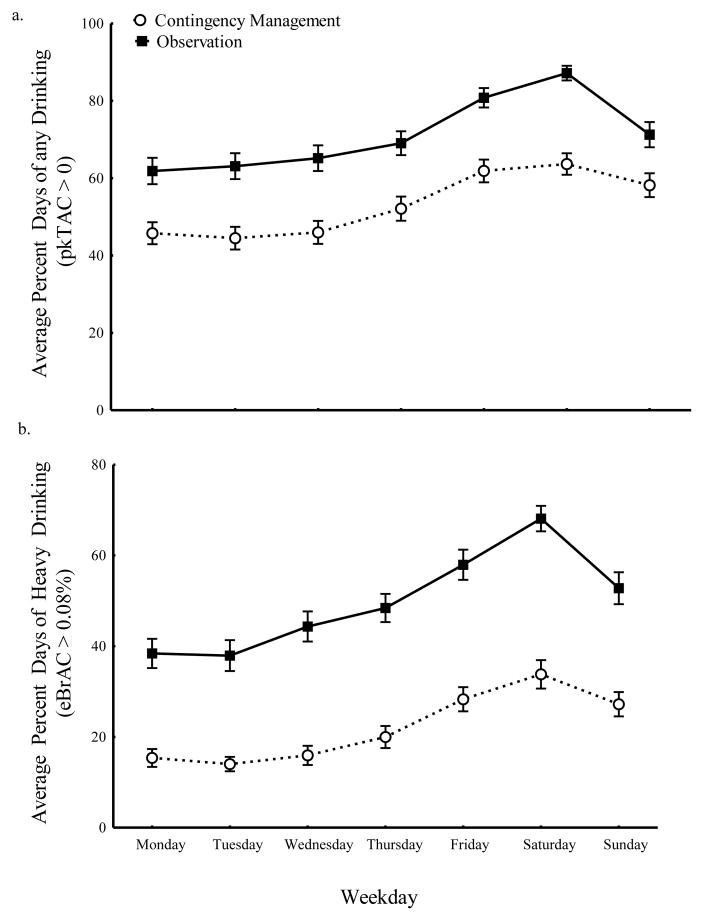
Because most heavy drinking occurs on weekends (e.g., Finlay et al., 2012; Haines et al., 2003; Kuntsche and Cooper, 2010), we also averaged, for each participant, the frequency of drinking at each classified level across weeks for each day of week. Figure 3 shows those results for “any drinking” (Figure 3a) and “heavy drinking” (Figure 3b). Any drinking revealed significant main effects of weekday (F(6, 1027) = 27.79, p < 0.001) and phase (F(1, 1027) = 210.46, p < 0.001), but the weekday by phase interaction was not significant (p = 0.45). Pairwise comparisons showed that any drinking was less frequent during the Contingency Management than during the Observation phase for each weekday (all p < 0.001); there was no evidence that any drinking on one weekday was less than another. Likewise, heavy drinking frequency (Figure 3b) showed significant main effects of weekday (F(6, 1027) = 32.94, p < 0.001) and phase (F(1, 1027) = 515.40, p < 0.001) and there was no weekday by phase interaction (p = 0.20). Pairwise comparison revealed that each weekday differed by phase with heavy drinking decreased during Contingency Management compared to the Observation phase (all p < 0.001).
Fig. 3.
Average percent days (by day of the week) that participants had (a) any drinking (pkTAC > 0) and (b) heavy drinking (eBrAC ≥ 0.08%) for both the Observation (black squares) and Contingency Management (white circles) phases.
3.5 Concordance of self-reported alcohol consumption with TAC contingency
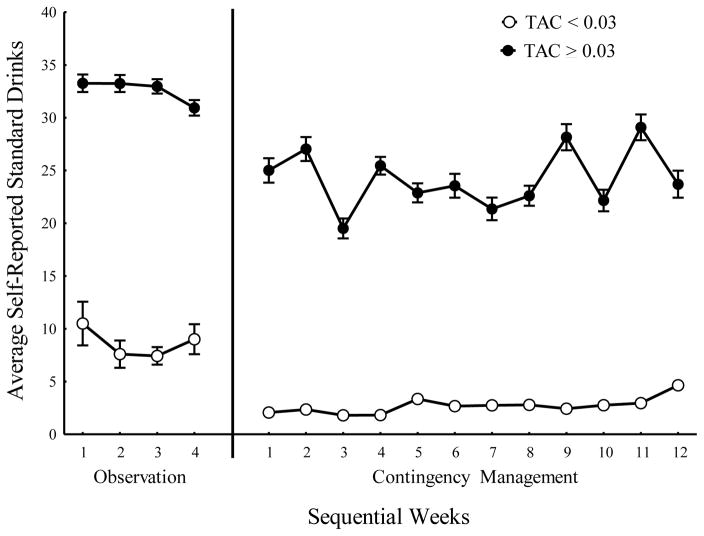
Overall, during the Follow-up phase, participants drank heavily on 22.19% of the days, moderately on 4.69% of the days, at low levels 5.13% of the days, and did not drink at all on 67.99% of the days. Because only self-reported data was collected during the Follow-up phase, a direct comparison between the self-reported data and TAC data is not possible. Figure 4 depicts the number of drinks per week self-reported by participants, for those weeks when they met the TAC contingency criteria and for those weeks when they failed to meet contingency criteria throughout the course of the study. Significant main effects of Phase (F(1,1086) = 86.50, p < 0.001) and meeting TAC contingency criteria (F(1, 1086) = 362.37, p < 0.001) were observed; and there was no significant effect of week within phase (F(14, 1086) = 1.2, p = 0.265). This clearly indicates that when participants failed to meet the contingency, they consistently also self-reported that indeed they had consumed significantly more standard drinks in that week (model based least squares mean = 27.86, SE = 1.09). Conversely, participants also reported drinking only a little (M = 8.62, SE = 1.32) during those weeks when they met criteria.
Fig. 4.
Average number of self-reported standard drinks when participants met (white circles) or failed (black circles) contingency criteria in both the Observation and Contingency Management phases.
4. DISCUSSION
The current report provides a detailed characterization of alcohol use observed by objectively-measured TAC data collected during a contingency management procedure which financially reinforced subjects for maintaining low TAC levels (< 0.03 g/dl) each day throughout each week over a 12-week contingency period. We found that compared to baseline, the contingency increased the likelihood that participants were able to keep their TAC levels below 0.03 g/dl each day throughout each week over a 12-week Contingency Management phase. We previously showed that this contingency worked for 4 weeks in a pilot study (Dougherty et al., 2014a). The current report demonstrates that we can avoid relying exclusively upon self-report and use objectively-measured TAC data to monitor drinking levels. First, we estimated BrAC levels from TAC data using previously-published methods (Hill-Kapturczak et al., 2014), and found that the contingency reduced the frequency of heavy drinking days (i.e., estimated BrAC > 0.08%) compared to the Observation phase, which included decreases on the weekends (see Figure 3). Second, we used raw TAC data only to estimate the frequency of abstinence (i.e., TAC = 0) and days of lower level drinking defined as TAC > 0 but less than the estimated BrAC level of 0.08% (i.e., both low and moderate drinking). Specifically, we found that the contingency increased days of TAC = 0 (i.e., no drinking) as well as the frequency of low-level drinking (defined as TAC > 0 but less than the 0.03 contingency level) and moderate drinking (TAC > 0.03 but less than estimated BrAC < 0.08%). These data clearly demonstrate that modest financial contingencies can be used to reduce the frequency of harmful drinking and that alcohol monitoring devices can be used both to implement the contingency as well as to monitor results.
Given the known power of contingency management (Hartzler et al., 2012; Prendergast et al., 2006), it is not surprising that a financial contingency of keeping TAC levels below 0.03 g/dl each day increased the frequency of days of abstinence and produced a shift from higher TAC level-classified drinking patterns to lower TAC levels and classified drinking patterns. The validity of these TAC-based conclusions are consistent with the previous report of a shift from heavier to lower-level drinking patterns (Dougherty et al., 2014b). Further, the current study found substantially more standard drinks per week were self-reported on those weeks in which participants failed to meet the contingency criteria than on weeks when they met the contingency. Though we cannot assume complete independence of TAC and self-report observations, we made every effort to limit the bias on self reporting of alcohol use by delivering contingencies prior to obtaining self-reported alcohol use by TLFB. However, regardless of the relationship between self-report and contingent reward, the real importance of this study is the demonstration that the contingency successfully produced less frequent positive TAC readings and/or lower TAC levels, necessarily indicating that heavy patterns of drinking (including weekend heavy drinking) were reduced in this sample of non-treatment seeking heavy drinkers.
We did observe a trend towards reduced drinking across the 4 weeks of observation before the contingency was applied which might be explained by an anticipation of the upcoming contingency. We also found a slight increasing trend in drinking across the 12 weeks of contingency management; however, substantial reductions in overall drinking were consistently maintained throughout the contingency phase.
Our previous report of reduced self-reported heavy drinking during the same 12-week contingency also showed that reduced drinking was reported to persist throughout a 3-month follow-up period where no contingencies were placed on drinking (Dougherty et al., 2014b). Although others such as McDonell et al. (2012) have been unable to show persistent effects of contingency management after contingencies were withdrawn, several factors may contribute to the contradictory finding. McDonell et al’s (2012) study was conducted in a small sample of, alcohol-dependent participants (n = 10) who were reinforced (vouchers on an escalating scale) twice weekly for negative breath alcohol and ethyl glucuronide tests across a 4-week period. This lack of a persistent effect could therefore be due to the study population (e.g., alcohol dependent), the limitations of alcohol monitoring procedures used or the length of the contingency management period (e.g., 4 weeks). These findings and others suggest that patients with more problematic drinking (including those with a diagnosis of dependence) are more likely to have problematic drinking after initial remission (Trim et al., 2013; Tuithof et al., 2014). Furthermore, longer treatments appear to have better short- and long-term outcomes than shorter ones (e.g., Moos and Moos, 2003; Simpson et al., 1997).
The current study demonstrates that TAC-based contingencies can be used to reduce heavy drinking patterns while permitting lower-level drinking. Barnett and colleagues (2011) previously used a financial contingency to reduce drinking using the SCRAM transdermal alcohol monitor, but that study used standard AMS criteria only to determine if a drinking event occurred or not in order to reinforce putative abstinence defined as the absence of an AMS confirmed drinking event. AMS criteria, however, are used to define a drinking event in judicial settings to monitor court-ordered abstinence. As such the criteria is relativley conservative and only reliably detects drinking events when 5 or more drinks are consumed (i.e., there must be 3 consecutive TAC readings > 0.02 g/dl and there are specific values for how fast TAC levels rise and fall; Barnett et al., 2014). In contrast, using TAC levels to quantify no, low, moderate and high drinking we showed that low-level drinking, revealed by low-level TAC readings, was actually increased during the contingency. Thus the objective of our contingency management procedure should be considered a harm-reduction approach which seeks to reduce heavy or problematic drinking while permitting lower level drinking with the objective to reduce the negative alcohol-related consequences of heavy drinking (Marlatt and Witkiewitz, 2002). Although abstinence may be appropriate for some groups (i.e., adolescents, pregnant or lactating mothers, or those with extreme alcohol dependence), others may benefit from an approach focused more on harm reduction (Marlatt and Witkiewitz, 2002). Such approaches are becoming a more common treatment option and are often considered preferable to abstinence-based methods by both patients and clinicians (Adamson et al., 2010; Adamson and Sellman, 2001; Ambrogne, 2002; Gastfriend et al., 2007; Marlatt and Witkiewitz, 2002; Rosenberg and Davis, 2014; Skewes and Gonzalez, 2013). In particular, some individuals with high self-efficacy and low physical dependence wish to reduce the high risks associated with heavy drinking, but wish to continue to drink moderately and avoid treatment programs that require abstinence (Marlatt and Witkiewitz, 2002). Indeed, studies have shown that preference for controlled-drinking treatment goals over abstinence range between 25.95% to 86.9% of treatment seekers (e.g., DeMartini et al., 2014; Engasser et al., 2015; Heather et al., 2010).
There are several limitations to the current study. First, our participants were non-treatment seeking and not alcohol dependent and so it is unknown whether or not contingency management procedures would work as well in a treatment seeking population. It is also possible that these effects may not be as robust in an alcohol-dependent population. Further research in more severe, alcohol-dependent populations should be conducted to determine the feasibility of using transdermal alcohol monitors. However, contingency management is a powerful technique that has been shown to be effective in opiate- and stimulant-dependent (e.g., Aklin et al., 2014; Chutuape et al., 1999; Garcia-Rodriguez et al., 2009; Higgins et al., 1991; Hser et al., 2011; Rogers et al., 2008) populations; this indirectly suggests that it also should work for alcohol dependence. Another limitation of this study is that participants were not randomized to control and treatment groups and our only control comparison was to the prior baseline phase (i.e., Observation Phase) where no contingency was applied. Future studies should include a randomized control group to further examine the effectiveness of a contingency management intervention. Though we previously reported reduced drinking in the three months after the removal of contingency management (Dougherty et al., 2014a), the absence of transdermal alcohol monitoring procedures limits our ability to objectively confirm these self-reports. Future studies should use transdermal alcohol monitoring to assess drinking behavior after the removal of contingency management. Furthermore, the use of transdermal alcohol monitoring devices can be costly. Although the cost-effectiveness of contingency management using transdermal alcohol monitors has yet to be examined, prior research has demonstrated the cost-effectiveness of contingency management procedures for a variety of substance (e.g., Sindelar et al., 2007a, 2007b). Therefore, the clinical significance of reducing at-risk drinking may outweigh the cost for using transdermal alcohol monitors.
Despite the limitations associated with transdermal alcohol monitoring, several advantages also exist. Specifically, the transdermal alcohol monitors allow for uninterrupted objective monitoring of alcohol consumption; therefore, the likelihood of detecting consumption over other monitoring devices such as breathalyzers attached to cell phones or biomarkers increases. Data from the monitors can also be downloaded automatically, without a direct connection to the monitor, by placing a modem in the individual’s home. Together, these factors could mean less frequent clinic visits for patients while they are wearing a transdermal alcohol monitor, reducing clinic personnel costs and increasing convenience for patients.
The results of this study highlight the utility of transdermal alcohol monitoring in a contingency management procedure that can reduce the risk of harmful, heavy drinking behavior. Further, we suggest that objectively-derived TAC data can be used to classify drinking levels and to promote harm-reduction without necessarily requiring complete abstinence. Further research will be required to demonstrate the utility of this procedure either as a clinical intervention in treatment-seeking patients or as an objective outcome measure in patients in treatment interventional studies.
Highlights.
Financial reinforcement for reduced heavy drinking was successful for 12 weeks
Contingencies based on transdermal alcohol concentration increased the number of no drinking days in heavy drinkers
Heavy drinking was reduced in non-treatment-seeking heavy drinkers
Transdermal alcohol monitoring has utility in contingency management procedures
Acknowledgments
Role of Funding Source:
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health [R01AA14988 to Donald Dougherty]. The research was also supported in part by the National Institute of Drug Abuse [T32DA031115 to Charles France] for postdoctoral training for Dr. Karns and Dr. Lake. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Dougherty also gratefully acknowledges support from a research endowment, the William and Marguerite Wurzbach Distinguished Professorship.
The authors appreciate the supportive functions performed for by our valued colleagues: Sharon Cates, Cameron Hunt, Krystal Shilling, and Phillip Brink.
Footnotes
Contributors:
All authors significantly contributed to this manuscript and have read and approved the final manuscript.
Conflicts of Interest:
None of the authors have conflicting interests concerning this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson SJ, Heather N, Morton V, Raistrick D, Team UR. Initial preference for drinking goal in the treatment of alcohol problems: II. Treatment outcomes. Alcohol Alcohol. 2010;45:136–142. doi: 10.1093/alcalc/agq005. [DOI] [PubMed] [Google Scholar]
- Adamson SJ, Sellman JD. Drinking goal selection and treatment outcome in out-patients with mild-moderate alcohol dependence. Drug Alcohol Rev. 2001;20:351–359. [Google Scholar]
- Aklin WM, Wong CJ, Hampton J, Svikis DS, Stitzer M, Bigelow GE, Silverman K. A therapeutic workplace for the long-term treatment of drug addiction and unemployment: Eight-year outcomes of a social business intervention. J Subst Abuse Treat. 2014;47:329–338. doi: 10.1016/j.jsat.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogne JA. Reduced-risk drinking as a treatment goal: what clinicians need to know. J Subst Abuse Treat. 2002;22:45–53. doi: 10.1016/s0740-5472(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Anton RF, Lieber C, Tabakoff B, Group CDS. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res. 2002;26:1215–1222. doi: 10.1097/01.ALC.0000023986.42254.F5. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM. Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MA, Silverman K, Stitzer ML. Use of methadone take-home contingencies with persistent opiate and cocaine abusers. J Subst Abuse Treat. 1999;16:23–30. doi: 10.1016/s0740-5472(97)00318-8. [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Devine EG, DiClemente CC, Martin DJ, Ray LA, O’Malley SS. Predictors of Pretreatment Commitment to Abstinence: Results from the COMBINE Study. J Stud Alcohol Drugs. 2014;75:438. doi: 10.15288/jsad.2014.75.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser RO, Birch JD. My cup runneth over: young people’s lack of knowledge of low-risk drinking guidelines. Drug Alcohol Rev. 2012;31:206–212. doi: 10.1111/j.1465-3362.2011.00371.x. [DOI] [PubMed] [Google Scholar]
- Devos-Comby L, Lange JE. Standardized measures of alcohol-related problems: a review of their use among college students. Psychol Addict Behav. 2008;22:349–361. doi: 10.1037/0893-164X.22.3.349. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr RM, Hill-Kapturczak N. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp Clin Psychopharmacol. 2012;20:373–381. doi: 10.1037/a0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, Mullen J, Roache JD. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend. 2014a;142:301–306. doi: 10.1016/j.drugalcdep.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Hill-Kapturczak N, Liang Y, Karns T, Mullen J, Roache JD. Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcohol Clin Exp Res. 2014b doi: 10.1111/acer.12687. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engasser JL, Hermos JA, Rubin A, Lachowicz M, Rybin D, Brief DJ, Roy M, Helmuth E, Rosenbloom D, Keane TM. Drinking goal choice and outcomes in a web-based alcohol intervention: results from VetChange. Addict Behav. 2015;42:63–68. doi: 10.1016/j.addbeh.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay AK, Ram N, Maggs JL, Caldwell LL. Leisure activities, the social weekend, and alcohol use: evidence from a daily study of firsty-year college students. J Stud Alcohol Drugs. 2012;73:250–259. doi: 10.15288/jsad.2012.73.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Givvon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- Garcia-Rodriguez O, Secades-Villa R, Higgins ST, Fernandez-Hermida JR, Carballo JL, Errasti Perez JM, Al-halabi Diaz S. Effects of voucher-based intervention on abstinence and retention in an outpatient treatment for cocaine addiction: a randomized controlled trial. Exp Clin Psychopharmacol. 2009;17:131–138. doi: 10.1037/a0015963. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR, Garbutt JC, Pettinati HM, Forman RF. Reduction in heavy drinking as a treatment outcome in alcohol dependence. J Subst Abuse Treat. 2007;33:71–80. doi: 10.1016/j.jsat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Haines PS, Hama MY, Guilkey DK, Popkin BM. Weekend eating in the United States is linked with greater energy, fat, and alcohol intake. Obesity Res. 2003;11:945–949. doi: 10.1038/oby.2003.130. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Lash SJ, Roll JM. Contingency management in substance abuse treatment: a structured review of the evidence for its transportability. Drug Alcohol Depend. 2012;122:1–10. doi: 10.1016/j.drugalcdep.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Adamson SJ, Raistrick D, Slegg GP. Initial preference for drinking goal in the treatment of alcohol problems: I. Baseline differences between abstinence and non-abstinence groups. Alcohol Alcohol. 2010;45:128–135. doi: 10.1093/alcalc/agp096. [DOI] [PubMed] [Google Scholar]
- Helander A, Jaeken J, Matthijs G, Eggertsen G. Asymptomatic phosphomannose isomerase deficiency (MPI-CDG) initially mistaken for excessive alcohol consumption. Clinica Chimica Acta. 2014;431:15–18. doi: 10.1016/j.cca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend. 1994;34:87–97. doi: 10.1016/0376-8716(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang YY, Karns TE, Cates SE, Dougherty DM. Accounting for sex-related differences in the estimation of breath alcohol levels using transdermal alcohol monitoring. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3644-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Li J, Jiang H, Zhang R, Du J, Zhang C, Zhang B, Evans E, Wu F, Chang YJ, Peng C, Huang D, Stitzer ML, Roll J, Zhao M. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction. 2011;106:1801–1809. doi: 10.1111/j.1360-0443.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Johnson BA. Current status of carbohydrate deficient transferrin, total serum sialic acid, sialic acid index of apolipoprotein J and serum beta-hexosaminidase as markers for alcohol consumption. Addiction. 2003;98:45–50. doi: 10.1046/j.1359-6357.2003.00582.x. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug Alcohol Rev. 2012;31:200–205. doi: 10.1111/j.1465-3362.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L DrugAbuse Sciences Naltrexone Depot Study G. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Cooper ML. Drinking to have fun and to get drunk: motives as predictors of weekend drinking over and above usual drinking habits. Drug Alcohol Depend. 2010;110:259–262. doi: 10.1016/j.drugalcdep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322–329. doi: 10.1016/j.cca.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Mann K, Bladstrom A, Torup L, Gual A, van den Brink W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry. 2013;73:706–713. doi: 10.1016/j.biopsych.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Markway EC, Baker SN. A review of the methods, interpretation, and limitations of the urine drug screen. Orthopedics. 2011;34:877–881. doi: 10.3928/01477447-20110922-22. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27:867–886. doi: 10.1016/s0306-4603(02)00294-0. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Res Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Howell DN, McPherson S, Cameron JM, Srebnik D, Roll JM, Ries RK. Voucher-based reinforcement for alcohol abstinence using the ethyl-glucuronide alcohol biomarker. J App Beh Analysis. 2012;45:161–165. doi: 10.1901/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R, Moos B. Long-term influence of duration and intensity of treatment on previously untreated individuals with alcohol use disorders. Addiction. 2003;98:325–337. doi: 10.1046/j.1360-0443.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- Muñiz-Hernández S, Velázquez-Fernández JB, Díaz-Chávez J, López-Sánchez RC, Hernández JA, Rendón-Ramírez A. Alcoholism: common and oxidative damage biomarkers. J Clin Toxicol. 2014;S7:S7–006. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Rethinking Drinking. U.S. Department of Health and Human Services; Washington, DC: 2010. NIH Publication No. 13–3770. [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, Dackis CA, O’Brien CP. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167:668–675. doi: 10.1176/appi.ajp.2009.08060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rogers RE, Higgins ST, Silverman K, Thomas CS, Badger GJ, Bigelow G, Stitzer M. Abstinence-contingent reinforcement and engagement in non-drug-related activities among illicit drug abusers. Psychol Addict Behav. 2008;22:544–550. doi: 10.1037/0893-164X.22.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, Blaine J, MacDonald M, DiMaria J, Lucero L, Kellogg S. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163:1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Davis AK. Differences in the acceptability of non-abstinence goals by type of drug among American substance abuse clinicians. J Subst Abuse Treat. 2014;46:214–218. doi: 10.1016/j.jsat.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007a;102:309–316. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007b;102:1463–1471. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Psychol Addict Behav. 1997;11:294–307. [Google Scholar]
- Skewes MC, Gonzalez VM. Attitudes toward harm reduction and abstinence-only approaches to alcohol misuse among Alaskan college students. Int J Circumpolar Health. 2013 doi: 10.3402/ijch.v72i0.21143. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Wilson VB, editors. Assessing Alcohol Problems: A guide for Clinicians and Researchers. 2. NIH; Bethesda: 2003. pp. 77–99. [Google Scholar]
- Stitzer M, Bigelow GE, Liebson I. Reducing drug use among methadone maintenance clients: contingent reinforcement for morphine-free urines. Addict Behav. 1980;5:8. doi: 10.1016/0306-4603(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Swift R. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcohol Clin Exp Res. 2000;24:422–423. [PubMed] [Google Scholar]
- Swift R. Direct measurement of alcohol and its metabolites. Addiction. 2003;98:73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. Predictors of initial and sustained remission from alcohol use disorders: findings from the 30-year follow-up of the San Diego Prospective Study. Alcohol Clin Exp Res. 2013;37:1424–1431. doi: 10.1111/acer.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuithof M, Have MT, van den Brink W, Vollebergh W, de Graaf R. The relationship between excessive alcohol consumption and alcohol use disorders according to DSM-IV and DSM-5. Alcohol Clin Exp Res. 2014;38:249–256. doi: 10.1111/acer.12248. [DOI] [PubMed] [Google Scholar]
- Weschler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation Harcourt Brace and Company; New York, NY: 1999. [Google Scholar]
- White AM, Kraus CL, McCracken LA, Swartzwelder HS. Do college students drink more than they think? Use of a free-pour paradigm to determine how college students define standard drinks. Alcohol Clin Exp Res. 2003;27:1750–1756. doi: 10.1097/01.ALC.0000095866.17973.AF. [DOI] [PubMed] [Google Scholar]